New Paradigms for the Study of Ocular Alphaherpesvirus Infections: Insights into the Use of Non-Traditional Host Model Systems
Abstract
:1. Introduction
2. In Vitro 2D Cell Culture Systems
2.1. Immortalized Cell Lines
2.2. Primary Corneal Epithelial Cells
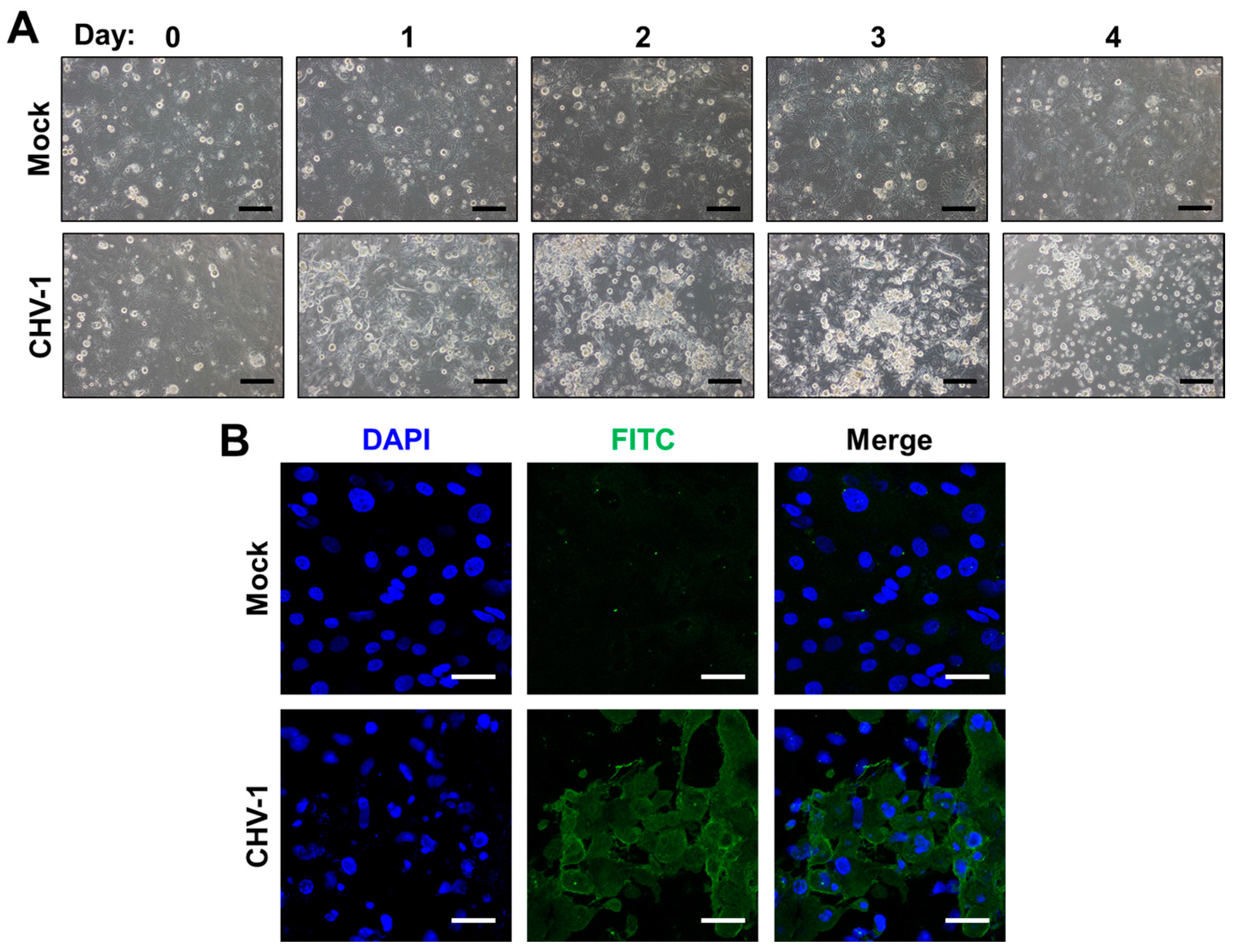
2.3. Limitations of 2D Cell Culture Systems
3. In Vitro 3D Cell Culture/Explant Systems
3.1. Corneal Facsimile
3.2. Explants
3.3. Limitations of 3D Cell Culture/Explant Systems
4. In Vivo Systems
4.1. Mice
4.2. Rabbits
4.3. Dogs
4.4. Cats
4.5. Limitations of Non-Traditional In Vivo Models
5. Conclusions and Future Prospects
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Gould, D. Feline herpesvirus-1: Ocular manifestations, diagnosis and treatment options. J. Feline Med. Surg. 2011, 13, 333–346. [Google Scholar] [CrossRef] [PubMed]
- Ledbetter, E.C. Canine herpesvirus-1 ocular diseases of mature dogs. N. Z. Vet. J. 2013, 61, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Rowe, A.M.; St Leger, A.J.; Jeon, S.; Dhaliwal, D.K.; Knickelbein, J.E.; Hendricks, R.L. Herpes keratitis. Prog. Retin. Eye Res. 2013, 32, 88–101. [Google Scholar] [CrossRef] [PubMed]
- Gaskell, R.; Dawson, S.; Radford, A.; Thiry, E. Feline herpesvirus. Vet. Res. 2007, 38, 337–354. [Google Scholar] [CrossRef] [PubMed]
- Maes, R. Felid herpesvirus type 1 infection in cats: A natural host model for alphaherpesvirus pathogenesis. ISRN Vet. Sci. 2012, 2012, 495830. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Zhu, H. Ocular herpes: The pathophysiology, management and treatment of herpetic eye diseases. Virol. Sin. 2014, 29, 327–342. [Google Scholar] [CrossRef] [PubMed]
- Kawakami, K.; Ogawa, H.; Maeda, K.; Imai, A.; Ohashi, E.; Matsunaga, S.; Tohya, Y.; Ohshima, T.; Mochizuki, M. Nosocomial outbreak of serious canine infectious tracheobronchitis (kennel cough) caused by canine herpesvirus infection. J. Clin. Microbiol. 2010, 48, 1176–1181. [Google Scholar] [CrossRef] [PubMed]
- Harman, R.M.; Bussche, L.; Ledbetter, E.C.; van de Walle, G.R. Establishment and characterization of an air-liquid canine corneal organ culture model to study acute herpes keratitis. J. Virol. 2014, 88, 13669–13677. [Google Scholar] [CrossRef] [PubMed]
- Pennington, M.R.; van de Walle, G.R. Electric cell-substrate impedance sensing to monitor viral growth and study cellular responses to infection with alphaherpesviruses in real time. mSphere 2017, 2, e00039-17. [Google Scholar] [CrossRef] [PubMed]
- Labetoulle, M.; Auquier, P.; Conrad, H.; Crochard, A.; Daniloski, M.; Bouée, S.; El Hasnaoui, A.; Colin, J. Incidence of herpes simplex virus keratitis in France. Ophthalmology 2005, 112, 888.e1–895.e1. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Sternberg, M.R.; Kottiri, B.J.; McQuillan, G.M.; Lee, F.K.; Nahmias, A.J.; Berman, S.M.; Markowitz, L.E. Trends in herpes simplex virus type 1 and type 2 seroprevalence in the United States. JAMA 2006, 296, 964–973. [Google Scholar] [CrossRef] [PubMed]
- Young, R.C.; Hodge, D.O.; Liesegang, T.J.; Baratz, K.H. Incidence, recurrence and outcomes of herpes simplex virus eye disease in Olmsted County, Minnesota, 1976–2007: The effect of oral antiviral prophylaxis. Arch. Ophthalmol. 2010, 128, 1178–1183. [Google Scholar] [CrossRef] [PubMed]
- Burcea, M.; Gheorghe, A.; Pop, M. Incidence of herpes simplex virus keratitis in HIV/AIDS patients compared with the general population. J. Med. Life 2015, 8, 62–63. [Google Scholar] [PubMed]
- Looker, K.J.; Magaret, A.S.; May, M.T.; Turner, K.M.; Vickerman, P.; Gottlieb, S.L. Global and regional estimates of prevalent and incident herpes simplex virus type 1 infections in 2012. PLoS ONE 2015, 10, e0140765. [Google Scholar] [CrossRef] [PubMed]
- Nöthling, J.O.; Hüssy, D.; Steckler, D.; Ackermann, M. Seroprevalence of canine herpesvirus in breeding kennels in the Gauteng Province of South Africa. Theriogenology 2008, 69, 276–282. [Google Scholar] [CrossRef] [PubMed]
- Babaei, H.; Akhtardanesh, B.; Ghanbarpour, R.; Namjoo, A. Serological evidence of canine herpesvirus-1 in dogs of Kerman city, south-east of Iran. Transbound. Emerg. Dis. 2010, 57, 348–351. [Google Scholar] [CrossRef] [PubMed]
- Ledbetter, E.C.; Kim, S.G.; Dubovi, E.J. Outbreak of ocular disease associated with naturally-acquired canine herpesvirus-1 infection in a closed domestic dog colony. Vet. Ophthalmol. 2009, 12, 242–247. [Google Scholar] [CrossRef] [PubMed]
- Krogenæs, A.; Rootwelt, V.; Larsen, S.; Sjøberg, E.K.; Akselsen, B.; Skår, T.M.; Myhre, S.S.; Renström, L.H.M.; Klingeborn, B.; Lund, A. A serologic study of canine herpesvirus-1 infection in the Norwegian adult dog population. Theriogenology 2012, 78, 153–158. [Google Scholar]
- Krogenæs, A.; Rootwelt, V.; Larsen, S.; Renström, L.; Farstad, W.; Lund, A. A serological study of canine herpesvirus-1 infection in a population of breeding bitches in Norway. Acta Vet. Scand. 2014, 56, 19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Larsen, R.W.; Kiupel, M.; Balzer, H.J.; Agerholm, J.S. Prevalence of canid herpesvirus-1 infection in stillborn and dead neonatal puppies in Denmark. Acta Vet. Scand. 2015, 57, 1. [Google Scholar] [CrossRef] [PubMed]
- Maggs, D.J.; Lappin, M.R.; Nasisse, M.P. Detection of feline herpesvirus-specific antibodies and DNA in aqueous humor from cats with or without uveitis. Am. J. Vet. Res. 1999, 60, 932–936. [Google Scholar] [PubMed]
- Kang, B.T.; Park, H.M. Prevalence of feline herpesvirus 1, feline calicivirus and Chlamydophila felis in clinically normal cats at a Korean animal shelter. J. Vet. Sci. 2008, 9, 207–209. [Google Scholar] [CrossRef] [PubMed]
- Henzel, A.; Brum, M.C.S.; Lautert, C.; Martins, M.; Lovato, L.T.; Weiblen, R. Isolation and identification of feline calicivirus and feline herpesvirus in Southern Brazil. Braz. J. Microbiol. 2012, 43, 560–568. [Google Scholar] [CrossRef] [PubMed]
