Characterization of a Proposed Dichorhavirus Associated with the Citrus Leprosis Disease and Analysis of the Host Response
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. RNA Isolation for High Throughput Sequencing
2.3. Bioinformatic Analysis
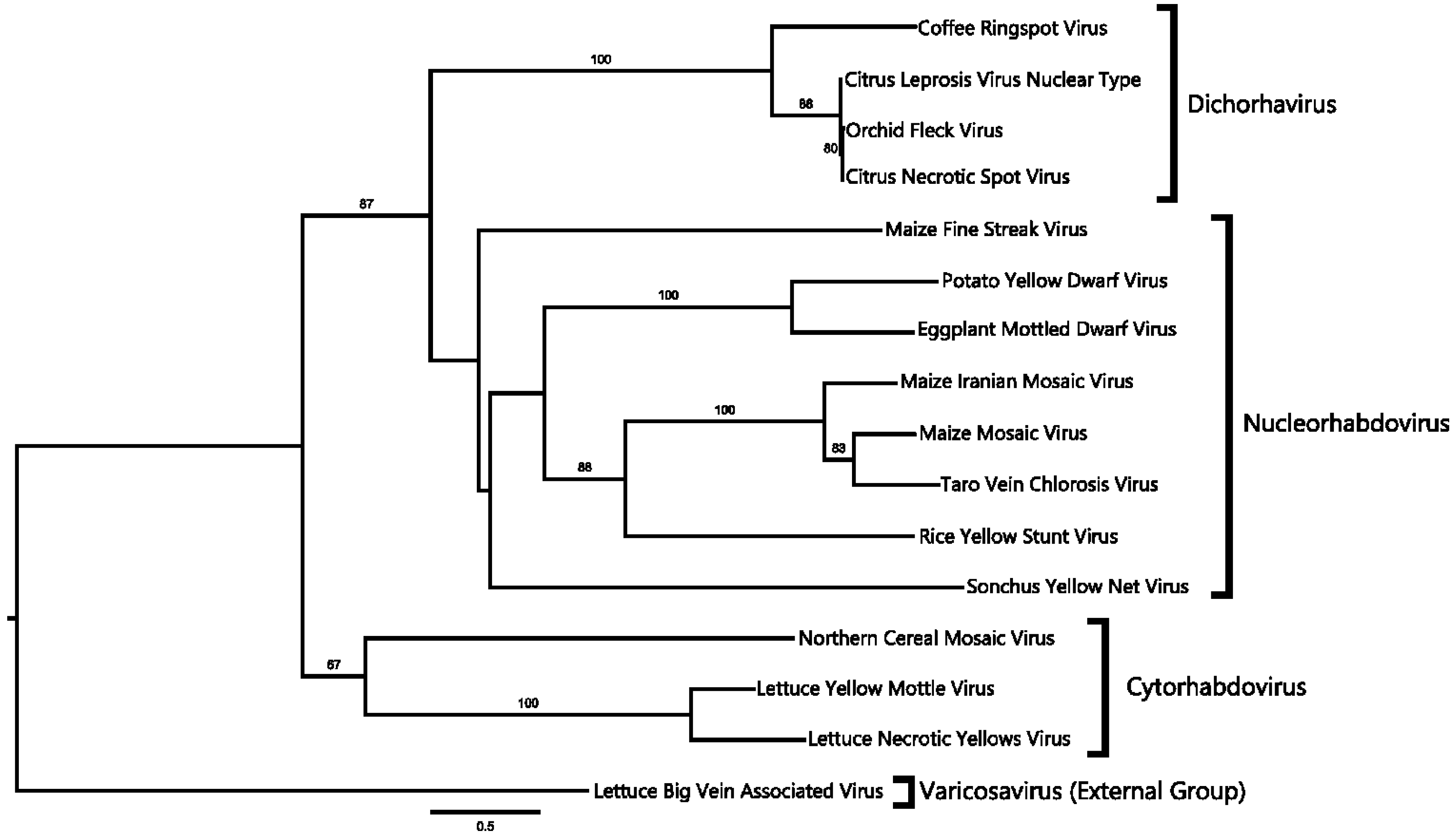
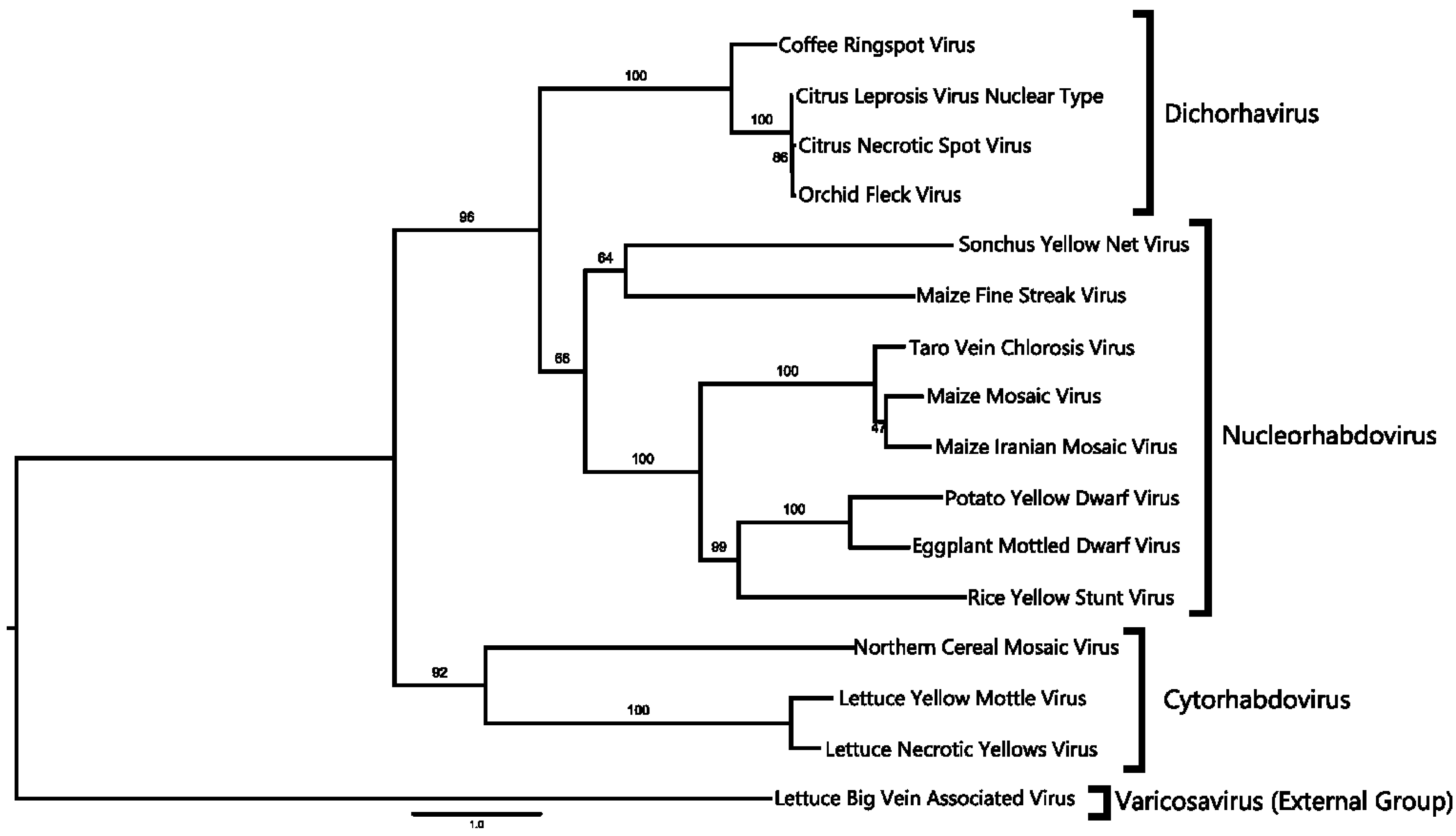
2.4. Phylogenetic Analysis
2.5. Differential Expression Analysis
2.6. RT-PCR Analysis
2.7. Validation of Differentially Gene Expression through Quantitative RT-PCR
2.8. Transmission Electron Microscopy
3. Results
3.1. Citrus Leprosis Symptomatology in Citrus Plants in Mexico
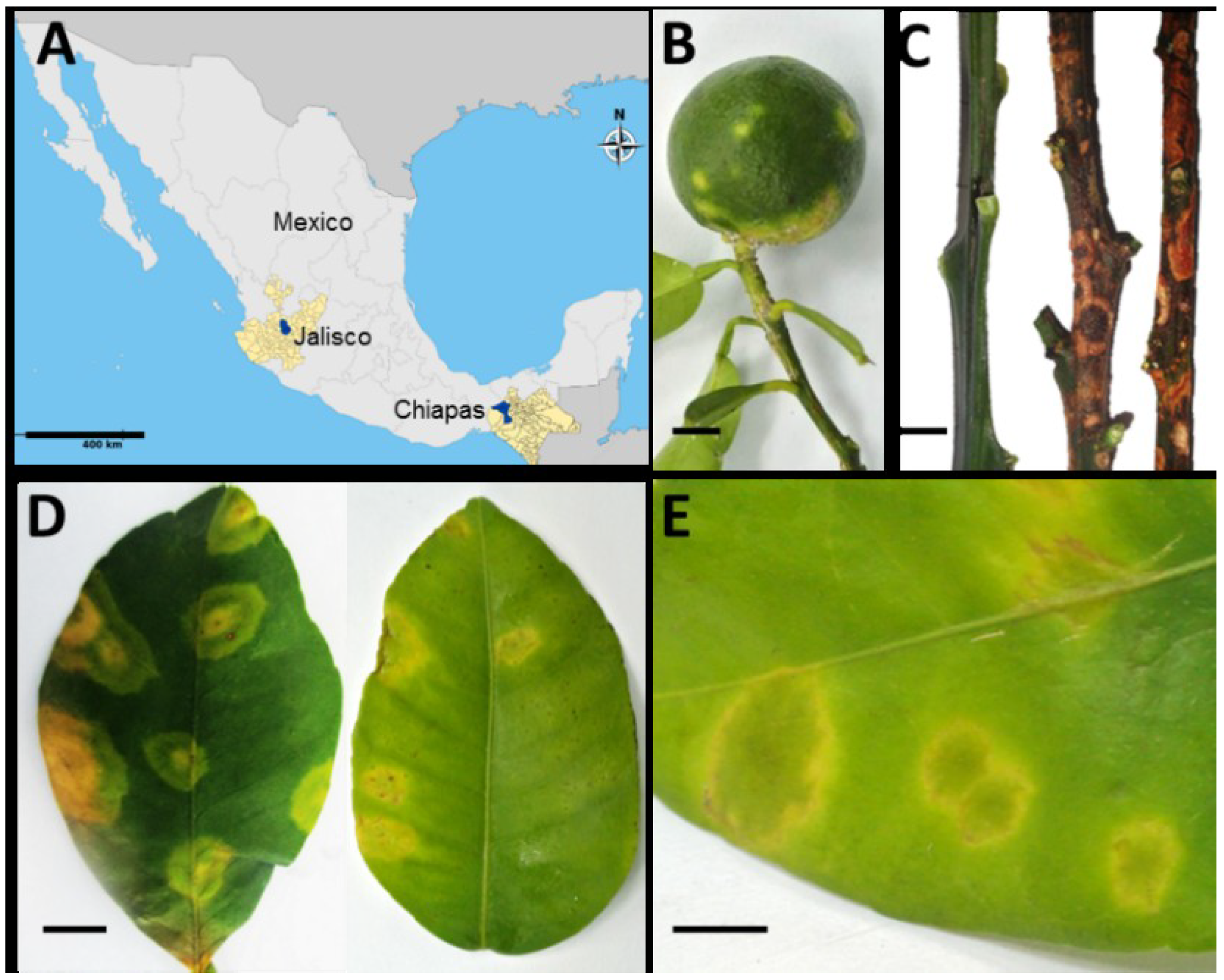
3.2. A Virus Belonging to the Proposed Dichorhavirus Genus is Associated with Citrus Leprosis Symptoms in Mexico
| Total no. of reads for symptomatic tissue | 7,737,481 |
| Total no. of reads for asymptomatic tissue | 7,291,897 |
| Total | 15,029,378 |
| Total no. of reads for RNA1 (6087 nt) | 12,083 |
| Average read | 196.87 nt |
| Total no. of reads for RNA2 (6015 nt) | 12,079 |
| Average read | 199.39 nt |
| Total length of coverage of cDNA reads for symptomatic tissue | 2.01 Gb |
| Total length of coverage of cDNA reads for asymptomatic tissue | 1.90 Gb |
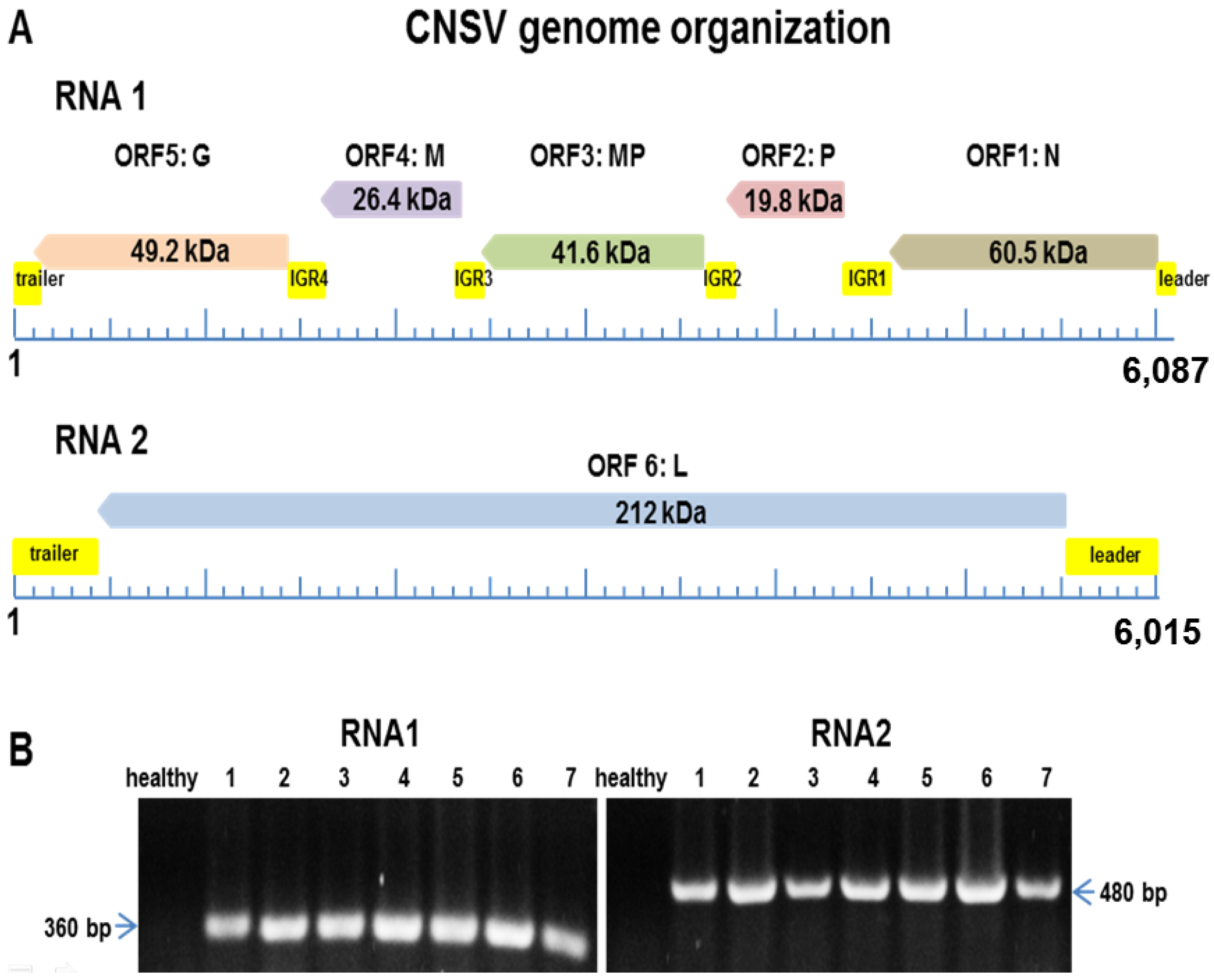
3.3. CNSV Particles Are Located in the Cytoplasm of Infected Cells

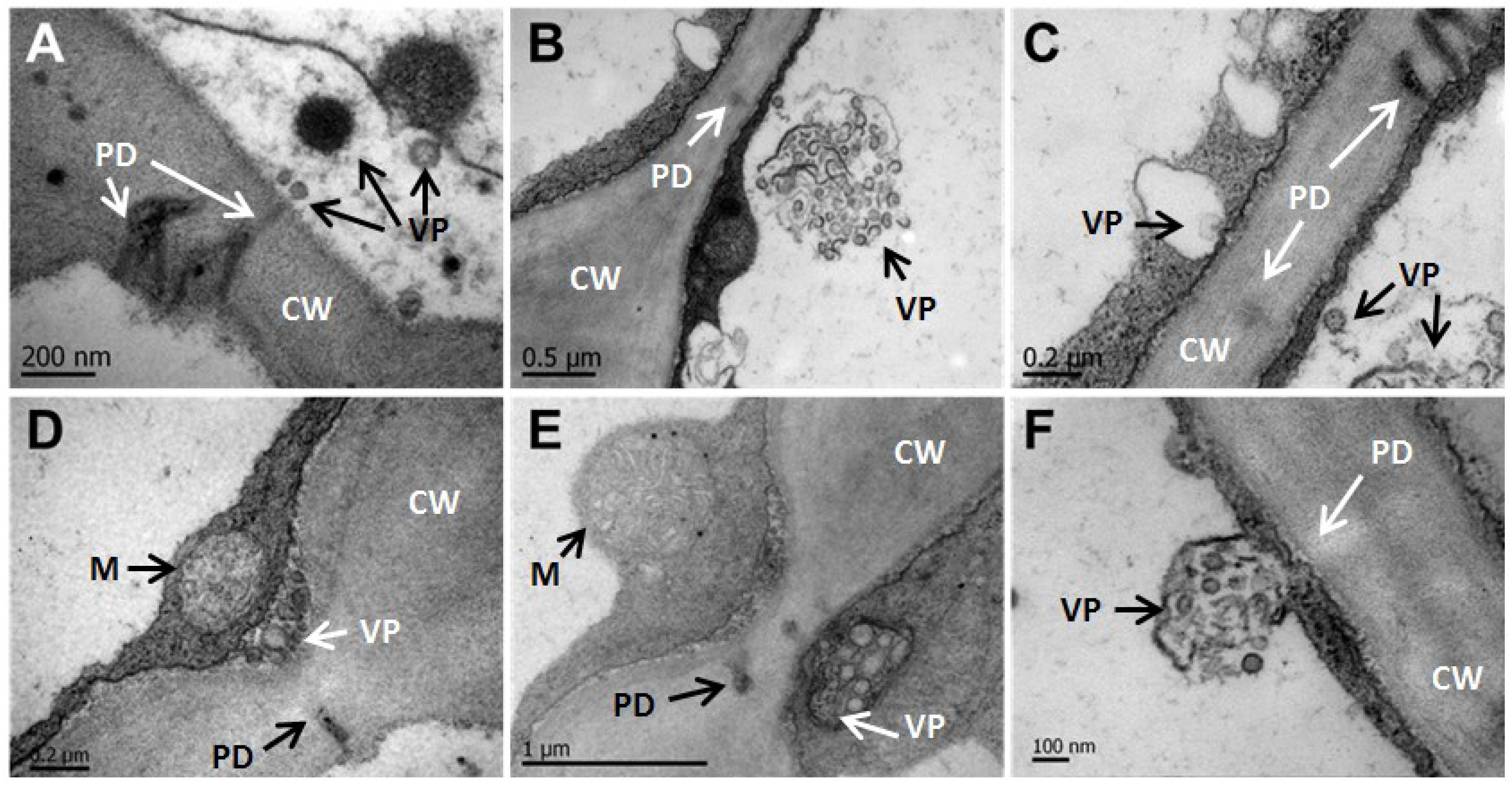
3.4. CNSV Particles Associate with Unmodified Plasmodesmata
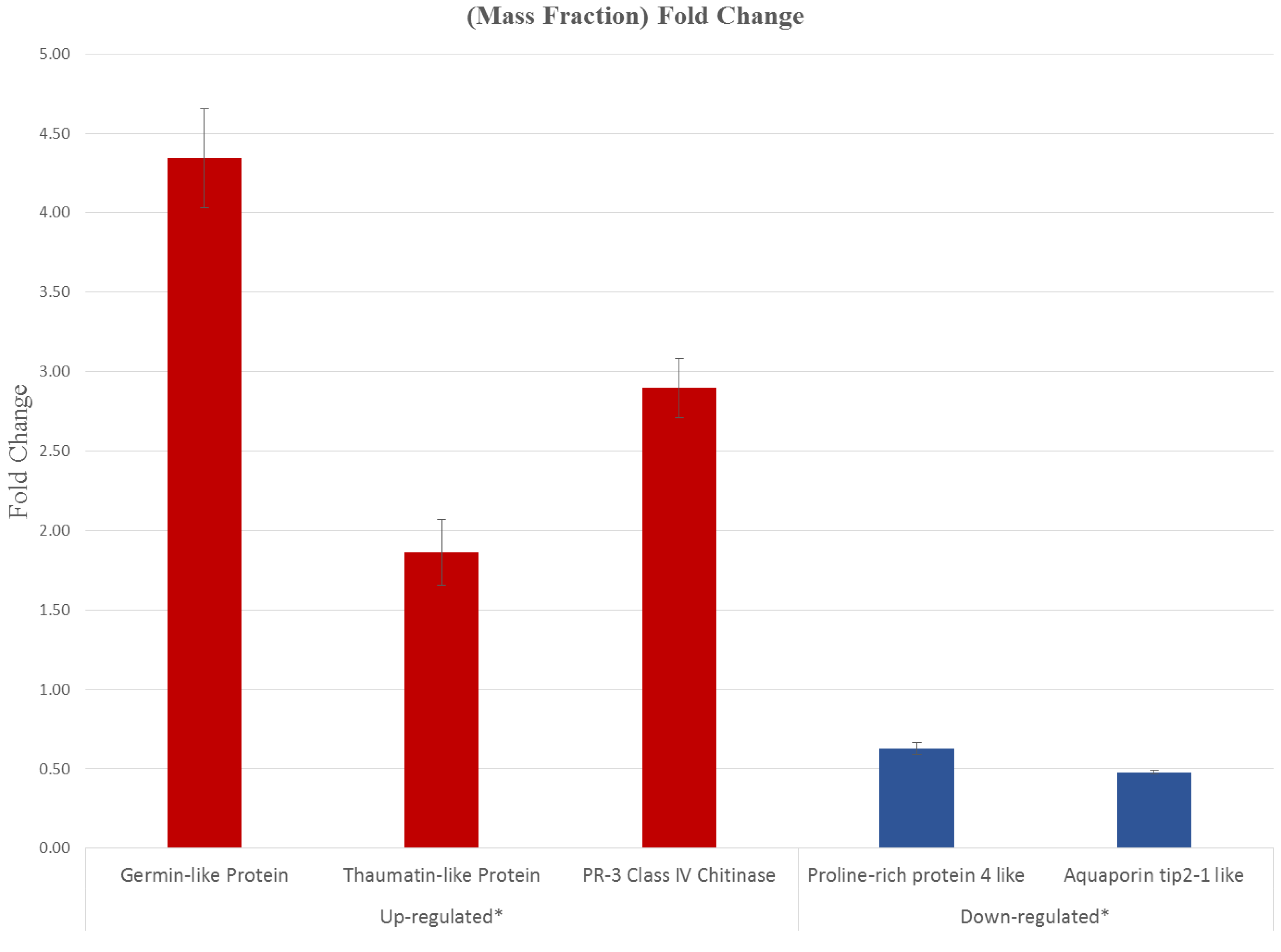
3.5. CNSV Elicits the Accumulation of Multiple Host Transcripts
4. Discussion
4.1. CNSV Belongs to the New Proposed Dichorhavirus Genus
4.2. CNSV Induces a Characteristic Set of Genes during Infection
Supplementary Files
Acknowledgments
Author Contributions
Conflicts of Interest
References and Notes
- Chagas, C.M. Leprosis and zonate chlorosis. In Compendium of Citrus Diseases, 2nd ed.; Timmer, L.W., Garnsey, S.M., Graham, J.H., Eds.; American Phytopathological Society Press: St. Paul, MN, USA, 2000; pp. 57–58. [Google Scholar]
- Childers, C.C.; Rodrigues, J.C.V.; Derrick, K.S.; Achor, D.S.; French, J.C.; Welbourn, W.C.; Ochoa, R.; Kitajima, E.W. Citrus leprosis and its status in Florida and Texas: Past and present. Exp. Appl. Acarol. 2003, 30, 181–202. [Google Scholar] [CrossRef]
