Broad-Spectrum Antiviral Activity of Influenza A Defective Interfering Particles against Respiratory Syncytial, Yellow Fever, and Zika Virus Replication In Vitro
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cells and Viruses
2.2. Co-Infection Experiments
2.3. Virus Quantification
2.4. Gene Expression Analysis
2.5. Quantification of Intracellular IAV vRNAs
3. Results
3.1. IFN-Dependent Inhibition of YFV Propagation by IAV DIP Co-Infection
3.2. IAV DIP Infection Inhibits ZIKV Replication via JAK/STAT Signaling
3.3. IFN-Dependent Inhibition of RSV Replication by IAV DIPs Relies on JAK/STAT Signaling
4. Discussion
5. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lozano, R.; Naghavi, M.; Foreman, K.; Lim, S.; Shibuya, K.; Aboyans, V.; Abraham, J.; Adair, T.; Aggarwal, R.; Ahn, S.Y.; et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012, 380, 2095–2128. [Google Scholar] [CrossRef]
- Ackerson, B.; Tseng, H.F.; Sy, L.S.; Solano, Z.; Slezak, J.; Luo, Y.; Fischetti, C.A.; Shinde, V. Severe Morbidity and Mortality Associated with Respiratory Syncytial Virus Versus Influenza Infection in Hospitalized Older Adults. Clin. Infect. Dis. 2019, 69, 197–203. [Google Scholar] [CrossRef] [PubMed]
- Falsey, A.R.; Hennessey, P.A.; Formica, M.A.; Cox, C.; Walsh, E.E. Respiratory Syncytial Virus Infection in Elderly and High-Risk Adults. N. Engl. J. Med. 2005, 352, 1749–1759. [Google Scholar] [CrossRef] [PubMed]
- PATH. RSV Vaccine and mAB Snapshot. Available online: https://www.path.org/resources/rsv-vaccine-and-mab-snapshot/ (accessed on 10 July 2023).
- EMA. Available online: https://www.ema.europa.eu/en/medicines/human/summaries-opinion/abrysvo (accessed on 31 July 2023).
- Simões, E.A.F.; Bont, L.; Manzoni, P.; Fauroux, B.; Paes, B.; Figueras-Aloy, J.; Checchia, P.A.; Carbonell-Estrany, X. Past, Present and Future Approaches to the Prevention and Treatment of Respiratory Syncytial Virus Infection in Children. Infect. Dis. Ther. 2018, 7, 87–120. [Google Scholar] [CrossRef] [PubMed]
- Trang, T.P.; Whalen, M.; Hilts-Horeczko, A.; Doernberg, S.B.; Liu, C. Comparative effectiveness of aerosolized versus oral ribavirin for the treatment of respiratory syncytial virus infections: A single-center retrospective cohort study and review of the literature. Transpl. Infect. Dis. 2018, 20, e12844. [Google Scholar] [CrossRef]
- Devincenzo, J.P. Therapy of respiratory syncytial virus infection. Pediatr. Infect. Dis. J. 2000, 19, 786–790. [Google Scholar] [CrossRef]
- Guerguerian, A.-M.; Gauthier, M.; Lebel, M.H.; Farrell, C.A.; Lacroix, J. Ribavirin in Ventilated Respiratory Syncytial Virus Bronchiolitis. Am. J. Respir. Crit. Care Med. 1999, 160, 829–834. [Google Scholar] [CrossRef]
- Domachowske, J.B.; Anderson, E.J.; Goldstein, M. The Future of Respiratory Syncytial Virus Disease Prevention and Treatment. Infect. Dis. Ther. 2021, 10, 47–60. [Google Scholar] [CrossRef]
- Daep, C.A.; Muñoz-Jordán, J.L.; Eugenin, E.A. Flaviviruses, an expanding threat in public health: Focus on dengue, West Nile, and Japanese encephalitis virus. J. NeuroVirol. 2014, 20, 539–560. [Google Scholar] [CrossRef]
- Chong, H.Y.; Leow, C.Y.; Abdul Majeed, A.B.; Leow, C.H. Flavivirus infection—A review of immunopathogenesis, immunological response, and immunodiagnosis. Virus Res. 2019, 274, 197770. [Google Scholar] [CrossRef]
