The Role of Human Papillomavirus in Human Immunodeficiency Virus Acquisition in Men who Have Sex with Men: A Review of the Literature
Abstract
:1. Introduction
2. Results and Discussion
2.1 Results
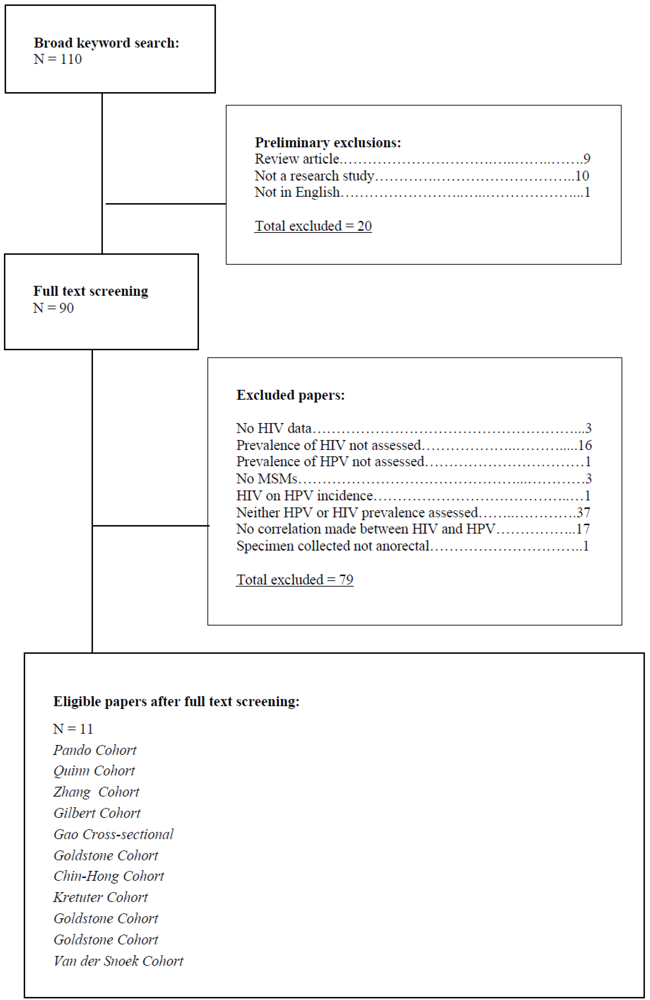
| Author | Type of study | Country | (N) | HIV Prevalence | HPV Prevalence | HPV types tested |
|---|---|---|---|---|---|---|
| Pando et al. | x sectional | Argentina | 496 | 17.30% | 83.5% | 36 |
| Quinn, et al. | x sectional | Peru | 105 | 36.50% | 77.1% | 7 |
| Zhang et al. | x sectional | China | 302 | 9.90% | 13.7% | 26 |
| Gilbert et al. | x sectional | Canada | 268 | 24.10% | 62.3% | 4 |
| Gao et al. | x sectional | China | 602 | 8.50% | 62.1% | 26 |
| Goldstone et al. | cohort | US | 412 | 31% | Over 2 yrs: stayed HPV+ (38%); became HPV+ (13%); | 13 |
| Chin-Hong et al. | cohort | US | 1409 | HIV acquisition 1.17/100 person-yrs | 56.8% | 22 |
| Kreuter, et al. | x sectional | Germany | 53 | 85% | 98% | 37 |
| Goldstone et al. | x sectional | US | 597 | 27% | 30% in HIV+ group, 14% in HIV- group | 18 |
| Goldstone, et al. | x sectional | US | 124 | 27.00% | 48% | 19 |
| VanDer Snoek, et al. | cohort | Nether-lands | 258 | 6.60% | Group A 3rd visit: 34.9% 6th visit, 36.6%. Group B 3rd visit 43.2% 6th visit, 49.1% | 26 |
2.2 Discussion
3. Conclusions
Acknowledgments
Conflict of Interest
References
- Chin-Hong, P.V. Anal human papillomavirus infection is associated with HIV acquisition in men who have sex with men. AIDS 2009, 23, 1135–1142. [Google Scholar] [CrossRef]
- Cranston, R.D. Anal human papillomavirus infection in a street-based sample of drug using HIV-positive men. International Journal of STD & AIDS 2012, 23, 195–200. [Google Scholar]
- Gao, L. Anal HPV infection in HIV-positive men who have sex with men from China. PLos ONE 2010, 5, e15256. [Google Scholar] [CrossRef]
- Anderson, J.S. A Randomized, placebo-controlled, dose-escalation study to determine the safety, tolerability, and immunogenicity of an HPV-16 therapeutic vaccine in HIV-positive participants with oncogenic HPV infection of the anus. J. Acquir. Immune. Defic. Syndr. 2009, 52, 371–381. [Google Scholar] [CrossRef]
- Kreuter, A. Human papillomavirus-associated induction of human b-defensins in anal intraepithelial neoplasia. Br. J. Dermatol. 2009, 160, 1197–1205. [Google Scholar] [CrossRef]
- Pando, M.A. HIV and other sexually transmitted infections among men who have sex with men recruited by RDS in Buenos Aires, Argentina: High HIV and HPV infection. PLoS ONE 2012, 7, e39834. [Google Scholar] [CrossRef]
- Ghosh, I. Prevalence of human papillomavirus and co-existent sexually transmitted infections among female sex workers, men having sex with men and injectable drug abusers from eastern India. Asian Pacific J. Cancer Prev. 2012, 13, 799–802. [Google Scholar] [CrossRef]
- Gilbert, M. Feasibility of incorporating self-collected rectal swabs into a community venue-based survey to measure the prevalence of HPV infection in men who have sex with men. Sex. Transm. Dis. 2011, 38, 964–969. [Google Scholar] [CrossRef]
