Chronic Bee Paralysis Virus in Honeybee Queens: Evaluating Susceptibility and Infection Routes
Abstract
:1. Introduction
2. Results and Discussion
2.1. Testing Susceptibility (Experiment 1)
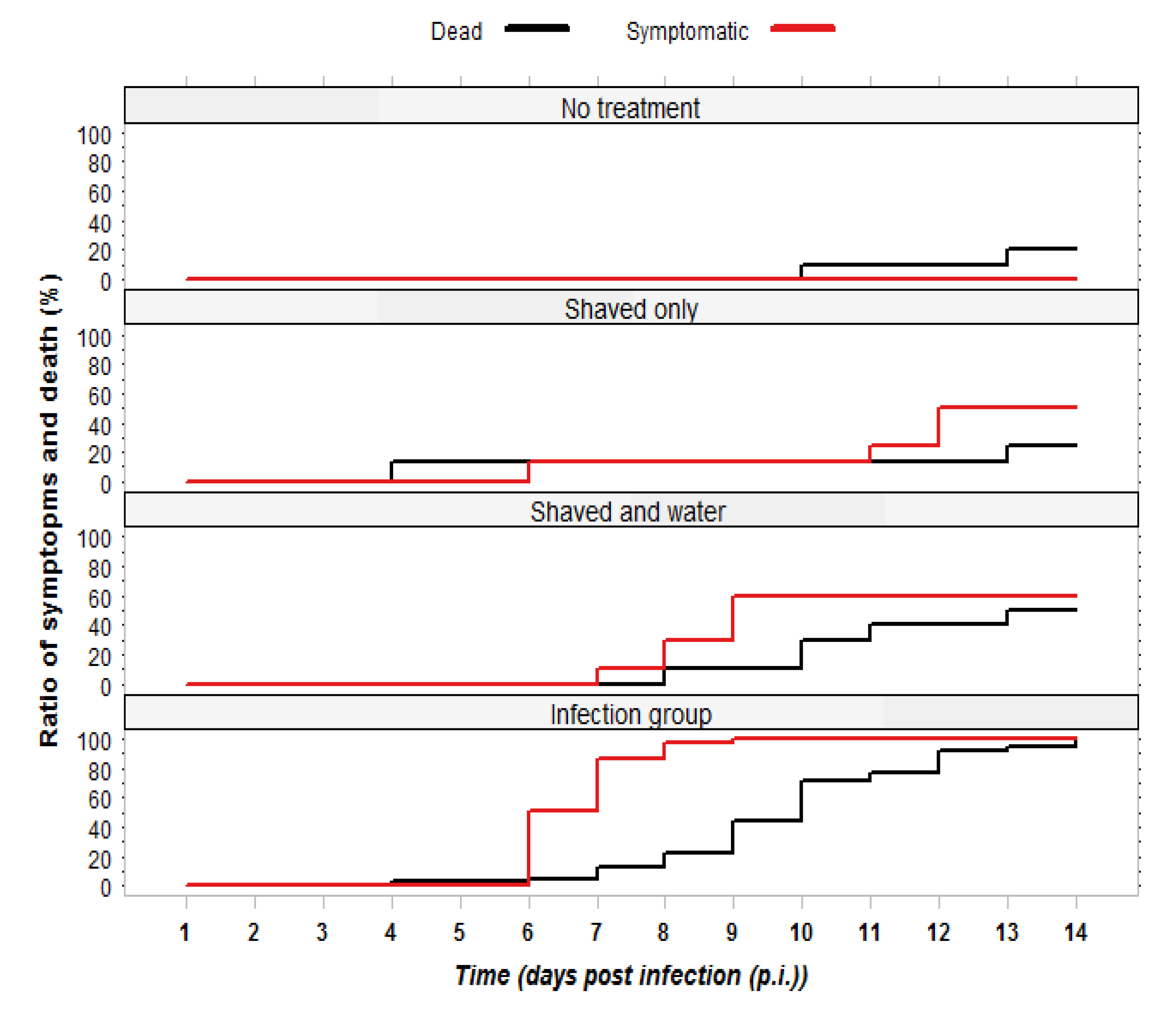
2.1.1. Observation of Symptoms and Mortality
2.1.2. RT-PCR Confirmation of Infection Status
| Source of variation | Degrees of freedom | Sum of squares | Mean of squares | F value | Pr (>F) |
|---|---|---|---|---|---|
| Groups of queens | 3 | 14,294 | 4764.8 | 28.18 | 0.000 |
| Residual | 63 | 10,653 | 169.1 |

2.2. Comparing Infection Routes (Experiment 2)
2.2.1. Observation of Symptoms and Mortality
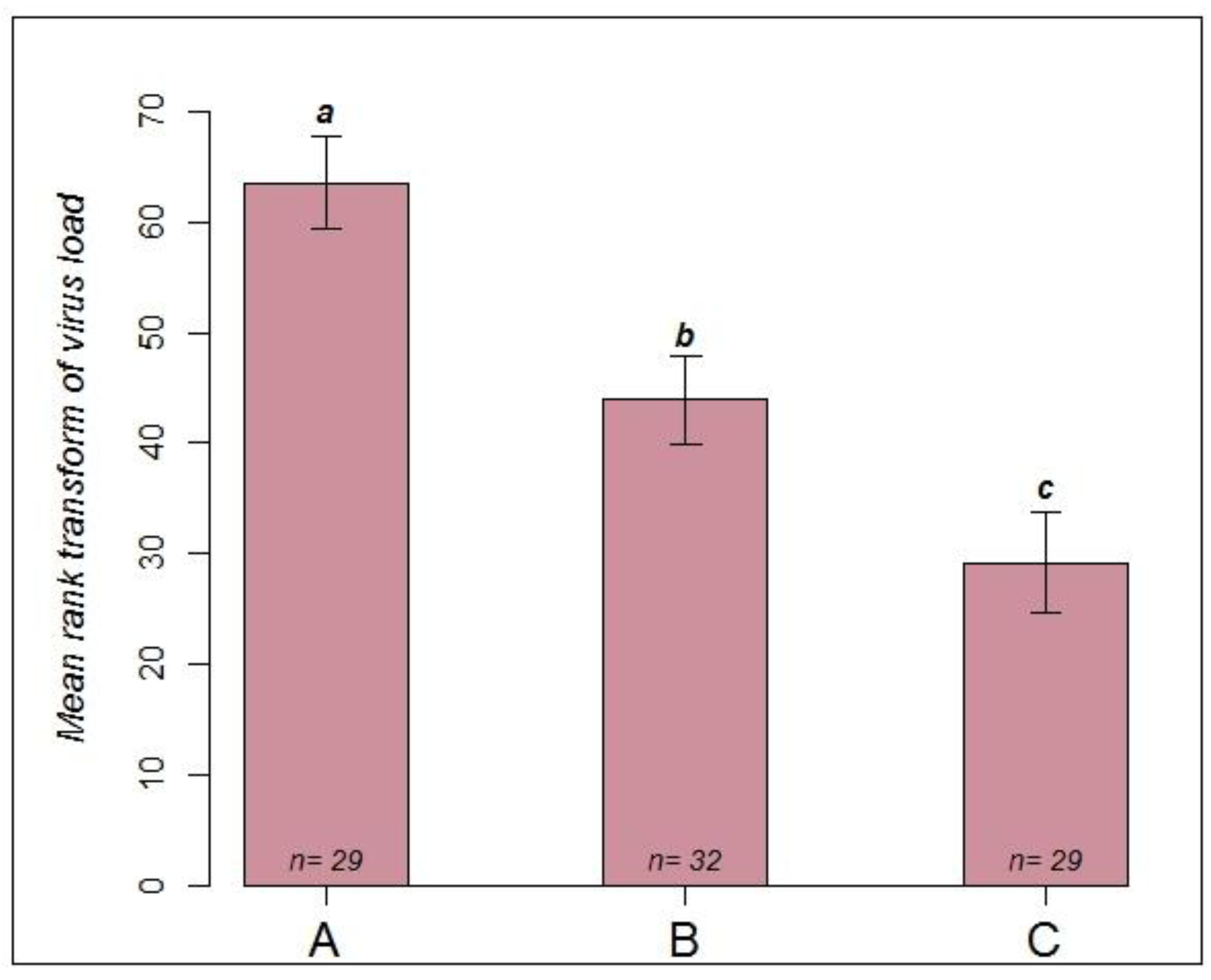
2.2.2. RT-PCR Confirmation of Infection Status
| Source of Variation | Degrees of freedom | Sum of squares | Mean of squares | F value | Pr (>F) |
|---|---|---|---|---|---|
| Groups of queens | 2 | 17,215 | 8607.6 | 17.2 | 0.000 |
| Residual | 87 | 43,527 | 500.3 |
3. Experimental Section
3.1. Virus Stock and Propagation
