Saikosaponin B2, Punicalin, and Punicalagin in Vitro Block Cellular Entry of Feline Herpesvirus-1
Abstract
:1. Introduction
2. Materials and Methods
2.1. Compounds
2.2. Chemicals and Reagents
2.3. Cells and Virus
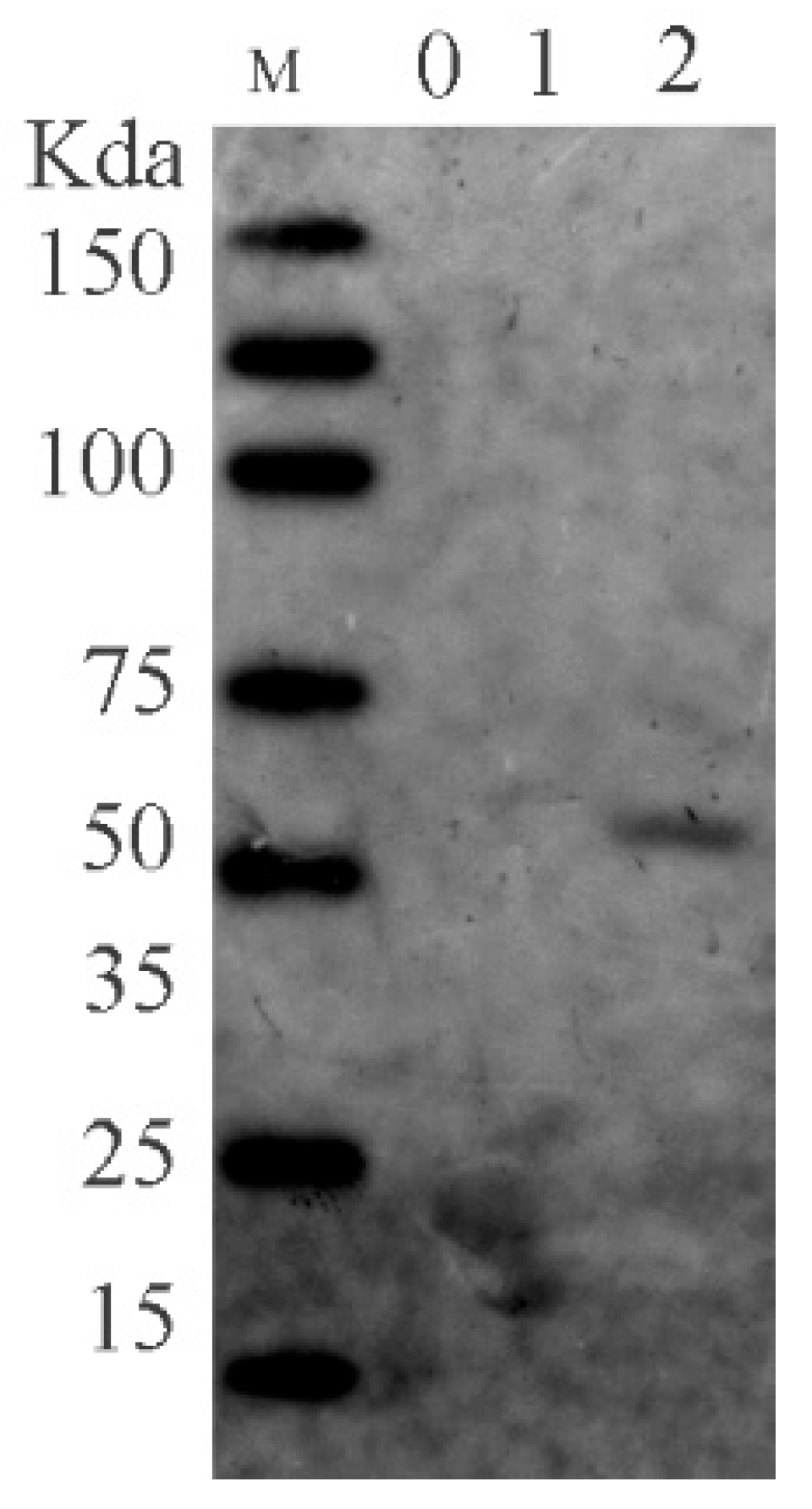
2.4. Expression and Purification of gB
2.5. Biolayer Interferometry (BLI) Assay
2.6. Cytotoxicity Assay
2.7. Cytopathic Effect (CPE) Inhibition Assay
2.8. Plaque Reduction Assay
2.9. Quantitative Real-Time PCR
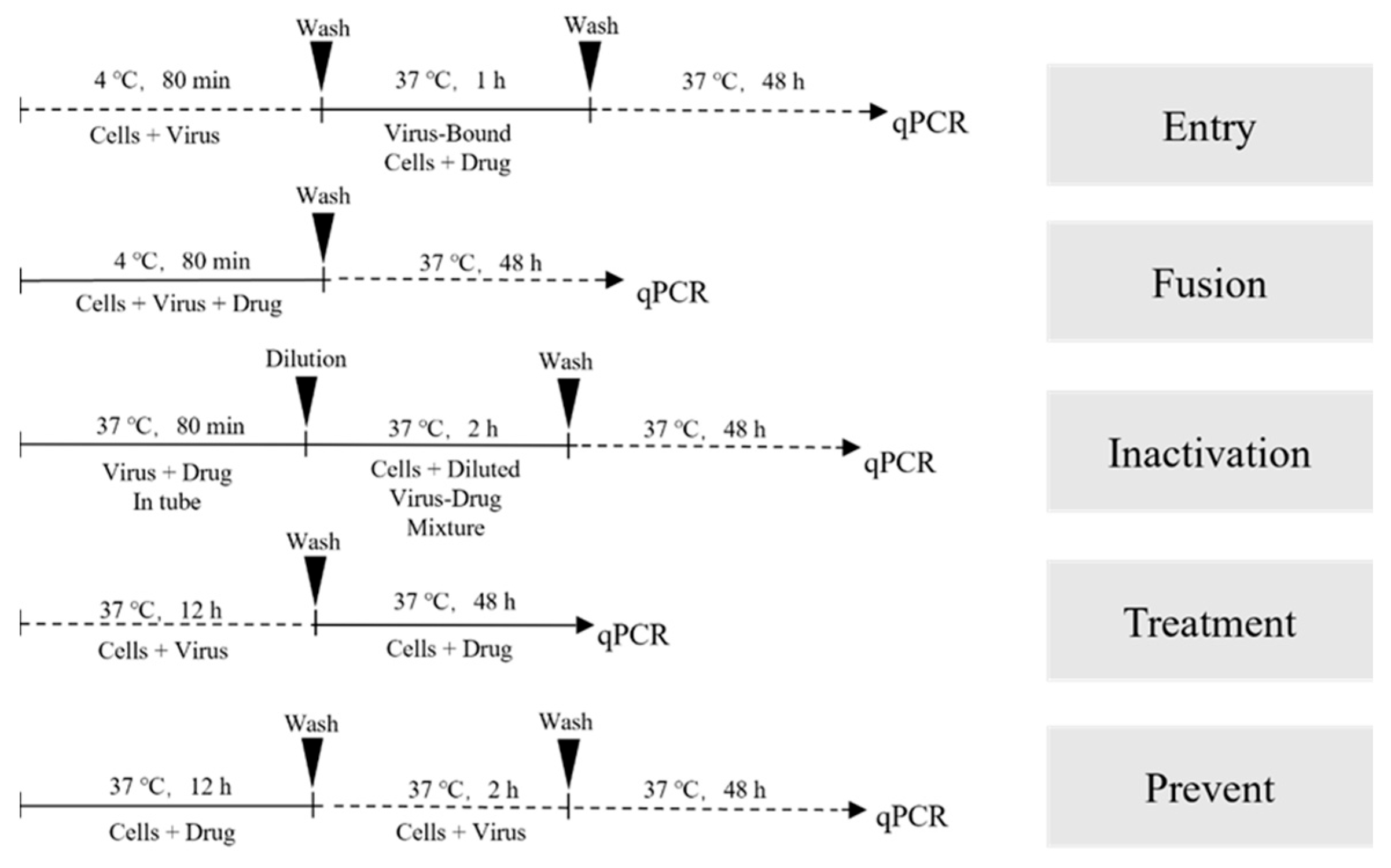
2.10. Time of Drug Addition Assay
2.11. Fusion and Entry Assays
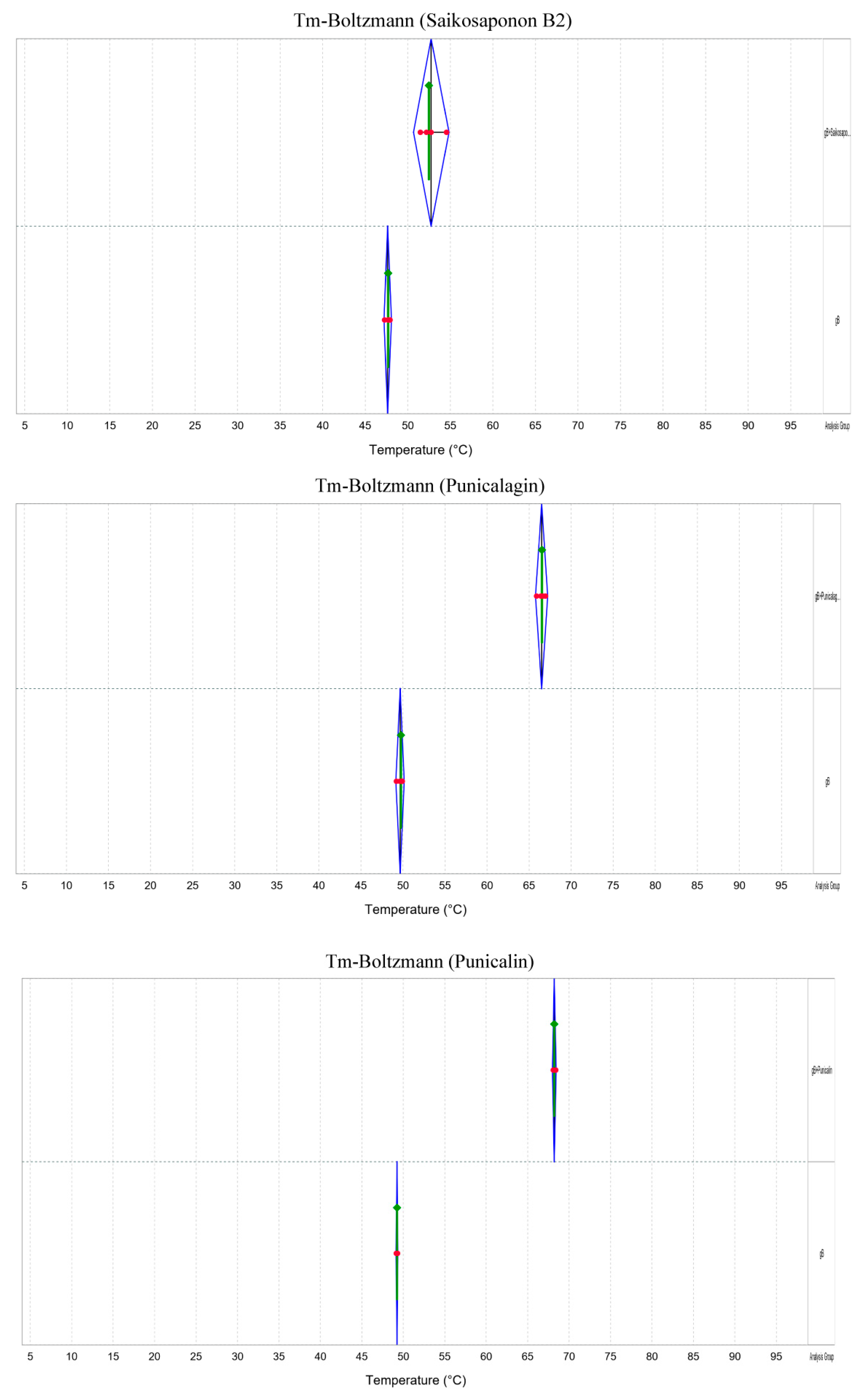
2.12. Protein Thermal Shift Assay
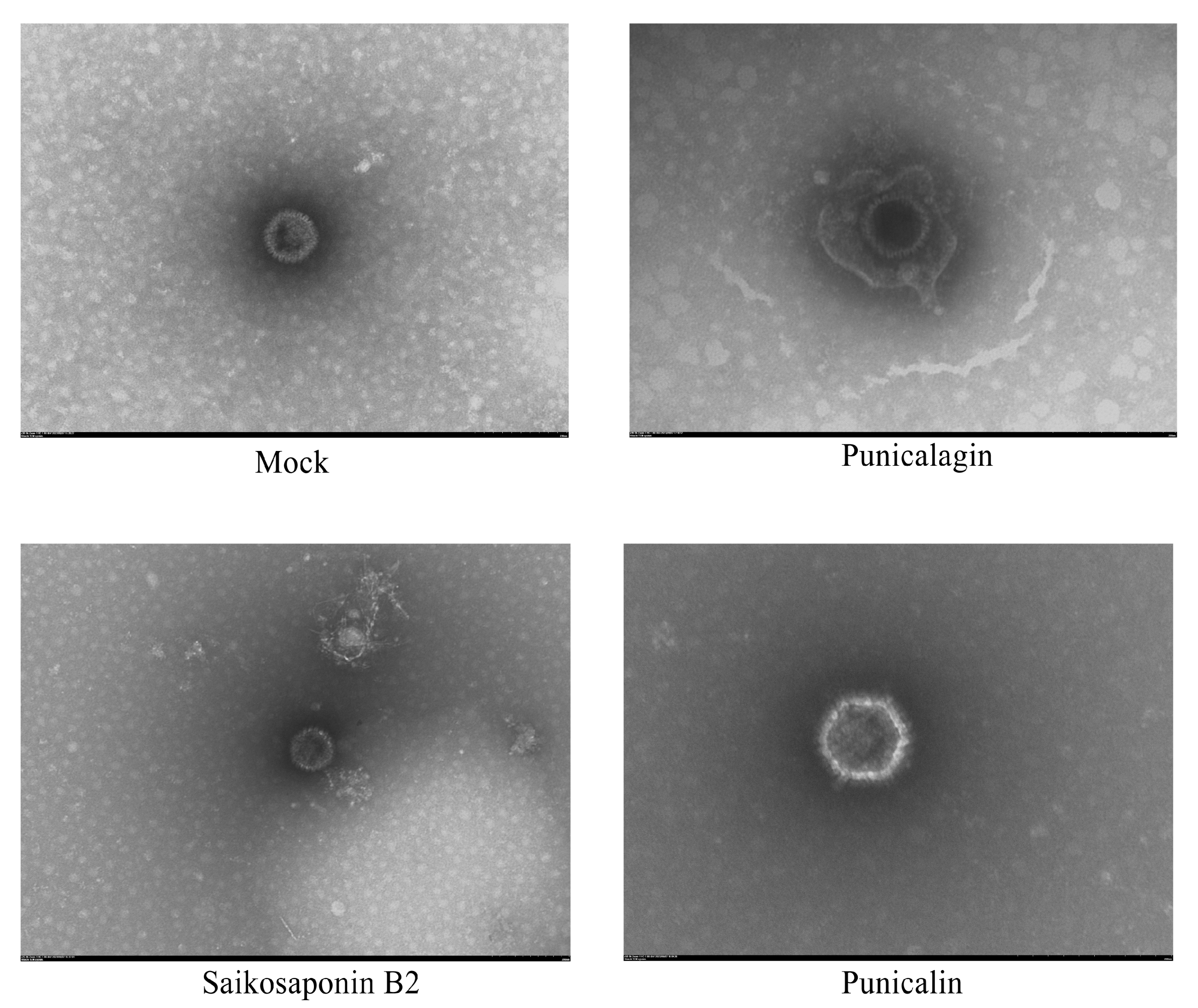
2.13. Transmission Electron Microscopy (TEM)
2.14. Statistical Analysis
3. Results
3.1. FHV-1 gB
3.2. Identification of Natural Small Molecule Inhibitors against FHV-1 gB

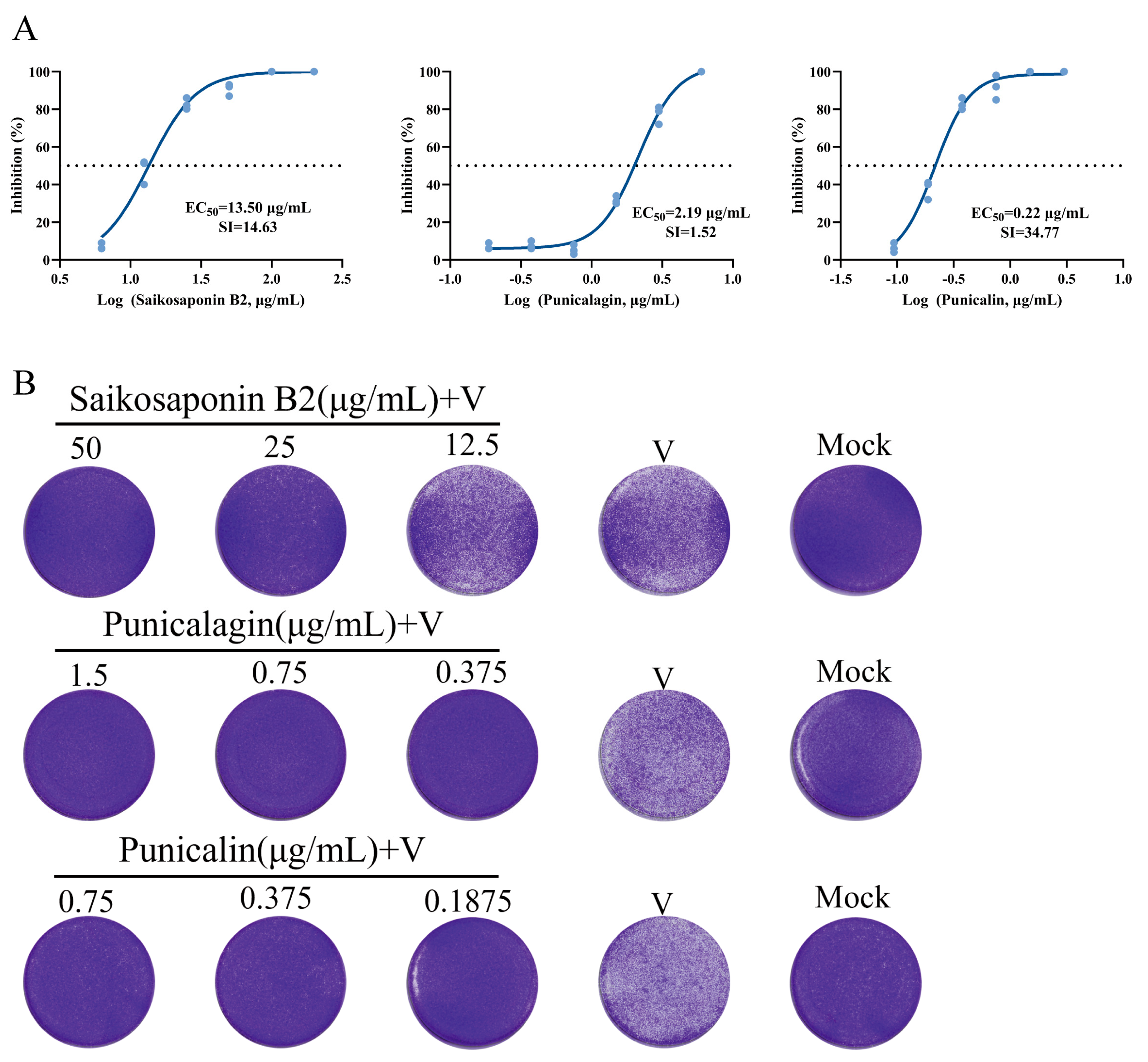
3.3. The Natural Compounds Saikosaponin B2, Punicalin, and Punicalagin Effectively Suppress Viral Infection of FHV-1 in CRFK Cells
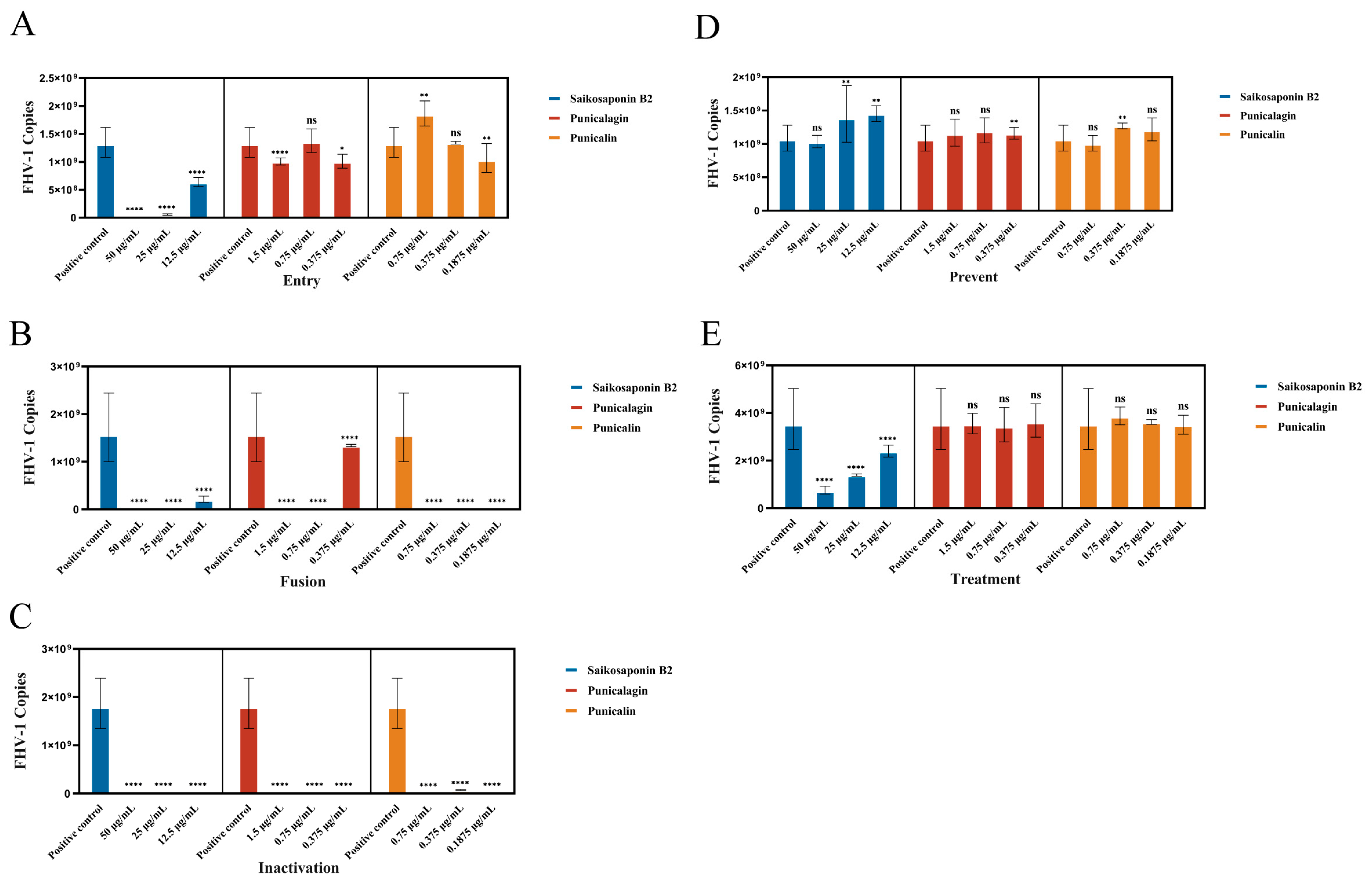
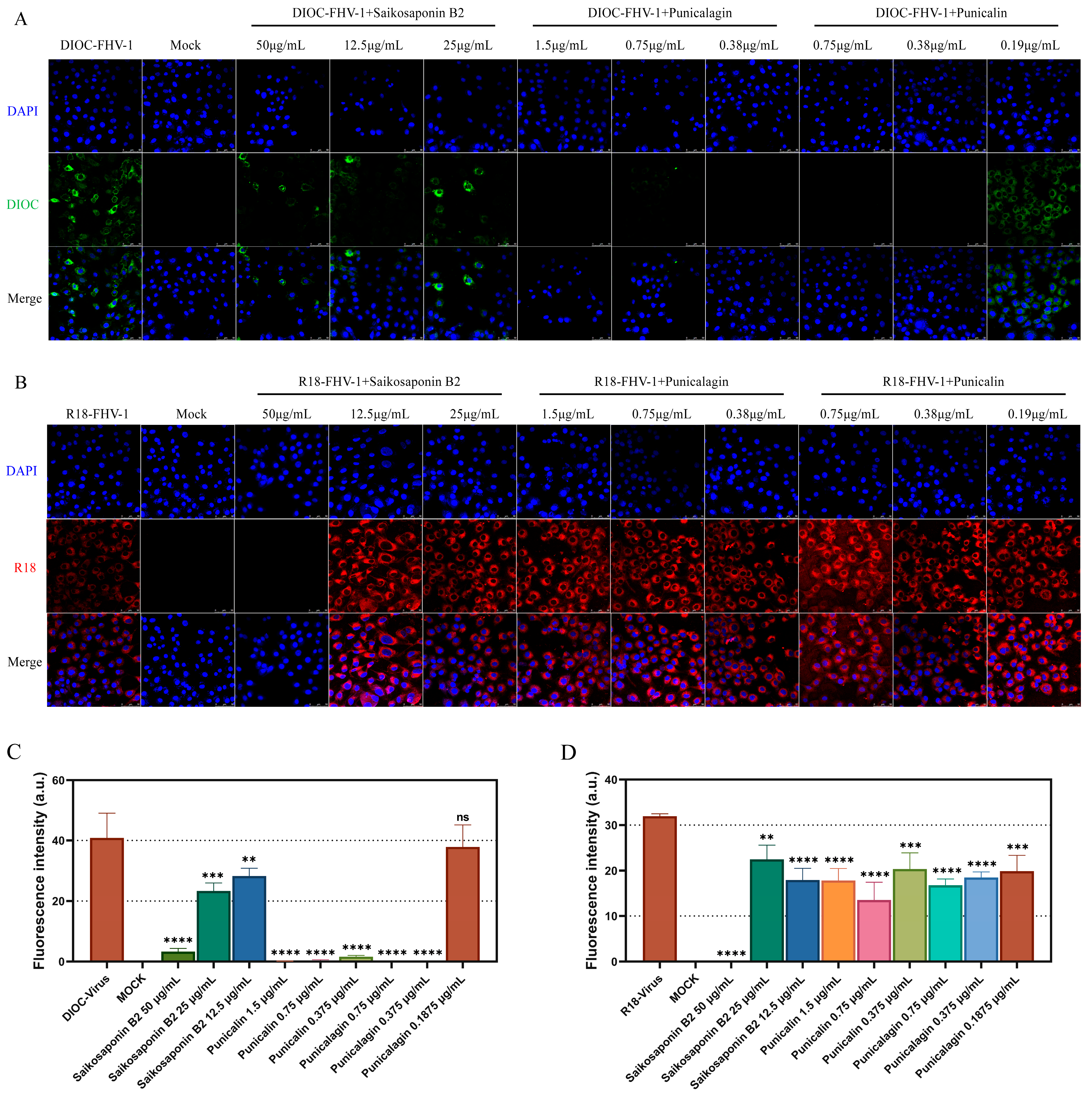
3.4. Saikosaponin B2, Punicalin, and Punicalagin Target the Early Viral Entry of FHV-1, Disrupting Both Viral Attachment and Entry/Fusion Events
3.5. The Drug Does Not Destroy the Structural Integrity of the Virus Particles
