How much of Virus-Specific CD8 T Cell Reactivity is Detected with a Peptide Pool when Compared to Individual Peptides?
Abstract
:1. Introduction
2. Results and Discussion
2.1 Individual Donor’s CD8 T cell Responses to Individual CEF Peptides
 |
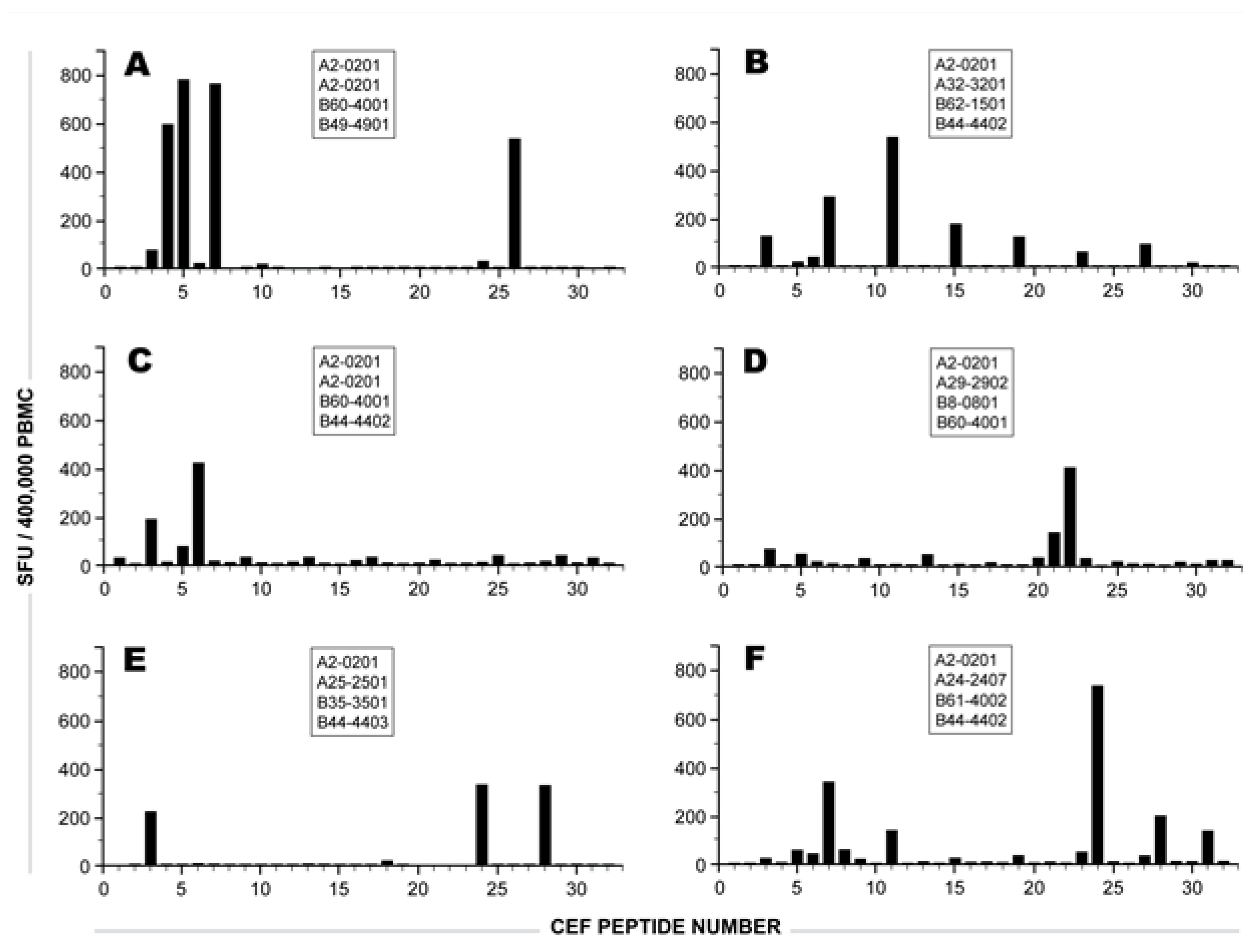
2.2 The Sum of CD8 T Cells Responding to Individual CEF Peptides Approximates the Number of CD8 T Cells Triggered by the CEF-Pool