- Goldstone, S.E. Hybrid capture II detection of oncogenic human papillomavirus: A useful tool when evaluating men who have sex with men with atypical squamous cells of undetermined significance on anal cytology. Dis. Colon. Rectum 2008, 51, 1130–1136. [Google Scholar] [CrossRef]
- Houlihan, C.F. HPV infection and increased risk of HIV acquisition. A systematic review and meta-analysis. AIDS 2012, 26, 2211–2222. [Google Scholar] [CrossRef]
- Goldstone, S. Prevalence of and risk factors for human papillomavirus (HPV) infection among HIV-seronegative men who have sex with men. J. Infect. Dis. 2011, 203, 66–74. [Google Scholar] [CrossRef]
- Van Der Snoek, E.M. Human Papillomavirus infection in men who have sex with men participating in a dutch gay-cohort study. Sex. Transm. Dis. 2003, 30, 639–644. [Google Scholar] [CrossRef]
- Goldstone, S.E. Detection of oncogenic human papillomavirus and other predictors of anal high-grade dysplasia in men who have sex with men with abnormal cytology. Dis. Colon. Rectum 2009, 52, 31–39. [Google Scholar] [CrossRef]
- Zhang, X. Prevalence and related risk behaviors of HIV, syphilis, and anal HPV infection among Men who have sex with men from Beijing, China. AIDS Behav 2011. [Google Scholar] [CrossRef]
- Quinn, R. Human papillomavirus infection in men who have sex with wen in Lima, Peru. AIDS Res. Hum. Retrovir. 2012, 28, 1–5. [Google Scholar] [CrossRef]
- Mateos, M. Prevalence of high risk genotypes of human papillomavirus in anal samples from men who have sex with men with abnormal cytology in Madrid. Enferm. Infec. Microbiol. Clin. 2011, 29, 778–786. [Google Scholar] [CrossRef]
- Parisi, S. Anal and oral human papillomavirus (HPV) infection in HIV-infected subjects in northern Italy: A longitudinal cohort study among men who have sex with men. BMC Infect. Dis. 2011, 11, 1–9. [Google Scholar]
- Van Der Snoek, E.M. Acquisition and clearance of perianal human papillomavirus infection in relation to HIV-positivity in men who have sex with wen in the Netherlands. Acta. Derm. Venereol. 2005, 85, 437–443. [Google Scholar] [CrossRef]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Brown, B.; Davtyan, M.; Galea, J.; Chow, E.; Leon, S.; Klausner, J.D. The Role of Human Papillomavirus in Human Immunodeficiency Virus Acquisition in Men who Have Sex with Men: A Review of the Literature. Viruses 2012, 4, 3851-3858. https://doi.org/10.3390/v4123851
Brown B, Davtyan M, Galea J, Chow E, Leon S, Klausner JD. The Role of Human Papillomavirus in Human Immunodeficiency Virus Acquisition in Men who Have Sex with Men: A Review of the Literature. Viruses. 2012; 4(12):3851-3858. https://doi.org/10.3390/v4123851
Chicago/Turabian StyleBrown, Brandon, Mariam Davtyan, Jerome Galea, Erica Chow, Segundo Leon, and Jeffrey D. Klausner. 2012. "The Role of Human Papillomavirus in Human Immunodeficiency Virus Acquisition in Men who Have Sex with Men: A Review of the Literature" Viruses 4, no. 12: 3851-3858. https://doi.org/10.3390/v4123851