- Bastianel, M.; Freitas-Astúa, J.; Nicolini, F.; Segatti, N.; Novelli, V.M.; Rodrigues, V.; Medina, C.L.; Machado, M.A. Response of mandarin cultivars and hybrids to Citrus leprosis virus. J. Plant Pathol. 2008, 90, 305–310. [Google Scholar]
- Bastianel, M.; Novelli, V.M.; Kitajima, E.W.; Kubo, K.S.; Bassanezi, R.B.; Machado, M.A.; Freitas-Astúa, J. Citrus leprosis: Centennial of an unusual mite–virus pathosystem. Plant Dis. 2010, 94, 284–292. [Google Scholar] [CrossRef]
- SENASICA (Servicio Nacional de Sanidad, Inocuidad y Calidad Agroalimentaria) Informe Sobre Situación de la leprosis de los cítricos. Available online: http://www.senasica.gob.mx/includes/asp/download.asp?IdDocumento=907&IdUrl=1519/ (accessed on 1 May 2014).
- Dietzgen, R.G.; Kuhn, J.H.; Clawson, A.N.; Freitas-Astúa, J.; Goodin, M.M.; Kitajima, E.W.; Kondo, H.; Wetzel, T.; Whitfield, A.E. Dichorhavirus: A proposed new genus for Brevipalpus mite-transmitted, nuclear, bacilliform, bipartite, negative-strand RNA plant viruses. Arch. Virol. 2014, 159, 607–619. [Google Scholar] [CrossRef]
- Locali-Fabris, E.C.; Freitas-Astúa, J.; Souza, A.A.; Takita, M.A.; Astúa-Monge, G.; Antonioli-Luizon, R.; Rodrigues, V.; Targon, M.L.; Machado, M.A. Complete nucleotide sequence, genomic organization and phylogenetic analysis of Citrus leprosis virus cytoplasmic type. J. Gen. Virol. 2006, 87, 2721–2729. [Google Scholar] [CrossRef]
- Prabha, K.; Baranwal, V.K. The genome sequence of an isolate of Indian citrus ringspot virus infecting the sweet orange in India. J. Virol. 2012, 86, 12446–12447. [Google Scholar] [CrossRef]
- Melzer, M.J.; Sether, D.M.; Borth, W.B.; Hu, J.S. Characterization of a virus infecting Citrus volkameriana with citrus leprosis-like symptoms. Phytopathology 2012, 102, 122–127. [Google Scholar] [CrossRef]
- Roy, A.; Stone, A.; Otero-Colina, G.; Wei, G.; Choudhary, N.; Achor, D.; Shao, J.; Levy, L.; Nakhla, M.K.; Hollingsworth, C.R.; et al. Genome assembly of citrus leprosis virus nuclear type reveals a close association with orchid fleck virus. Genome Announc. 2013, 1, e00519–e00513. [Google Scholar]
- Roy, A.; Choudhary, N.; Guillermo, L.M.; Shao, J.; Govindarajulu, A.; Achor, D.; Wei, G.; Picton, D.D.; Levy, L.; Nakhla, M.K.; et al. A novel virus of the genus Cilevirus causing symptoms similar to citrus leprosis. Phytopathology 2012, 103, 488–500. [Google Scholar]