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Andrew, S.E. Ocular manifestations of feline herpesvirus. J. Feline Med. Surg. 2001, 3, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Gaskell, R.; Dawson, S.; Radford, A.; Thiry, E. Feline herpesvirus. Vet. Res. 2007, 38, 337–354. [Google Scholar] [CrossRef] [PubMed]
- Maggs, D.J.; Clarke, H.E. In vitro efficacy of ganciclovir, cidofovir, penciclovir, foscarnet, idoxuridine, and acyclovir against feline herpesvirus type-1. Am. J. Vet. Res. 2004, 65, 399–403. [Google Scholar] [CrossRef] [PubMed]
- Maeda, K.; Horimoto, T.; Mikami, T. Properties and functions of feline herpesvirus type 1 glycoproteins. J. Vet. Med. Sci. 1998, 60, 881–888. [Google Scholar] [CrossRef] [PubMed]
- Synowiec, A.; Dąbrowska, A.; Pachota, M.; Baouche, M.; Owczarek, K.; Niżański, W.; Pyrc, K. Feline herpesvirus 1 (FHV-1) enters the cell by receptor-mediated endocytosis. J. Virol. 2023, 97, e0068123. [Google Scholar] [CrossRef] [PubMed]
- Hilterbrand, A.T.; Daly, R.E.; Heldwein, E.E. Contributions of the Four Essential Entry Glycoproteins to HSV-1 Tropism and the Selection of Entry Routes. mBio 2021, 12, e00143-21. [Google Scholar] [CrossRef] [PubMed]
- Atanasiu, D.; Saw, W.T.; Gallagher, J.R.; Hannah, B.P.; Matsuda, Z.; Whitbeck, J.C.; Cohen, G.H.; Eisenberg, R.J. Dual split protein-based fusion assay reveals that mutations to herpes simplex virus (HSV) glycoprotein gB alter the kinetics of cell-cell fusion induced by HSV entry glycoproteins. J. Virol. 2013, 87, 11332–11345. [Google Scholar] [CrossRef] [PubMed]