- Yuan, J.; Yu, J.; Huang, Y.; He, Z.; Luo, J.; Wu, Y.; Zheng, Y.; Wu, J.; Zhu, X.; Wang, H.; et al. Antibiotic fidaxomicin is an RdRp inhibitor as a potential new therapeutic agent against Zika virus. BMC Med. 2020, 18, 204. [Google Scholar] [CrossRef] [PubMed]
- Kamiyama, N.; Soma, R.; Hidano, S.; Watanabe, K.; Umekita, H.; Fukuda, C.; Noguchi, K.; Gendo, Y.; Ozaki, T.; Sonoda, A.; et al. Ribavirin inhibits Zika virus (ZIKV) replication in vitro and suppresses viremia in ZIKV-infected STAT1-deficient mice. Antivir. Res. 2017, 146, 1–11. [Google Scholar] [CrossRef]
- Ngono, A.E.; Shresta, S. Immune Response to Dengue and Zika. Annu. Rev. Immunol. 2018, 36, 279–308. [Google Scholar] [CrossRef]
- Hijano, D.R.; Vu, L.D.; Kauvar, L.M.; Tripp, R.A.; Polack, F.P.; Cormier, S.A. Role of Type I Interferon (IFN) in the Respiratory Syncytial Virus (RSV) Immune Response and Disease Severity. Front. Immunol. 2019, 10, 566. [Google Scholar] [CrossRef] [PubMed]
- Caine, E.A.; Scheaffer, S.M.; Arora, N.; Zaitsev, K.; Artyomov, M.N.; Coyne, C.B.; Moley, K.H.; Diamond, M.S. Interferon lambda protects the female reproductive tract against Zika virus infection. Nat. Commun. 2019, 10, 280. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.A.; Cooke, G.S.; Day, J.N.; Flower, B.; Phuong, L.T.; Hung, T.M.; Dung, N.T.; Khoa, D.B.; Hung, L.M.; Kestelyn, E.; et al. The direct-medical costs associated with interferon-based treatment for Hepatitis C in Vietnam. Wellcome Open Res. 2019, 4, 129. [Google Scholar] [CrossRef]
- Kirkwood, J.M.; Bender, C.; Agarwala, S.; Tarhini, A.; Shipe-Spotloe, J.; Smelko, B.; Donnelly, S.; Stover, L. Mechanisms and management of toxicities associated with high-dose interferon alfa-2b therapy. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2002, 20, 3703–3718. [Google Scholar] [CrossRef]
- Borden, E.C.; Parkinson, D. A perspective on the clinical effectiveness and tolerance of interferon-alpha. Semin. Oncol. 1998, 25, 3–8. [Google Scholar]
- Hauschild, A.; Gogas, H.; Tarhini, A.; Middleton, M.R.; Testori, A.; Dréno, B.; Kirkwood, J.M. Practical guidelines for the management of interferon-alpha-2b side effects in patients receiving adjuvant treatment for melanoma: Expert opinion. Cancer 2008, 112, 982–994. [Google Scholar] [CrossRef]
- Rand, U.; Kupke, S.Y.; Shkarlet, H.; Hein, M.D.; Hirsch, T.; Marichal-Gallardo, P.; Cicin-Sain, L.; Reichl, U.; Bruder, D. Antiviral Activity of Influenza A Virus Defective Interfering Particles against SARS-CoV-2 Replication In Vitro through Stimulation of Innate Immunity. Cells 2021, 10, 1756. [Google Scholar] [CrossRef] [PubMed]
- Easton, A.J.; Scott, P.D.; Edworthy, N.L.; Meng, B.; Marriott, A.C.; Dimmock, N.J. A novel broad-spectrum treatment for respiratory virus infections: Influenza-based defective interfering virus provides protection against pneumovirus infection in vivo. Vaccine 2011, 29, 2777–2784. [Google Scholar] [CrossRef] [PubMed]
- Scott, P.D.; Meng, B.; Marriott, A.C.; Easton, A.J.; Dimmock, N.J. Defective interfering influenza A virus protects in vivo against disease caused by a heterologous influenza B virus. J. Gen. Virol. 2011, 92, 2122–2132. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.H.; Li, D.; Tang, B.; Li, L.; Suhrbier, A.; Harrich, D. Defective Interfering Particles with Broad-Acting Antiviral Activity for Dengue, Zika, Yellow Fever, Respiratory Syncytial and SARS-CoV-2 Virus Infection. Microbiol. Spectr. 2022, 10, e0394922. [Google Scholar] [CrossRef] [PubMed]