 |
2.3 Functional Avidity of CEF Peptide-Specific T Cells.
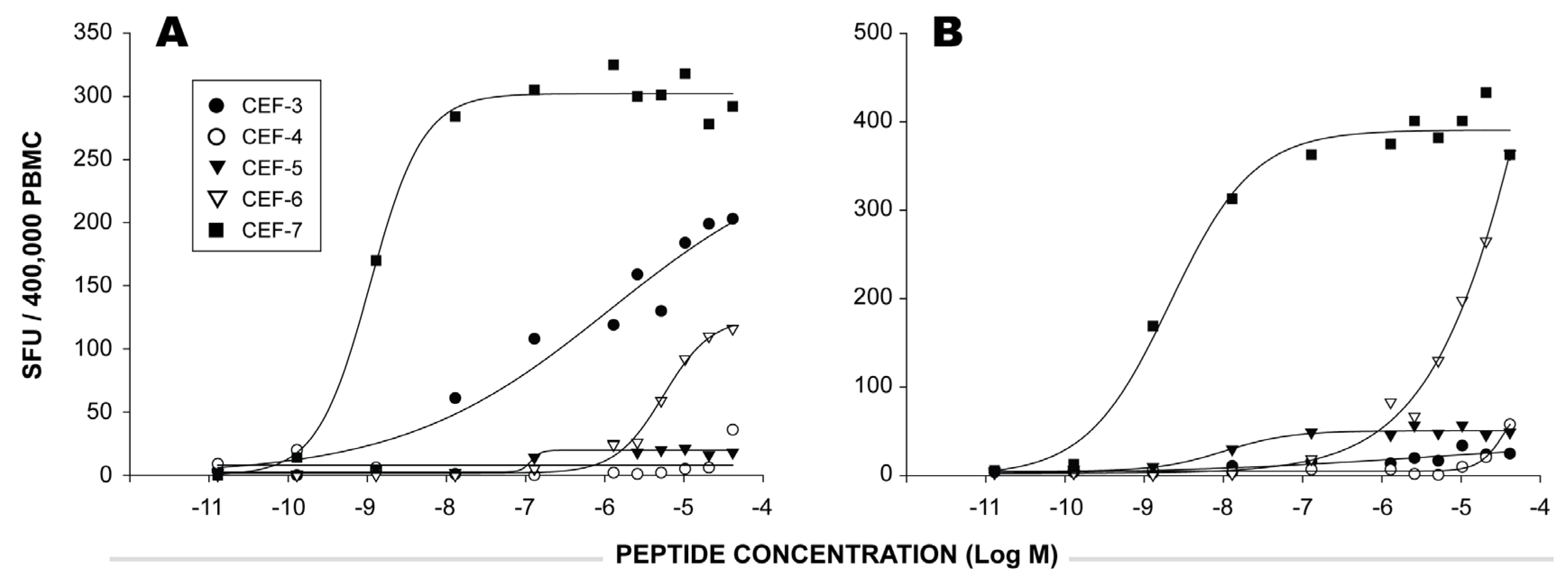
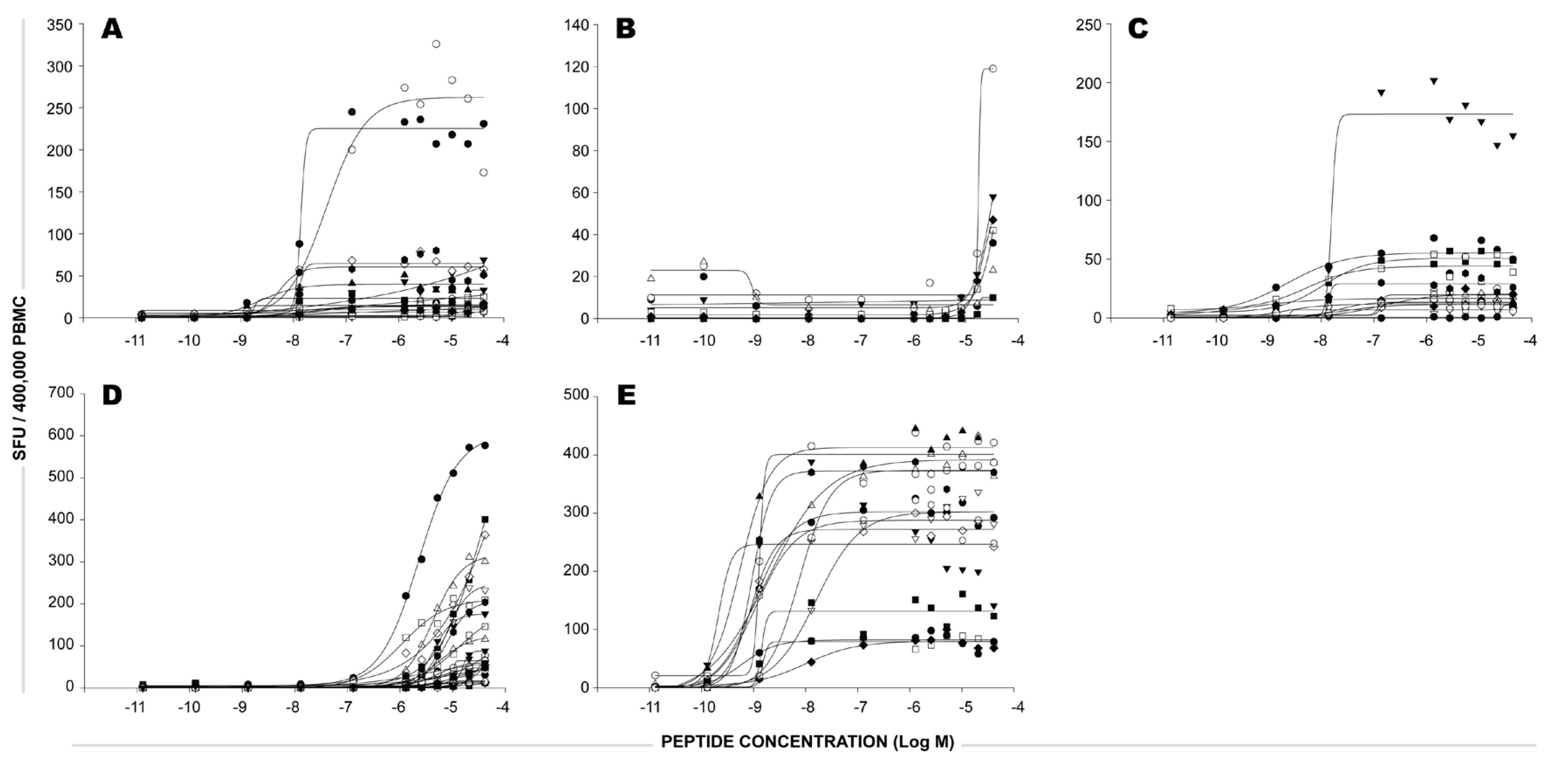
3. Experimental Section
3.1. Thawing and Handling of Cryopreserved PBMC and HLA Typing
3.2 CEF Peptides
3.3. Human Interferon-γ ELISPOT Assay
4. Conclusions
Acknowledgments
Conflict of Interest
References
- Raghavan, M.; Del Cid, N.; Rizvi, S.M.; Peters, L.R. MHC class I assembly: out and about. Trends Immunol. 2008, 29, 436–443. [Google Scholar] [CrossRef]
- Engelhard, V.H. Structure of peptides associated with class I and class II MHC molecules. Annu. Rev. Immunol. 1994, 12, 181–207. [Google Scholar]
- Doytchinova, I.A.; Flower, D.R. Class I T-cell epitope prediction: improvements using a combination of proteasome cleavage, TAP affinity, and MHC binding. Mol. Immunol. 2006, 43, 2037–2044. [Google Scholar] [CrossRef]
- Ben Dror, L.; Barnea, E.; Beer, I.; Mann, M.; Admon, A. The HLA-B*2705 peptidome. Arthritis Rheum. 2010, 62, 420–429. [Google Scholar]
- Johnson, K.L.; Ovsyannikova, I.G.; Mason, C.J.; Bergen, H.R. Discovery of naturally processed and HLA-presented class I peptides from vaccinia virus infection using mass spectrometry for vaccine development. Vaccine 2009, 28, 38–47. [Google Scholar] [CrossRef]
- Chen, Y.; Sidney, J.; Southwood, S.; Cox, A.L.; Sakaguchi, K.; Henderson, R.A.; Appella, E.; Hunt, D.F.; Sette, A.; Engelhard, V.H. Naturally processed peptides longer than nine amino acid residues bind to the class I MHC molecule HLA-A2.1 with high affinity and in different conformations. J. Immunol. 1994, 152, 2874–2881. [Google Scholar]
- Germain, R.N. The biochemistry and cell biology of antigen presentation by MHC class I and class II molecules. Implications for development of combination vaccines. Ann. NY Acad .Sci. 1995, 754, 114–125. [Google Scholar] [CrossRef]