- McManus, C.M.; Levy, J.K.; Andersen, L.A.; McGorray, S.P.; Leutenegger, C.M.; Gray, L.K.; Hilligas, J.; Tucker, S.J. Prevalence of upper respiratory pathogens in four management models for unowned cats in the Southeast United States. Vet. J. 2014, 201, 196–201. [Google Scholar] [CrossRef] [PubMed]
- Rypuła, K.; Płoneczka-Janeczko, K.; Bierowiec, K.; Kumala, A.; Sapikowski, G. Prevalence of viral infections in cats in southwestern Poland in the years 2006 to 2010. Berl Munch Tierarztl Wochenschr. 2014, 127, 163–165. [Google Scholar] [PubMed]
- Shaikh, S.; Ta, C.N. Evaluation and management of herpes zoster ophthalmicus. Am. Fam. Physician 2002, 66, 1723–1730. [Google Scholar] [PubMed]
- Borkar, D.S.; Tham, V.M.; Esterberg, E.; Ray, K.J.; Vinoya, A.C.; Parker, J.V.; Uchida, A.; Acharya, N.R. Incidence of herpes zoster ophthalmicus: Results from the pacific ocular inflammation study. Ophthalmology 2013, 120, 451–456. [Google Scholar] [CrossRef] [PubMed]
- Johnson, R.W.; Alvarez-Pasquin, M.J.; Bijl, M.; Franco, E.; Gaillat, J.; Clara, J.G.; Labetoulle, M.; Michel, J.P.; Naldi, L.; Sanmarti, L.S.; et al. Herpes zoster epidemiology, management and disease and economic burden in Europe: A multidisciplinary perspective. Ther. Adv. Vaccines 2015, 3, 109–120. [Google Scholar] [CrossRef] [PubMed]
- Allen, G.P.; Bolin, D.C.; Bryant, U.; Carter, C.N.; Giles, R.C.; Harrison, L.R.; Hong, C.B.; Jackson, C.B.; Poonacha, K.; Wharton, R.; et al. Prevalence of latent, neuropathogenic equine herpesvirus-1 in the thoroughbred broodmare population of central Kentucky. Equine Vet. J. 2008, 40, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Lunn, D.P.; Davis-Poynter, N.; Flaminio, M.J.B.F.; Horohov, D.W.; Osterrieder, K.; Pusterla, N.; Townsend, H.G.G. Equine herpesvirus-1 consensus statement. J. Vet. Intern. Med. 2009, 23, 450–461. [Google Scholar] [CrossRef] [PubMed]
- Hussey, G.S.; Goehring, L.S.; Lunn, D.P.; Hussey, S.B.; Huang, T.; Osterrieder, N.; Powell, C.; Hand, J.; Holz, C.; Slater, J. Experimental infection with equine herpesvirus type 1 (EHV-1) induces chorioretinal lesions. Vet. Res. 2013, 44, 118. [Google Scholar] [CrossRef] [PubMed]
- Yildirim, Y.; Yilmaz, V.; Kirmizigul, A.H. Equine herpesvirus type 1 (EHV-1) and 4 (EHV-4) infections in horses and donkeys in northeastern Turkey. Iran. J. Vet. Res. 2015, 16, 341–344. [Google Scholar] [PubMed]
- Collinson, P.N.; O’Rielly, J.L.; Ficorilli, N.; Studdert, M.J. Isolation of equine herpesvirus type 2 (equine gammaherpesvirus 2) from foals with keratoconjunctivitis. J. Am. Vet. Med. Assoc. 1994, 205, 329–331. [Google Scholar] [PubMed]
- Borchers, K.; Wolfinger, U.; Goltz, M.; Broll, H.; Ludwig, H. Distribution and relevance of equine herpesvirus type 2(EHV-2) infections. Arch. Virol. 1997, 142, 917–928. [Google Scholar] [CrossRef] [PubMed]
- Borchers, K.; Frölich, K.; Ludwig, H. Detection of equine herpesvirus types 2 and 5 (EHV-2 and EHV-5) in Przewalski’s wild horses. Arch. Virol. 1999, 144, 771–780. [Google Scholar] [CrossRef] [PubMed]
- Kershaw, O.; von Oppen, T.; Glitz, F.; Deegen, E.; Ludwig, H.; Borchers, K. Detection of equine herpesvirus type 2 (EHV-2) in horses with keratoconjunctivitis. Virus Res. 2001, 80, 93–99. [Google Scholar] [CrossRef]
- Craig, M.I.; Barrandeguy, M.E.; Fernández, F.M. Equine herpesvirus 2 (EHV-2) infection in thoroughbred horses in Argentina. BMC Vet. Res. 2005, 1, 9. [Google Scholar] [CrossRef] [PubMed]
- Borchers, K.; Ebert, M.; Fetsch, A.; Hammond, T.; Sterner-Kock, A. Prevalence of equine herpesvirus type 2 (EHV-2) DNA in ocular swabs and its cell tropism in equine conjunctiva. Vet Microbiol. 2006, 118, 260–266. [Google Scholar] [CrossRef] [PubMed]
- Webber, J.J.; Selby, L.A. Risk factors related to the prevalence of infectious bovine keratoconjunctivitis. J. Am. Vet. Med. Assoc. 1981, 179, 823–826. [Google Scholar] [PubMed]
- Nandi, S.; Kumar, M.; Manohar, M.; Chauhan, R.S. Bovine herpesvirus infections in cattle. Anim. Health Res. Rev. 2009, 10, 85–98. [Google Scholar] [CrossRef] [PubMed]
- Raaperi, K.; Orro, T.; Viltrop, A. Epidemiology and control of bovine herpesvirus 1 infection in Europe. Vet. J. 2014, 201, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Bartha, A.; Juhász, M.; Liebermann, H. Isolation of a bovine herpesvirus from calves with respiratory disease and keratoconjunctivitis. A preliminary report. Acta Vet. Acad. Sci. Hung. 1966, 16, 357–358. [Google Scholar] [PubMed]
- Graham, D.A.; McNeill, G.J.; Calvert, V.; Mawhinney, K.; Curran, W.; Ball, N.W.; Todd, D. Virological and serological evidence of bovine herpesvirus type 4 in cattle in Northern Ireland. Vet. Rec. 2005, 157, 539–543. [Google Scholar] [CrossRef] [PubMed]
- Aslan, M.E.; Azkur, A.K.; Gazyagci, S. Epidemiology and genetic characterization of BVDV, BHV-1, BHV-4, BHV-5 and Brucella spp. infections in cattle in Turkey. J. Vet. Med. Sci. 2015, 77, 1371–1377. [Google Scholar] [CrossRef] [PubMed]
- Cvetojević, Đ.; Savić, B.; Milićević, V.; Kureljušić, B.; Jezdimirović, N.; Jakić-Dimić, D.; Pavlović, M.; Spalević, L. Prevalence of Bovine herpesvirus type 4 in aborting dairy cows. Pol. J. Vet. Sci. 2016, 19, 731–736. [Google Scholar] [CrossRef] [PubMed]
- Seal, B.S.; Heuschele, W.P.; Klieforth, R.B. Prevalence of antibodies to alcelaphine herpesvirus-1 and nucleic acid hybridization analysis of viruses isolated from captive exotic ruminants. Am. J. Vet. Res. 1989, 50, 1447–1453. [Google Scholar] [PubMed]
- Russell, G.C.; Stewart, J.P.; Haig, D.M. Malignant catarrhal fever: A review. Vet. J. 2009, 179, 324–335. [Google Scholar] [CrossRef] [PubMed]
