Culicoides Midge Abundance across Years: Modeling Inter-Annual Variation for an Avian Feeder and a Candidate Vector of Hemorrhagic Diseases in Farmed Wildlife
Abstract
:1. Introduction
2. Materials and Methods
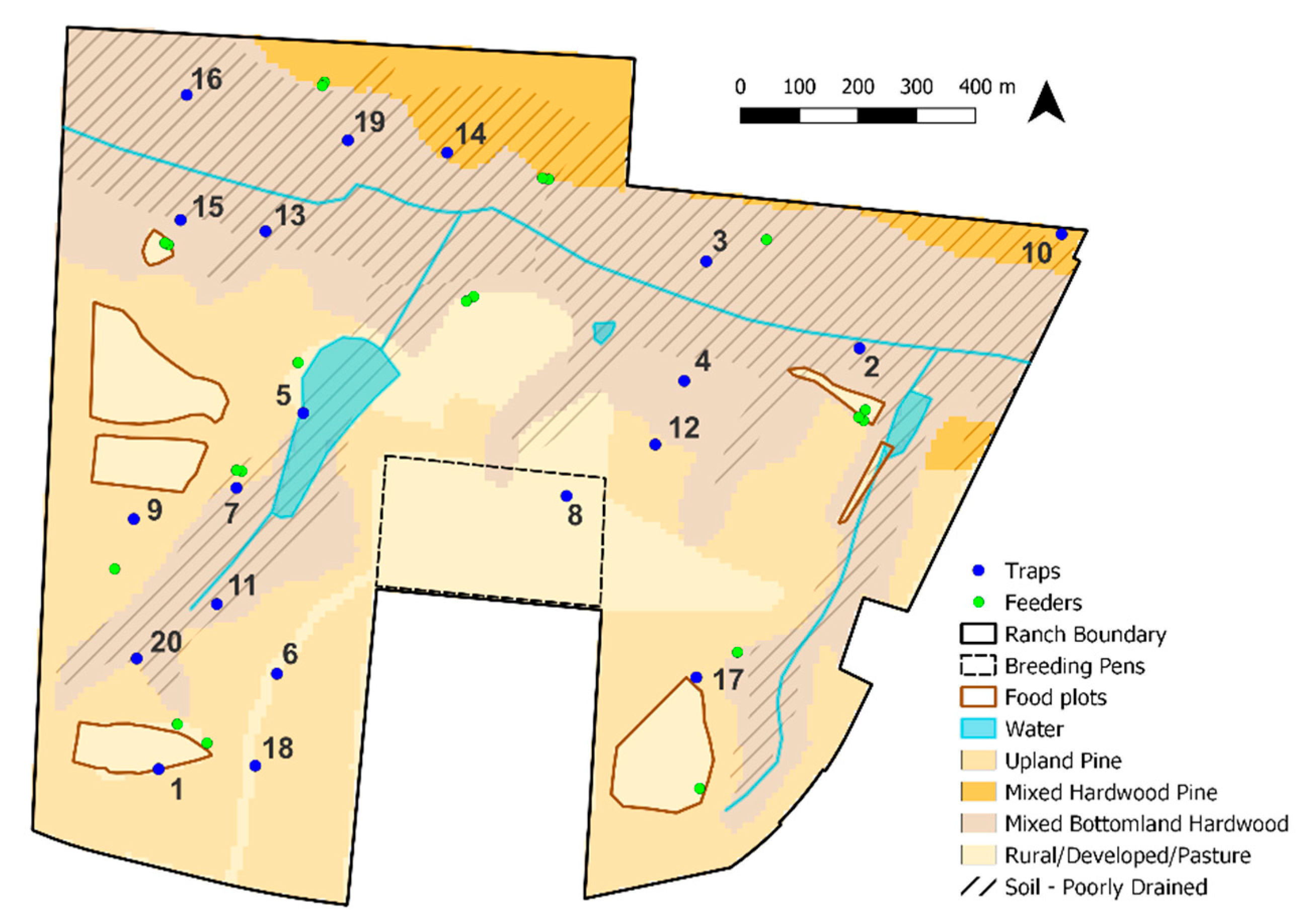
2.1. Study Area
2.2. Entomological Sampling
2.3. Environmental Data
2.4. Model Construction
3. Results
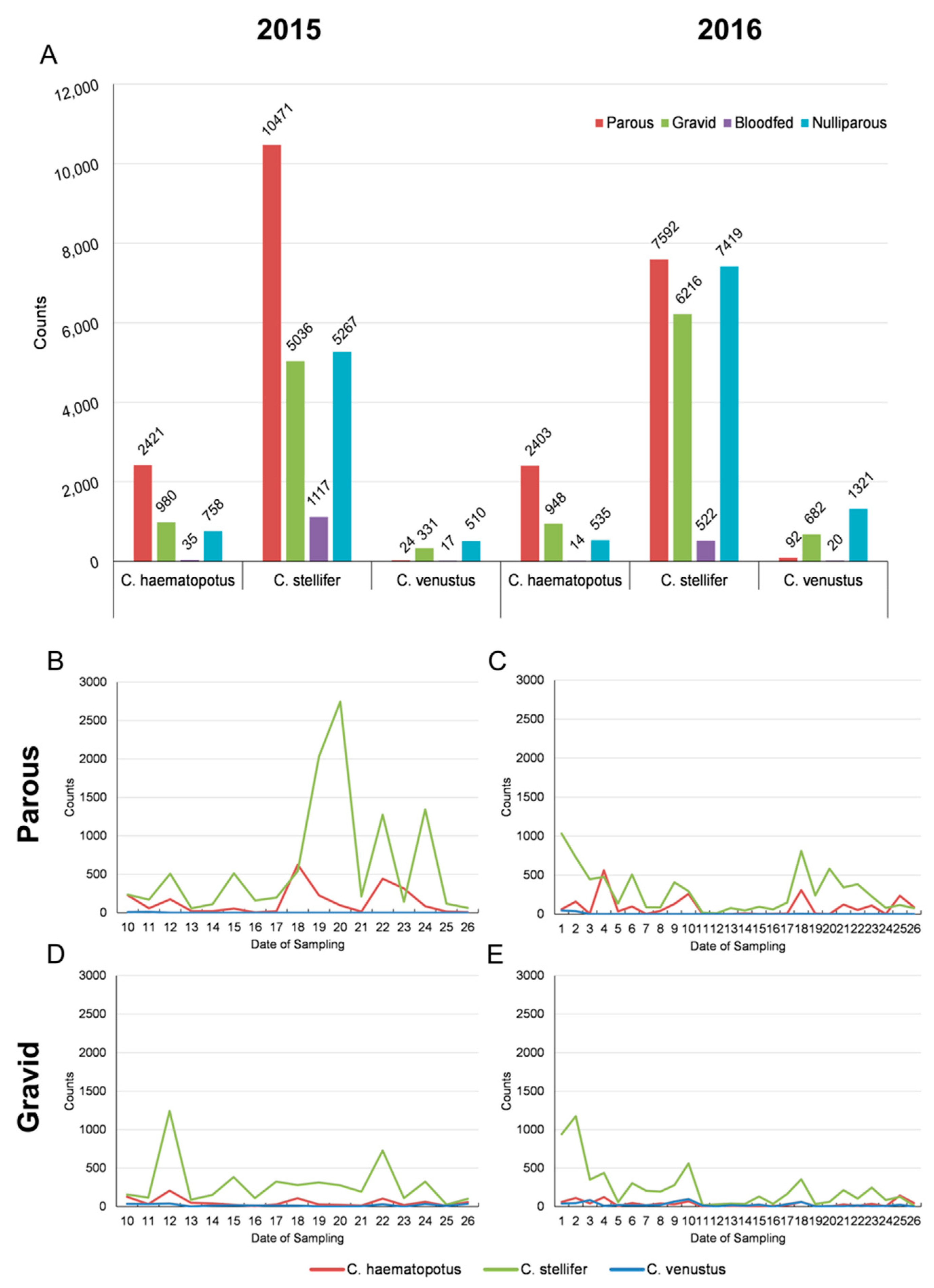
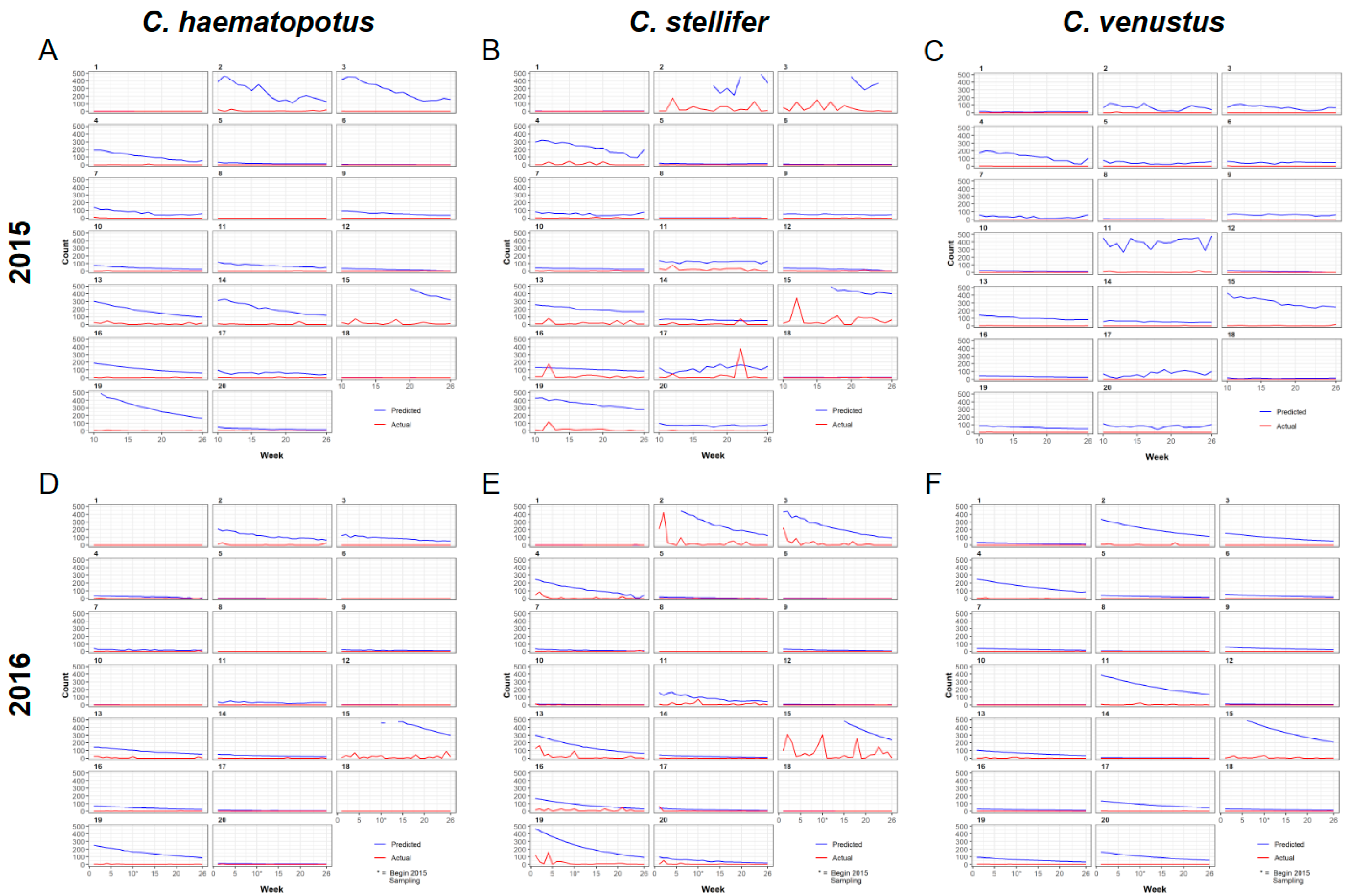
3.1. Entomological Sampling
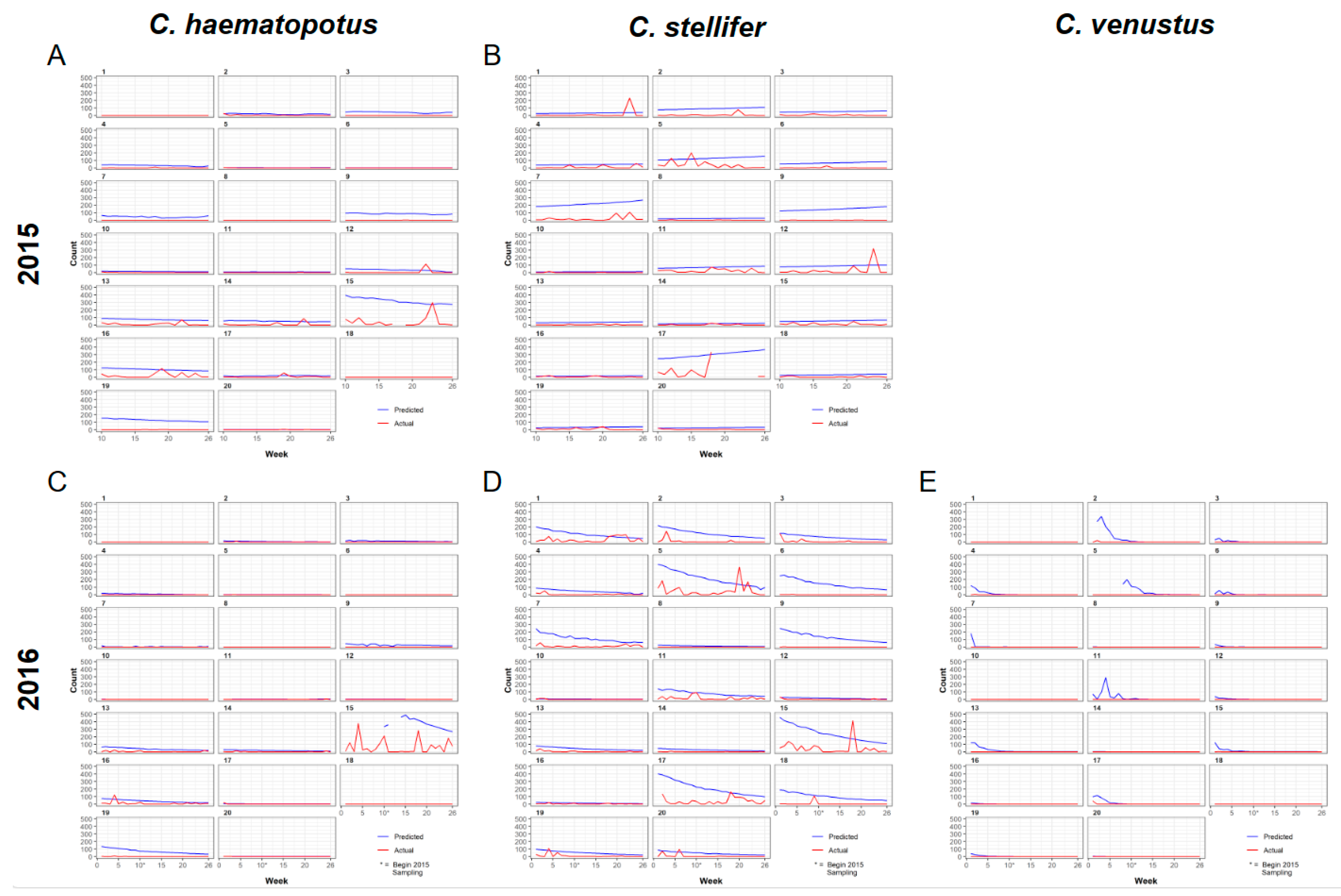
3.2. Universal Model for Culicoides Species Abundance
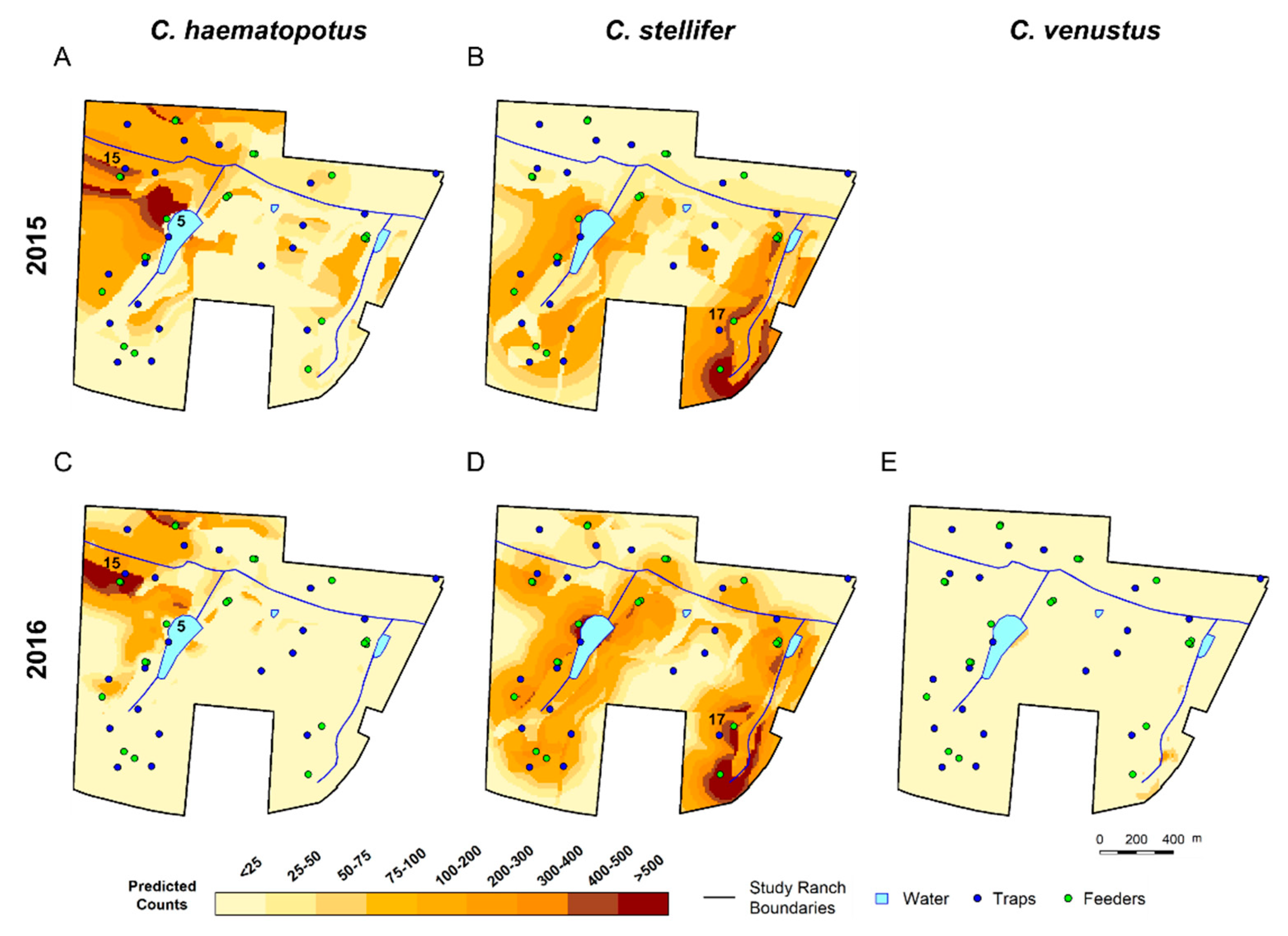
3.3. Spatial Predictions Using the Universal Model
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stevens, G.; McCluskey, B.; King, A.; O’Hearn, E.; Mayr, G. Review of the 2012 Epizootic Hemorrhagic Disease Outbreak in Domestic Ruminants in the United States. PLoS ONE 2015, 10, e0133359. [Google Scholar] [CrossRef] [PubMed]
- Wilson, A.; Mellor, P. Bluetongue in Europe: Vectors, Epidemiology and Climate Change. Parasitol. Res. 2008, 103, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Ruder, M.G.; Lysyk, T.J.; Stallknecht, D.E.; Foil, L.D.; Johnson, D.J.; Chase, C.C.; Dargatz, D.A.; Gibbs, E.P.J. Transmission and Epidemiology of Bluetongue and Epizootic Hemorrhagic Disease in North America: Current Perspectives, Research Gaps, and Future Directions. Vector-Borne Zoonotic Dis. 2015, 15, 348–363. [Google Scholar] [CrossRef] [PubMed]
- Savini, G.; Afonso, A.; Mellor, P.; Aradaib, I.; Yadin, H.; Sanaa, M.; Wilson, W.; Monaco, F.; Domingo, M. Epizootic Haemorragic Disease. Res. Vet. Sci. 2011, 91, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Stair, E.L.; Robinson, R.M.; Jones, L.P. Spontaneous Bluetongue in Texas White-Tailed Deer. Pathol. Vet. 1968, 5, 164–173. [Google Scholar] [CrossRef] [PubMed]
- Howerth, E.W.; Stallknecht, D.E.; Kirkland Peter, D. Bluetongue, epizootic hemorrhagic disease, and other orbivirus-related diseases. In Infectious Diseases of Wild Mammals; Williams, E.S., Barker, I.K., Eds.; John Wiley & Sons, Incorporated: Ames, IA, USA, 2000; pp. 77–97. ISBN 978-0-470-34481-1. [Google Scholar]
- Nol, P.; Kato, C.; Reeves, W.K.; Rhyan, J.; Spraker, T.; Gidlewski, T.; VerCauteren, K.; Salman, M. Epizootic Hemorrhagic Disease Outbreak in a Captive Facility Housing White-Tailed Deer (Odocoileus virginianus), Bison (Bison bison), Elk (Cervus elaphus), Cattle (Bos taurus), and Goats (Capra hircus) in Colorado, U.S.A. J. Zoo Wildl. Med. 2010, 41, 510–515. [Google Scholar] [CrossRef]