- Connolly, S.A.; Jardetzky, T.S.; Longnecker, R. The structural basis of herpesvirus entry. Nat. Rev. Microbiol. 2021, 19, 110–121. [Google Scholar] [CrossRef]
- Laquerre, S.; Anderson, D.B.; Argnani, R.; Glorioso, J.C. Herpes simplex virus type 1 glycoprotein B requires a cysteine residue at position 633 for folding, processing, and incorporation into mature infectious virus particles. J. Virol. 1998, 72, 4940–4949. [Google Scholar] [CrossRef]
- Groth, A.D.; Contreras, M.T.; Kado-Fong, H.K.; Nguyen, K.Q.; Thomasy, S.M.; Maggs, D.J. In vitro cytotoxicity and antiviral efficacy against feline herpesvirus type 1 of famciclovir and its metabolites. Vet. Ophthalmol. 2014, 17, 268–274. [Google Scholar] [CrossRef]
- Kopecny, L.; Maggs, D.J.; Leutenegger, C.M.; Johnson, L.R. Effects of famciclovir in cats with spontaneous acute upper respiratory tract disease. J. Feline Med. Surg. 2020, 22, 492–499. [Google Scholar] [CrossRef] [PubMed]
- Ledbetter, E.C.; Badanes, Z.I.; Chan, R.X.; Donohue, L.K.; Hayot, N.L.; Harman, R.M.; Van de Walle, G.R.; Mohammed, H.O. Comparative Efficacy of Topical Ophthalmic Ganciclovir and Oral Famciclovir in Cats with Experimental Ocular Feline Herpesvirus-1 Epithelial Infection. J. Ocul. Pharmacol. Ther. Off. J. Assoc. Ocul. Pharmacol. Ther. 2022, 38, 339–347. [Google Scholar] [CrossRef] [PubMed]