- Kitajima, E.W.; Chagas, C.M.; Rodrigues, J.C. Brevipalpus-transmitted plant virus and virus-like diseases: Cytopathology and some recent cases. Exp. Appl. Acarol. 2003, 30, 135–160. [Google Scholar] [CrossRef]
- Rodrigues, J.C.; Childers, C.C. Brevipalpus mites (Acari: Tenuipalpidae): Vectors of invasive, non-systemic cytoplasmic and nuclear viruses in plants. Exp. Appl. Acarol. 2013, 59, 165–175. [Google Scholar]
- Baker, E.W.; Tuttle, D.M.; Abbatiello, M.J. The False Spider Mites of Northwestern and North Central Mexico (Acarina: Tenuipalpidae). In Smithsonian Contributions to Zoology; Smithsonian Institution Press: Washington, DC, USA, 1975; No. 194. [Google Scholar]
- Colariccio, A.; Lovisolo, O.; Chagas, C.M.; Galletti, S.R.; Rossetti, V.V.; Kitajima, E.W. Mechanical transmission and ultrastructural aspects of citrus leprosis disease. Fitopatol. Bras. 1995, 20, 208–213. [Google Scholar]
- Jackson, A.O.; Dietzgen, R.G.; Goodin, M.M.; Bragg, J.N.; Deng, M. Biology of plant rhabdoviruses. Annu. Rev. Phytopathol. 2005, 43, 623–660. [Google Scholar] [CrossRef]
- Redinbaugh, M.G.; Hogenhout, S.A. Plant rhabdoviruses. Curr. Top. Microbiol. Immunol. 2005, 292, 143–163. [Google Scholar]
- Radford, A.D.; Chapman, D.; Dixon, L.; Chantrey, J.; Darby, A.C.; Hall, N. Application of next-generation sequencing technologies in virology. J. Gen. Virol. 2012, 93, 1853–1868. [Google Scholar] [CrossRef]
- Wang, Z.; Gerstein, M.; Snyder, M. RNA-Seq: A revolutionary tool for transcriptomics. Nat. Rev. Genet. 2009, 10, 57–63. [Google Scholar]
- Marguerat, S.; Bähler, J. RNA-seq: From technology to biology. Cell. Mol. Life Sci. 2010, 67, 569–579. [Google Scholar] [CrossRef]
- Comité Estatal de Sanidad Vegetal de Jalisco. Available online: http://www.cesavejal.org.mx/ (accessed on 6 June 2013).
- Comité Estatal de Sanidad Vegetal de Chiapas. Available online: http://cesavechiapas.org.mx/ (accessed on 6 June 2013).
- Nagalakshmi, U.; Waern, K.; Snyder, M. RNA-Seq: A method for comprehensive transcriptome analysis. In Current Protocols in Molecular Biology; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2010; Suppl. 89, pp. 4.11.1–4.11.13. [Google Scholar]