- Bremer, C.W. The prevalence of ovine herpesvirus-2 in 4 sheep breeds from different regions in South Africa. J. S. Afr. Vet. Assoc. 2010, 81, 93–96. [Google Scholar] [CrossRef] [PubMed]
- O’Toole, D.; Li, H. The pathology of malignant catarrhal fever, with an emphasis on ovine herpesvirus 2. Vet. Pathol. 2014, 51, 437–452. [Google Scholar] [CrossRef] [PubMed]
- Thiry, J.; Keuser, V.; Muylkens, B.; Meurens, F.; Gogev, S.; Vanderplasschen, A.; Thiry, E. Ruminant alphaherpesviruses related to bovine herpesvirus 1. Vet. Res. 2006, 37, 169–190. [Google Scholar] [CrossRef] [PubMed]
- Tryland, M.; Das Neves, C.G.; Sunde, M.; Mork, T. Cervid herpesvirus 2, the primary agent in an outbreak of infectious keratoconjunctivitis in semidomesticated reindeer. J. Clin. Microbiol. 2009, 47, 3707–3713. [Google Scholar] [CrossRef] [PubMed]
- Evans, A.L.; das Neves, C.G.; Finstad, G.F.; Beckmen, K.B.; Skjerve, E.; Nymo, I.H.; Tryland, M. Evidence of alphaherpesvirus infections in Alaskan caribou and reindeer. BMC Vet. Res. 2012, 8, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Squires, R.; Wilson, P.; Whelan, N.; Johnstone, A.; Ayanegui-Alcérreca, M.; Castillo-Alcala, F.; Knight, D. Alpha and gamma herpesvirus detection in two herds of farmed red deer (Cervus elaphus) in New Zealand. N. Z. Vet. J. 2012, 60, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Rola, J.; Larska, M.; Socha, W.; Rola, J.G.; Materniak, M.; Urban-Chmiel, R.; Thiry, E.; Żmudziński, J.F. Seroprevalence of bovine herpesvirus 1 related alphaherpesvirus infections in free-living and captive cervids in Poland. Vet. Microbiol. 2017, 204, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Tryland, M.; Romano, J.S.; Marcin, N.; Nymo, I.H.; Josefsen, T.D.; Sørensen, K.K.; Mørk, T. Cervid herpesvirus 2 and not Moraxella bovoculi caused keratoconjunctivitis in experimentally inoculated semi-domesticated Eurasian tundra reindeer. Acta Vet. Scand. 2017, 59, 23. [Google Scholar] [CrossRef] [PubMed]
- Wright, E.P.; Waugh, L.F.; Goldstein, T.; Freeman, K.S.; Kelly, T.R.; Wheeler, E.A.; Smith, B.R.; Gulland, F.M.; Goldstein, T. Evaluation of viruses and their association with ocular lesions in pinnipeds in rehabilitation. Vet. Ophthalmol. 2015, 18, 148–159. [Google Scholar] [CrossRef] [PubMed]
- Webre, J.M.; Hill, J.M.; Nolan, N.M.; Clement, C.; McFerrin, H.E.; Bhattacharjee, P.S.; Hsia, V.; Neumann, D.M.; Foster, T.P.; Lukiw, W.J.; et al. Rabbit and mouse models of HSV-1 latency, reactivation and recurrent eye diseases. J. Biomed. Biotechnol. 2012, 2012, 612316. [Google Scholar] [CrossRef] [PubMed]
- Bean, A.G.D.; Baker, M.L.; Stewart, C.R.; Cowled, C.; Deffrasnes, C.; Wang, L.F.; Lowenthal, J.W. Studying immunity to zoonotic diseases in the natural host—keeping it real. Nat. Rev. Immunol. 2013, 13, 851–861. [Google Scholar] [CrossRef] [PubMed]
- Menne, S.; Cote, P.J. The woodchuck as an animal model for pathogenesis and therapy of chronic hepatitis B virus infection. World J. Gastroenterol. 2007, 13, 104–124. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, K.; Jacobson, I.M.; Tennant, B.C. The role of the woodchuck model in the treatment of hepatitis B virus infection. Clin. Liver Dis. 2007, 11, 707–725. [Google Scholar] [CrossRef] [PubMed]
- Domingo, E.; Parrish, C.R.; Holland, J.J. Origin and Evolution of Viruses; Elsevier Academic Press: New York, NY, USA, 2008; ISBN 9780080564968. [Google Scholar]
- White, D.W.; Suzanne Beard, R.; Barton, E.S. Immune modulation during latent herpesvirus infection. Immunol. Rev. 2012, 245, 189–208. [Google Scholar] [CrossRef] [PubMed]
- Wertheim, J.O.; Smith, M.D.; Smith, D.M.; Scheffler, K.; Kosakovsky Pond, S.L. Evolutionary origins of human herpes simplex viruses 1 and 2. Mol. Biol. Evol. 2014, 31, 2356–2364. [Google Scholar] [CrossRef] [PubMed]
- Field, H.J.; Huang, M.L.; Lay, E.M.; Mickleburgh, I.; Zimmermann, H.; Birkmann, A. Baseline sensitivity of HSV-1 and HSV-2 clinical isolates and defined acyclovir-resistant strains to the helicase—primase inhibitor pritelivir. Antivir. Res. 2013, 100, 297–299. [Google Scholar] [CrossRef] [PubMed]
- Piret, J.; Boivin, G. Antiviral drug resistance in herpesviruses other than cytomegalovirus. Rev. Med. Virol. 2014, 24, 186–218. [Google Scholar] [CrossRef] [PubMed]
- Kongyingyoes, B.; Priengprom, T.; Pientong, C.; Aromdee, C.; Suebsasana, S.; Ekalaksananan, T. 3,19-isopropylideneandrographolide suppresses early gene expression of drug-resistant and wild type herpes simplex viruses. Antivir. Res. 2016, 132, 281–286. [Google Scholar] [CrossRef] [PubMed]
- Novoa, B.; Romero, A.; Álvarez, Á.L.; Moreira, R.; Pereiro, P.; Costa, M.M.; Dios, S.; Estepa, A.; Parra, F.; Figueras, A. Antiviral activity of myticin C peptide from mussel: An ancient defense against herpesviruses. J. Virol. 2016, 90, 7692–7702. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Yang, K.; Wills, E.; Tang, L.; Baines, J.D. A mutation in the DNA polymerase accessory factor of herpes simplex virus 1 restores viral DNA replication in the presence of raltegravir. J. Virol. 2014, 88, 11121–11129. [Google Scholar] [CrossRef] [PubMed]
- Maggs, D.J.; Clarke, H.E. In vitro efficacy of ganciclovir, cidofovir, penciclovir, foscarnet, idoxuridine and acyclovir against feline herpesvirus type-1. Am. J. Vet. Res. 2004, 65, 399–403. [Google Scholar] [CrossRef] [PubMed]
- Van der Meulen, K.; Garré, B.; Croubels, S.; Nauwynck, H. In vitro comparison of antiviral drugs against feline herpesvirus 1. BMC Vet. Res. 2006, 2, 13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ledbetter, E.C.; Spertus, C.B.; Pennington, M.R.; van de Walle, G.R.; Judd, B.E.; Mohammed, H.O. In vitro and in vivo evaluation of cidofovir as a topical ophthalmic antiviral for ocular canine herpesvirus-1 infections in dogs. J. Ocul. Pharmacol. Ther. 2015, 31, 642–649. [Google Scholar] [CrossRef] [PubMed]