- Lewin, A.C.; Ineck, N.E.; Mironovich, M.A.; Marino, M.E.; Liu, C.C.; Emelogu, U.; Mills, E.P.; Camacho-Luna, P.; Carter, R.T. Surveillance for feline herpesvirus type 1 mutation and development of resistance in cats treated with antiviral medications. Front. Vet. Sci. 2023, 10, 1197249. [Google Scholar] [CrossRef] [PubMed]
- Mironovich, M.A.; Yoon, A.; Marino, M.E.; Ineck, N.E.; Liu, C.C.; Carter, R.T.; Lewin, A.C. Evaluation of compounded cidofovir, famciclovir, and ganciclovir for the treatment of feline herpesvirus ocular surface disease in shelter-housed cats. Vet. Ophthalmol. 2023, 26 (Suppl. S1), 143–153. [Google Scholar] [CrossRef] [PubMed]
- Takakusa, H.; Iwazaki, N.; Nishikawa, M.; Yoshida, T.; Obika, S.; Inoue, T. Drug Metabolism and Pharmacokinetics of Antisense Oligonucleotide Therapeutics: Typical Profiles, Evaluation Approaches, and Points to Consider Compared with Small Molecule Drugs. Nucleic Acid Ther. 2023, 33, 83–94. [Google Scholar] [CrossRef] [PubMed]
- Hsu, W.C.; Chang, S.P.; Lin, L.C.; Li, C.L.; Richardson, C.D.; Lin, C.C.; Lin, L.T. Limonium sinense and gallic acid suppress hepatitis C virus infection by blocking early viral entry. Antivir. Res. 2015, 118, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Yuan, X.; Sun, Y.; Mao, X.; Meng, C.; Tan, L.; Song, C.; Qiu, X.; Ding, C.; Liao, Y. Infectious bronchitis virus entry mainly depends on clathrin mediated endocytosis and requires classical endosomal/lysosomal system. Virology 2019, 528, 118–136. [Google Scholar] [CrossRef] [PubMed]
- Bergmann, M.; Ballin, A.; Schulz, B.; Dörfelt, R.; Hartmann, K. Treatment of acute viral feline upper respiratory tract infections. Tierarztl. Praxis. Ausg. K Kleintiere/Heimtiere 2019, 47, 98–109. [Google Scholar] [CrossRef]
- Piret, J.; Boivin, G. Resistance of herpes simplex viruses to nucleoside analogues: Mechanisms, prevalence, and management. Antimicrob. Agents Chemother. 2011, 55, 459–472. [Google Scholar] [CrossRef]
- Raza, S.; Shahin, F.; Zhai, W.; Li, H.; Alvisi, G.; Yang, K.; Chen, X.; Chen, Y.; Chen, J.; Hu, C.; et al. Ivermectin Inhibits Bovine Herpesvirus 1 DNA Polymerase Nuclear Import and Interferes with Viral Replication. Microorganisms 2020, 8, 409. [Google Scholar] [CrossRef]
- van der Meulen, K.; Garré, B.; Croubels, S.; Nauwynck, H. In vitro comparison of antiviral drugs against feline herpesvirus 1. BMC Vet. Res. 2006, 2, 13. [Google Scholar] [CrossRef]