- Trapnell, C.; Roberts, A.; Goff, L.; Pertea, G.; Kim, D.; Kelley, D.R.; Pimentel, H.; Salzberg, S.L.; Rinn, J.L.; Pachter, L. Differential gene and transcript expression analysis of RNA-seq experiments with TopHat and Cufflinks. Nat. Protoc. 2012, 17, 562–578. [Google Scholar]
- Goodstein, D.M.; Shu, S.; Howson, R.; Neupane, R.; Hayes, R.D.; Fazo, J.; Mitros, T.; Dirks, W.; Hellsten, U.; Putnam, N.; et al. Phytozome: A comparative platform for green plant genomics. Nucleic Acids Res. 2012, 40, 1178–1186. [Google Scholar] [CrossRef]
- Kim, D.; Pertea, G.; Trapnell, C.; Pimentel, H.; Kelley, R.; Salzberg, S.L. TopHat2: Accurate alignment of transcriptomes in the presence of insertions, deletions and gene fusions. Genome Biol. 2013, 14, R36. [Google Scholar] [CrossRef]
- Cock, P.J.; Fields, C.J.; Goto, N.; Heuer, M.L.; Rice, P.M. The Sanger FASTQ file format for sequences with quality scores, and the Solexa/Illumina FASTQ variants. Nucleic Acids Res. 2010, 38, 1767–1771. [Google Scholar] [CrossRef]
- Langmead, B. Aligning short sequencing reads with Bowtie. In Current Protocols in Bioinformatics; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2010; Suppl. 32, pp. 11.7.1–11.7.14. [Google Scholar]
- Langmead, B.; Trapnell, C.; Pop, M.; Salzberg, S.L. Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome Biol. 2009, 10, R25. [Google Scholar] [CrossRef]
- Mutz, K.O.; Heilkenbrinker, A.; Lönne, M.; Walter, J.G.; Stahl, F. Transcriptome analysis using next-generation sequencing. Curr. Opin. Biotechnol. 2012, 24, 22–30. [Google Scholar]
- Trapnell, C.; Williams, B.A.; Pertea, G.; Mortazavi, A.; Kwan, G.; van Baren, M.J.; Salzberg, S.L.; Wold, B.J.; Pachter, L. Transcript assembly and quantification by RNA-Seq reveals unannotated transcripts and isoform switching during cell differentiation. Nat. Biotechnol. 2010, 28, 511–515. [Google Scholar] [CrossRef]
- Johnson, M.; Zaretskaya, I.; Raytselis, Y.; Merezhuk, Y.; McGinnis, S.; Madden, T.L. NCBI BLAST: A better web interface. Nucleic Acids Res. 2008, 36, W5–W9. [Google Scholar]
- Chevreux, B.; Pfisterer, T.; Drescher, B.; Driesel, A.J.; Müller, W.E.G.; Wetter, T.; Suhai, S. Using the miraEST assembler for reliable and automated mRNA transcript assembly and SNP detection in sequenced ESTs. Genome Res. 2004, 14, 1147–1159. [Google Scholar] [CrossRef]