- Pennington, M.R.; Fort, M.W.; Ledbetter, E.C.; van de Walle, G.R. A novel corneal explant model system to evaluate antiviral drugs against feline herpesvirus type 1 (FHV-1). J. Gen. Virol. 2016, 97, 1414–1425. [Google Scholar] [CrossRef] [PubMed]
- Ledbetter, E.C.; Nicklin, A.M.; Spertus, C.B.; Pennington, M.R.; van de Walle, G.R.; Mohammed, H.O. Evaluation of topical ophthalmic ganciclovir gel in the treatment of experimentally induced ocular canine herpesvirus-1 infection. Am. J. Vet. Res. 2017, in press. [Google Scholar]
- Kim, H.S.; Jun Song, X.; de Paiva, C.S.; Chen, Z.; Pflugfelder, S.C.; Li, D.Q. Phenotypic characterization of human corneal epithelial cells expanded ex vivo from limbal explant and single cell cultures. Exp. Eye Res. 2004, 79, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Sandmeyer, L.S.; Keller, C.B.; Bienzle, D. Culture of feline corneal epithelial cells and infection with feline herpesvirus-1 as an investigative tool. Am. J. Vet. Res. 2005, 66, 205–209. [Google Scholar] [CrossRef] [PubMed]
- Werner, A.; Braun, M.; Kietzmann, M. Isolation and cultivation of canine corneal cells for in vitro studies on the anti-inflammatory effects of dexamethasone. Vet. Ophthalmol. 2008, 11, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.; Farooq, A.V.; Tiwari, V.; Kim, M.J.; Shukla, D. HSV-1 infection of human corneal epithelial cells: Receptor-mediated entry and trends of re-infection. Mol. Vis. 2010, 16, 2476–2486. [Google Scholar] [PubMed]
- García-Posadas, L.; Arranz-Valsero, I.; López-García, A.; Soriano-Romaní, L.; Diebold, Y. A new human primary epithelial cell culture model to study conjunctival inflammation. Investig. Ophthalmol. Vis. Sci. 2013, 54, 7143–7152. [Google Scholar] [CrossRef] [PubMed]
- Robertson, D.M.; Li, L.; Fisher, S.; Pearce, V.P.; Shay, J.W.; Wright, W.E.; Cavanagh, H.D.; Jester, J.V. Characterization of growth and differentiation in a telomerase-immortalized human corneal epithelial cell line. Investig. Opthalmol. Vis. Sci. 2005, 46, 470–478. [Google Scholar] [CrossRef] [PubMed]
- Araki-Sasaki, K.; Ohashi, Y.; Sasabe, T.; Hayashi, K.; Watanabe, H.; Tano, Y.; Handa, H. An SV40-immortalized human corneal epithelial cell line and its characterization. Investig. Ophthalmol. Vis. Sci. 1995, 36, 614–621. [Google Scholar]
- Rolinski, J.; Hus, I. Immunological aspects of acute and recurrent herpes simplex keratitis. J. Immunol. Res. 2014, 2014, 513560. [Google Scholar] [CrossRef] [PubMed]
- Giménez, F.; Suryawanshi, A.; Rouse, B.T. Pathogenesis of herpes stromal keratitis—A focus on corneal neovascularization. Prog. Retin. Eye Res. 2013, 33, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Astashkina, A.; Mann, B.; Grainger, D.W. A critical evaluation of in vitro cell culture models for high-throughput drug screening and toxicity. Pharmacol. Ther. 2012, 134, 82–106. [Google Scholar] [CrossRef] [PubMed]
- Nasisse, M.P.; Guy, J.S.; Davidson, M.G.; Sussman, W.; de Clercq, E. In vitro susceptibility of feline herpesvirus-1 to vidarabine, idoxuridine, trifluridine, acyclovir, or bromovinyldeoxyuridine. Am. J. Vet. Res. 1989, 50, 158–160. [Google Scholar] [PubMed]
- Stiles, J. Treatment of cats with ocular disease attributable to herpesvirus infection: 17 cases (1983–1993). J. Am. Vet. Med. Assoc. 1995, 207, 599–603. [Google Scholar] [PubMed]
- Nasisse, M.P.; Dorman, D.C.; Jamison, K.C.; Weigler, B.J.; Hawkins, E.C.; Stevens, J.B. Effects of valacyclovir in cats infected with feline herpesvirus 1. Am. J. Vet. Res. 1997, 58, 1141–1144. [Google Scholar] [PubMed]
- Williams, D.L.; Robinson, J.C.; Lay, E.; Field, H. Efficacy of topical aciclovir for the treatment of feline herpetic keratitis: Results of a prospective clinical trial and data from in vitro investigations. Vet. Rec. 2005, 157, 254–257. [Google Scholar] [CrossRef] [PubMed]
- Sandmeyer, L.S.; Keller, C.B.; Bienzle, D. Effects of cidofovir on cell death and replication of feline herpesvirus-1 in cultured feline corneal epithelial cells. Am. J. Vet. Res. 2005, 66, 217–222. [Google Scholar] [CrossRef] [PubMed]
- Fontenelle, J.P.; Powell, C.C.; Veir, J.K.; Radecki, S.V.; Lappin, M.R. Effect of topical ophthalmic application of cidofovir on experimentally induced primary ocular feline herpesvirus-1 infection in cats. Am. J. Vet. Res. 2008, 69, 289–293. [Google Scholar] [CrossRef] [PubMed]
- Thiry, E.; Addie, D.; Belák, S.; Boucraut-Baralon, C.; Egberink, H.; Frymus, T.; Gruffydd-Jones, T.; Hartmann, K.; Hosie, M.J.; Lloret, A.; et al. Feline herpesvirus infection ABCD guidelines on prevention and management. J. Feline Med. Surg. 2009, 11, 547–555. [Google Scholar] [CrossRef] [PubMed]
- Rajaiya, J.; Zhou, X.; Barequet, I.; Gilmore, M.S.; Chodosh, J. Novel model of innate immunity in corneal infection. In Vitro Cell. Dev. Biol. Anim. 2015, 51, 827–834. [Google Scholar] [CrossRef] [PubMed]
- Ghezzi, C.E.; Rnjak-Kovacina, J.; Kaplan, D.L. Corneal tissue engineering: Recent advances and future perspectives. Tissue Eng. Part B. Rev. 2015, 21, 278–287. [Google Scholar] [CrossRef] [PubMed]
- Lai, J.Y.; Li, Y.T.; Cho, C.H.; Yu, T.C. Nanoscale modification of porous gelatin scaffolds with chondroitin sulfate for corneal stromal tissue engineering. Int. J. Nanomedicine 2012, 7, 1101–1114. [Google Scholar] [CrossRef] [PubMed]
- Shafiq, M.A.; Gemeinhart, R.A.; Yue, B.Y.J.T.; Djalilian, A.R. Decellularized human cornea for reconstructing the corneal epithelium and anterior stroma. Tissue Eng. Part C Methods 2012, 18, 340–348. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, Y.; Funamoto, S.; Sasaki, S.; Honda, T.; Hattori, S.; Nam, K.; Kimura, T.; Mochizuki, M.; Fujisato, T.; Kobayashi, H.; et al. Preparation and characterization of decellularized cornea using high-hydrostatic pressurization for corneal tissue engineering. Biomaterials 2010, 31, 3941–3948. [Google Scholar] [CrossRef] [PubMed]