- Vignuzzi, M.; Lopez, C.B. Defective viral genomes are key drivers of the virus-host interaction. Nat. Microbiol. 2019, 4, 1075–1087. [Google Scholar] [CrossRef] [PubMed]
- Meir, M.; Harel, N.; Miller, D.; Gelbart, M.; Eldar, A.; Gophna, U.; Stern, A. Competition between social cheater viruses is driven by mechanistically different cheating strategies. Sci. Adv. 2020, 6, eabb7990. [Google Scholar] [CrossRef]
- Ziegler, C.M.; Botten, J.W. Defective Interfering Particles of Negative-Strand RNA Viruses. Trends Microbiol. 2020, 28, 554–565. [Google Scholar] [CrossRef]
- Levi, L.I.; Rezelj, V.V.; Henrion-Lacritick, A.; Erazo, D.; Boussier, J.; Vallet, T.; Bernhauerova, V.; Suzuki, Y.; Carrau, L.; Weger-Lucarelli, J.; et al. Defective viral genomes from chikungunya virus are broad-spectrum antivirals and prevent virus dissemination in mosquitoes. PLoS Pathog. 2021, 17, e1009110. [Google Scholar] [CrossRef]
- Rezelj, V.V.; Carrau, L.; Merwaiss, F.; Levi, L.I.; Erazo, D.; Tran, Q.D.; Henrion-Lacritick, A.; Gausson, V.; Suzuki, Y.; Shengjuler, D.; et al. Defective viral genomes as therapeutic interfering particles against flavivirus infection in mammalian and mosquito hosts. Nat. Commun. 2021, 12, 2290. [Google Scholar] [CrossRef]
- Sun, Y.; Jain, D.; Koziol-White, C.J.; Genoyer, E.; Gilbert, M.; Tapia, K.; Panettieri, R.A., Jr.; Hodinka, R.L.; Lopez, C.B. Immunostimulatory Defective Viral Genomes from Respiratory Syncytial Virus Promote a Strong Innate Antiviral Response during Infection in Mice and Humans. PLoS Pathog. 2015, 11, e1005122. [Google Scholar] [CrossRef]
- Welch, S.R.; Tilston, N.L.; Lo, M.K.; Whitmer, S.L.M.; Harmon, J.R.; Scholte, F.E.M.; Spengler, J.R.; Duprex, W.P.; Nichol, S.T.; Spiropoulou, C.F. Inhibition of Nipah Virus by Defective Interfering Particles. J. Infect. Dis. 2020, 221, S460–S470. [Google Scholar] [CrossRef]
- Xiao, Y.; Lidsky, P.V.; Shirogane, Y.; Aviner, R.; Wu, C.T.; Li, W.; Zheng, W.; Talbot, D.; Catching, A.; Doitsh, G.; et al. A defective viral genome strategy elicits broad protective immunity against respiratory viruses. Cell 2021, 184, 6037–6051.e14. [Google Scholar] [CrossRef] [PubMed]
- Yao, S.; Narayanan, A.; Majowicz, S.A.; Jose, J.; Archetti, M. A synthetic defective interfering SARS-CoV-2. PeerJ 2021, 9, e11686. [Google Scholar] [CrossRef] [PubMed]
- Felt, S.A.; Sun, Y.; Jozwik, A.; Paras, A.; Habibi, M.S.; Nickle, D.; Anderson, L.; Achouri, E.; Feemster, K.A.; Cardenas, A.M.; et al. Detection of respiratory syncytial virus defective genomes in nasal secretions is associated with distinct clinical outcomes. Nat. Microbiol. 2021, 6, 672–681. [Google Scholar] [CrossRef] [PubMed]
- Girgis, S.; Xu, Z.; Oikonomopoulos, S.; Fedorova, A.D.; Tchesnokov, E.P.; Gordon, C.J.; Schmeing, T.M.; Götte, M.; Sonenberg, N.; Baranov, P.V.; et al. Evolution of naturally arising SARS-CoV-2 defective interfering particles. Commun. Biol. 2022, 5, 1140. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Chan, K.W.K.; Naripogu, K.B.; Swarbrick, C.M.D.; Aaskov, J.; Vasudevan, S.G. Subgenomic RNA from Dengue Virus Type 2 Suppresses Replication of Dengue Virus Genomes and Interacts with Virus-Encoded NS3 and NS5 Proteins. ACS Infect. Dis. 2020, 6, 436–446. [Google Scholar] [CrossRef]
- Hein, M.D.; Arora, P.; Marichal-Gallardo, P.; Winkler, M.; Genzel, Y.; Pöhlmann, S.; Schughart, K.; Kupke, S.Y.; Reichl, U. Cell culture-based production and in vivo characterization of purely clonal defective interfering influenza virus particles. BMC Biol. 2021, 19, 91. [Google Scholar] [CrossRef]