- Rutherford, K.; Parkhill, J.; Crook, J.; Horsnell, T.; Rice, P.; Rajandream, M.A.; Barrell, B. Artemis: Sequence visualization and annotation. Bioinformatics 2000, 10, 944–945. [Google Scholar]
- Galtier, N.; Gouy, M.; Gautier, C. SEAVIEW and PHYLO_WIN: Two graphic tools for sequence alignment and molecular phylogeny. Comput. Appl. Biosci. 1996, 12, 543–548. [Google Scholar]
- Larkin, M.A.; Blackshields, G.; Brown, N.P.; Chenna, R.; McGettigan, P.A.; McWilliam, H.; Valentin, F.; Wallace, I.M.; Wilm, A.; Lopez, R.; et al. lustal W and Clustal X version 2.0.0. Bioinformatics 2007, 23, 2947–2948. [Google Scholar] [CrossRef]
- Abascal, F.; Zardoya, R.; Posada, D. ProtTest: Selection of best-fit models of protein evolution. Bioinformatics 2005, 21, 2104–2105. [Google Scholar] [CrossRef]
- Felsenstein, J. PHYLIP-Phylogeny inference package (version 3.2). Cladistics 1989, 5, 164–166. [Google Scholar]
- Felsenstein, J. PHYLIP (Phylogeny Inference Package), version 3.6; Distributed by the author; Department of Genome Sciences, University of Washington: Seattle, WA, USA, 2005. [Google Scholar]
- Guindon, S.; Dufayard, J.F.; Lefort, V.; Anisimova, M.; Hordijk, W.; Gascuel, O. New algorithms and methods to estimate maximum-likelihood phylogenies: Assessing the performance of PhyML 3.0. Syst. Biol. 2010, 59, 307–321. [Google Scholar] [CrossRef]
- Molecular Evolution, Phylogenetics and Epidemiology. Available online: http://tree.bio.ed.ac.uk/software/figtree/ (accessed on 5 December 2012).
- Oshlack, A.; Robinson, M.; Young, M. From RNA-seq reads to differential expression results. Genome Biol. 2009, 11, 220. [Google Scholar] [CrossRef]
- Conesa, A.; Götz, S.; García-Gómez, J.M.; Terol, J.; Talón, M.; Robles, M. Blast2GO: A universal tool for annotation, visualization and analysis in functional genomics research. Bioinformatics 2005, 21, 3674–3676. [Google Scholar] [CrossRef]
- Conesa, A.; Götz, S. Blast2GO: A comprehensive suite for functional analysis in plant genomics. Int. J. Plant Genomics 2008, 2008, 619832. [Google Scholar]
