Pre-Treatment HIV Drug Resistance and Genetic Diversity in Cameroon: Implications for First-Line Regimens
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design, Sites, and Population
2.2. Selection Criteria
2.3. Data Collection
2.4. Ethical Considerations
2.5. Genotypic Resistance Test
2.6. Sequence Analysis for HIVDR Profiling, Drug Efficacy, and Subtyping
2.7. Data Entry and Statistical Analysis
3. Results
3.1. General Characteristics
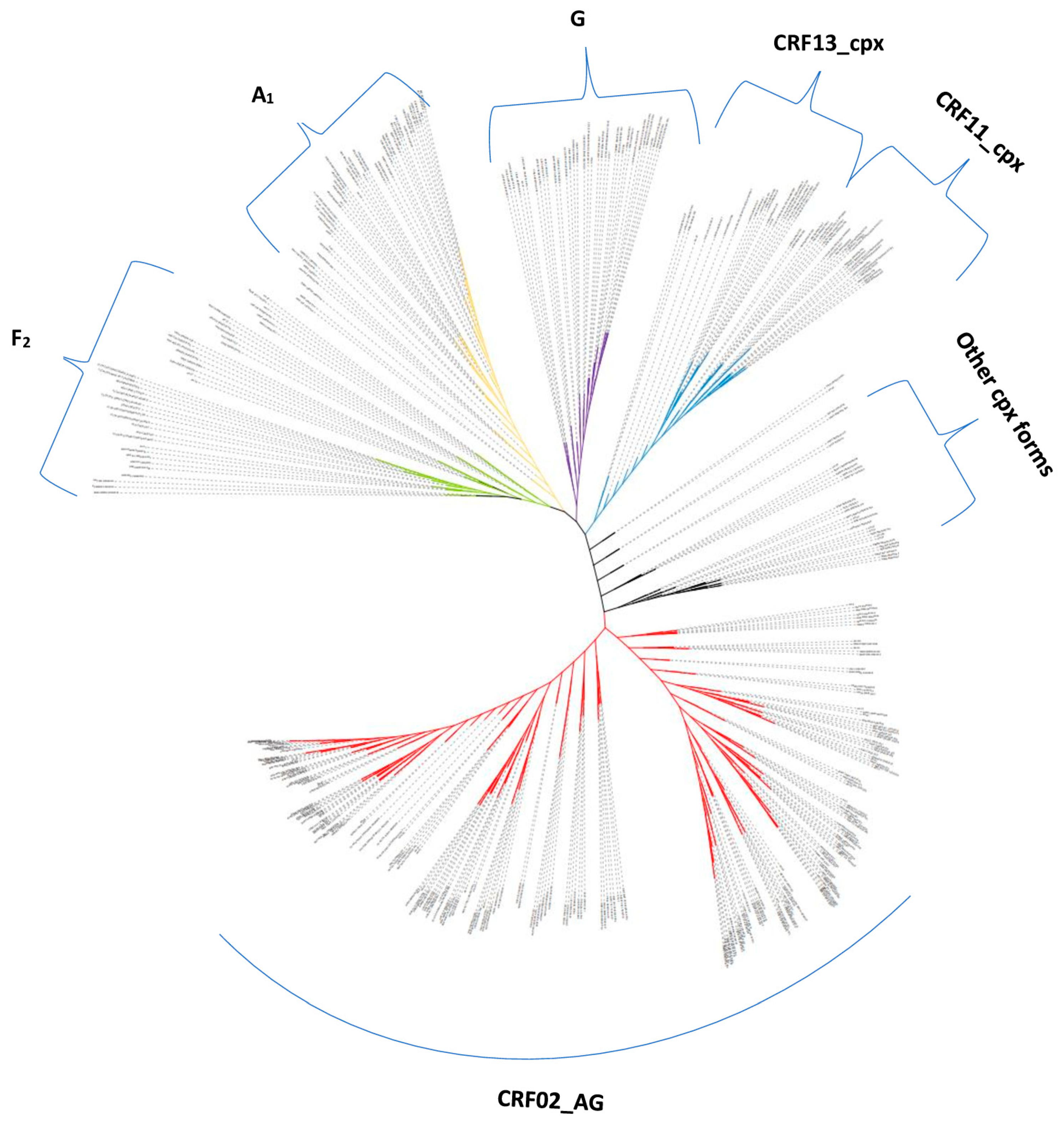
3.2. Genetic Diversity
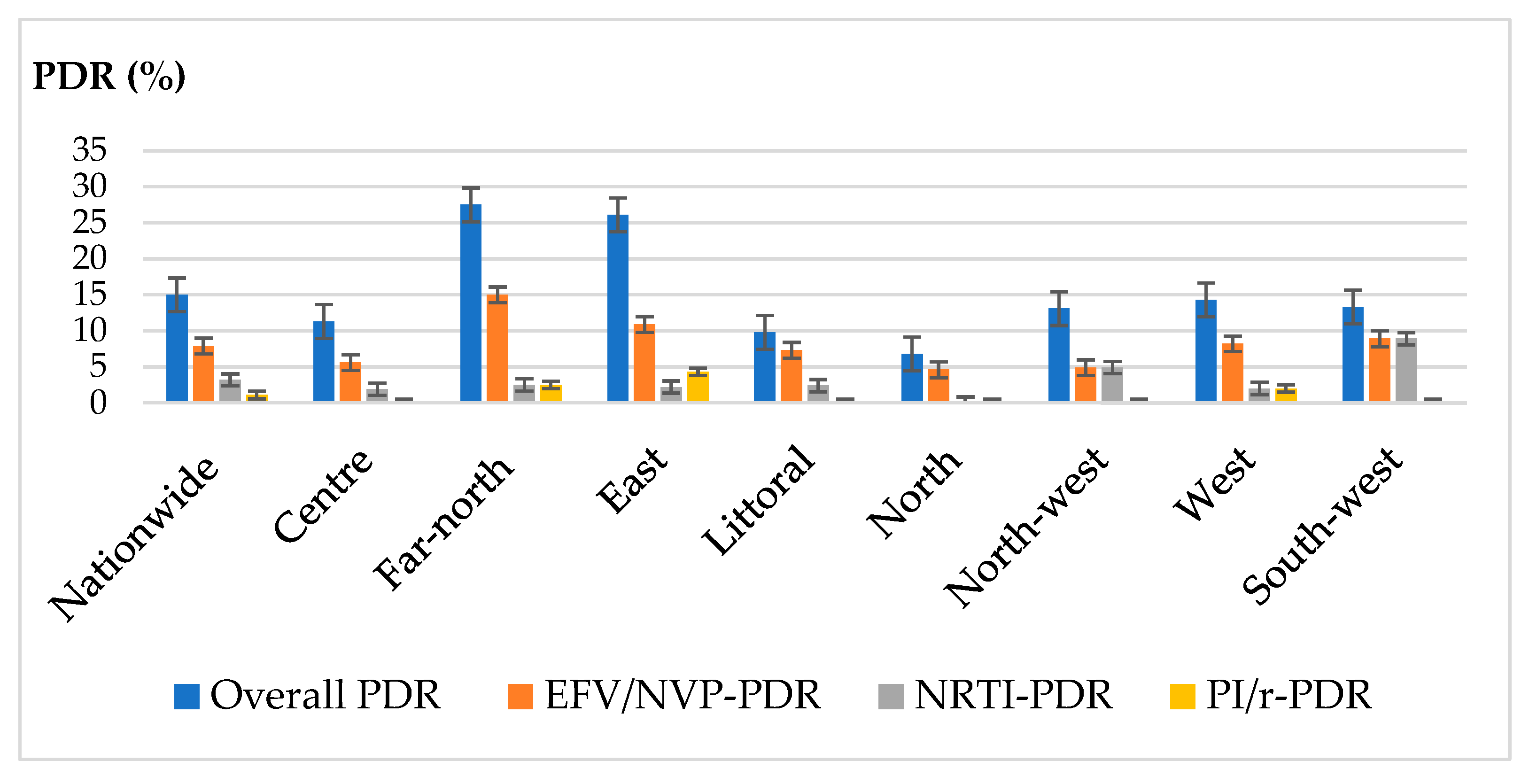
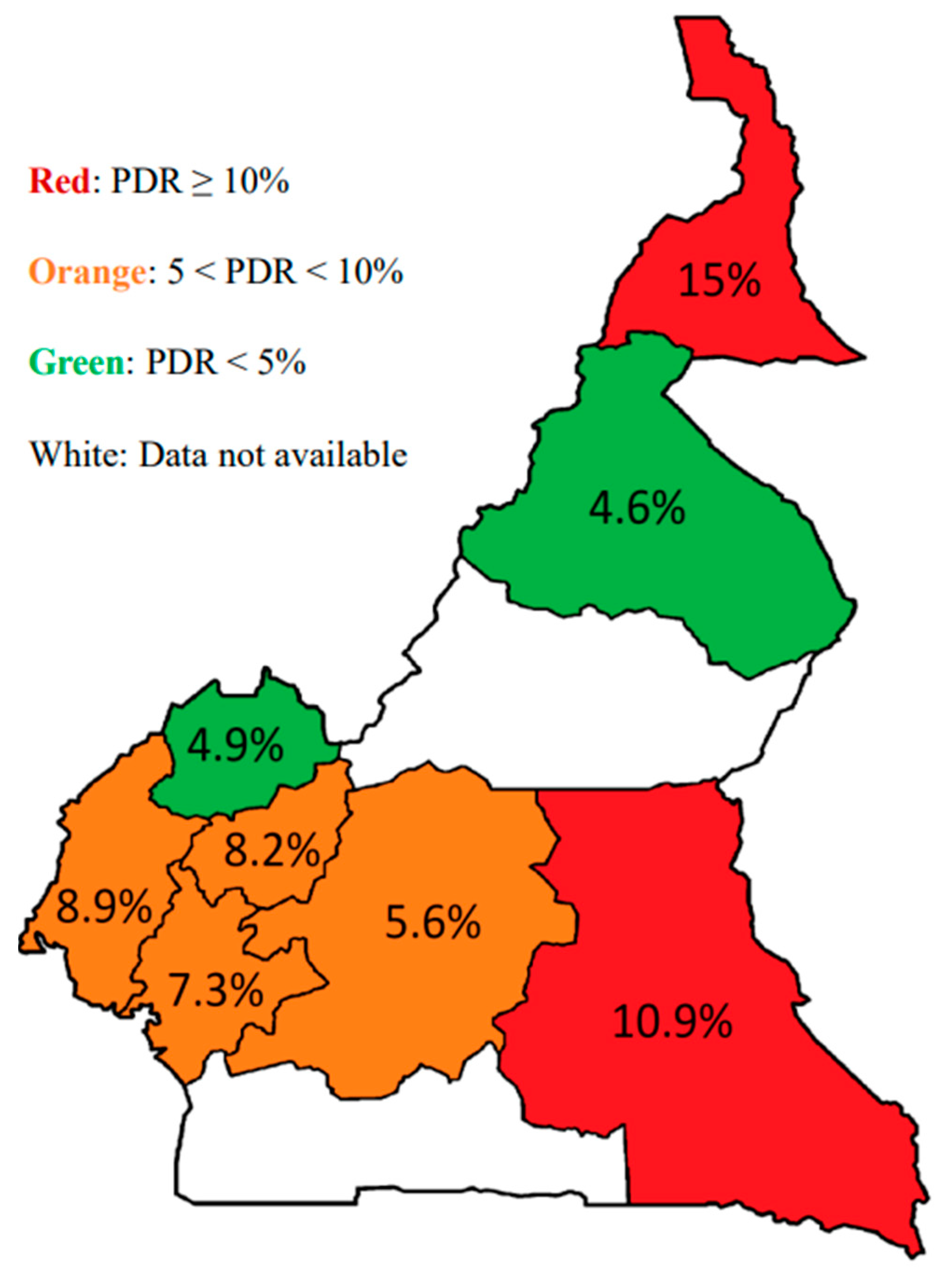
3.3. Genotypic Resistance Profile
3.4. Association between Pre-Treatment Drug Resistance, HIV-1 Genetic Diversity, Clinical and Demographics Parameters of the Study Participants
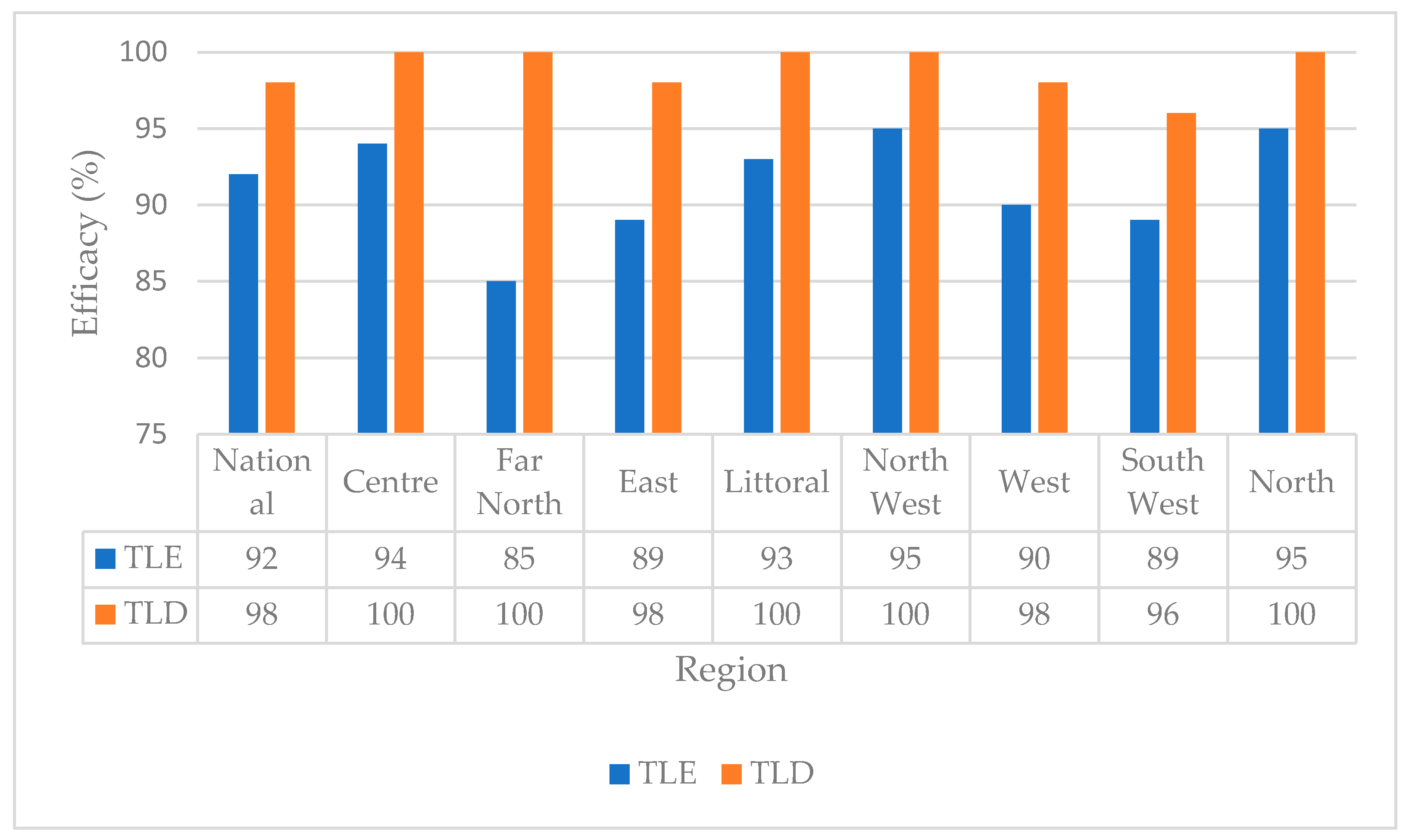
3.5. Predictive Efficacy of TLE versus TLD
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Joint United Nations Programme on HIV/AIDS. Global and Regional Statiatics—2018. 2019. Available online: https://www.unaids.org/sites/default/files/media_asset/UNAIDS_FactSheet_en.pdf (accessed on 2 February 2021).
- Joint United Nations Programme on HIV/AIDS. Countries Statistics—2018. 2019. Available online: https://www.unaids.org/fr/regionscountries/countries/cameroon (accessed on 2 February 2021).
- WHO. Consolidated Guidelines on the Use of Antiretroviral Drugs for Treating and Preventing HIV Infection—Recommendations for a Public Health Approach—Second Edition. 2016. Available online: http://apps.who.int/iris/bitstream/10665/208825/1/9789241549684_eng.pdf (accessed on 2 February 2021).
- CAMPHIA. CAMPHIA Results. 2018. Available online: https://phia.icap.columbia.edu/wp-content/uploads/2017/02/CAMPHIA-Summary-Sheet-EN_ARV-adjusted_Feb2020.pdf (accessed on 19 June 2023).