3.2. Experimental Assay
3.2.1. Infection Experiment 1: Testing Susceptibility
- Infection group (n = 39): a small area on the thorax was carefully shaved and 4 µL of virus suspension was topically applied. Thus, each queens received approximately 2 × 109 virus copies.
- Control group 1 (n = 10): the queens were immobilized, a small area on the thorax was shaved and 4 µL of distilled water was applied.
- Control group 2 (n = 8): the queens were immobilized, and a small area was shaved, but nothing was applied.
- Control group 3 (n = 10): the queens were immobilized, but no treatment was applied.
3.2.2. Infection Experiment 2: Comparing Infection Routes
- Group A (n = 29): to simulate a natural infection via feeding and contact, queens were kept in metal cages together with about 30 workers each who all suffered from a natural infection with CBPV. These workers were symptomatic and most likely each contained more than 107 virus copies [6]. To minimise the spread of virus via feces, a piece of paper was placed on the floor of each cage and exchanged daily.
- Group B (n = 32): queens were kept with about 30 healthy bees each in metal cages. Following immobilization on ice, 4 µL of virus suspension (5 × 108 copies/µL) was applied to a small shaved area on the thorax of the queens. After the virus suspension was absorbed, each queen was returned to her cage. All cages were supplied with sucrose syrup, sugar candy and pollen cake.
- Group C (n = 29): queens were kept in plastic queen shipping cages [39] together with 10 workers showing symptoms of a natural infection with CBPV. Each cage was placed into separate mating nucs [39] containing approximately 1500 healthy bees. Thus, the queens were in close contact with infected bees, but could be fed by workers outside the queen cage. Symptoms and mortality were recorded daily. After observing symptoms in the first queens on day 9 after caging, all queens were collected in individual tubes and immediately frozen at −80 °C for virus quantification.