- Thimm, O.; Blaesing, O.; Gibon, Y.; Nagel, A.; Meyer, S.; Krüger, P.; Selbig, J.; Müller, L.A.; Rhee, S.Y.; Stitt, M. MAPMAN: A user-driven tool to display genomics data sets onto diagrams of metabolic pathways and other biological processes. Plant J. 2004, 37, 914–939. [Google Scholar] [CrossRef]
- Logemann, J.; Schell, J.; Willmitzer, L. Improved method for the isolation of RNA from plant tissues. Anal. Biochem. 1987, 163, 16–20. [Google Scholar] [CrossRef]
- Plants Profile for Citrus × aurantium [Maxima × Reticulata] (Sour Orange). Available online: http://plants.usda.gov/java/profile?symbol=CIAU8/ (accessed on 1 June 2013).
- Hiraguri, A.; Hibino, H.; Hayashi, T.; Netsu, O.; Shimizu, T.; Uehara-Ichiki, T.; Omura, T.; Sasaki, N.; Nyunoya, H.; Sasaya, T. The movement protein encoded by gene 3 of rice transitory yellowing virus is associated with virus particles. J. Gen. Virol. 2012, 93, 2290–2298. [Google Scholar] [CrossRef]
- Huang, Y.W.; Geng, Y.F.; Ying, X.B.; Chen, X.Y.; Fang, R.X. Identification of a movement protein of rice yellow stunt rhabdovirus. J. Virol. 2005, 79, 2108–2114. [Google Scholar] [CrossRef]
- Peng, W.; Zheng, G.H.; Zheng, Z.Z.; Tong, Q.X.; Ming, Y.L. Orchid fleck virus: An unclassified bipartite, negative-sense RNA plant virus. Arch. Virol. 2013, 158, 313–323. [Google Scholar] [CrossRef]
- Martelli, G.P.; Russo, M. Plant virus inclusion bodies. Adv. Virus Res. 1977, 21, 175–266. [Google Scholar] [CrossRef]
- Moshe, A.; Gorovits, R. Virus-induced aggregates in infected cells. Viruses 2012, 4, 2218–2232. [Google Scholar] [CrossRef]
- Lin, N.S.; Chen, C.C. Association of Bamboo Mosaic Virus (BoMV) and BoMV-specific electron-dense crystalline bodies with chloroplasts. Phytopathology 1991, 81, 1551–1555. [Google Scholar] [CrossRef]
- Chang, M.U.; Arai, K.; Doi, Y.; Yora, K. Morphology and intracellular appearance of orchid fleck virus. Ann. Phytopathol. Soc. Jpn. 1976, 42, 156–167. [Google Scholar] [CrossRef]
- Kitajima, E.W.; Kondo, H.; Mackenzie, A.; Rezende, J.A.M.; Gioria, R.; Gibbs, A.; Tamada, T. Comparative cytopathology and immunocytochemistry of Japanese, Australian and Brazilian isolates of Orchid fleck virus. J. Gen. Plant Pathol. 2001, 67, 231–237. [Google Scholar] [CrossRef]
- Jones, J.D.; Dangl, J.L. The plant immune system. Nature 2006, 444, 323–329. [Google Scholar] [CrossRef]