- Bayyoud, T.; Thaler, S.; Hofmann, J.; Maurus, C.; Spitzer, M.S.; Bartz-Schmidt, K.U.; Szurman, P.; Yoeruek, E. Decellularized bovine corneal posterior lamellae as carrier matrix for cultivated human corneal endothelial cells. Curr. Eye Res. 2012, 37, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Kong, B.; Sun, W.; Chen, G.; Tang, S.; Li, M.; Shao, Z.; Mi, S. Tissue-engineered cornea constructed with compressed collagen and laser-perforated electrospun mat. Sci. Rep. 2017, 7, 970. [Google Scholar] [CrossRef] [PubMed]
- Resau, J.H.; Sakamoto, K.; Cottrell, J.R.; Hudson, E.A.; Meltzer, S.J. Explant organ culture: A review. Cytotechnology 1991, 7, 137–149. [Google Scholar] [CrossRef] [PubMed]
- Russell, W.M.S.; Burch, R.L. The Principles of Humane Experimental Technique; Methuen: London, UK, 1959. [Google Scholar]
- Armitage, W.J. Preservation of human cornea. Transfus. Med. Hemother. 2011, 38, 143–147. [Google Scholar] [CrossRef] [PubMed]
- Richard, N.R.; Anderson, J.A.; Weiss, J.L.; Binder, P.S. Air/liquid corneal organ culture: A light microscopic study. Curr. Eye Res. 1991, 10, 739–749. [Google Scholar] [CrossRef] [PubMed]
- Collin, H.B.; Anderson, J.A.; Richard, N.R.; Binder, P.S. In vitro model for corneal wound healing; organ-cultured human corneas. Curr. Eye Res. 1995, 14, 331–339. [Google Scholar] [CrossRef] [PubMed]
- Alekseev, O.; Tran, A.H.; Azizkhan-Clifford, J. Ex vivo organotypic corneal model of acute epithelial herpes simplex virus type I infection. J. Vis. Exp. 2012, e3631. [Google Scholar] [CrossRef] [PubMed]
- Alekseev, O.; Limonnik, V.; Donovan, K.; Azizkhan-Clifford, J. Activation of checkpoint kinase 2 is critical for herpes simplex virus type 1 replication in corneal epithelium. Ophthalmic Res. 2015, 53, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Drevets, P.; Chucair-Elliott, A.; Shrestha, P.; Jinkins, J.; Karamichos, D.; Carr, D.J.J. The use of human cornea organotypic cultures to study herpes simplex virus type 1 (HSV-1)-induced inflammation. Graefe’s Arch. Clin. Exp. Ophthalmol. 2015, 253, 1721–1728. [Google Scholar] [CrossRef] [PubMed]
- Thakkar, N.; Jaishankar, D.; Agelidis, A.; Yadavalli, T.; Mangano, K.; Patel, S.; Tekin, S.Z.; Shukla, D. Cultured corneas show dendritic spread and restrict herpes simplex virus infection that is not observed with cultured corneal cells. Sci. Rep. 2017, 7, 42559. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; van Cleemput, J.; Qiu, Y.; Reddy, V.R.; Mateusen, B.; Nauwynck, H.J. Ex vivo modeling of feline herpesvirus replication in ocular and respiratory mucosae, the primary targets of infection. Virus Res. 2015, 210, 227–231. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, S.B.; Jensen, S.B.; Nielsen, C.; Quartin, E.; Kato, H.; Chen, Z.J.; Silverman, R.H.; Akira, S.; Paludan, S.R. Herpes simplex virus infection is sensed by both toll-like receptors and retinoic acid-inducible gene-like receptors, which synergize to induce type I interferon production. J. Gen. Virol. 2009, 90, 74–78. [Google Scholar] [CrossRef] [PubMed]
- Thompson, M.R.; Kaminski, J.J.; Kurt-Jones, E.A.; Fitzgerald, K.A. Pattern recognition receptors and the innate immune response to viral infection. Viruses 2011, 3, 920–940. [Google Scholar] [CrossRef] [PubMed]
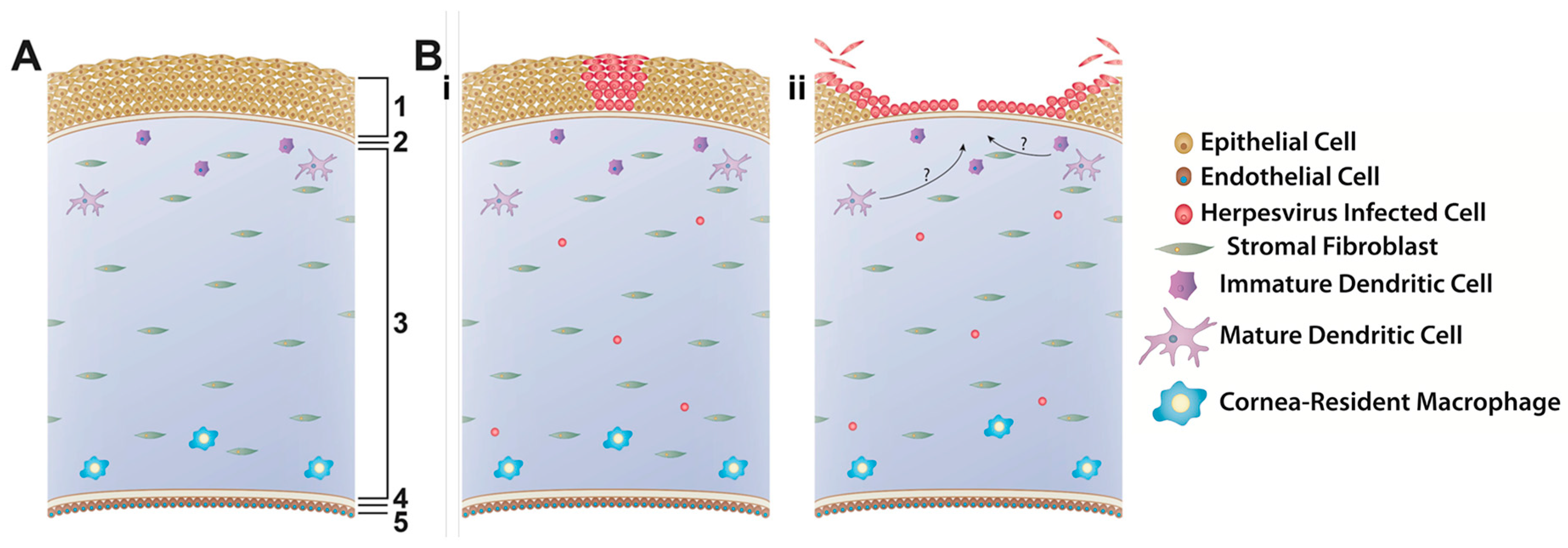
- Hamrah, P.; Huq, S.O.; Liu, Y.; Zhang, Q.; Dana, M.R. Corneal immunity is mediated by heterogeneous population of antigen-presenting cells. J. Leukoc. Biol. 2003, 74, 172–178. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, A.R.R.; Naranjo, C.; Leiva, M.; Fondevila, D.; Iborra, A.; Martinez, P.; Peña, T. Canine normal corneal epithelium bears a large population of CD45-positive cells. Vet. J. 2009, 179, 437–442. [Google Scholar] [CrossRef] [PubMed]
- Morgan, R.V.; Abrams, K.L.; Kern, T.J. Feline eosinophilic keratitis: A retrospective study of 54 cases: (1989–1994). Vet. Comp. Ophthalmol. 1996, 6, 131–134. [Google Scholar]
- Novak, N.; Peng, W.M. Dancing with the enemy: The interplay of herpes simplex virus with dendritic cells. Clin. Exp. Immunol. 2005, 142, 405–410. [Google Scholar] [CrossRef] [PubMed]
- Kaye, S.; Choudhary, A. Herpes simplex keratitis. Prog. Retin. Eye Res. 2006, 25, 355–380. [Google Scholar] [CrossRef] [PubMed]
- Röck, T.; Hofmann, J.; Thaler, S.; Bramkamp, M.; Bartz-Schmidt, K.U.; Yoeruek, E.; Röck, D. Factors that influence the suitability of human organ-cultured corneas. Graefe’s Arch. Clin. Exp. Ophthalmol. 2016, 254, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Hagenah, M.; Böhnke, M.; Engelmann, K.; Winter, R. Incidence of bacterial and fungal contamination of donor corneas preserved by organ culture. Cornea 1995, 14, 423–426. [Google Scholar] [CrossRef] [PubMed]
- Armitage, W.J.; Easty, D.L. Factors influencing the suitability of organ-cultured corneas for transplantation. Investig. Ophthalmol. Vis. Sci. 1997, 38, 16–24. [Google Scholar]
- Gain, P.; Thuret, G.; Chiquet, C.; Vautrin, A.C.; Carricajo, A.; Acquart, S.; Maugery, J.; Aubert, G. Use of a pair of blood culture bottles for sterility testing of corneal organ culture media. Br. J. Ophthalmol. 2001, 85, 1158–1162. [Google Scholar] [CrossRef] [PubMed]
- Pels, L. Organ culture: The method of choice for preservation of human donor corneas. Br. J. Ophthalmol. 1997, 81, 523–525. [Google Scholar] [CrossRef] [PubMed]
- Spelsberg, H.; Reinhard, T.; Sengler, U.; Daeubener, W.; Sundmacher, R. Organ-cultured corneal grafts from septic donors: A retrospective study. Eye 2002, 16, 622–627. [Google Scholar] [CrossRef] [PubMed]
