A Viral Noncoding RNA Complements a Weakened Viral RNA Silencing Suppressor and Promotes Efficient Systemic Host Infection
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plasmids
2.2. In Vitro Transcription, Plant Infection, Protein, and RNA Extractions
2.3. Agroinfiltration of N. benthamiana 16C
3. Results
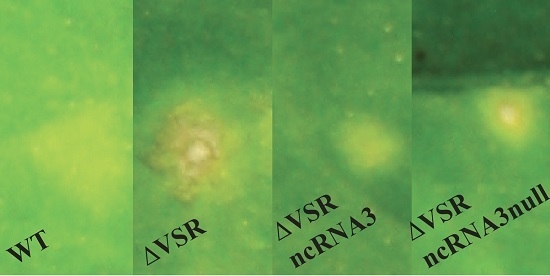
3.1. The ncRNA3 Complements the Absence of BNYVV VSR in C. quinoa
3.2. The ncRNA3 Promotes Systemic Movement of p14BA2 VSR Mutant in N. benthamiana
3.3. Silencing of N. benthamiana RDR6 Allows Systemic Movement of VSR BA2 Mutant Independently of the Presence of RNA3
3.4. BNYVV p14 Inhibits the Systemic Spread of RNA Silencing
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Incarbone, M.; Dunoyer, P. RNA silencing and its suppression: Novel insights from in planta analyses. Trends Plant Sci. 2013, 18, 382–392. [Google Scholar] [CrossRef] [PubMed]
- Ding, S.W.; Voinnet, O. Antiviral immunity directed by small RNAs. Cell 2007, 130, 413–426. [Google Scholar] [CrossRef] [PubMed]
- Mermigka, G.; Verret, F.; Kalantidis, K. RNA silencing movement in plants. J. Integr. Plant Biol. 2016, 58, 328–342. [Google Scholar] [CrossRef] [PubMed]
- Blevins, T.; Rajeswaran, R.; Aregger, M.; Borah, B.K.; Schepetilnikov, M.; Baerlocher, L.; Farinelli, L.; Meins, F.; Hohn, T.; Pooggin, M.M. Massive production of small RNAs from a non-coding region of cauliflower mosaic virus in plant defense and viral counter-defense. Nucleic Acids Res. 2011, 39, 5003–5014. [Google Scholar] [CrossRef] [PubMed]
- Hohn, T. RNA based viral silencing suppression in plant pararetroviruses. Front Plant Sci. 2015, 6, 398. [Google Scholar] [CrossRef] [PubMed]
- Miller, W.A.; Shen, R.; Staplin, W.; Kanodia, P. Noncoding RNAs of plant viruses and viroids: Sponges of host translation and RNA interference machinery. Mol. Plant Microbe Interact. 2016, 29, 156–164. [Google Scholar] [CrossRef] [PubMed]
- Derrien, B.; Baumberger, N.; Schepetilnikov, M.; Viotti, C.; De Cillia, J.; Ziegler-Graff, V.; Isono, E.; Schumacher, K.; Genschik, P. Degradation of the antiviral component argonaute1 by the autophagy pathway. Proc. Natl. Acad. Sci. USA 2012, 109, 15942–15946. [Google Scholar] [CrossRef] [PubMed]
- Chiba, S.; Hleibieh, K.; Delbianco, A.; Klein, E.; Ratti, C.; Ziegler-Graff, V.; Bouzoubaa, S.; Gilmer, D. The benyvirus RNA silencing suppressor is essential for long-distance movement, requires both zinc-finger and NoLs basic residues but not a nucleolar localization for its silencing-suppression activity. Mol. Plant Microbe Interact. 2013, 26, 168–181. [Google Scholar] [CrossRef] [PubMed]
- Gilmer, D.; Ratti, C. Benyvirus. In Virus Taxonomy: Classification and Nomenclature of Viruses: Ninth Report of the International Committee on Taxonomy of Viruses; King, A.M.Q., Adams, M.J., Carstens, E.B., Lefkowitz, E.J., Eds.; Elsevier: San Diego, CA, USA, 2012; pp. 1133–1138. [Google Scholar]
- Jupin, I.; Guilley, H.; Richards, K.E.; Jonard, G. Two proteins encoded by beet necrotic yellow vein virus RNA 3 influence symptom phenotype on leaves. EMBO J. 1992, 11, 479–488. [Google Scholar] [PubMed]
- Peltier, C.; Schmidlin, L.; Klein, E.; Taconnat, L.; Prinsen, E.; Erhardt, M.; Heintz, D.; Weyens, G.; Lefebvre, M.; Renou, J.P.; et al. Expression of the Beet necrotic yellow vein virus p25 protein induces hormonal changes and a root branching phenotype in Arabidopsis thaliana. Transgenic Res. 2011, 20, 443–466. [Google Scholar] [CrossRef] [PubMed]