- WHO. Surveillance of HIV Drug Resistance in Populations Initiating Antiretroviral Therapy (Pre-Treatment HIV Drug Resistance). 2014. Available online: http://apps.who.int/iris/bitstream/10665/112802/1/9789241507196_eng.pdf (accessed on 2 February 2021).
- WHO. HIV Drug Resistance Report. 2017. Available online: http://apps.who.int/iris/bitstream/10665/255896/1/9789241512831-eng.pdf (accessed on 2 February 2021).
- Fact Sheet: HIV Drug Resistance. Available online: https://www.who.int/news-room/fact-sheets/detail/hiv-drug-resistance (accessed on 10 June 2021).
- HIV Drug Resistance Report. 2021. Available online: https://apps.who.int/iris/bitstream/handle/10665/349340/9789240038608-eng.pdf?sequence=1&isAllowed=y (accessed on 19 June 2023).
- Organisation mondiale de la Santé, O.M. Traitement du VIH; Transition Vers de Nouveaux Antiretroviraux dans les Programmes de Lutte Contre le VIH [Internet]. 2017. Available online: https://medicinespatentpool.org/uploads/2017/07/Policy-brief-Transition-to-new-antiretroviral-drugs-in-HIV-programmes-fr-web.pdf (accessed on 2 February 2021).
- WHO. HIV Drug Resistance Report. 2019. Available online: https://www.who.int/hiv/pub/drugresistance/hivdr-report-2019/en/ (accessed on 2 February 2021).
- Gupta, R.K.; Jordan, M.R.; Sultan, B.J.; Hill, A.; Davis, D.H.J.; Gregson, J.; Sawyer, A.W.; Hamers, R.L.; Ndembi, N.; Pillay, D.; et al. Global trends in antiretroviral resistance in treatment-naive individuals with HIV after rollout of antiretroviral treatment in resource-limited settings: A global collaborative study and meta-regression analysis. Lancet 2012, 380, 1250–1258. [Google Scholar] [CrossRef] [Green Version]
- Hamers, R.L.; Wallis, C.L.; Kityo, C.; Siwale, M.; Mandaliya, K.; Conradie, F.; Botes, M.E.; Wellington, M.; Osibogun, A.; Sigaloff, K.C.; et al. HIV-1 drug resistance in antiretroviral-naive individuals in sub-Saharan Africa after rollout of antiretroviral therapy: A multicentre observational study. Lancet Infect Dis. 2011, 11, 750–759. [Google Scholar] [CrossRef]
- WHO. Global Action Plan on HIV Drug Resistance 2017–2021, 2018 Progress Report. Available online: http://apps.who.int/iris/bitstream/handle/10665/255883/9789241512848-eng.pdf?sequence=1 (accessed on 19 June 2022).
- Tchouwa, G.F.; Eymard-Duvernay, S.; Cournil, A.; Lamare, N.; Serrano, L.; Butel, C.; Bertagnolio, S.; Mpoudi-Ngole, E.; Raizes, E.; Aghokeng, A.F.; et al. Prevalence of pretreatment HIV drug resistance in Cameroon following a nationally representative WHO survey. J. Antimicrob. Chemother. 2018, 73, 2468–2474. [Google Scholar] [CrossRef]
- WHO. Updated Recommendations on HIV Prevention, Infant Diagnosis, Antiretroviral Initiationo and Monitoring [Internet]. 2021. Available online: https://apps.who.int/iris/rest/bitstreams/1336192/retrieve (accessed on 19 June 2022).