- Zheng, Z.; Qualley, A.; Fan, B.; Dudareva, N.; Chen, Z. An important role of a BAHD acyl transferase-like protein in plant innate immunity. Plant J. 2009, 57, 1040–1053. [Google Scholar] [CrossRef]
- Schreiber, K.; Desveaux, D. Message in a bottle: Chemical biology of induced disease resistance in plants. Plant Pathol. J. 2008, 24, 245–268. [Google Scholar] [CrossRef]
- Dao, T.T.; Linthorst, H.J.; Verpoorte, R. Chalcone synthase and its functions in plant resistance. Phytochem. Rev. 2011, 10, 397–412. [Google Scholar] [CrossRef]
- Wang, T.; Chen, X.; Zhu, F.; Li, H.; Li, L.; Yang, Q.; Chi, X.; Yu, S.; Liang, X. Characterization of peanut germin-like proteins, AhGLPs in plant development and defense. PLoS One 2013, 8, e61722. [Google Scholar]
- Rowland, O.; Ludwig, A.A.; Merrick, C.J.; Baillieul, F.; Tracy, F.E.; Durrant, W.E.; Fritz-Laylin, L.; Nekrasov, V.; Sjölander, K.; Yoshioka, H.; et al. Functional analysis of Avr9/Cf-9 rapidly elicited genes identifies a protein kinase, ACIK1, that is essential for full Cf-9-dependent disease resistance in tomato. Plant Cell 2005, 17, 295–310. [Google Scholar] [CrossRef]
- Jones, J.B.; Vallad, G.E.; Iriarte, F.B.; Obradovic, A.; Wernsing, M.; Jackson, L.E.; Balogh, B.; Hong, J.C.; Momol, MT. Considerations for using bacteriophages for plant disease control. Bacteriophage 2012, 2, 208–214. [Google Scholar] [CrossRef]
- Kondo, H.; Maeda, T.; Shirako, Y.; Tamada, T. Orchid fleck virus is a rhabdovirus with an unusual bipartite genome. J. Gen. Virol. 2006, 87, 2413–2421. [Google Scholar] [CrossRef]
- Kondo, H.; Chiba, S.; Andika, I.B.; Maruyama, K.; Tamada, T.; Suzuki, N. Orchid fleck virus structural proteins N and P form intranuclear viroplasm-like structures in the absence of viral infection. J. Virol. 2013, 87, 7423–7434. [Google Scholar] [CrossRef]
- Ruiz-Medrano, R.; Xoconostle-Cázares, B.; Lucas, W.J. The phloem as a conduit for inter-organ communication. Curr. Opin. Plant Biol. 2001, 4, 202–209. [Google Scholar] [CrossRef]
- Ham, B.K.; Li, G.; Kang, B.H.; Zeng, F.; Lucas, W.J. Overexpression of Arabidopsis plasmodesmata germin-like proteins disrupts root growth and development. Plant Cell 2012, 24, 3630–3648. [Google Scholar] [CrossRef]