- Doherty, P.C.; Biddison, W.E.; Bennink, J.R.; Knowles, B.B. Cytotoxic T-cell responses in mice infected with influenza and vaccinia viruses vary in magnitude with H-2 genotype. J. Exp. Med. 1978, 148, 534–543. [Google Scholar]
- Zinkernagel, R.M.; Althage, A.; Cooper, S.; Kreeb, G.; Klein, P.A.; Sefton, B.; Flaherty, L.; Stimpfling, J.; Shreffler, D.; Klein, J. Ir-genes in H-2 regulate generation of anti-viral cytotoxic T cells. Mapping to K or D and dominance of unresponsiveness. J. Exp. Med. 1978, 148, 592–606. [Google Scholar] [CrossRef]
- Nagy, Z.A.; Lehmann, P.V.; Falcioni, F.; Muller, S.; Adorini, L. Why peptides? Their possible role in the evolution of MHC-restricted T-cell recognition. Immunol. Today 1989, 10, 132–138. [Google Scholar] [CrossRef]
- Lehmann, P.V.; Cardinaux, F.; Appella, E.; Muller, S.; Falcioni, F.; Adorini, L.; Nagy, Z.A. Inhibition of T cell response with peptides is influenced by both peptide-binding specificity of major histocompatibility complex molecules and susceptibility of T cells to blocking. Eur. J. Immunol. 1989, 19, 1071–1077. [Google Scholar] [CrossRef]
- Currier, J.R.; Kuta, E.G.; Turk, E.; Earhart, L.B.; Loomis-Price, L.; Janetzki, S.; Ferrari, G.; Birx, D.L.; Cox, J.H. A panel of MHC class I restricted viral peptides for use as a quality control for vaccine trial ELISPOT assays. J. Immunol. Methods 2002, 260, 157–172. [Google Scholar] [CrossRef]
- Rao, X.; Hoof, I.; Costa, A.I.; van Baarle, D.; Kesmir, C. HLA class I allele promiscuity revisited. Immunogenetics 2011, 63, 691–701. [Google Scholar] [CrossRef]
- Frahm, N.; Yusim, K.; Suscovich, T.J.; Adams, S.; Sidney, J.; Hraber, P.; Hewitt, H.S.; Linde, C.H.; Kavanagh, D.G.; Woodberry, T.; Henry, L.M.; Faircloth, K.; Listgarten, J.; Kadie, C.; Jojic, N.; Sango, K.; Brown, N.V.; Pae, E.,; Zaman, M.T.; Bihl, F.; Khatri, A.; John, M.; Mallal, S.; Marincola, F.M.; Walker, B.D.; Sette, A.; Heckerman, D.; Korber, B.T.; Brander, C. Extensive HLA class I allele promiscuity among viral CTL epitopes. Eur. J. Immunol. 2007, 37, 2419–2433. [Google Scholar]