- Thorne, E.T.; Williams, E.S.; Spraker, T.R.; Helms, W.; Segerstrom, T. Bluetongue in free-ranging pronghorn antelope (antilocapra americana) in wyoming: 1976 and 1984. JWDI 1988, 24, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Dubay, S.A.; Noon, T.H.; deVos, J.C.; Ockenfels, R.A. Serologic Survey for Pathogens Potentially Affecting Pronghorn (Antilocapra Americana) Fawn Recruitment in Arizona, USA. JWDI 2006, 42, 844–848. [Google Scholar] [CrossRef]
- Robinson, R.M.; Hailey, T.L.; Livingston, C.W.; Thomas, J.W. Bluetongue in the Desert Bighorn Sheep. J. Wildl. Manag. 1967, 31, 165–168. [Google Scholar] [CrossRef]
- Ruder, M.G.; Allison, A.B.; Stallknecht, D.E.; Mead, D.G.; McGraw, S.M.; Carter, D.L.; Kubiski, S.V.; Batten, C.A.; Klement, E.; Howerth, E.W. Susceptibility of white-tailed deer (Odocoileus virginianus) to experimental infection with epizootic hemorrhagic disease virus serotype 7. J. Wildl. Dis. 2012, 48, 676–685. [Google Scholar] [CrossRef]
- Allison, A.B.; Goekjian, V.H.; Potgieter, A.C.; Wilson, W.C.; Johnson, D.J.; Mertens, P.P.C.; Stallknecht, D.E. Detection of a Novel Reassortant Epizootic Hemorrhagic Disease Virus (EHDV) in the USA Containing RNA Segments Derived from Both Exotic (EHDV-6) and Endemic (EHDV-2) Serotypes. J. Gen. Virol. 2010, 91, 430–439. [Google Scholar] [CrossRef] [PubMed]
- Johnson, D.J.; Ostlund, E.N.; Stallknecht, D.E.; Goekjian, V.H.; Jenkins-Moore, M.; Harris, S.C. First Report of Bluetongue Virus Serotype 1 Isolated from a White-Tailed Deer in the United States. J. Vet. Diagn. Investig. 2006, 18, 398–401. [Google Scholar] [CrossRef] [PubMed]
- Drolet, B.S.; Reister, L.M.; Rigg, T.D.; Nol, P.; Podell, B.K.; Mecham, J.O.; VerCauteren, K.C.; van Rijn, P.A.; Wilson, W.C.; Bowen, R.A. Experimental Infection of White-Tailed Deer (Odocoileus virginianus) with Northern European Bluetongue Virus Serotype 8. Vet. Microbiol. 2013, 166, 347–355. [Google Scholar] [CrossRef] [PubMed]
- Gaydos, J.K.; Crum, J.M.; Davidson, W.R.; Cross, S.S.; Owen, S.F.; Stallknecht, D.E. Epizootiology of an epizootic hemorrhagic disease outbreak in West Virginia. JWDI 2004, 40, 383–393. [Google Scholar] [CrossRef] [PubMed]
- Anderson, D.P.; Frosch, B.J.; Outlaw, J.L. Economic Impact of the United States Cervid Farming Industry; Texas A&M University Agricultural and Food Policy Center: College Station, TX, USA, 2007. [Google Scholar]