- Dimmock, N.J.; Rainsford, E.W.; Scott, P.D.; Marriott, A.C. Influenza virus protecting RNA: An effective prophylactic and therapeutic antiviral. J. Virol. 2008, 82, 8570–8578. [Google Scholar] [CrossRef]
- Yang, Y.; Lyu, T.; Zhou, R.; He, X.; Ye, K.; Xie, Q.; Zhu, L.; Chen, T.; Shen, C.; Wu, Q.; et al. The Antiviral and Antitumor Effects of Defective Interfering Particles/Genomes and Their Mechanisms. Front. Microbiol. 2019, 10, 1852. [Google Scholar] [CrossRef]
- Zhao, H.; To, K.K.W.; Chu, H.; Ding, Q.; Zhao, X.; Li, C.; Shuai, H.; Yuan, S.; Zhou, J.; Kok, K.H.; et al. Dual-functional peptide with defective interfering genes effectively protects mice against avian and seasonal influenza. Nat. Commun. 2018, 9, 2358. [Google Scholar] [CrossRef]
- Huo, C.; Cheng, J.; Xiao, J.; Chen, M.; Zou, S.; Tian, H.; Wang, M.; Sun, L.; Hao, Z.; Hu, Y. Defective Viral Particles Produced in Mast Cells Can Effectively Fight Against Lethal Influenza A Virus. Front. Microbiol. 2020, 11, 553274. [Google Scholar] [CrossRef]
- Mendes, M.; Russell, A.B. Library-based analysis reveals segment and length dependent characteristics of defective influenza genomes. PLoS Pathog. 2021, 17, e1010125. [Google Scholar] [CrossRef] [PubMed]
- Alnaji, F.G.; Brooke, C.B. Influenza virus DI particles: Defective interfering or delightfully interesting? PLoS Pathog. 2020, 16, e1008436. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Zhou, E.; Sheng, R.; Fu, X.; Li, J.; Jiang, C.; Su, W. Defective Interfering Particles of Influenza Virus and Their Characteristics, Impacts, and Use in Vaccines and Antiviral Strategies: A Systematic Review. Viruses 2022, 14, 2773. [Google Scholar] [CrossRef] [PubMed]
- Alnaji, F.G.; Reiser, W.K.; Rivera-Cardona, J.; Velthuis, A.J.W.t.; Brooke, C.B. Influenza A Virus Defective Viral Genomes Are Inefficiently Packaged into Virions Relative to Wild-Type Genomic RNAs. mBio 2021, 12, e0295921. [Google Scholar] [CrossRef]
- Rüdiger, D.; Pelz, L.; Hein, M.D.; Kupke, S.Y.; Reichl, U. Multiscale model of defective interfering particle replication for influenza A virus infection in animal cell culture. PLoS Comput. Biol. 2021, 17, e1009357. [Google Scholar] [CrossRef]
- Rüdiger, D.; Kupke, S.Y.; Laske, T.; Zmora, P.; Reichl, U. Multiscale modeling of influenza A virus replication in cell cultures predicts infection dynamics for highly different infection conditions. PLoS Comput. Biol. 2019, 15, e1006819. [Google Scholar] [CrossRef]
- Nayak, D.P.; Chambers, T.M.; Akkina, R.K. Defective-Interfering (DI) RNAs of Influenza Viruses: Origin, Structure, Expression, and Interference. In Current Topics in Microbiology and Immunology; Cooper, M., Eisen, H., Goebel, W., Hofschneider, P.H., Koprowski, H., Melchers, F., Oldstone, M., Rott, R., Schweiger, H.G., Vogt, P.K., et al., Eds.; Springer: Berlin/Heidelberg, Germany, 1985; pp. 103–151. [Google Scholar]
- Marriott, A.C.; Dimmock, N.J. Defective interfering viruses and their potential as antiviral agents. Rev. Med. Virol. 2010, 20, 51–62. [Google Scholar] [CrossRef]
- Laske, T.; Heldt, F.S.; Hoffmann, H.; Frensing, T.; Reichl, U. Modeling the intracellular replication of influenza A virus in the presence of defective interfering RNAs. Virus Res. 2016, 213, 90–99. [Google Scholar] [CrossRef] [PubMed]