- Axelsson-Robertson, R.; Weichold, F.; Sizemore, D.; Wulf, M.; Skeiky, Y.A.; Sadoff, J.; Maeurer, M.J. Extensive major histocompatibility complex class I binding promiscuity for Mycobacterium tuberculosis TB10.4 peptides and immune dominance of human leucocyte antigen (HLA)-B*0702 and HLA-B*0801 alleles in TB10.4 CD8 T-cell responses. Immunology 2010, 129, 496–505. [Google Scholar]
- Nakagawa, M.; Kim, K.H.; Gillam, T.M.; Moscicki, A.B. HLA class I binding promiscuity of the CD8 T-cell epitopes of human papillomavirus type 16 E6 protein. J. Virol. 2007, 81, 1412–1423. [Google Scholar] [CrossRef]
- Rosette, C.; Werlen, G.; Daniels, M.A.; Holman, P.O.; Alam, S.M.; Travers, P.J.; Gascoigne, N.R.; Palmer, E.; Jameson, S.C. The impact of duration versus extent of TCR occupancy on T cell activation: a revision of the kinetic proofreading model. Immunity 2001, 15, 59–70. [Google Scholar] [CrossRef]
- Valitutti, S.; Muller, S.; Cella, M.; Padovan, E.; Lanzavecchia, A. Serial triggering of many T-cell receptors by a few peptide-MHC complexes. Nature 1995, 375, 148–151. [Google Scholar] [CrossRef]
- Manz, B.N.; Jackson, B.L.; Petit, R.S.; Dustin, M.L.; Groves, J. T-cell triggering thresholds are modulated by the number of antigen within individual T-cell receptor clusters. Proc. Natl. Acad. Sci. USA 2011, 108, 9089–9094. [Google Scholar]
- Zhang, J.; Bardos, T.; Li, D.; Gal, I.; Vermes, C.; Xu, J.; Mikecz, K.; Finnegan, A.; Lipkowitz, S.; Glant, T.T. Cutting edge: regulation of T cell activation threshold by CD28 costimulation through targeting Cbl-b for ubiquitination. J. Immunol. 2002, 169, 2236–2240. [Google Scholar]
- Targoni, O.S.; Lehmann, P.V. Endogenous myelin basic protein inactivates the high avidity T cell repertoire. J. Exp. Med. 1998, 187, 2055–2063. [Google Scholar] [CrossRef]