- Anderson, D.P.; Outlaw, J.L.; Earle, M.L.; Richardson, J.W. Economic Impact of Texas Deer Breeding and Hunting Operations; Texas A&M University Agricultural and Food Policy Center: College Station, TX, USA, 2017; pp. 1–20. [Google Scholar]
- Stallknecht, D.E.; Luttrell, M.P.; Smith, K.E.; Nettles, V.F. Hemorrhagic Disease in White-Tailed Deer in Texas: A Case for Enzootic Stability. JWDI 1996, 32, 695–700. [Google Scholar] [CrossRef] [PubMed]
- McGregor, B.L.; Sloyer, K.E.; Sayler, K.A.; Goodfriend, O.; Krauer, J.M.C.; Acevedo, C.; Zhang, X.; Mathias, D.; Wisely, S.M.; Burkett-Cadena, N.D. Field Data Implicating Culicoides Stellifer and Culicoides Venustus (Diptera: Ceratopogonidae) as Vectors of Epizootic Hemorrhagic Disease Virus. Parasites Vectors 2019, 12, 258. [Google Scholar] [CrossRef] [PubMed]
- Cottingham, S.L.; White, Z.S.; Wisely, S.M.; Campos-Krauer, J.M. A Mortality-Based Description of EHDV and BTV Prevalence in Farmed White-Tailed Deer (Odocoileus virginianus) in Florida, USA. Viruses 2021, 13, 1443. [Google Scholar] [CrossRef]
- Purse, B.V.; Mellor, P.S.; Rogers, D.J.; Samuel, A.R.; Mertens, P.P.C.; Baylis, M. Climate Change and the Recent Emergence of Bluetongue in Europe. Nat. Rev. Microbiol. 2005, 3, 171–181. [Google Scholar] [CrossRef]
- Noronha, L.E.; Cohnstaedt, L.W.; Richt, J.A.; Wilson, W.C. Perspectives on the Changing Landscape of Epizootic Hemorrhagic Disease Virus Control. Viruses 2021, 13, 2268. [Google Scholar] [CrossRef]
- Allen, S.E.; Rothenburger, J.L.; Jardine, C.M.; Ambagala, A.; Hooper-McGrevy, K.; Colucci, N.; Furukawa-Stoffer, T.; Vigil, S.; Ruder, M.; Nemeth, N.M. Epizootic Hemorrhagic Disease in White-Tailed Deer, Canada. Emerg. Infect. Dis. 2019, 25, 832–834. [Google Scholar] [CrossRef]
- Allen, S.E.; Jardine, C.M.; Hooper-McGrevy, K.; Ambagala, A.; Bosco-Lauth, A.M.; Kunkel, M.R.; Mead, D.G.; Nituch, L.; Ruder, M.G.; Nemeth, N.M. Serologic Evidence of Arthropod-Borne Virus Infections in Wild and Captive Ruminants in Ontario, Canada. Am. J. Trop. Med. Hyg. 2020, 103, 2100–2107. [Google Scholar] [CrossRef] [PubMed]