3.3. Virus Quantification
3.4. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References and Notes
- Chen, Y.P.; Siede, R. Honey bee viruses. Adv. Virus Res. 2007, 70, 33–80. [Google Scholar] [CrossRef]
- Maori, E.; Lavi, S.; Mozes-Koch, R.; Gantman, Y.; Peretz, Y.; Edelbaum, O.; Tanne, E.; Sela, I. Isolation and characterization of Israeli acute paralysis virus, a dicistrovirus affecting honeybees in Israel: Evidence for diversity due to intra- and inter-species recombination. J. Gen. Virol. 2007, 88, 3428–3438. [Google Scholar] [CrossRef]
- Runckel, C.; Flenniken, M.L.; Engel, J.C.; Ruby, J.G.; Ganem, D.; Andino, R.; DeRisi, J.L. Temporal analysis of the honey bee microbiome reveals four novel viruses and seasonal prevalence of known viruses, Nosema, and Crithidia. PLoS One 2011, 6, e20656. [Google Scholar]
- Bailey, L.; Gibbs, A.J.; Woods, R.D. Two viruses from adult honey bees (Apis mellifera Linnaeus). Virology 1963, 21, 390–395. [Google Scholar] [CrossRef]
- Bailey, L.; Gibbs, A.J.; Woods, R.D. The purification and properties of Chronic bee-paralysis virus. J. Gen. Virol. 1968, 2, 251–260. [Google Scholar] [CrossRef]
- Blanchard, P.; Ribiere, M.; Celle, O.; Lallemand, P.; Schurr, F.; Olivier, V.; Iscache, A.L.; Faucon, J.P. Evaluation of a real-time two-step RT-PCR assay for quantitation of Chronic bee paralysis virus (CBPV) genome in experimentally-infected bee tissues and in life stages of a symptomatic colony. J. Virol. Methods 2007, 141, 7–13. [Google Scholar] [CrossRef]
- Bailey, L. The incidence of virus diseases in the honey bee. Ann. Appl. Biol. 1967, 60, 43–48. [Google Scholar] [CrossRef]
- Ribiere, M.; Olivier, V.; Blanchard, P. Chronic bee paralysis: a disease and a virus like no other? J. Invertebr. Pathol. 2010, 103, S120–S131. [Google Scholar] [CrossRef]
- Allen, M.; Ball, B. The incidence and world distribution of honey beeviruses. Bee World 1996, 77, 141–162. [Google Scholar]
- Bailey, L.; Milne, R.G. The multiplication regions and interaction of Acute and Chronic bee-paralysis viruses in adult honey bees. J. Gen. Virol. 1969, 4, 9–14. [Google Scholar] [CrossRef]
- Olivier, V.; Blanchard, P.; Chaouch, S.; Lallemand, P.; Schurr, F.; Celle, O.; Dubois, E.; Tordo, N.; Thiery, R.; Houlgatte, R.; et al. Molecular characterisation and phylogenetic analysis of Chronic bee paralysis virus, a honey bee virus. Virus Res. 2008, 132, 59–68. [Google Scholar] [CrossRef]
- Bailey, L.; Ball, B.V.; Perry, J.N. Honeybee paralysis—Its iatural ipread and its diminished incidence in England and Wales. J. Apicult. Res. 1983, 22, 191–195. [Google Scholar]
- Ribiere, M.; Lallemand, P.; Iscache, A.L.; Schurr, F.; Celle, O.; Blanchard, P.; Olivier, V.; Faucon, J.P. Spread of infectious Chronic bee paralysis virus by honeybee (Apis mellifera L.) feces. Appl. Environ. Microbiol. 2007, 73, 7711–7716. [Google Scholar] [CrossRef]
- Bailey, L. The occurrence of Chronic and Acute bee paralysis viruses in bees outside Britain. J. Invertebr. Pathol. 1965, 7, 167–169. [Google Scholar] [CrossRef]
- Rinderer, T.E.; Rothenbuhler, W.C. Characteristic field symptoms comprising honeybee Hairless-black syndrome induced in laboratory by a virus. J. Invertebr. Pathol. 1976, 27, 215–219. [Google Scholar] [CrossRef]