- Kupke, S.Y.; Riedel, D.; Frensing, T.; Zmora, P.; Reichl, U. A Novel Type of Influenza A Virus-Derived Defective Interfering Particle with Nucleotide Substitutions in Its Genome. J. Virol. 2019, 93, e01786-18. [Google Scholar] [CrossRef]
- Dimmock, N.J.; Dove, B.K.; Scott, P.D.; Meng, B.; Taylor, I.; Cheung, L.; Hallis, B.; Marriott, A.C.; Carroll, M.W.; Easton, A.J. Cloned defective interfering influenza virus protects ferrets from pandemic 2009 influenza A virus and allows protective immunity to be established. PLoS ONE 2012, 7, e49394. [Google Scholar] [CrossRef]
- Frensing, T.; Pflugmacher, A.; Bachmann, M.; Peschel, B.; Reichl, U. Impact of defective interfering particles on virus replication and antiviral host response in cell culture-based influenza vaccine production. Appl. Microbiol. Biotechnol. 2014, 98, 8999–9008. [Google Scholar] [CrossRef] [PubMed]
- Penn, R.; Tregoning, J.S.; Flight, K.E.; Baillon, L.; Frise, R.; Goldhill, D.H.; Johansson, C.; Barclay, W. Levels of Influenza A Virus Defective Viral Genomes Determine Pathogenesis in the BALB/c Mouse Model. J. Virol. 2022, 96, e0117822. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Lin, M.H.; Rawle, D.J.; Jin, H.; Wu, Z.; Wang, L.; Lor, M.; Hussain, M.; Aaskov, J.; Harrich, D. Dengue virus-free defective interfering particles have potent and broad anti-dengue virus activity. Commun. Biol. 2021, 4, 557. [Google Scholar] [CrossRef]
- Baum, A.; García-Sastre, A. Differential recognition of viral RNA by RIG-I. Virulence 2011, 2, 166–169. [Google Scholar] [CrossRef] [PubMed]
- Rehwinkel, J.; Tan, C.P.; Goubau, D.; Schulz, O.; Pichlmair, A.; Bier, K.; Robb, N.; Vreede, F.; Barclay, W.; Fodor, E.; et al. RIG-I Detects Viral Genomic RNA during Negative-Strand RNA Virus Infection. Cell 2010, 140, 397–408. [Google Scholar] [CrossRef]
- Dimmock, N.J.; Dove, B.K.; Meng, B.; Scott, P.D.; Taylor, I.; Cheung, L.; Hallis, B.; Marriott, A.C.; Carroll, M.W.; Easton, A.J. Comparison of the protection of ferrets against pandemic 2009 influenza A virus (H1N1) by 244 DI influenza virus and oseltamivir. Antivir. Res 2012, 96, 376–385. [Google Scholar] [CrossRef]
- Hein, M.D.; Kollmus, H.; Marichal-Gallardo, P.; Püttker, S.; Benndorf, D.; Genzel, Y.; Schughart, K.; Kupke, S.Y.; Reichl, U. OP7, a novel influenza A virus defective interfering particle: Production, purification, and animal experiments demonstrating antiviral potential. Appl. Microbiol. Biotechnol. 2021, 105, 129–146. [Google Scholar] [CrossRef]
- Marichal-Gallardo, P.; Pieler, M.M.; Wolff, M.W.; Reichl, U. Steric exclusion chromatography for purification of cell culture-derived influenza A virus using regenerated cellulose membranes and polyethylene glycol. J. Chromatogr. A 2017, 1483, 110–119. [Google Scholar] [CrossRef]
- Marichal-Gallardo, P.; Börner, K.; Pieler, M.M.; Sonntag-Buck, V.; Obr, M.; Bejarano, D.; Wolff, M.W.; Krausslich, H.G.; Reichl, U.; Grimm, D. Single-Use Capture Purification of Adeno-Associated Viral Gene Transfer Vectors by Membrane-Based Steric Exclusion Chromatography. Hum. Gene Ther. 2021, 32, 959–974. [Google Scholar] [CrossRef]
- Kalbfuss, B.; Knöchlein, A.; Kröber, T.; Reichl, U. Monitoring influenza virus content in vaccine production: Precise assays for the quantitation of hemagglutination and neuraminidase activity. Biologicals 2008, 36, 145–161. [Google Scholar] [CrossRef]