- Allison, A.B.; Holmes, E.C.; Potgieter, A.C.; Wright, I.M.; Sailleau, C.; Breard, E.; Ruder, M.G.; Stallknecht, D.E. Segmental Configuration and Putative Origin of the Reassortant orbivirus, Epizootic Hemorrhagic Disease Virus Serotype 6, Strain Indiana. Virology 2012, 424, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, S.; McArthur, C.; Selby, R.; Ward, R.; Nolan, D.V.; Luntz, A.J.M.; Dallas, J.F.; Tripet, F.; Mellor, P.S. Experimental Infection Studies of UK Culicoides Species Midges with Bluetongue Virus Serotypes 8 and 9. Vet. Rec. 2008, 163, 589–592. [Google Scholar] [CrossRef] [PubMed]
- Elbers, A.R.W.; Backx, A.; Meroc, E.; Gerbier, G.; Staubach, C.; Hendrickx, G.; van der Spek, A.; Mintiens, K. Field Observations during the Bluetongue Serotype 8 Epidemic in 2006: I. Detection of First Outbreaks and Clinical Signs in Sheep and Cattle in Belgium, France and the Netherlands. Prev. Vet. Med. 2008, 87, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Maan, S.; Maan, N.S.; Ross-smith, N.; Batten, C.A.; Shaw, A.E.; Anthony, S.J.; Samuel, A.R.; Darpel, K.E.; Veronesi, E.; Oura, C.A.L.; et al. Sequence Analysis of Bluetongue Virus Serotype 8 from the Netherlands 2006 and Comparison to Other European Strains. Virology 2008, 377, 308–318. [Google Scholar] [CrossRef] [PubMed]
- Maan, S.; Maan, N.S.; Rijn, P.A.V.; van Gennip, R.G.; Sanders, A.; Wright, I.M.; Batten, C.; Hoffmann, B.; Eschbaumer, M.; Oura, C.A.L.; et al. Full Genome Characterisation of Bluetongue Virus Serotype 6 from the Netherlands 2008 and Comparison to Other Field and Vaccine Strains. PLoS ONE 2010, 5, e10323. [Google Scholar] [CrossRef] [PubMed]
- Elbers, A.R.W.; Koenraadt, C.; Meiswinkel, R. Mosquitoes and Culicoides Biting Midges: Vector Range and the Influence of Climate Change: -EN- -FR-Les Moustiques et Les Moucherons Piqueurs Culicoides: Diversité Des Vecteurs et Influence Du Changement Climatique-ES-Mosquitos y Jejenes Culicoides: Distribución de Los Vectores e Influencia Del Cambio Climático. Rev. Sci. Tech. OIE 2015, 34, 123–137. [Google Scholar] [CrossRef] [PubMed]
- Foxi, C.; Delrio, G.; Falchi, G.; Marche, M.G.; Satta, G.; Ruiu, L. Role of Different Culicoides Vectors (Diptera: Ceratopogonidae) in Bluetongue Virus Transmission and Overwintering in Sardinia (Italy). Parasites Vectors 2016, 9, 440. [Google Scholar] [CrossRef] [PubMed]
- Calvo, J.H.; Berzal, B.; Calvete, C.; Miranda, M.A.; Estrada, R.; Lucientes, J. Host Feeding Patterns of Culicoides Species (Diptera: Ceratopogonidae) within the Picos de Europa National Park in Northern Spain. Bull. Entomol. Res. 2012, 102, 692–697. [Google Scholar] [CrossRef]