- Lauber, E.; Guilley, H.; Tamada, T.; Richards, K.E.; Jonard, G. Vascular movement of beet necrotic yellow vein virus in Beta macrocarpa is probably dependent on an RNA 3 sequence domain rather than a gene product. J. Gen. Virol. 1998, 79, 385–393. [Google Scholar] [CrossRef] [PubMed]
- Peltier, C.; Klein, E.; Hleibieh, K.; D’Alonzo, M.; Hammann, P.; Bouzoubaa, S.; Ratti, C.; Gilmer, D. Beet necrotic yellow vein virus subgenomic RNA3 is a cleavage product leading to stable non-coding RNA required for long-distance movement. J. Gen. Virol. 2012, 93, 1093–1102. [Google Scholar] [CrossRef] [PubMed]
- Ratti, C.; Hleibieh, K.; Bianchi, L.; Schirmer, A.; Autonell, C.R.; Gilmer, D. Beet soil-borne mosaic virus RNA-3 is replicated and encapsidated in the presence of BNYVV RNA-1 and -2 and allows long distance movement in Beta macrocarpa. Virology 2009, 385, 392–399. [Google Scholar] [CrossRef] [PubMed]
- Quillet, L.; Guilley, H.; Jonard, G.; Richards, K. In vitro synthesis of biologically active beet necrotic yellow vein virus RNA. Virology 1989, 172, 293–301. [Google Scholar] [PubMed]
- Schwach, F.; Vaistij, F.E.; Jones, L.; Baulcombe, D.C. An RNA-dependent RNA polymerase prevents meristem invasion by potato virus X and is required for the activity but not the production of a systemic silencing signal. Plant Physiol. 2005, 138, 1842–1852. [Google Scholar] [CrossRef] [PubMed]
- Guilley, H.; Bortolamiol, D.; Jonard, G.; Bouzoubaa, S.; Ziegler-Graff, V. Rapid screening of RNA silencing suppressors by using a recombinant virus derived from beet necrotic yellow vein virus. J. Gen. Virol. 2009, 90, 2536–2541. [Google Scholar] [CrossRef] [PubMed]
- Klein, E.; Link, D.; Schirmer, A.; Erhardt, M.; Gilmer, D. Sequence variation within Beet necrotic yellow vein virus p25 protein influences its oligomerization and isolate pathogenicity on Tetragonia expansa. Virus Res. 2007, 126, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Veidt, I.; Bouzoubaa, S.E.; Leiser, R.M.; Ziegler-Graff, V.; Guilley, H.; Richards, K.; Jonard, G. Synthesis of full-length transcripts of beet western yellows virus RNA: messenger properties and biological activity in protoplasts. Virology 1992, 186, 192–200. [Google Scholar] [CrossRef]
- Jupin, I.; Bouzoubaa, S.; Richards, K.; Jonard, G.; Guilley, H. Multiplication of beet necrotic yellow vein virus RNA 3 lacking a 3’ poly(A) tail is accompanied by reappearance of the poly(A) tail and a novel short U-rich tract preceding it. Virology 1990, 178, 281–284. [Google Scholar] [CrossRef]
- Kozlowska-Makulska, A.; Guilley, H.; Szyndel, M.S.; Beuve, M.; Lemaire, O.; Herrbach, E.; Bouzoubaa, S. P0 proteins of European beet-infecting poleroviruses display variable RNA silencing suppression activity. J. Gen. Virol. 2010, 91, 1082–1091. [Google Scholar] [CrossRef] [PubMed]
- Ellis, E.L.; Delbruck, M. The growth of bacteriophage. J. Gen. Physiol. 1939, 22, 365–384. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wang, X.; Li, D.; Han, C.; Zhai, Y.; Yu, J. Two virus-encoded RNA silencing suppressors, p14 of beet necrotic yellow vein virus and s6 of rice black streak dwarf virus. Chin. Sci. Bull. 2005, 50, 305–310. [Google Scholar]
- Wang, X.B.; Wu, Q.; Ito, T.; Cillo, F.; Li, W.X.; Chen, X.; Yu, J.L.; Ding, S.W. RNAi-mediated viral immunity requires amplification of virus-derived siRNAs in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 2010, 107, 484–489. [Google Scholar] [CrossRef] [PubMed]