- Chiang, L.C.; Ng, L.T.; Liu, L.T.; Shieh, D.E.; Lin, C.C. Cytotoxicity and anti-hepatitis B virus activities of saikosaponins from Bupleurum species. Planta Medica 2003, 69, 705–709. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.T.; Chung, C.Y.; Hsu, W.C.; Chang, S.P.; Hung, T.C.; Shields, J.; Russell, R.S.; Lin, C.C.; Li, C.F.; Yen, M.H.; et al. Saikosaponin b2 is a naturally occurring terpenoid that efficiently inhibits hepatitis C virus entry. J. Hepatol. 2015, 62, 541–548. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.S.; Im, H.T.; Lee, K.T. Saikosaponin B2 Suppresses Inflammatory Responses Through IKK/IκBα/NF-κB Signaling Inactivation in LPS-Induced RAW 264.7 Macrophages. Inflammation 2019, 42, 342–353. [Google Scholar] [CrossRef] [PubMed]
- Wijayasinghe, Y.S.; Bhansali, P.; Viola, R.E.; Kamal, M.A.; Poddar, N.K. Natural Products: A Rich Source of Antiviral Drug Lead Candidates for the Management of COVID-19. Curr. Pharm. Des. 2021, 27, 3526–3550. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Cai, D.; Zhang, L.; Tang, W.; Yan, R.; Guo, H.; Chen, X. Identification of hydrolyzable tannins (punicalagin, punicalin and geraniin) as novel inhibitors of hepatitis B virus covalently closed circular DNA. Antivir. Res. 2016, 134, 97–107. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Du, R.; Chen, Z.; Wang, Y.; Zhan, P.; Liu, X.; Kang, D.; Chen, Z.; Zhao, X.; Wang, L.; et al. Punicalagin is a neuraminidase inhibitor of influenza viruses. J. Med. Virol. 2021, 93, 3465–3472. [Google Scholar] [CrossRef]