- Vasić, A.; Zdravković, N.; Aniță, D.; Bojkovski, J.; Marinov, M.; Mathis, A.; Niculaua, M.; Oșlobanu, E.L.; Pavlović, I.; Petrić, D.; et al. Species Diversity, Host Preference and Arbovirus Detection of Culicoides (Diptera: Ceratopogonidae) in South-Eastern Serbia. Parasites Vectors 2019, 12, 61. [Google Scholar] [CrossRef]
- Dinh, E.T.N.; Gomez, J.P.; Orange, J.P.; Morris, M.A.; Sayler, K.A.; McGregor, B.L.; Blosser, E.M.; Burkett-Cadena, N.D.; Wisely, S.M.; Blackburn, J.K. Modeling Abundance of Culicoides Stellifer, a Candidate Orbivirus Vector, Indicates Nonrandom Hemorrhagic Disease Risk for White-Tailed Deer (Odocoileus virginianus). Viruses 2021, 13, 1328. [Google Scholar] [CrossRef] [PubMed]
- Purse, B.V.; Carpenter, S.; Venter, G.J.; Bellis, G.; Mullens, B.A. Bionomics of Temperate and Tropical Culicoides Midges: Knowledge Gaps and Consequences for Transmission of Culicoides-Borne Viruses. Annu. Rev. Entomol. 2015, 60, 373–392. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, S.; Wilson, A.; Mellor, P.S. Culicoides and the Emergence of Bluetongue Virus in Northern Europe. Trends Microbiol. 2009, 17, 172–178. [Google Scholar] [CrossRef] [PubMed]
- Cauvin, A.; Dinh, E.T.N.; Orange, J.P.; Shuman, R.M.; Blackburn, J.K.; Wisely, S.M. Antibodies to Epizootic Hemorrhagic Disease Virus (EHDV) in Farmed and Wild Florida White-Tailed Deer (Odocoileus virginianus). J. Wildl. Dis. 2020, 56, 208–213. [Google Scholar] [CrossRef] [PubMed]
- McGregor, B.L.; Stenn, T.; Sayler, K.A.; Blosser, E.M.; Blackburn, J.K.; Wisely, S.M.; Burkett-Cadena, N.D. Host Use Patterns of Culicoides Spp. Biting Midges at a Big Game Preserve in Florida, U.S.A., and Implications for the Transmission of Orbiviruses. Med. Vet. Entomol. 2019, 33, 110–120. [Google Scholar] [CrossRef] [PubMed]
- Blanton, F.S.; Wirth, W.W. The Sand Flies (Culicoides) of Florida (Diptera: Ceratopogonidae). In Arthropods of Florida and Neighboring Land Areas; Florida Department of Agriculture and COnsumer Affairs, Division of Plant Industry: Gainesville, FL, USA, 1979; Volume 10, pp. 188–200. [Google Scholar]
- Holbrook, F.R. Biting MIdges And The Agents They Transmit. In The Biology of Disease Vectors; Beaty, B.J., Marquardt, W.C., Eds.; University Press of Colorado: Niwot, CO, USA, 1996; pp. 110–116. ISBN 0-87081-411-7. [Google Scholar]
- Cooperative Land Cover, Version 3.5—Published November 2021. Available online: https://myfwc.com/research/gis/regional-projects/cooperative-land-cover/ (accessed on 8 July 2022).