- Borderie, V.M.; Laroche, L. Microbiologic study of organ-cultured donor corneas. Transplantation 1998, 66, 120–123. [Google Scholar] [CrossRef] [PubMed]
- Röck, D.; Wude, J.; Bartz-Schmidt, K.U.; Yoeruek, E.; Thaler, S.; Röck, T. Factors influencing the contamination rate of human organ-cultured corneas. Acta Ophthalmol. 2017, in press. [Google Scholar]
- Gruenert, A.K.; Rosenbaum, K.; Geerling, G.; Fuchsluger, T.A. The influence of donor factors on corneal organ culture contamination. Acta Ophthalmol. 2017, in press. [Google Scholar] [CrossRef] [PubMed]
- Seiler, T.G.; Tschopp, M.; Zimmerli, S.; Tappeiner, C.; Wittwer, V.V.; Frueh, B.E. Time course of antibiotic and antifungal concentrations in corneal organ culture. Cornea 2016, 35, 127–131. [Google Scholar] [CrossRef] [PubMed]
- Hill, J.M.; Clement, C. Herpes simplex virus type 1 DNA in human corneas: What are the virological and clinical implications? J. Infect. Dis. 2009, 200, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Kaufman, H.E.; Azcuy, A.M.; Varnell, E.D.; Sloop, G.D.; Thompson, H.W.; Hill, J.M. HSV-1 DNA in tears and saliva of normal adults. Investig. Ophthalmol. Vis. Sci. 2005, 46, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Farooq, A.V.; Shukla, D. Corneal latency and transmission of herpes simplex virus-1. Future Virol. 2011, 6, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Cleator, G.M.; Klapper, P.E.; Dennett, C.; Sullivan, A.L.; Bonshek, R.E.; Marcyniuk, B.; Tullo, A.B. Corneal donor infection by herpes simplex virus: Herpes simplex virus DNA in donor corneas. Cornea 1994, 13, 294–304. [Google Scholar] [CrossRef] [PubMed]
- Openshaw, H.; McNeill, J.I.; Lin, X.H.; Niland, J.; Cantin, E.M. Herpes simplex virus DNA in normal corneas: Persistence without viral shedding from ganglia. J. Med. Virol. 1995, 46, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Garweg, J.G.; Boehnke, M. Low rate shedding of HSV-1 DNA but not of infectious virus from human donor corneae into culture media. J. Med. Virol. 1997, 52, 320–325. [Google Scholar] [CrossRef]
- Van Gelderen, B.E.; van der Lelij, A.; Treffers, W.F.; van der Gaag, R. Detection of herpes simplex virus type 1, 2 and varicella zoster virus DNA in recipient corneal buttons. Br. J. Ophthalmol. 2000, 84, 1238–1243. [Google Scholar] [CrossRef] [PubMed]
- Broniek, G.; Langwińska-Wośko, E.; Sybilska, M.; Szaflik, J.P.; Przybylski, M.; Wróblewska, M. Occurrence of viral DNA in paired samples of corneal rim and cornea preservation fluid. J. Med. Virol. 2016, 89, 732–736. [Google Scholar] [CrossRef] [PubMed]
- Sengler, U.; Reinhard, T.; Adams, O.; Krempe, C.; Sundmacher, R. Herpes simplex virus infection in the media of donor corneas during organ culture: Frequency and consequences. Eye (Lond.) 2001, 15, 644–647. [Google Scholar] [CrossRef] [PubMed]
- Stiles, J.; Pogranichniy, R. Detection of virulent feline herpesvirus-1 in the corneas of clinically normal cats. J. Feline Med. Surg. 2008, 10, 154–159. [Google Scholar] [CrossRef] [PubMed]
- Sawtell, N.M.; Poon, D.K.; Tansky, C.S.; Thompson, R.L. The latent herpes simplex virus type 1 genome copy number in individual neurons is virus strain specific and correlates with reactivation. J. Virol. 1998, 72, 5343–5350. [Google Scholar] [PubMed]
- Mulik, S.; Xu, J.; Reddy, P.B.J.; Rajasagi, N.K.; Gimenez, F.; Sharma, S.; Lu, P.Y.; Rouse, B.T. Role of miR-132 in angiogenesis after ocular infection with herpes simplex virus. Am. J. Pathol. 2012, 18, 525–534. [Google Scholar] [CrossRef] [PubMed]
- Matundan, H.; Mott, K.R.; Ghiasi, H. Role of CD8+ T cells and lymphoid dendritic cells in protection from ocular herpes simplex virus 1 challenge in immunized mice. J. Virol. 2014, 88, 8016–8027. [Google Scholar] [CrossRef] [PubMed]
- Chucair-Elliott, A.J.; Zheng, M.; Carr, D.J.J. Degeneration and regeneration of corneal nerves in response to HSV-1 infection. Investig. Ophthalmol. Vis. Sci. 2015, 56, 1097–1107. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Yin, X.; Stuart, P.M.; Leib, D.A. Dendritic cell autophagy contributes to herpes simplex virus-driven stromal keratitis and immunopathology. mBio 2015, 6, e01426-15. [Google Scholar] [CrossRef] [PubMed]
- Royer, D.J.; Zheng, M.; Conrady, C.D.; Carr, D.J.J. Granulocytes in ocular HSV-1 infection: Opposing roles of mast cells and neutrophils. Investig. Ophthalmol. Vis. Sci. 2015, 56, 3763–3775. [Google Scholar] [CrossRef] [PubMed]
- Feldman, L.T.; Ellison, A.R.; Voytek, C.C.; Yang, L.; Krause, P.; Margolis, T.P. Spontaneous molecular reactivation of herpes simplex virus type 1 latency in mice. Proc. Natl. Acad. Sci. USA 2002, 99, 978–983. [Google Scholar] [CrossRef] [PubMed]
- Gebhardt, B.M.; Halford, W.P. Evidence that spontaneous reactivation of herpesvirus does not occur in mice. Virol. J. 2005, 2, 67. [Google Scholar] [CrossRef] [PubMed]
- Margolis, T.P.; Elfman, F.L.; Leib, D.; Pakpour, N.; Apakupakul, K.; Imai, Y.; Voytek, C. Spontaneous reactivation of herpes simplex virus type 1 in latently infected murine sensory ganglia. J. Virol. 2007, 81, 11069–11074. [Google Scholar] [CrossRef] [PubMed]
- Jester, J.V.; Morishige, N.; BenMohamed, L.; Brown, D.J.; Osorio, N.; Hsiang, C.; Perng, G.C.; Jones, C.; Wechsler, S.L. Confocal microscopic analysis of a rabbit eye model of high-incidence recurrent herpes stromal keratitis. Cornea 2016, 35, 81–88. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Cosby, R.; Hill, J.M.; Bazan, H.E.P. Changes in corneal innervation after HSV-1 latency established with different reactivation phenotypes. Curr. Eye Res. 2016, 42, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, R.; Dervillez, X.; Khan, A.A.; Chentoufi, A.A.; Chilukuri, S.; Shukr, N.; Fazli, Y.; Ong, N.N.; Afifi, R.E.; Osorio, N.; et al. The herpes simplex virus latency-associated transcript gene is associated with a broader repertoire of virus-specific exhausted CD8+ T cells retained within the trigeminal ganglia of latently infected HLA transgenic rabbits. J. Virol. 2016, 90, 3913–3928. [Google Scholar] [CrossRef] [PubMed]