- Li, S.; Ye, M.; Chen, Y.; Zhang, Y.; Li, J.; Liu, W.; Li, H.; Peng, K. Screening of a Small Molecule Compound Library Identifies Toosendanin as an Inhibitor Against Bunyavirus and SARS-CoV-2. Front. Pharmacol. 2021, 12, 735223. [Google Scholar] [CrossRef]
- Saadh, M.J.; Almaaytah, A.M.; Alaraj, M.; Dababneh, M.F.; Sa’adeh, I.; Aldalaen, S.M.; Kharshid, A.M.; Alboghdadly, A.; Hailat, M.; Khaleel, A.; et al. Punicalagin and zinc (II) ions inhibit the activity of SARS-CoV-2 3CL-protease in vitro. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 3908–3913. [Google Scholar] [CrossRef]
- Jean-Gilles, D.; Li, L.; Vaidyanathan, V.G.; King, R.; Cho, B.; Worthen, D.R.; Chichester, C.O., 3rd; Seeram, N.P. Inhibitory effects of polyphenol punicalagin on type-II collagen degradation in vitro and inflammation in vivo. Chem.-Biol. Interact. 2013, 205, 90–99. [Google Scholar] [CrossRef]
- Pinheiro Silva, L.; Damacena de Angelis, C.; Bonamin, F.; Kushima, H.; José Mininel, F.; Campaner Dos Santos, L.; Karina Delella, F.; Luis Felisbino, S.; Vilegas, W.; Regina Machado da Rocha, L.; et al. Terminalia catappa L.: A medicinal plant from the Caribbean pharmacopeia with anti-Helicobacter pylori and antiulcer action in experimental rodent models. J. Ethnopharmacol. 2015, 159, 285–295. [Google Scholar] [CrossRef]