- Dinh, E.T.N. Epizootic Hemorrhagic Disease Virus Transmission Risk in North Florida Free-Ranging Ranched and Wild White-Tailed Deer. Ph.D. Thesis, University of Florida, Gainesville, FL, USA, 2019. [Google Scholar]
- Web Soil Survey—Home. Available online: https://websoilsurvey.sc.egov.usda.gov/App/HomePage.htm (accessed on 8 July 2022).
- Erram, D.; Burkett-Cadena, N. Laboratory Studies on the Oviposition Stimuli of Culicoides Stellifer (Diptera: Ceratopogonidae), a Suspected Vector of Orbiviruses in the United States. Parasites Vectors 2018, 11, 300. [Google Scholar] [CrossRef] [PubMed]
- Erram, D.; Blosser, E.M.; Burkett-Cadena, N. Habitat Associations of Culicoides Species (Diptera: Ceratopogonidae) Abundant on a Commercial Cervid Farm in Florida, USA. Parasites Vectors 2019, 12, 367. [Google Scholar] [CrossRef] [PubMed]
- Worton, B.J. Kernel Methods for Estimating the Utilization Distribution in Home-Range Studies. Ecology 1989, 70, 164–168. [Google Scholar] [CrossRef]
- Getz, W.M.; Wilmers, C.C. A Local Nearest-Neighbor Convex-Hull Construction of Home Ranges and Utilization Distributions. Ecography 2004, 27, 489–505. [Google Scholar] [CrossRef]
- Dinh, E.T.N.; Cauvin, A.; Orange, J.P.; Shuman, R.M.; Wisely, S.M.; Blackburn, J.K. Living La Vida T-LoCoH: Site Fidelity of Florida Ranched and Wild White-Tailed Deer (Odocoileus virginianus) during the Epizootic Hemorrhagic Disease Virus (EHDV) Transmission Period. Mov. Ecol. 2020, 8, 14. [Google Scholar] [CrossRef]
- Fiske, I.; Chandler, R. Unmarked: An R Package for Fitting Hierarchical Models of Wildlife Occurrence and Abundance. J. Stat. Softw. 2011, 43, 1–23. [Google Scholar] [CrossRef]
- Fiske, I.; Chandler, R. Overview of Unmarked: An R Package for the Analysis of Data from Unmarked Animals. Available online: https://citeseerx.ist.psu.edu/document?repid=rep1&type=pdf&doi=a6120f7b6305fdd39c429c8738f2eec24196b2be (accessed on 8 July 2022).
- A Method for Estimating Insect Abundance and Patch Occupancy with Potential Applications in Large-Scale Monitoring Programmes. Available online: https://journals.co.za/doi/10.10520/EJC32718 (accessed on 8 July 2022).
- Royle, J.A. N-Mixture Models for Estimating Population Size from Spatially Replicated Counts. Biometrics 2004, 60, 108–115. [Google Scholar] [CrossRef] [PubMed]
- McGregor, B.L.; Runkel, A.E.; Wisely, S.M.; Burkett-Cadena, N.D. Vertical Stratification of Culicoides Biting Midges at a Florida Big Game Preserve. Parasites Vectors 2018, 11, 505. [Google Scholar] [CrossRef] [PubMed]
- CHeRI HD Cases: Dashboard Experience. Available online: https://experience.arcgis.com/experience/3a08f867439043b1a71e280f73495037/ (accessed on 17 August 2022).
- Smith, K.E.; Stallknecht, D.E. Culicoides (Diptera: Ceratopogonidae) Collected During Epizootics of Hemorrhagic Disease among Captive White-Tailed Deer. J. Med. Entomol. 1996, 33, 507–510. [Google Scholar] [CrossRef] [PubMed]
- Smith, K.E.; Stallknecht, D.E.; Sewell, C.T.; Iii, E.A.R.; Mullen, G.R.; Anderson, R.R. Monitoring of Culicoides Spp. at a site enzootic for hemorrhagic disease in white-tailed deer in Georgia, USA. JWDI 1996, 32, 627–642. [Google Scholar] [CrossRef] [PubMed]
- Climate at a Glance|National Centers for Environmental Information (NCEI). Available online: https://www.ncei.noaa.gov/access/monitoring/climate-at-a-glance/county/rankings/FL-039/tavg/201608 (accessed on 3 August 2022).