- Nikolay, A.; Castilho, L.R.; Reichl, U.; Genzel, Y. Propagation of Brazilian Zika virus strains in static and suspension cultures using Vero and BHK cells. Vaccine 2018, 36, 3140–3145. [Google Scholar] [CrossRef] [PubMed]
- Hierholzer, J.; Killington, R. Virus isolation and quantitation. In Virology Methods Manual; Elsevier: Amsterdam, The Netherlands, 1996; pp. 25–46. [Google Scholar]
- Sun, Y.; Lopez, C.B. Preparation of Respiratory Syncytial Virus with High or Low Content of Defective Viral Particles and Their Purification from Viral Stocks. Bio Protoc. 2016, 6, e1820. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Jiang, J.; Li, J.; Fan, W.; Zheng, W.; Yu, M.; Chen, C.; Sun, L.; Bi, Y.; Ding, C.; Gao, G.F.; et al. Robust Lys63-Linked Ubiquitination of RIG-I Promotes Cytokine Eruption in Early Influenza B Virus Infection. J. Virol. 2016, 90, 6263–6275. [Google Scholar] [CrossRef] [PubMed]
- Busnadiego, I.; Fernbach, S.; Pohl, M.O.; Karakus, U.; Huber, M.; Trkola, A.; Stertz, S.; Hale, B.G. Antiviral Activity of Type I, II, and III Interferons Counterbalances ACE2 Inducibility and Restricts SARS-CoV-2. mBio 2020, 11, e01928-20. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Chen, L.; Luo, J.; He, H. NP and NS1 proteins of H5N1 virus significantly upregulated IFITM1, IFITM2, and IFITM3 in A549 cells. Afr. Health Sci. 2019, 19, 1402–1410. [Google Scholar] [CrossRef]
- Frensing, T.; Kupke, S.Y.; Bachmann, M.; Fritzsche, S.; Gallo-Ramirez, L.E.; Reichl, U. Influenza virus intracellular replication dynamics, release kinetics, and particle morphology during propagation in MDCK cells. Appl. Microbiol. Biotechnol. 2016, 100, 7181–7192. [Google Scholar] [CrossRef] [PubMed]
- Kawakami, E.; Watanabe, T.; Fujii, K.; Goto, H.; Watanabe, S.; Noda, T.; Kawaoka, Y. Strand-specific real-time RT-PCR for distinguishing influenza vRNA, cRNA, and mRNA. J. Virol. Methods 2011, 173, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Diaz, M.O.; Ziemin, S.; Le Beau, M.M.; Pitha, P.; Smith, S.D.; Chilcote, R.R.; Rowley, J.D. Homozygous deletion of the alpha- and beta 1-interferon genes in human leukemia and derived cell lines. Proc. Natl. Acad. Sci. USA 1988, 85, 5259–5263. [Google Scholar] [CrossRef]
- Vester, D.; Rapp, E.; Kluge, S.; Genzel, Y.; Reichl, U. Virus–host cell interactions in vaccine production cell lines infected with different human influenza A virus variants: A proteomic approach. J. Proteom. 2010, 73, 1656–1669. [Google Scholar] [CrossRef] [PubMed]
- Chew, T.; Noyce, R.; Collins, S.E.; Hancock, M.H.; Mossman, K.L. Characterization of the interferon regulatory factor 3-mediated antiviral response in a cell line deficient for IFN production. Mol. Immunol. 2009, 46, 393–399. [Google Scholar] [CrossRef] [PubMed]
- Emeny, J.M.; Morgan, M.J. Regulation of the interferon system: Evidence that Vero cells have a genetic defect in interferon production. J. Gen. Virol. 1979, 43, 247–252. [Google Scholar] [CrossRef]
- Desmyter, J.; Melnick, J.L.; Rawls, W.E. Defectiveness of interferon production and of rubella virus interference in a line of African green monkey kidney cells (Vero). J. Virol. 1968, 2, 955–961. [Google Scholar] [CrossRef]
- Osada, N.; Kohara, A.; Yamaji, T.; Hirayama, N.; Kasai, F.; Sekizuka, T.; Kuroda, M.; Hanada, K. The genome landscape of the african green monkey kidney-derived vero cell line. DNA Res. 2014, 21, 673–683. [Google Scholar] [CrossRef]