| Combining Drugs | CC50 (μg/mL) | Anti-FHV-1 |
|---|---|---|
| Saikosaponin B2 | 197.5 ± 0.52 | √ |
| Baicalin | 65.80 ± 2.117 | × |
| 4,5-Dicaffeoylquinic acid | 54.52 ± 2.89 | × |
| Ivermectin | 1.81 ± 0.06 | × |
| Hyperoside | >50 | × |
| Isoquercetin | 289.35 ± 5.69 | × |
| Dihydromyricetin | 3.22 ± 0.43 | × |
| Diosmin | 449.75 ± 0.21 | × |
| Verbascoside | 79.56 ± 0.99 | × |
| Allicin | 84.91 ± 0.87 | × |
| Sennoside A | 130.82 ± 8.86 | × |
| Cynarine | 60.08 ± 1.86 | × |
| Punicalin | 7.65 ± 0.68 | √ |
| Punicalagin | 3.32 ± 0.28 | √ |
| Scutellarin | 185.2 ± 3.37 | × |
| Isochlorgenic acid A | 100.71 ± 8.45 | × |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, B.; Jiao, X.-Q.; Dong, X.-F.; Guo, P.; Wang, S.-B.; Qin, Z.-H. Saikosaponin B2, Punicalin, and Punicalagin in Vitro Block Cellular Entry of Feline Herpesvirus-1. Viruses 2024, 16, 231. https://doi.org/10.3390/v16020231
Liu B, Jiao X-Q, Dong X-F, Guo P, Wang S-B, Qin Z-H. Saikosaponin B2, Punicalin, and Punicalagin in Vitro Block Cellular Entry of Feline Herpesvirus-1. Viruses. 2024; 16(2):231. https://doi.org/10.3390/v16020231
Chicago/Turabian StyleLiu, Bin, Xiao-Qian Jiao, Xu-Feng Dong, Pei Guo, Shu-Bai Wang, and Zhi-Hua Qin. 2024. "Saikosaponin B2, Punicalin, and Punicalagin in Vitro Block Cellular Entry of Feline Herpesvirus-1" Viruses 16, no. 2: 231. https://doi.org/10.3390/v16020231