- Kollias, C.M.; Huneke, R.B.; Wigdahl, B.; Jennings, S.R. Animal models of herpes simplex virus immunity and pathogenesis. J. Neurovirol. 2015, 21, 8–23. [Google Scholar] [CrossRef] [PubMed]
- Chentoufi, A.A.; Dasgupta, G.; Christensen, N.D.; Hu, J.; Choudhury, Z.S.; Azeem, A.; Jester, J.V.; Nesburn, A.B.; Wechsler, S.L.; BenMohamed, L. A novel HLA (HLA-A*0201) transgenic rabbit model for preclinical evaluation of human CD8+ T cell epitope-based vaccines against ocular herpes. J. Immunol. 2010, 184, 2561–2571. [Google Scholar] [CrossRef] [PubMed]
- Evermann, J.F.; Ledbetter, E.C.; Maes, R.K. Canine reproductive, respiratory and ocular diseases due to canine herpesvirus. Vet. Clin. N. Am. Small Anim. Pract. 2011, 41, 1097–1120. [Google Scholar] [CrossRef] [PubMed]
- Wright, N.G.; Cornwell, H.J.C. The susceptibility of six-week old puppies to canine jerpes virus. J. Small Anim. Pract. 1969, 10, 669–674. [Google Scholar] [CrossRef]
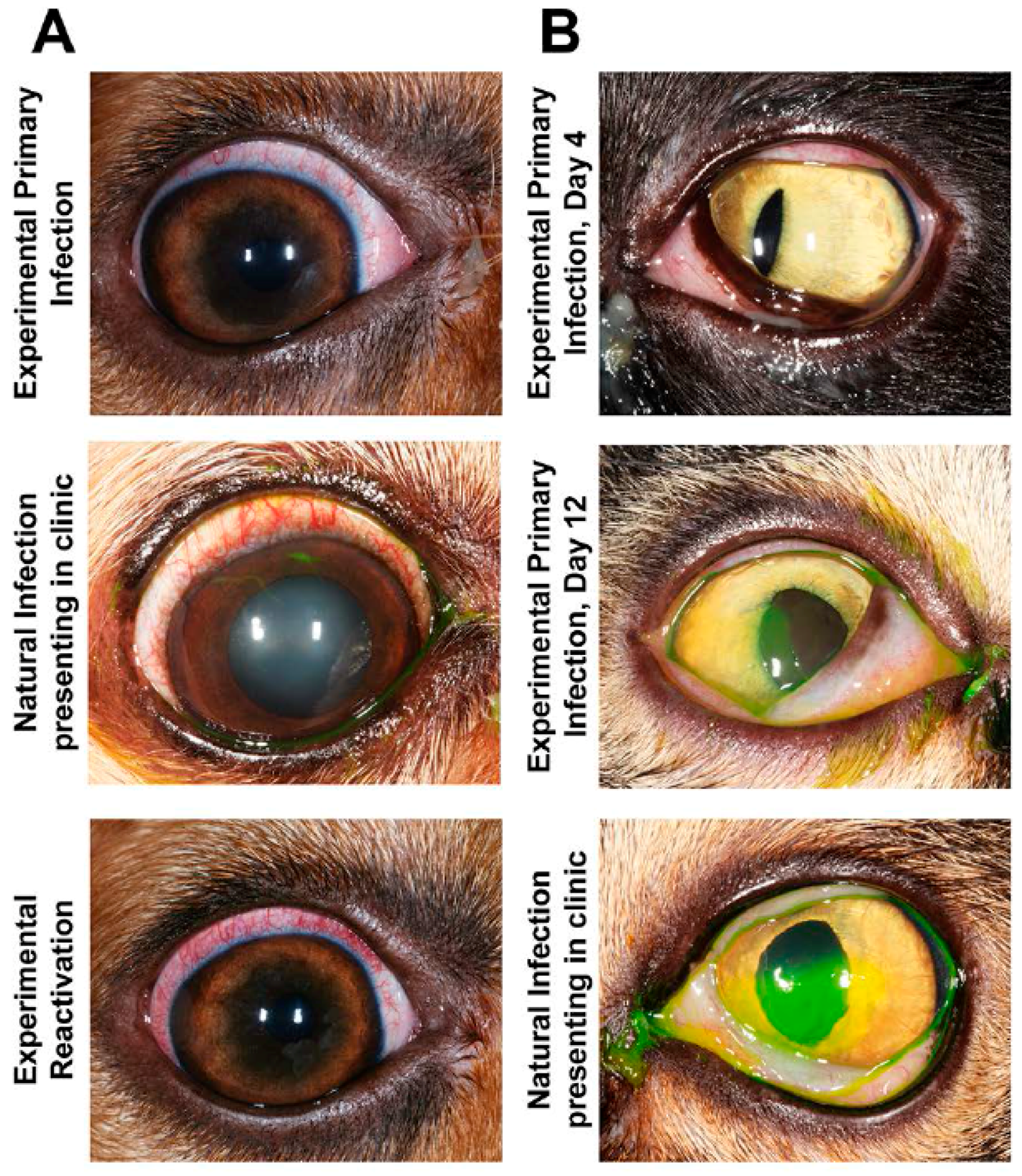
- Ledbetter, E.C.; Dubovi, E.J.; Kim, S.G.; Maggs, D.J.; Bicalho, R.C. Experimental primary ocular canine herpesvirus-1 infection in adult dogs. Am. J. Vet. Res. 2009, 70, 513–521. [Google Scholar] [CrossRef] [PubMed]
- Ledbetter, E.C.; Kim, S.G.; Dubovi, E.J.; Bicalho, R.C. Experimental reactivation of latent canine herpesvirus-1 and induction of recurrent ocular disease in adult dogs. Vet. Microbiol. 2009, 138, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Ledbetter, E.C.; Kice, N.C.; Matusow, R.B.; Dubovi, E.J.; Kim, S.G. The effect of topical ocular corticosteroid administration in dogs with experimentally induced latent canine herpesvirus-1 infection. Exp. Eye Res. 2010, 90, 711–717. [Google Scholar] [CrossRef] [PubMed]
- Ledbetter, E.C.; da Silva, E.C.; Kim, S.G.; Dubovi, E.J.; Schwark, W.S. Frequency of spontaneous canine herpesvirus-1 reactivation and ocular viral shedding in latently infected dogs and canine herpesvirus-1 reactivation and ocular viral shedding induced by topical administration of cyclosporine and systemic administration of corticosteroids. Am. J. Vet. Res. 2012, 73, 1079–1084. [Google Scholar] [PubMed]
- Mundy, P.; da Silva, E.C.; Ledbetter, E.C. Effects of cyclophosphamide myelosuppression in adult dogs with latent canine herpesvirus-1 infection. Vet. Microbiol. 2012, 159, 230–235. [Google Scholar] [CrossRef] [PubMed]
- Nicklin, A.M.; McEntee, M.C.; Ledbetter, E.C. Effects of ocular surface strontium-90 β radiotherapy in dogs latently infected with canine herpesvirus-1. Vet. Microbiol. 2014, 174, 433–437. [Google Scholar] [CrossRef] [PubMed]
- Spertus, C.B.; Mohammed, H.O.; Ledbetter, E.C. Effects of topical ocular application of 1% trifluridine ophthalmic solution in dogs with experimentally induced recurrent ocular canine herpesvirus-1 infection. Am. J. Vet. Res. 2016, 77, 1140–1147. [Google Scholar] [CrossRef] [PubMed]
- Ledbetter, E.C.; Kim, K.; Dubovi, E.J.; Mohammed, H.O.; Felippe, M.J.B. Clinical and immunological assessment of therapeutic immunization with a subunit vaccine for recurrent ocular canine herpesvirus-1 infection in dogs. Vet. Microbiol. 2016, 197, 102–110. [Google Scholar] [CrossRef] [PubMed]
- Nasisse, M.P.; Guy, J.S.; Davidson, M.G.; Sussman, W.A.; Fairley, N.M. Experimental ocular herpesvirus infection in the cat. Sites of virus replication, clinical features and effects of corticosteroid administration. Investig. Ophthalmol. Vis. Sci. 1989, 30, 1758–1768. [Google Scholar]
- Haid, C.; Kaps, S.; Gönczi, E.; Hässig, M.; Metzler, A.; Spiess, B.M.; Richter, M. Pretreatment with feline interferon omega and the course of subsequent infection with feline herpesvirus in cats. Vet. Ophthalmol. 2007, 10, 278–284. [Google Scholar] [CrossRef] [PubMed]
- Thomasy, S.M.; Lim, C.C.; Reilly, C.M.; Kass, P.H.; Lappin, M.R.; Maggs, D.J. Evaluation of orally administered famciclovir in cats experimentally infected with feline herpesvirus type-1. Am. J. Vet. Res. 2011, 72, 85–95. [Google Scholar] [CrossRef] [PubMed]
- Hamano, M.; Maeda, K.; Mizukoshi, F.; Une, Y.; Mochizuki, M.; Tohya, Y.; Akashi, H.; Kai, K. Experimental infection of recent field isolates of feline herpesvirus type 1. J. Vet. Med. Sci. 2003, 65, 939–943. [Google Scholar] [CrossRef] [PubMed]
- Vaz, P.K.; Job, N.; Horsington, J.; Ficorilli, N.; Studdert, M.J.; Hartley, C.A.; Gilkerson, J.R.; Browning, G.F.; Devlin, J.M. Low genetic diversity among historical and contemporary clinical isolates of felid herpesvirus 1. BMC Genomics 2016, 17, 704. [Google Scholar] [CrossRef] [PubMed]
- Maggs, D.J.; Nasisse, M.P.; Kass, P.H. Efficacy of oral supplementation with l-lysine in cats latently infected with feline herpesvirus. Am. J. Vet. Res. 2003, 64, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Driscoll, J.W. Attitudes toward Animals: Species Ratings. Soc. Anim. 1995, 3, 139–150. [Google Scholar] [CrossRef]
- Baumans, V. Use of animals in experimental research: An ethical dilemma? Gene Ther. 2004, 11, S64–S66. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.J.; Lee, H.S.; Yu, X.F.; Choi, E.; Koo, B.C.; Kwon, M.S.; Lee, Y.S.; Cho, S.J.; Jin, G.Z.; Kim, L.H.; et al. Generation of cloned transgenic cats expressing red fluorescence protein. Biol. Reprod. 2008, 78, 425–431. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.G.; Kim, M.K.; Jang, G.; Oh, H.J.; Park, J.E.; Kang, J.T.; Koo, O.J.; Kim, T.; Kwon, M.S.; Koo, B.C.; et al. Generation of red fluorescent protein transgenic dogs. Genesis 2009, 47, 314–322. [Google Scholar] [CrossRef] [PubMed]