- Lam, L.K.M.; Watson, A.M.; Ryman, K.D.; Klimstra, W.B. Gamma-interferon exerts a critical early restriction on replication and dissemination of yellow fever virus vaccine strain 17D-204. NPJ Vaccines 2018, 3, 5. [Google Scholar] [CrossRef] [PubMed]
- Bernatchez, J.A.; Yang, Z.; Coste, M.; Li, J.; Beck, S.; Liu, Y.; Clark, A.E.; Zhu, Z.; Luna, L.A.; Sohl, C.D.; et al. Development and Validation of a Phenotypic High-Content Imaging Assay for Assessing the Antiviral Activity of Small-Molecule Inhibitors Targeting Zika Virus. Antimicrob. Agents Chemother. 2018, 62, e00725-18. [Google Scholar] [CrossRef] [PubMed]
- Frumence, E.; Roche, M.; Krejbich-Trotot, P.; El-Kalamouni, C.; Nativel, B.; Rondeau, P.; Misse, D.; Gadea, G.; Viranaicken, W.; Despres, P. The South Pacific epidemic strain of Zika virus replicates efficiently in human epithelial A549 cells leading to IFN-beta production and apoptosis induction. Virology 2016, 493, 217–226. [Google Scholar] [CrossRef]
- Hamel, R.; Dejarnac, O.; Wichit, S.; Ekchariyawat, P.; Neyret, A.; Luplertlop, N.; Perera-Lecoin, M.; Surasombatpattana, P.; Talignani, L.; Thomas, F.; et al. Biology of Zika Virus Infection in Human Skin Cells. J. Virol. 2015, 89, 8880–8896. [Google Scholar] [CrossRef] [PubMed]
- Cinatl, J.; Morgenstern, B.; Bauer, G.; Chandra, P.; Rabenau, H.; Doerr, H.W. Treatment of SARS with human interferons. Lancet 2003, 362, 293–294. [Google Scholar] [CrossRef]
- Pires de Mello, C.P.; Drusano, G.L.; Rodriquez, J.L.; Kaushik, A.; Brown, A.N. Antiviral Effects of Clinically-Relevant Interferon-alpha and Ribavirin Regimens against Dengue Virus in the Hollow Fiber Infection Model (HFIM). Viruses 2018, 10, 317. [Google Scholar] [CrossRef]
- Remsen, J.F.; Miller, N.; Cerutti, P.A. Photohydration of Uridine in the RNA of Coliphage R17, II. The Relationship between Ultraviolet Inactivation and Uridine Photohydration. Proc. Natl. Acad. Sci. USA 1970, 65, 460–466. [Google Scholar] [CrossRef]
- Coahran, D.R.; Buzzell, A.; Lauffer, M.A. The effect of ultraviolet irradiation on nucleic acid isolated from tobacco mosaic virus. Biochim. Et. Biophys. Acta 1962, 55, 755–767. [Google Scholar] [CrossRef]
- Jericevic, Z.; Kucan, I.; Chambers, R.W. Photochemical cleavage of phosphodiester bonds in oligoribonucleotides. Biochemistry 1982, 21, 6563–6567. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Forst, C.V.; Chou, T.W.; Geber, A.; Wang, M.; Hamou, W.; Smith, M.; Sebra, R.; Zhang, B.; Zhou, B.; et al. Cell-to-Cell Variation in Defective Virus Expression and Effects on Host Responses during Influenza Virus Infection. mBio 2020, 11, e02880-19. [Google Scholar] [CrossRef]
- Wang, C.; Honce, R.; Salvatore, M.; Chow, D.; Randazzo, D.; Yang, J.; Twells, N.M.; Mahal, L.K.; Schultz-Cherry, S.; Ghedin, E. Influenza Defective Interfering Virus Promotes Multiciliated Cell Differentiation and Reduces the Inflammatory Response in Mice. J. Virol. 2023, 97, e0049323. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.-X.; You, C.-H.; Qi, Z.-T.; Wang, X.-M.; Sun, P.-Q.; Bi, W.-S.; Qian, Y.; Ding, R.-L.; Du, P.; He, Y. Children’s respiratory viral diseases treated with interferon aerosol. Chin. Med. J. 1987, 100, 162–166. [Google Scholar] [CrossRef] [PubMed]
- Sung, R.Y.; Yin, J.; Oppenheimer, S.J.; Tam, J.S.; Lau, J. Treatment of respiratory syncytial virus infection with recombinant interferon alfa-2a. Arch. Dis. Child. 1993, 69, 440–442. [Google Scholar] [CrossRef]