- Fokam, J.; Salpini, R.; Santoro, M.M.; Cento, V.; D’Arrigo, R.; Gori, C.; Perno, C.F.; Colizzi, V.; Nanfack, A.; Gwom, L.C.; et al. Performance evaluation of an in-house human immunodeficiency virus type-1 protease-reverse transcriptase genotyping assay in Cameroon. Arch. Virol. 2011, 156, 1235–1243. [Google Scholar] [CrossRef] [Green Version]
- Semengue, E.; Armenia, D.; Inzaule, S.; Santoro, M.; Dambaya, B.; Takou, D.; Teto, G.; Nka, A.; Bouba, Y.; Fabeni, L.; et al. Baseline integrase drug resistance mutations and conserved regions across HIV-1 clades in Cameroon: Implications for transition to dolutegravir in resource-limited settings. J. Antimicrob. Chemother. 2021, 76, 1277–1285. [Google Scholar] [CrossRef]
- Taffa, N.; Roscoe, C.; Sawadogo, S.; De Klerk, M.; Baughman, A.L.; Wolkon, A.; Mutenda, N.; DeVos, J.; Zheng, D.P.; Wagar, N.; et al. Pretreatment HIV drug resistance among adults initiating ART in Namibia. J. Antimicrob. Chemother. 2018, 73, 3137–3142. [Google Scholar] [CrossRef]
- Yendewa, G.A.; Sahr, F.; Lakoh, S.; Ruiz, M.; Patiño, L.; Tabernilla, A.; Deen, G.F.; Sesay, M.; Salata, R.A.; Poveda, E. Prevalence of drug resistance mutations among ART-naive and -experienced HIV-infected patients in Sierra Leone. J. Antimicrob. Chemother. 2019, 74, 2024–2029. [Google Scholar] [CrossRef] [Green Version]
- Fokam, J.; Takou, D.; Teto, G.; Nforbih, S.E.; Kome, O.P.; Santoro, M.M.; Ngoufack, E.S.; Eyongetah, M.; Palmer, D.; Fokunang, E.T.; et al. Pre-treatment drug resistance and HIV-1 genetic diversity in the rural and urban settings of Northwest-Cameroon. PLoS ONE 2020, 15, e0235958. [Google Scholar] [CrossRef]
- Silverman, R.A.; Beck, I.A.; Kiptinness, C.; Levine, M.; Milne, R.; McGrath, C.J.; Bii, S.; Richardson, B.A.; John-Stewart, G.; Chohan, B.; et al. Prevalence of Pre-antiretroviral-Treatment Drug Resistance by Gender, Age, and Other Factors in HIV-Infected Individuals Initiating Therapy in Kenya, 2013-2014. J. Infect. Dis. 2017, 216, 1569–1578. [Google Scholar] [CrossRef] [Green Version]
- Little, S.J.; Frost, S.D.W.; Wong, J.K.; Smith, D.M.; Pond, S.L.K.; Ignacio, C.C.; Parkin, N.T.; Petropoulos, C.J.; Richman, D.D. Persistence of transmitted drug resistance among subjects with primary human immunodeficiency virus infection. J. Virol. 2008, 82, 5510–5518. [Google Scholar] [CrossRef] [Green Version]
- Ruelle, J.; Ingels, M.-G.; Jnaoui, K.; Ausselet, N.; Vincent, A.; Sasse, A.; Verhofstede, C.; Goubau, P. Transmission network of an HIV type 1 strain with K103N in young Belgian patients from different risk groups. AIDS Res. Hum. Retrovir. 2013, 29, 1306–1309. [Google Scholar] [CrossRef]
- Mazzuti, L.; Melengu, T.; Falasca, F.; Calabretto, M.; Cella, E.; Ciccozzi, M.; Mezzaroma, I.; Iaiani, G.; Spaziante, M.; d’Ettorre, G.; et al. Transmitted drug resistance mutations and trends of HIV-1 subtypes in treatment-naïve patients: A single-centre experience. J. Glob. Antimicrob. Resist. 2020, 20, 298–303. [Google Scholar] [CrossRef]
- Billong, S.C.; Fokam, J.; Aghokeng, A.F.; Milenge, P.; Kembou, E.; Abessouguie, I.; Meva’a-Onglene, F.B.; Bissek, A.C.Z.K.; Colizzi, V.; Mpoudi, E.N.; et al. Population-based monitoring of emerging HIV-1 drug resistance on antiretroviral therapy and associated factors in a sentinel site in Cameroon: Low levels of resistance but poor programmatic performance. PLoS ONE 2013, 8, e72680. [Google Scholar] [CrossRef]
- Lal, R.B.; Chakrabarti, S.; Yang, C. Impact of genetic diversity of HIV-1 on diagnosis, antiretroviral therapy & vaccine development. Indian J. Med. Res. 2005, 121, 287–314. [Google Scholar]