- Pope, C.E.; Keller, G.L.; Dresser, B.L. In vitro fertilization in domestic and non-domestic cats including sequences of early nuclear events, development in vitro, cryopreservation and successful intra- and interspecies embryo transfer. J. Reprod. Fertil. Suppl. 1993, 47, 189–201. [Google Scholar] [PubMed]
- Nagashima, J.B.; Sylvester, S.R.; Nelson, J.L.; Cheong, S.H.; Mukai, C.; Lambo, C.; Flanders, J.A.; Meyers-Wallen, V.N.; Songsasen, N.; Travis, A.J. Live births from domestic dog (Canis familiaris) embryos produced by in vitro rertilization. PLoS ONE 2015, 10, e0143930. [Google Scholar] [CrossRef] [PubMed]
- Robert-Tissot, C.; Rüegger, V.L.; Cattori, V.; Meli, M.L.; Riond, B.; Gomes-Keller, M.A.; Vögtlin, A.; Wittig, B.; Juhls, C.; Hofmann-Lehmann, R.; et al. The innate antiviral immune system of the cat: Molecular tools for the measurement of its state of activation. Vet. Immunol. Immunopathol. 2011, 143, 269–281. [Google Scholar] [CrossRef] [PubMed]
- Decker, B.; Parker, H.G.; Dhawan, D.; Kwon, E.M.; Karlins, E.; Davis, B.W.; Ramos-Vara, J.A.; Bonney, P.L.; McNiel, E.A.; Knapp, D.W.; et al. Homologous mutation to human BRAF V600E is common in naturally occurring canine bladder cancer—Evidence for a relevant model system and urine-based diagnostic test. Mol. Cancer Res. 2015, 13, 993–1002. [Google Scholar] [CrossRef] [PubMed]
- Brachelente, C.; Cappelli, K.; Capomaccio, S.; Porcellato, I.; Silvestri, S.; Bongiovanni, L.; de Maria, R.; Verini Supplizi, A.; Mechelli, L.; Sforna, M. Transcriptome analysis of canine cutaneous melanoma and melanocytoma reveals a modulation of genes regulating extracellular matrix metabolism and cell cycle. Sci. Rep. 2017, 7, 6386. [Google Scholar] [CrossRef] [PubMed]
- Pomari, E.; Stefanon, B.; Colitti, M. Effect of Arctium lappa (burdock) extract on canine dermal fibroblasts. Vet. Immunol. Immunopathol. 2013, 156, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Ertl, R.; Klein, D. Transcriptional profiling of the host cell response to feline immunodeficiency virus infection. Virol. J. 2014, 11, 52. [Google Scholar] [CrossRef] [PubMed]
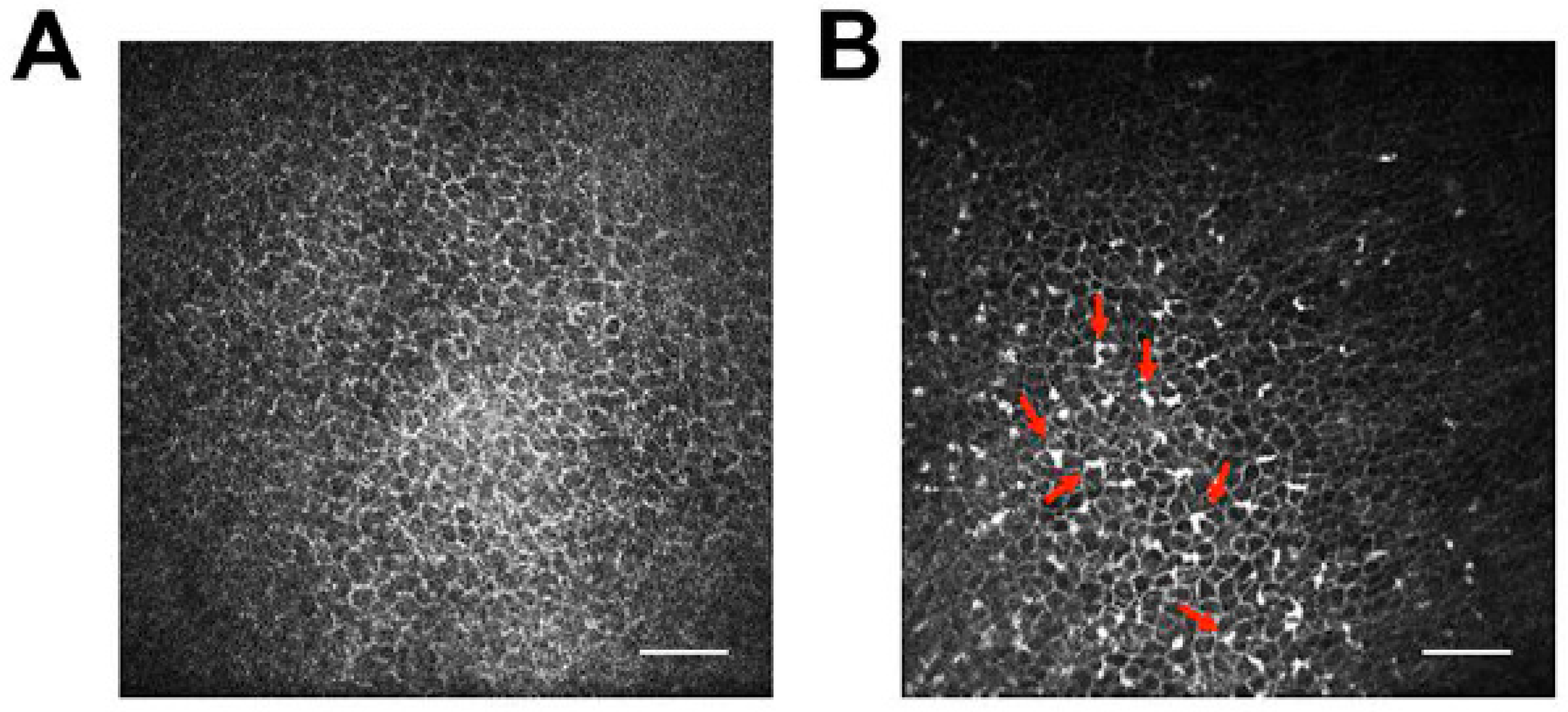
- Kafarnik, C.; Fritsche, J.; Reese, S. In vivo confocal microscopy in the normal corneas of cats, dogs and birds. Vet. Ophthalmol. 2007, 10, 222–230. [Google Scholar] [CrossRef] [PubMed]
- Kafarnik, C.; Fritsche, J.; Reese, S. Corneal innervation in mesocephalic and brachycephalic dogs and cats: Assessment using in vivo confocal microscopy. Vet. Ophthalmol. 2008, 11, 363–367. [Google Scholar] [CrossRef] [PubMed]
- Hughes, D.J.; Kipar, A.; Sample, J.T.; Stewart, J.P. Pathogenesis of a model gammaherpesvirus in a natural host. J. Virol. 2010, 84, 3949–3961. [Google Scholar] [CrossRef] [PubMed]
- McHugh, K.J.; Saint-Geniez, M.; Tao, S.L. Topographical control of ocular cell types for tissue engineering. J. Biomed. Mater. Res. B. Appl. Biomater. 2013, 101, 1571–1584. [Google Scholar] [CrossRef] [PubMed]
- Taylor, A.W. Ocular immune privilege and transplantation. Front. Immunol. 2016, 7, 37. [Google Scholar] [CrossRef] [PubMed]

| Virus | Abbreviations | Subfamily | Associated Ocular Diseases | Overall Prevalence | Herpesvirus-Associated Ocular Disease Prevalence | References |
|---|---|---|---|---|---|---|
| Human alphaherpesvirus 1 | HHV-1/HSV-1 | Simplexvirus | Corneal lesions, stromal & epithelial keratitis, conjunctivitis | 67–90% | 12–36/100,000 | [3,10,11,12,13,14] |
| Canid alphaherpesvirus 1 | CHV-1 | Varicellovirus | Corneal lesions, stromal & epithelial keratitis, conjunctivitis | 21–98% | Unknown | [2,15,16,17,18,19,20] |
| Felid alphaherpesvirus 1 | FHV-1 | Varicellovirus | Corneal lesions, stromal & epithelial keratitis, conjunctivitis | 40–97% | Unknown | [21,22,23,24,25] |
| Human alphaherpesvirus 3 | HHV-3/VZV | Varicellovirus | Herpes zoster ophthalmicus | > 95% | 19–31/100,000 | [26,27,28] |
| Equid alphaherpesvirus 1 | EHV-1 | Varicellovirus | Chorioretinitis | 52%-“endemic” | 50–90% of choroidal lesions in experimental infection | [29,30,31,32] |
| Equid gammaherpesvirus 2 | EHV-2 | Percavirus | Keratoconjunctivitis | 51–93% | 8–60% of keratoconjunctivitis cases tested | [33,34,35,36,37,38] |
| Bovine alphaherpesvirus 1 | BoHV-1 | Varicellovirus | Keratoconjunctivitis | 20–97% | 4.95/100 | [39,40,41] |
| Bovine gammaherpesvirus 4 | BoHV-4 | Rhadinovirus | Keratoconjunctivitis & ocular discharge | 21–35% | Unknown | [42,43,44,45] |
| Alcelpahine gammaherpesvirus 1 & Ovine gammaherpesvirus 2 | AlHV-1 OvHV-2 | Macavirus | Ocular discharge | 29–77% | Typical symptom of malignant catarrhal fever | [46,47,48,49] |
| Cervid alphaherpesvirus 1 & 2 | CvHV-1 CvHV-2 | Varicellovirus | Keratoconjunctivitis & keratitis | 18–47% | ~5% in free-ranging, 30% in animals | [50,51,52,53,54,55] |
| Otariid herpesviruses & Phocid herpesviruses (Various species) | OtHV PhHV | Gammaherpes-viruses | Corneal lesions, keratoconjunctivitis | 26–76% | Unknown | [56] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pennington, M.R.; Ledbetter, E.C.; Van de Walle, G.R. New Paradigms for the Study of Ocular Alphaherpesvirus Infections: Insights into the Use of Non-Traditional Host Model Systems. Viruses 2017, 9, 349. https://doi.org/10.3390/v9110349
Pennington MR, Ledbetter EC, Van de Walle GR. New Paradigms for the Study of Ocular Alphaherpesvirus Infections: Insights into the Use of Non-Traditional Host Model Systems. Viruses. 2017; 9(11):349. https://doi.org/10.3390/v9110349
Chicago/Turabian StylePennington, Matthew R., Eric C. Ledbetter, and Gerlinde R. Van de Walle. 2017. "New Paradigms for the Study of Ocular Alphaherpesvirus Infections: Insights into the Use of Non-Traditional Host Model Systems" Viruses 9, no. 11: 349. https://doi.org/10.3390/v9110349