- Bailey, L. Paralysis of the honey bee, Apis melliera Linnaeus. J. Invertebr. Pathol. 1965, 7, 132–140. [Google Scholar] [CrossRef]
- Chevin, A.; Schurr, F.; Blanchard, P.; Thiéry, R.; Ribière, M. Experimental infection of the honeybee (Apis mellifera L.) with the chronic bee paralysis virus (CBPV): Infectivity of naked CBPV RNAs. Virus Res. 2012, 167, 173–178. [Google Scholar] [CrossRef]
- Toplak, I.; Jamnikar Ciglenečki, U.; Aronstein, K.; Gregorc, A. Chronic bee paralysis virus and Nosema ceranae experimental co-infection of winter honey bee workers (Apis mellifera L.). Viruses 2013, 5, 2282–2297. [Google Scholar] [CrossRef]
- Bailey, L.; Ball, B.V.; Perry, J.N. Association of viruses with two protozoal pathogens of the honey bee. Ann. Appl. Biol. 1983, 103, 13–20. [Google Scholar] [CrossRef]
- Tentcheva, D.; Gauthier, L.; Zappulla, N.; Dainat, B.; Cousserans, F.; Colin, M.E.; Bergoin, M. Prevalence and seasonal variations of six bee viruses in Apis mellifera L. and Varroa destructor mite populations in France. Appl. Environ. Microbiol. 2004, 70, 7185–7191. [Google Scholar] [CrossRef]
- Bowen-Walker, P.L.; Martin, S.J.; Gunn, A. The transmission of Deformed wing virus between honeybees (Apis mellifera L.) by the ectoparasitic mite Varroa jacobsoni Oud. J. Invertebr. Pathol. 1999, 73, 101–106. [Google Scholar] [CrossRef]
- Yue, C.; Genersch, E. RT-PCR analysis of Deformed wing virus in honeybees (Apis mellifera) and mites (Varroa destructor). J. Gen. Virol. 2005, 86, 3419–3424. [Google Scholar] [CrossRef]
- Bakonyi, T.; Grabensteiner, E.; Kolodziejek, J.; Rusvai, M.; Topolska, G.; Ritter, W.; Nowotny, N. Phylogenetic analysis of Acute bee paralysis virus strains. Appl. Environ. Microbiol. 2002, 68, 6446–6450. [Google Scholar] [CrossRef]
- Ribiere, M.; Triboulot, C.; Mathieu, L.; Aurieres, C.; Faucon, J.P.; Pepin, M. Molecular diagnosis of Chronic bee paralysis virus infection. Apidologie 2002, 33, 339–351. [Google Scholar] [CrossRef]
- Ball, B.V.; Allen, M.F. The prevalence of pathogens in honey bee (Apis mellifera) colonies infested with the parasitic mite Varroa jacobsoni. Ann. Appl. Biol. 1988, 113, 237–244. [Google Scholar] [CrossRef]
- Celle, O.; Blanchard, P.; Olivier, V.; Schurr, F.; Cougoule, N.; Faucon, J.-P.; Ribière, M. Detection of Chronic bee paralysis virus (CBPV) genome and its replicative RNA form in various hosts and possible ways of spread. Virus Res. 2008, 133, 280–284. [Google Scholar] [CrossRef]
- Chen, Y.; Pettis, J.S.; Feldlaufer, M.F. Detection of multiple viruses in queens of the honey bee Apis mellifera L. J. Invertebr. Pathol. 2005, 90, 118–121. [Google Scholar] [CrossRef]
- Chen, Y.P.; Pettis, J.S.; Collins, A.; Feldlaufer, M.F. Prevalence and transmission of honeybee viruses. Appl. Environ. Microbiol. 2006, 72, 606–611. [Google Scholar] [CrossRef]
- Winston, M.L. The Biology of the Honey Bee; Harvard University Press: Cambridge, MA, USA/London, UK, 1987. [Google Scholar]
- Kulincevic, J.M.; Rothenbuhler, W.C. The effects of artificial infection with Chronic bee paralysis virus on queens from strains of honeybee resistant or susceptible to Hairless-black syndrome. J. Apicult. Res. 1989, 28, 79–80. [Google Scholar]