- Sanders, C.J.; Shortall, C.R.; England, M.; Harrington, R.; Purse, B.; Burgin, L.; Carpenter, S.; Gubbins, S. Long-Term Shifts in the Seasonal Abundance of Adult Culicoides Biting Midges and Their Impact on Potential Arbovirus Outbreaks. J. Appl. Ecol. 2019, 56, 1649–1660. [Google Scholar] [CrossRef] [PubMed]
- West, R.G.; Mathias, D.R.; Day, J.F.; Boohene, C.K.; Unnasch, T.R.; Burkett-Cadena, N.D. Vectorial Capacity of Culiseta Melanura (Diptera: Culicidae) Changes Seasonally and Is Related to Epizootic Transmission of Eastern Equine Encephalitis Virus in Central Florida. Front. Ecol. Evol. 2020, 8, 270. [Google Scholar] [CrossRef]
- Stokes, J.E.; Carpenter, S.; Sanders, C.; Gubbins, S. Emergence Dynamics of Adult Culicoides Biting Midges at Two Farms in South-East England. Parasites Vectors 2022, 15, 251. [Google Scholar] [CrossRef] [PubMed]
- Sayler, K.; Blosser, E.; McGregor, B.; Burkett-Cadena, N.; Wisely, S.M. Overwintering of Epizootic Hemorrhagic Disease Virus in White-Tailed Deer in Florida, USA: Unanticipated Seroconversion and the Case for Alternative Vectors. Int. J. Infect. Dis. 2016, 53, 65–66. [Google Scholar] [CrossRef]
- Schmidtmann, E.T.; Bobian, R.J.; Belden, R.P. Soil Chemistries Define Aquatic Habitats with Immature Populations of the Culicoides Variipennis Complex (Diptera: Ceratopogonidae). J. Med. Entomol. 2000, 37, 58–64. [Google Scholar] [CrossRef]
- Mandujano, S.; Gallina, S.; JAS, S.-R.; Arceo, G.; Silva-Villilobos, G. Habitat Use by White-Tailed Deer in a Tropical Forest; Deer & Elk Workshop, Fish & Wildlife Department: Rio Rico, AZ, USA, 1997. [Google Scholar]
- Dryden, G. McL. Quantitative Nutrition of Deer: Energy, Protein and Water. Anim. Prod. Sci. 2011, 51, 292. [Google Scholar] [CrossRef]
- Atchley, W.R.; Wirth, W.W. A Review of the Culicoides Haematopotus Group in North America (Diptera: Ceratopogonidae). J. Kans. Entomol. Soc. 1979, 52, 524–545. [Google Scholar]
- Swanson, D.A.; Turnbull, M.W. Molecular Identification of Bloodmeals from Culicoides Latreille (Diptera: Ceratopogonidae) in the Southeastern U.S.A. Proc. Entomol. Soc. Wash. 2014, 116, 354–357. [Google Scholar] [CrossRef]
- McGregor, B.L.; Blackburn, J.K.; Wisely, S.M.; Burkett-Cadena, N.D. Culicoides (Diptera: Ceratopogonidae) Communities Differ Between a Game Preserve and Nearby Natural Areas in Northern Florida. J. Med. Entomol. 2021, 58, 450–457. [Google Scholar] [CrossRef]
- Hopken, M.W.; Ryan, B.M.; Huyvaert, K.P.; Piaggio, A.J. Picky Eaters Are Rare: DNA-Based Blood Meal Analysis of Culicoides (Diptera: Ceratopogonidae) Species from the United States. Parasites Vectors 2017, 10, 169. [Google Scholar] [CrossRef]

| Variable | Description |
|---|---|
| Week | Week in which samples were collected |
| Latitude | Insect trap location latitude |
| Longitude | Insect trap location longitude |
| Feeder | Euclidian distance from each feeder |
| Water | Euclidian distance from major water bodies |
| Habitat | Upland pine, mixed hardwood pine, mixed bottomland Hardwoods, rural/developed/pasture |
| Soil | Poorly drained, well-drained |
| Utilization distribution (UD) | Weekly probability density of deer presence based on GPS collar data collected during the study |
| Covariates | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Year | Species | Life Stage | Universal Model ΔAIC | Best Model ID | Week | Lat | Long | Hab | Feeder | Water | Soil | UD |
| 2015 | C. haematopotus | Parous | 2.03 | G3 | X | X | X | X | - | X | X | - |
| Gravid | 0 | GlobalE | X | X | X | X | X | X | X | X | ||
| C. stellifer | Parous | 2.23 | B3 | X | X | X | X | X | X | X | - | |
| Gravid | 0.5 | B2 | X | X | X | X | X | X | X | X | ||
| C. venustus | Parous | 8.3 | H2 | X | X | X | - | - | X | X | - | |
| Gravid | 0.88 | F1 | X | X | X | X | - | X | X | X | ||
| 2016 | C. haematopotus | Parous | 0 | GlobalE | X | X | X | X | X | X | X | X |
| Gravid | 0 | GlobalE | X | X | X | X | X | X | X | X | ||
| C. stellifer | Parous | 3.543 | F4 | X | X | X | - | X | X | X | X | |
| Gravid | 0 | GlobalE | X | X | X | X | X | X | X | X | ||
| C. venustus | Parous | 7.17 | C2 | X | X | X | - | - | X | X | X | |
| Gravid | 1.99 | F3 | X | X | X | X | X | X | X | - | ||
| 2015 | 2016 | |||||||
|---|---|---|---|---|---|---|---|---|
| Species | Life Stage | Variable | Estimate | Lower CI | Upper CI | Estimate | Lower CI | Upper CI |
| C. haematopotus | Parous | Intercept | 3.9283 | 1.9899 | 5.8667 | 1.3792 | 0.4080 | 2.3504 |
| Week | −0.0251 | −0.0976 | 0.0474 | −0.0565 | −0.0890 | −0.0240 | ||
| Latitude | −0.2257 | −0.7686 | 0.3172 | −0.7474 | −1.1451 | −0.3497 | ||
| Longitude | 1.2017 | 0.5921 | 1.8113 | 1.0429 | 0.5847 | 1.5011 | ||
| Hardwood Pine | −1.5468 | −3.1324 | 0.0388 | 0.0905 | −1.3615 | 1.5425 | ||
| Mixed Bottomland Hardwoods | −1.1175 | −2.3249 | 0.0899 | 0.5947 | −0.4557 | 1.6451 | ||
| Rural/Developed/Pasture | −3.4974 | −4.8400 | −2.1548 | −2.6068 | −4.0055 | −1.2081 | ||
| Distance to Feeder | −0.2925 | −0.8570 | 0.2720 | −0.7749 | −1.3125 | −0.2373 | ||
| Distance to Water | −0.5942 | −1.4037 | 0.2153 | −0.7226 | −1.4507 | 0.0055 | ||
| Soil—Well-Drained | 1.1964 | 0.1792 | 2.2136 | 1.7574 | 0.9146 | 2.6002 | ||
| UD | −0.2319 | −0.6396 | 0.1758 | −0.8701 | −1.4940 | −0.2462 | ||