- Hesse, M.D.; Karulin, A.Y.; Boehm, B.O.; Lehmann, P.V.; Tary-Lehmann, M. A T cell clone's avidity is a function of its activation state. J. Immunol. 2001, 167, 1353–1361. [Google Scholar]
- Touvrey, C.; Derre, L.; Devevre, E.; Corthesy, P.; Romero, P.; Rufer, N.; Speiser, D.E. Dominant human CD8 T cell clonotypes persist simultaneously as memory and effector cells in memory phase. J. Immunol. 2009, 182, 6718–6726. [Google Scholar]
- Iancu, E.M.; Corthesy, P.; Baumgaertner, P.; Devevre, E.; Voelter, V.; Romero, P.; Speiser, D.E.; Rufer, N. Clonotype selection and composition of human CD8 T cells specific for persistent herpes viruses varies with differentiation but is stable over time. J. Immunol. 2009, 183, 319–331. [Google Scholar] [CrossRef]
- Staras, S.A.; Dollard, S.C.; Radford, K.W.; Flanders, W.D.; Pass, R.F.; Cannon, M.J. Seroprevalence of cytomegalovirus infection in the United States, 1988-1994. Clin. Infect. Dis. 2006, 43, 1143–1151. [Google Scholar] [CrossRef]
- Evans, A.S.; Niederman, J.C. Epstein-Barr Virus. In Viral Infections of Humans, Epidemiology and Control; Evans, A.S., Ed.; Plenum Publishing Corporation: New York, 1989; pp. 265–292. [Google Scholar]
- Sauerbrei, A.; Schmidt-Ott, R.; Hoyer, H.; Wutzler, P. Seroprevalence of influenza A and B in German infants and adolescents. Med Microbiol Immunol 2009, 198, 93–101. [Google Scholar] [CrossRef]
- Griesemer, A.D.; Sorenson, E.C.; Hardy, M.A. The role of the thymus in tolerance. Transplantation 2010, 90, 465–474. [Google Scholar]
- Targoni, O.S.; Baus, J.; Hofstetter, H.H.; Hesse, M.D.; Karulin, A.Y.; Boehm, B.O.; Forsthuber, T.G.; Lehmann, P.V. Frequencies of neuroantigen-specific T cells in the central nervous system versus the immune periphery during the course of experimental allergic encephalomyelitis. J. Immunol. 2001, 166, 4757–4764. [Google Scholar]