- Silhavy, D.; Molnar, A.; Lucioli, A.; Szittya, G.; Hornyik, C.; Tavazza, M.; Burgyan, J. A viral protein suppresses RNA silencing and binds silencing-generated, 21- to 25-nucleotide double-stranded RNAs. EMBO J. 2002, 21, 3070–3080. [Google Scholar] [CrossRef] [PubMed]
- Baumberger, N.; Tsai, C.H.; Lie, M.; Havecker, E.; Baulcombe, D.C. The polerovirus silencing suppressor p0 targets argonaute proteins for degradation. Curr. Biol. 2007, 17, 1609–1614. [Google Scholar] [CrossRef] [PubMed]
- Pfeffer, S.; Dunoyer, P.; Heim, F.; Richards, K.E.; Jonard, G.; Ziegler-Graff, V. P0 of beet Western yellows virus is a suppressor of posttranscriptional gene silencing. J. Virol. 2002, 76, 6815–6824. [Google Scholar] [CrossRef] [PubMed]
- Flobinus, A.; Chevigny, N.; Seissler, T.; Klein, E.; Bleykasten-Grosshans, C.; Ratti, C.; Bouzoubaa, A.; Gilmer, D. Benyvirus noncoding RNA production depends on an XRN activity. J. Gen. Virol. Unpublished, In revision.
- Landeo-Rios, Y.; Navas-Castillo, J.; Moriones, E.; Canizares, M.C. The p22 RNA silencing suppressor of the crinivirus Tomato chlorosis virus preferentially binds long dsRNAs preventing them from cleavage. Virology 2016, 488, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Iwakawa, H.O.; Mizumoto, H.; Nagano, H.; Imoto, Y.; Takigawa, K.; Sarawaneeyaruk, S.; Kaido, M.; Mise, K.; Okuno, T. A viral noncoding RNA generated by cis-element-mediated protection against 5’->3’ RNA decay represses both cap-independent and cap-dependent translation. J. Virol. 2008, 82, 10162–10174. [Google Scholar] [CrossRef] [PubMed]
- Pijlman, G.P.; Funk, A.; Kondratieva, N.; Leung, J.; Torres, S.; van der Aa, L.; Liu, W.J.; Palmenberg, A.C.; Shi, P.Y.; Hall, R.A.; et al. A highly structured, nuclease-resistant, noncoding RNA produced by flaviviruses is required for pathogenicity. Cell Host Microbe 2008, 4, 579–591. [Google Scholar] [CrossRef] [PubMed]
- Schnettler, E.; Sterken, M.G.; Leung, J.Y.; Metz, S.W.; Geertsema, C.; Goldbach, R.W.; Vlak, J.M.; Kohl, A.; Khromykh, A.A.; Pijlman, G.P. Noncoding flavivirus RNA displays RNA interference suppressor activity in insect and mammalian cells. J. Virol. 2012, 86, 13486–13500. [Google Scholar] [CrossRef] [PubMed]
- Andersson, M.G.; Haasnoot, P.C.; Xu, N.; Berenjian, S.; Berkhout, B.; Akusjarvi, G. Suppression of RNA interference by adenovirus virus-associated RNA. J. Virol. 2005, 79, 9556–9565. [Google Scholar] [CrossRef] [PubMed]





© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Flobinus, A.; Hleibieh, K.; Klein, E.; Ratti, C.; Bouzoubaa, S.; Gilmer, D. A Viral Noncoding RNA Complements a Weakened Viral RNA Silencing Suppressor and Promotes Efficient Systemic Host Infection. Viruses 2016, 8, 272. https://doi.org/10.3390/v8100272
Flobinus A, Hleibieh K, Klein E, Ratti C, Bouzoubaa S, Gilmer D. A Viral Noncoding RNA Complements a Weakened Viral RNA Silencing Suppressor and Promotes Efficient Systemic Host Infection. Viruses. 2016; 8(10):272. https://doi.org/10.3390/v8100272
Chicago/Turabian StyleFlobinus, Alyssa, Kamal Hleibieh, Elodie Klein, Claudio Ratti, Salah Bouzoubaa, and David Gilmer. 2016. "A Viral Noncoding RNA Complements a Weakened Viral RNA Silencing Suppressor and Promotes Efficient Systemic Host Infection" Viruses 8, no. 10: 272. https://doi.org/10.3390/v8100272