- Chipps, B.E.; Sullivan, W.F.; Portnoy, J.M. Alpha-2A-interferon for treatment of bronchiolitis caused by respiratory syncytial virus. Pediatr. Infect. Dis. J. 1993, 12, 653–658. [Google Scholar] [CrossRef]
- Higgins, P.G.; Barrow, G.I.; Tyrrell, D.A.J.; Isaacs, D.; Gauci, C.L. The efficacy of intranasal interferonα-2a in respiratory syncytial virus infection in volunteers. Antivir. Res. 1990, 14, 3–10. [Google Scholar] [CrossRef]
- Atreya, P.L.; Kulkarni, S. Respiratory Syncytial Virus Strain A2 Is Resistant to the Antiviral Effects of Type I Interferons and Human MxA. Virology 1999, 261, 227–241. [Google Scholar] [CrossRef]
- Hein, M.D.; Chawla, A.; Cattaneo, M.; Kupke, S.Y.; Genzel, Y.; Reichl, U. Cell culture-based production of defective interfering influenza A virus particles in perfusion mode using an alternating tangential flow filtration system. Appl. Microbiol. Biotechnol. 2021, 105, 7251–7264. [Google Scholar] [CrossRef] [PubMed]
- Dimmock, N.J.; Easton, A.J. Cloned Defective Interfering Influenza RNA and a Possible Pan-Specific Treatment of Respiratory Virus Diseases. Viruses 2015, 7, 3768–3788. [Google Scholar] [CrossRef] [PubMed]

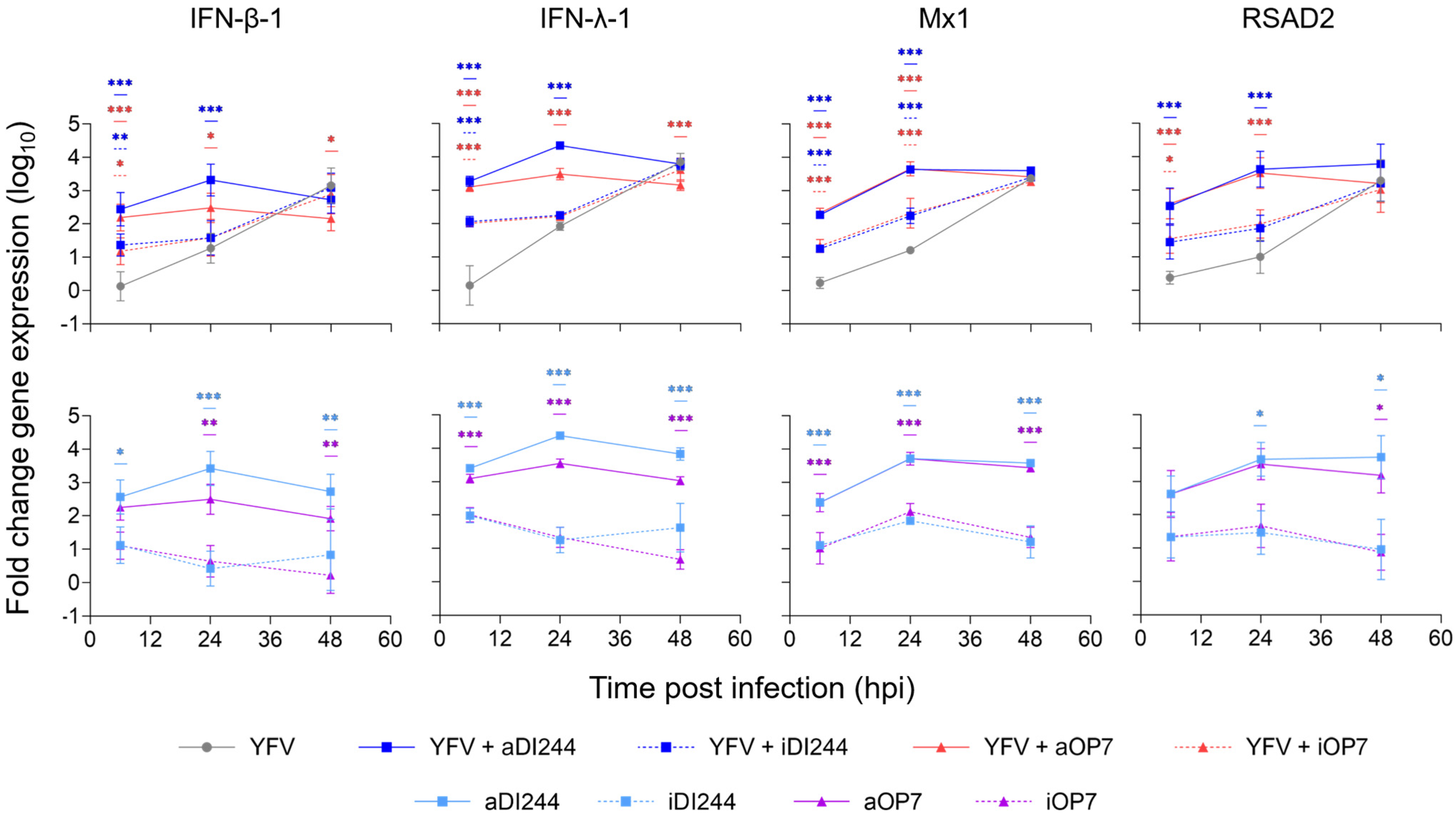



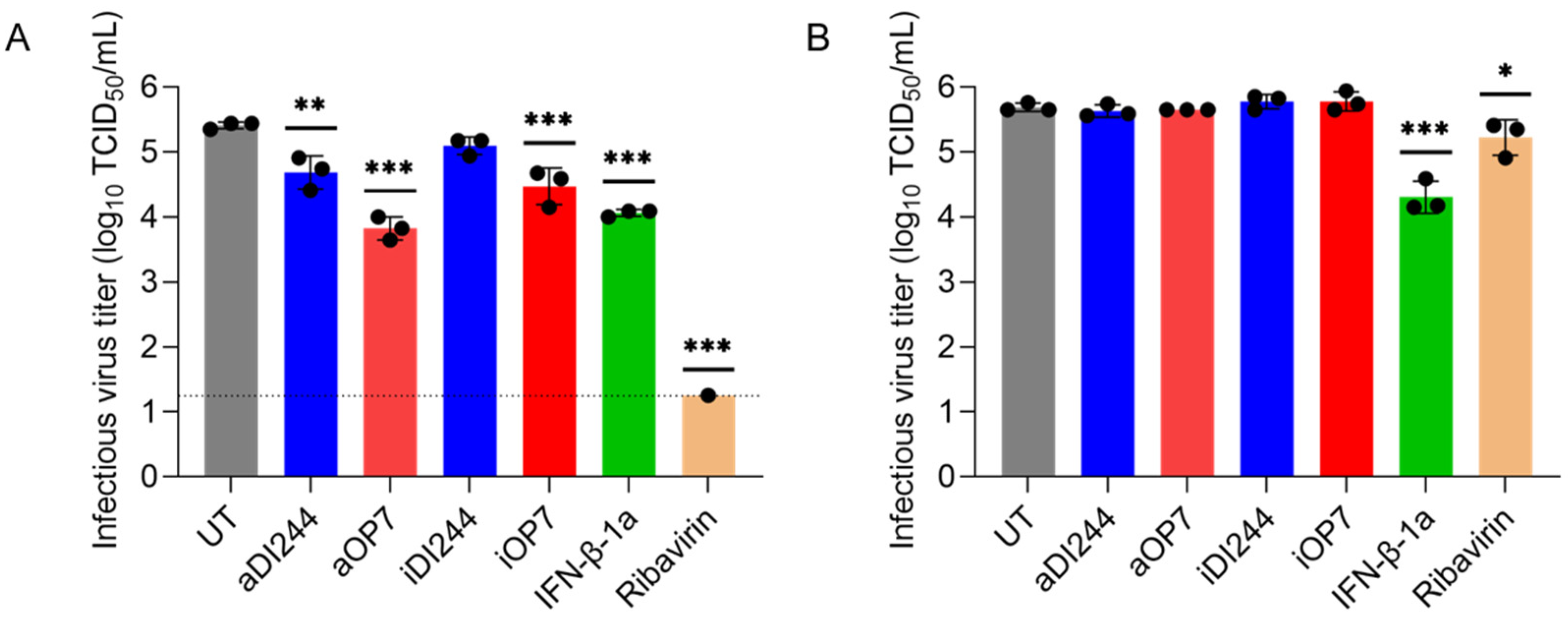
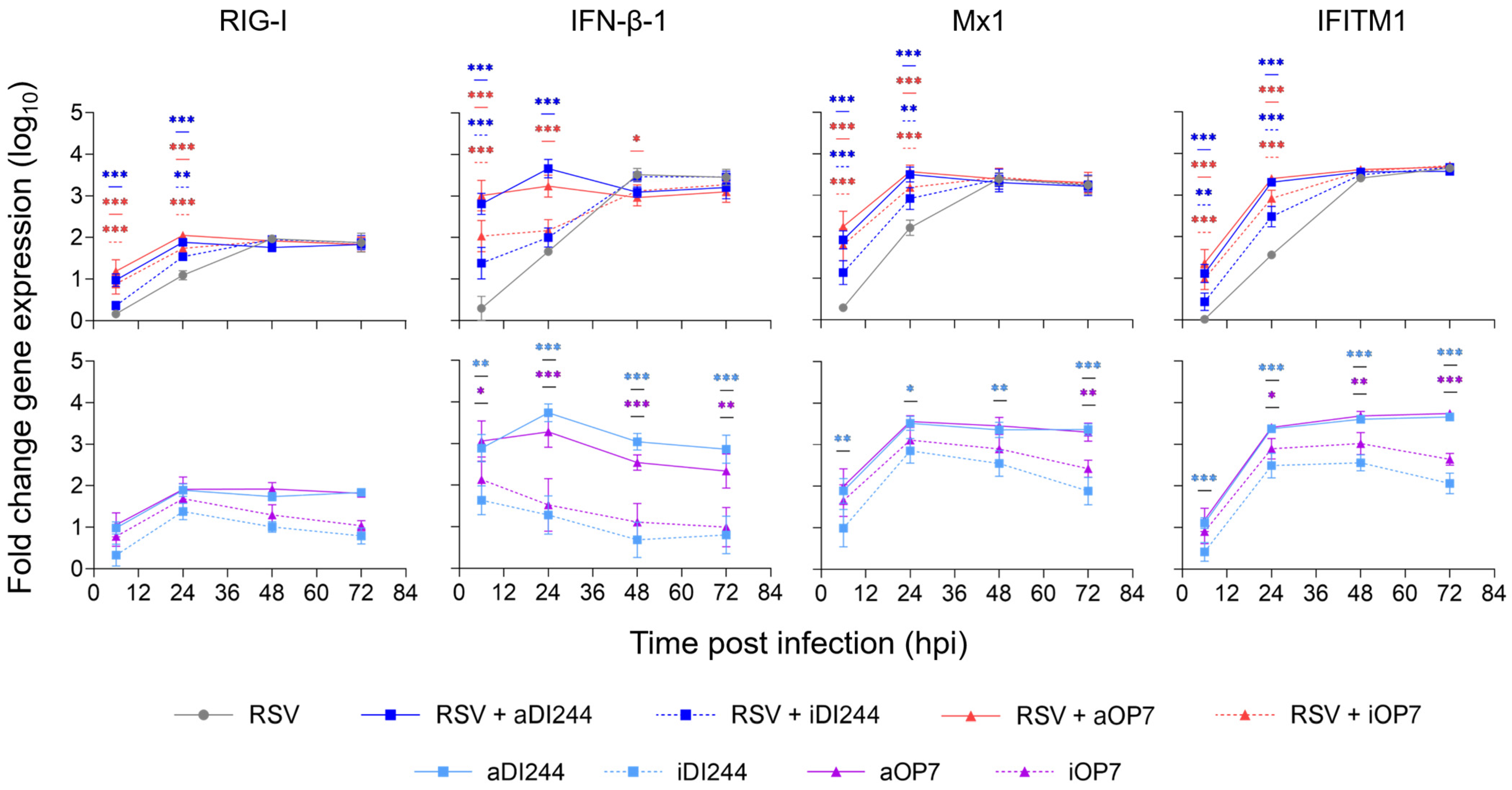
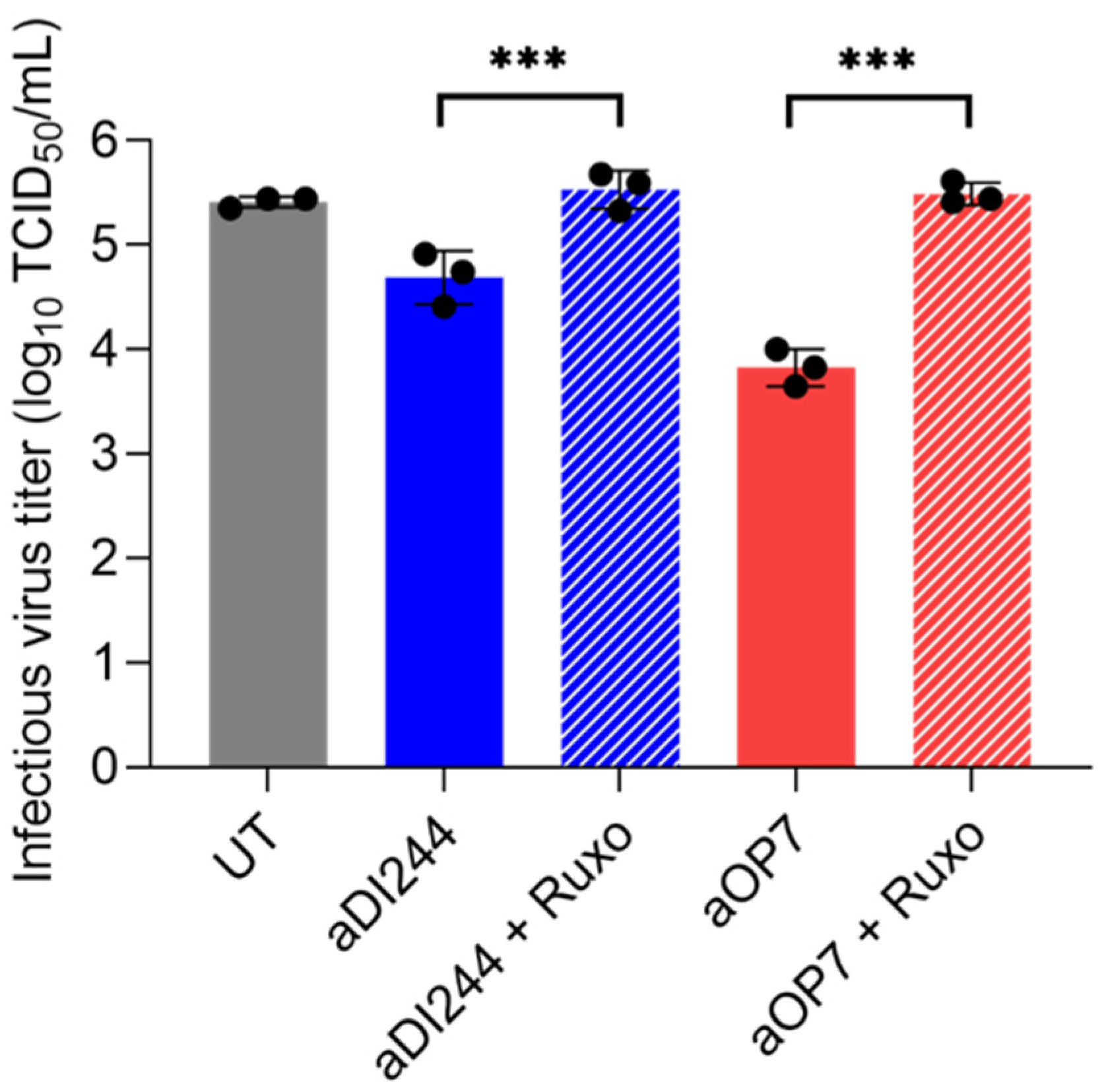
| Description | HA Titer 1 (log10 HAU/100 µL) | DI vRNA Concentration (DI vRNAs/mL) 2 |
|---|---|---|
| Active OP7 (8 min UV) | 3.53 | 1.60 × 1011 |
| Inactive OP7 (24 min UV) | 3.52 | 2.85 × 1010 |
| Active DI244 (no UV) | 3.89 | 8.89 × 1010 |
| Inactive DI244 (24 min UV) | 3.85 | 1.03 × 109 |
| Target Gene | Primer Name | Sequence (5′→3′) |
|---|---|---|
| RIG-I | RIG-I for | GGACGTGGCAAAACAAATCAG |
| [68] | RIG-I rev | GCAATGTCAATGCCTTCATCA |
| IFN-β-1 | IFN-β-1 for | CATTACCTGAAGGCCAAGGA |
| [69] | IFN-β-1 rev | CAGCATCTGCTGGTTGAAGA |
| IFN-λ-1 | IFN-λ-1 for | GGTGACTTTGGTGCTAGGCT |
| [69] | IFN-λ-1 rev | TGAGTGACTCTTCCAAGGCG |
| Mx1 | Mx1 for | GTATCACAGAGCTGTTCTCCTG |
| [69] | Mx1 rev | CTCCCACTCCCTGAAATCTG |
| IFITM1 | IFITM1 for | ATCAACATCCACAGCGAGAC |
| [70] | IFITM1 rev | CAGAGCCGAATACCAGTAACAG |
| RSAD2 | RSAD2 for | CCCCAACCAGCGTCAACTAT |
| [69] | RSAD2 rev | TGATCTTCTCCATACCAGCTTCC |
| GAPDH | GAPDH for | CTGGCGTCTTCACCACCATGG |
| [69] | GAPDH rev | CATCACGCCACAGTTTCCCGG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pelz, L.; Piagnani, E.; Marsall, P.; Wynserski, N.; Hein, M.D.; Marichal-Gallardo, P.; Kupke, S.Y.; Reichl, U. Broad-Spectrum Antiviral Activity of Influenza A Defective Interfering Particles against Respiratory Syncytial, Yellow Fever, and Zika Virus Replication In Vitro. Viruses 2023, 15, 1872. https://doi.org/10.3390/v15091872
Pelz L, Piagnani E, Marsall P, Wynserski N, Hein MD, Marichal-Gallardo P, Kupke SY, Reichl U. Broad-Spectrum Antiviral Activity of Influenza A Defective Interfering Particles against Respiratory Syncytial, Yellow Fever, and Zika Virus Replication In Vitro. Viruses. 2023; 15(9):1872. https://doi.org/10.3390/v15091872
Chicago/Turabian StylePelz, Lars, Elena Piagnani, Patrick Marsall, Nancy Wynserski, Marc Dominique Hein, Pavel Marichal-Gallardo, Sascha Young Kupke, and Udo Reichl. 2023. "Broad-Spectrum Antiviral Activity of Influenza A Defective Interfering Particles against Respiratory Syncytial, Yellow Fever, and Zika Virus Replication In Vitro" Viruses 15, no. 9: 1872. https://doi.org/10.3390/v15091872