- Wang, D.-I.; Moeller, F.E. Division of labor and queen attendance behavior of nosema-infected worker honey bees. J. Econ. Entom. 1970, 63, 1540–1541. [Google Scholar]
- Rueppell, O.; Hayworth, M.K.; Ross, N.P. Altruistic self-removal of health-compromised honey bee workers from their hive. J. Evol. Biol. 2010, 23, 1538–1546. [Google Scholar] [CrossRef]
- de Miranda, J.R.; Bailey, L.; Ball, B.V.; Blanchard, P.; Budge, G.E.; Chejanovsky, N.; Chen, Y.-P.; Gauthier, L.; Genersch, E.; de Graaf, D.C.; et al. Standard methods for virus research in Apis mellifera. J. Apicult. Res. 2013, 52, 1–56. [Google Scholar]
- Ribière, M.; Faucon, J.-P.; Pépin, M. Detection of Chronic honey bee (Apis mellifera L.) paralysis virus infection: application to a field survey. Apidologie 2000, 31, 567–577. [Google Scholar] [CrossRef]
- Lindström, M. Detection of honey bee viruses in Apis mellifera and Apis cerana. Ph.D. Thesis, Uppsala University, Uppsala, Sweden, 2011. [Google Scholar]
- Gauthier, L.; Tentcheva, D.; Tournaire, M.; Dainat, B.; Cousserans, F.; Colin, M.E.; Bergoin, M. Viral load estimation in asymptomatic honey bee colonies using the quantitative RT-PCR technique. Apidologie 2007, 38, 426–435. [Google Scholar] [CrossRef]
- Grabensteiner, E.; Ritter, W.; Carter, M.J.; Davison, S.; Pechhacker, H.; Kolodziejek, J.; Boecking, O.; Derakhshifar, I.; Moosbeckhofer, R.; Licek, E.; et al. Sacbrood virus of the honeybee (Apis mellifera): rapid identification and phylogenetic analysis using reverse transcription-PCR. Clin. Diagn. Lab. Immunol. 2001, 8, 93–104. [Google Scholar]
- Francis, R.; Kryger, P. Single assay detection of Acute bee paralysis virus, Kashmir bee virus and Israeli acute paralysis virus. J. Apicult. Sci. 2012, 56, 137. [Google Scholar]
- Büchler, R.; Andonov, S.; Bienefeld, K.; Costa, C.; Hatjina, F.; Kezic, N.; Kryger, P.; Spivak, M.; Uzunov, A.; Wilde, J. Standard methods for rearing and selection of Apis mellifera queens. J. Apicult. Res. 2013, 52. [Google Scholar] [CrossRef]
- Bustin, S.A.; Benes, V.; Garson, J.A.; Hellemans, J.; Huggett, J.; Kubista, M.; Mueller, R.; Nolan, T.; Pfaffl, M.W.; Shipley, G.L.; et al. The MIQE Guidelines: Minimum Information for Publication of Quantitative Real-Time PCR Experiments. Clin. Chem. 2009, 55, 611–622. [Google Scholar] [CrossRef]
- Francis, R.M.; Nielsen, S.L.; Kryger, P. Patterns of viral infection in honey bee queens. J. Gen. Virol. 2013, 94, 668–676. [Google Scholar] [CrossRef]
- R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2010.
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Amiri, E.; Meixner, M.; Büchler, R.; Kryger, P. Chronic Bee Paralysis Virus in Honeybee Queens: Evaluating Susceptibility and Infection Routes. Viruses 2014, 6, 1188-1201. https://doi.org/10.3390/v6031188
Amiri E, Meixner M, Büchler R, Kryger P. Chronic Bee Paralysis Virus in Honeybee Queens: Evaluating Susceptibility and Infection Routes. Viruses. 2014; 6(3):1188-1201. https://doi.org/10.3390/v6031188
Chicago/Turabian StyleAmiri, Esmaeil, Marina Meixner, Ralph Büchler, and Per Kryger. 2014. "Chronic Bee Paralysis Virus in Honeybee Queens: Evaluating Susceptibility and Infection Routes" Viruses 6, no. 3: 1188-1201. https://doi.org/10.3390/v6031188