- Lehmann, P.V.; Targoni, O.S.; Forsthuber, T.G. Shifting T-cell activation thresholds in autoimmunity and determinant spreading. Immunol. Rev. 1998, 164, 53–61. [Google Scholar] [CrossRef]
- Ramachandran, H.; Laux, J.; Moldovan, I.; Caspell, R.; Lehmann, P.V.; Subbramanian, R.A. Optimal thawing of cryopreserved peripheral blood mononuclear cells for use in high-throughput human immune monitoring studies. Cells 2012, 1, 313–324. [Google Scholar] [CrossRef]
- Kuerten, S.F.; Batoulis, H.; Recks, M.S.; Karacsony, E.; Zhang, W.; Subbramanian, R.A.; Lehmann, P.V. Resting of cryopreserved PBMC does not in general benefit the performance of antigen-specific T cell ELISPOT assays. Cells 2012, 1, 409–427. [Google Scholar] [CrossRef]
- Lehmann, P.V. Image analysis and data management of ELISPOT assay results. Methods Mol. Biol. 2005, 302, 117–132. [Google Scholar]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Zhang, W.; Moldovan, I.; Targoni, O.S.; Subbramanian, R.A.; Lehmann, P.V. How much of Virus-Specific CD8 T Cell Reactivity is Detected with a Peptide Pool when Compared to Individual Peptides? Viruses 2012, 4, 2636-2649. https://doi.org/10.3390/v4112636
Zhang W, Moldovan I, Targoni OS, Subbramanian RA, Lehmann PV. How much of Virus-Specific CD8 T Cell Reactivity is Detected with a Peptide Pool when Compared to Individual Peptides? Viruses. 2012; 4(11):2636-2649. https://doi.org/10.3390/v4112636
Chicago/Turabian StyleZhang, Wenji, Ioana Moldovan, Oleg S. Targoni, Ramu A. Subbramanian, and Paul V. Lehmann. 2012. "How much of Virus-Specific CD8 T Cell Reactivity is Detected with a Peptide Pool when Compared to Individual Peptides?" Viruses 4, no. 11: 2636-2649. https://doi.org/10.3390/v4112636
APA StyleZhang, W., Moldovan, I., Targoni, O. S., Subbramanian, R. A., & Lehmann, P. V. (2012). How much of Virus-Specific CD8 T Cell Reactivity is Detected with a Peptide Pool when Compared to Individual Peptides? Viruses, 4(11), 2636-2649. https://doi.org/10.3390/v4112636