- Guevara-Olvera, L.; Ruíz-Nito, M.L.; Rangel-Cano, R.M.; Torres-Pacheco, I.; Rivera-Bustamante, R.F.; Muñoz-Sánchez, C.I.; González-Chavira, M.M.; Cruz-Hernandez, A.; Guevara-González, R.G. Expression of a germin-like protein gene (CchGLP) from a geminivirus-resistant pepper (Capsicum chinense Jacq.) enhances tolerance to geminivirus infection in transgenic tobacco. Physiol. Mol. Plant Pathol. 2012, 78, 45–50. [Google Scholar] [CrossRef]
- Whitham, S.A.; Yang, C.; Goodin, M.M. Global impact: Elucidating plant responses to viral infection. Mol. PlantMicrobe Interact. 2006, 19, 1207–1215. [Google Scholar] [CrossRef]
- Dorokhov, Y.L.; Komarova, T.V.; Petrunia, I.V.; Frolova, O.Y.; Pozdyshev, D.V.; Gleba, Y.Y. Airborne signals from a wounded leaf facilitate viral spreading and induce antibacterial resistance in neighboring plants. PLoS Pathog. 2012, 8, e1002640. [Google Scholar]
- Postnikova, O.A.; Nemchinov, L.G. Comparative analysis of microarray data in Arabidopsis transcriptome during compatible interactions with plant viruses. Virol. J. 2012, 9, 101. [Google Scholar]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Cruz-Jaramillo, J.L.; Ruiz-Medrano, R.; Rojas-Morales, L.; López-Buenfil, J.A.; Morales-Galván, O.; Chavarín-Palacio, C.; Ramírez-Pool, J.A.; Xoconostle-Cázares, B. Characterization of a Proposed Dichorhavirus Associated with the Citrus Leprosis Disease and Analysis of the Host Response. Viruses 2014, 6, 2602-2622. https://doi.org/10.3390/v6072602
Cruz-Jaramillo JL, Ruiz-Medrano R, Rojas-Morales L, López-Buenfil JA, Morales-Galván O, Chavarín-Palacio C, Ramírez-Pool JA, Xoconostle-Cázares B. Characterization of a Proposed Dichorhavirus Associated with the Citrus Leprosis Disease and Analysis of the Host Response. Viruses. 2014; 6(7):2602-2622. https://doi.org/10.3390/v6072602
Chicago/Turabian StyleCruz-Jaramillo, José Luis, Roberto Ruiz-Medrano, Lourdes Rojas-Morales, José Abel López-Buenfil, Oscar Morales-Galván, Claudio Chavarín-Palacio, José Abrahán Ramírez-Pool, and Beatriz Xoconostle-Cázares. 2014. "Characterization of a Proposed Dichorhavirus Associated with the Citrus Leprosis Disease and Analysis of the Host Response" Viruses 6, no. 7: 2602-2622. https://doi.org/10.3390/v6072602