| Gravid | Intercept | 4.7592 | 3.7126 | 5.8058 | 1.8442 | 0.9157 | 2.7727 | |
| Week | −0.0728 | −0.1134 | −0.0322 | −0.0422 | −0.0692 | −0.0152 | ||
| Latitude | 0.1728 | −0.1518 | 0.4974 | −0.2125 | −0.5377 | 0.1127 | ||
| Longitude | 0.4277 | 0.0402 | 0.8152 | 0.5066 | 0.0411 | 0.9721 | ||
| Hardwood Pine | 0.7949 | −0.3094 | 1.8992 | 0.7308 | −0.5557 | 2.0173 | ||
| Mixed Bottomland Hardwoods | 0.6951 | −0.0179 | 1.4081 | 1.5035 | 0.6950 | 2.3120 | ||
| Rural/Developed/Pasture | −1.9882 | −2.9521 | −1.0243 | −2.8471 | −4.6562 | −1.0380 | ||
| Distance to Feeder | −0.6294 | −1.0098 | −0.2490 | −0.8668 | −1.3474 | −0.3862 | ||
| Distance to Water | −0.6552 | −1.2097 | −0.1007 | −1.2174 | −1.9851 | −0.4497 | ||
| Soil—Well-Drained | 0.7038 | 0.0339 | 1.3737 | 1.0071 | 0.3672 | 1.6470 | ||
| UD | −0.2136 | −0.4911 | 0.0639 | −0.3294 | −0.8327 | 0.1739 | ||
| C. stellifer | Parous | Intercept | 4.221 | 3.2388 | 5.2032 | 3.9575 | 3.3369 | 4.5780 |
| Week | 0.0238 | −0.0156 | 0.0632 | −0.0577 | −0.0787 | −0.0367 | ||
| Latitude | 0.153 | −0.1383 | 0.4443 | −0.0024 | −0.2684 | 0.2636 | ||
| Longitude | −0.3199 | −0.5865 | −0.0533 | −0.4059 | −0.6548 | −0.1569 | ||
| Hardwood Pine | −1.7376 | −2.6582 | −0.8170 | 0.0597 | −0.8493 | 0.9688 | ||
| Mixed Bottomland Hardwoods | −1.2134 | −1.8455 | −0.5813 | 0.2423 | −0.3174 | 0.8021 | ||
| Rural/Developed/Pasture | −0.8437 | −1.4811 | −0.2063 | 0.4621 | −0.1151 | 1.0393 | ||
| Distance to Feeder | −0.2166 | −0.4673 | 0.0341 | −0.7959 | −1.0442 | −0.5475 | ||
| Distance to Water | −0.6428 | −0.9499 | −0.3357 | −0.7113 | −0.9806 | −0.4420 | ||
| Soil—Well-Drained | 0.4181 | −0.2209 | 1.0571 | 1.0961 | 0.5663 | 1.6259 | ||
| UD | −0.0101 | −0.2290 | 0.2088 | −0.1003 | −0.3035 | 0.1030 | ||
| Gravid | Intercept | 4.1836 | 3.1201 | 5.2471 | 2.5868 | 2.0634 | 3.1102 | |
| Week | −0.0293 | −0.0734 | 0.0148 | −0.0650 | −0.0832 | −0.0468 | ||
| Latitude | 0.5101 | 0.2461 | 0.7741 | 0.0947 | −0.0945 | 0.2839 | ||
| Longitude | 0.0741 | −0.1995 | 0.3477 | 0.2074 | −0.0086 | 0.4234 | ||
| Hardwood Pine | −0.1702 | −1.1055 | 0.7651 | 0.5949 | −0.2403 | 1.4301 | ||
| Mixed Bottomland Hardwoods | 1.3422 | 0.6578 | 2.0266 | 2.5083 | 2.0242 | 2.9924 | ||
| Rural/Developed/Pasture | −1.6848 | −2.4184 | −0.9512 | −0.9730 | −1.5591 | −0.3869 | ||
| Distance to Feeder | −0.6471 | −0.9144 | −0.3798 | −0.6411 | −0.8746 | −0.4076 | ||
| Distance to Water | −0.3603 | −0.7431 | 0.0225 | −0.6112 | −0.9108 | −0.3116 | ||
| Soil—Well-Drained | 0.4308 | −0.2076 | 1.0692 | 0.8486 | 0.4276 | 1.2696 | ||
| UD | −0.3187 | −0.5208 | −0.1166 | −0.1660 | −0.3421 | 0.0101 | ||
| C. venustus | Parous | Intercept | 3.2275 | 0.0268 | 6.4282 | |||
| Week | −0.4112 | −0.6425 | −0.1799 | |||||
| Latitude | −0.2923 | −1.7231 | 1.1385 | |||||
| Longitude | −1.2482 | −3.1435 | 0.6471 | |||||
| Hardwood Pine | −7.9021 | −75.5848 | 59.7806 | |||||
| Mixed Bottomland Hardwoods | −0.3547 | −3.5319 | 2.8225 | |||||
| Rural/Developed/Pasture | 0.0806 | −3.9727 | 4.1339 | |||||
| Distance to Feeder | 0.6702 | −1.1683 | 2.5087 | |||||
| Distance to Water | −3.6087 | −6.6193 | −0.5981 | |||||
| Soil—Well-Drained | 1.1208 | −1.3998 | 3.6414 | |||||
| UD | −1.9747 | −3.9974 | 0.0480 | |||||
| Gravid | Intercept | 3.1712 | 1.6934 | 4.6490 | 2.3449 | 1.3514 | 3.3384 | |
| Week | −0.0375 | −0.0794 | 0.0044 | −0.0435 | −0.0696 | −0.0174 | ||
| Latitude | −0.2011 | −0.4847 | 0.0825 | 0.0413 | −0.1767 | 0.2593 | ||
| Longitude | −0.8125 | −1.1620 | −0.4630 | −0.6835 | −0.9936 | −0.3734 | ||
| Hardwood Pine | 1.6433 | 0.4056 | 2.8810 | 0.0416 | −1.6707 | 1.7539 | ||
| Mixed Bottomland Hardwoods | 1.4783 | 0.7313 | 2.2253 | 2.3591 | 1.7362 | 2.9820 | ||
| Rural/Developed/Pasture | −0.4192 | −1.2796 | 0.4412 | −0.0916 | −0.8611 | 0.6779 | ||
| Distance to Feeder | −0.1861 | −0.5326 | 0.1604 | −0.5462 | −0.8476 | −0.2448 | ||
| Distance to Water | −1.1374 | −1.5835 | −0.6913 | −0.8012 | −1.1938 | −0.4086 | ||
| Soil—Well-Drained | 1.1702 | 0.5363 | 1.8041 | 1.4401 | 0.9021 | 1.9781 | ||
| UD | −0.5041 | −0.8694 | −0.1388 | −0.0125 | −0.2385 | 0.2135 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Benn, J.S.; Orange, J.P.; Gomez, J.P.; Dinh, E.T.N.; McGregor, B.L.; Blosser, E.M.; Burkett-Cadena, N.D.; Wisely, S.M.; Blackburn, J.K. Culicoides Midge Abundance across Years: Modeling Inter-Annual Variation for an Avian Feeder and a Candidate Vector of Hemorrhagic Diseases in Farmed Wildlife. Viruses 2024, 16, 766. https://doi.org/10.3390/v16050766
Benn JS, Orange JP, Gomez JP, Dinh ETN, McGregor BL, Blosser EM, Burkett-Cadena ND, Wisely SM, Blackburn JK. Culicoides Midge Abundance across Years: Modeling Inter-Annual Variation for an Avian Feeder and a Candidate Vector of Hemorrhagic Diseases in Farmed Wildlife. Viruses. 2024; 16(5):766. https://doi.org/10.3390/v16050766
Chicago/Turabian StyleBenn, Jamie S., Jeremy P. Orange, Juan Pablo Gomez, Emily T. N. Dinh, Bethany L. McGregor, Erik M. Blosser, Nathan D. Burkett-Cadena, Samantha M. Wisely, and Jason K. Blackburn. 2024. "Culicoides Midge Abundance across Years: Modeling Inter-Annual Variation for an Avian Feeder and a Candidate Vector of Hemorrhagic Diseases in Farmed Wildlife" Viruses 16, no. 5: 766. https://doi.org/10.3390/v16050766