- Zash, R.; Makhema, J.; Shapiro, R.L. Neural-Tube Defects with Dolutegravir Treatment from the Time of Conception. N. Engl. J. Med. 2018, 379, 979–981. [Google Scholar] [CrossRef]
- Zash, R.; Holmes, L.; Diseko, M.; Jacobson, D.L.; Brummel, S.; Mayondi, G.; Isaacson, A.; Davey, S.; Mabuta, J.; Mmalane, M.; et al. Neural-Tube Defects and Antiretroviral Treatment Regimens in Botswana. N. Engl. J. Med. 2019, 381, 827–840. [Google Scholar] [CrossRef]
- NAMSAL ANRS 12313 Study Group; Kouanfack, C.; Mpoudi-Etame, M.; Bassega, P.O.; Eymard-Duvernay, S.; Leroy, S.; Boyer, S.; Peeters, M.; Calmy, A.; Delaporte, E. Dolutegravir-Based or Low-Dose Efavirenz–Based Regimen for the Treatment of HIV-1. N. Engl. J. Med. 2019, 381, 816–826. [Google Scholar] [CrossRef]
- Esber, A.L.; Chang, D.; Iroezindu, M.; Bahemana, E.; Kibuuka, H.; Owuoth, J.; Singoei, V.; Maswai, J.; Dear, N.F.; Crowell, T.A.; et al. Weight gain during the dolutegravir transition in the African Cohort Study. J. Int. AIDS Soc. 2022, 25, e25899. [Google Scholar] [CrossRef]
- Chouchana, L.; Pariente, A.; Pannier, E.; Tsatsaris, V.; Treluyer, J.-M. Dolutegravir and neural tube defects: A new insight. Lancet Infect. Dis. 2020, 20, 405–406. [Google Scholar] [CrossRef]
- Dolutegravir No Longer Linked to Higher Risk of Neural Tube Defects: Latest Update from the Tsepamo Study|HTB|HIV i-Base. Available online: https://i-base.info/htb/43791 (accessed on 19 June 2023).
| Region | Health Facility |
|---|---|
| Center | Yaoundé Central Hospital 1, Mbalmayo District Hospital 2 |
| Littoral | Bonassama Hospital 1, Edea District Hospital 2 |
| West | Bafoussam Regional Hospital 1, MIFI District Hospital 1, Foumban District Hospital 2, Bangou District Hospital 2 |
| Northwest | Bamenda Regional Hospital 1, Mbingo Baptist Hospital 2 |
| Southwest | Buea Regional Hospital 1, Mount Mary Hospital Buea 1, Buea Road Integrated Health Center 1, Ekona Medicalized Health Center 2, Muyuka District Hospital 2 |
| North | Garoua Regional Hospital 1, Guider District Hospital 2 |
| Far North | Maroua Regional Hospital 1, Kolofata District Hospital 2 |
| Variable | Subtype | Proportion with PDR (%) | OR | p Value |
|---|---|---|---|---|
| CRF02_AG vs. Non-CRF02_AG clades | CRF02-AG (N = 38) | 16.0 | 0.8 | 0.4 |
| Non CRF02-AG (N = 26) | 19.6 | |||
| Pure vs. recombinant clades | Pure Clades (N = 15) | 17.4 | 1 | 1.0 |
| Recombinant (N = 49) | 17.2 | |||
| Complex clades vs. other clades | Complex recombinants (N = 10) | 23.3 | 1.5 | 0.3 |
| Others (N = 54) | 16.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fokam, J.; Chenwi, C.A.; Tala, V.; Takou, D.; Santoro, M.M.; Teto, G.; Dambaya, B.; Anubodem, F.; Semengue, E.N.J.; Beloumou, G.; et al. Pre-Treatment HIV Drug Resistance and Genetic Diversity in Cameroon: Implications for First-Line Regimens. Viruses 2023, 15, 1458. https://doi.org/10.3390/v15071458
Fokam J, Chenwi CA, Tala V, Takou D, Santoro MM, Teto G, Dambaya B, Anubodem F, Semengue ENJ, Beloumou G, et al. Pre-Treatment HIV Drug Resistance and Genetic Diversity in Cameroon: Implications for First-Line Regimens. Viruses. 2023; 15(7):1458. https://doi.org/10.3390/v15071458
Chicago/Turabian StyleFokam, Joseph, Collins Ambe Chenwi, Valère Tala, Désiré Takou, Maria Mercedes Santoro, George Teto, Beatrice Dambaya, Felix Anubodem, Ezechiel Ngoufack Jagni Semengue, Grace Beloumou, and et al. 2023. "Pre-Treatment HIV Drug Resistance and Genetic Diversity in Cameroon: Implications for First-Line Regimens" Viruses 15, no. 7: 1458. https://doi.org/10.3390/v15071458





