Classical Swine Fever—An Updated Review
Abstract
:1. Introduction
2. Virus Properties
2.1. Virus Organization and Replication
2.2. Tenacity and Virus Inactivation
2.3. Genetic Diversity and Virulence Factors
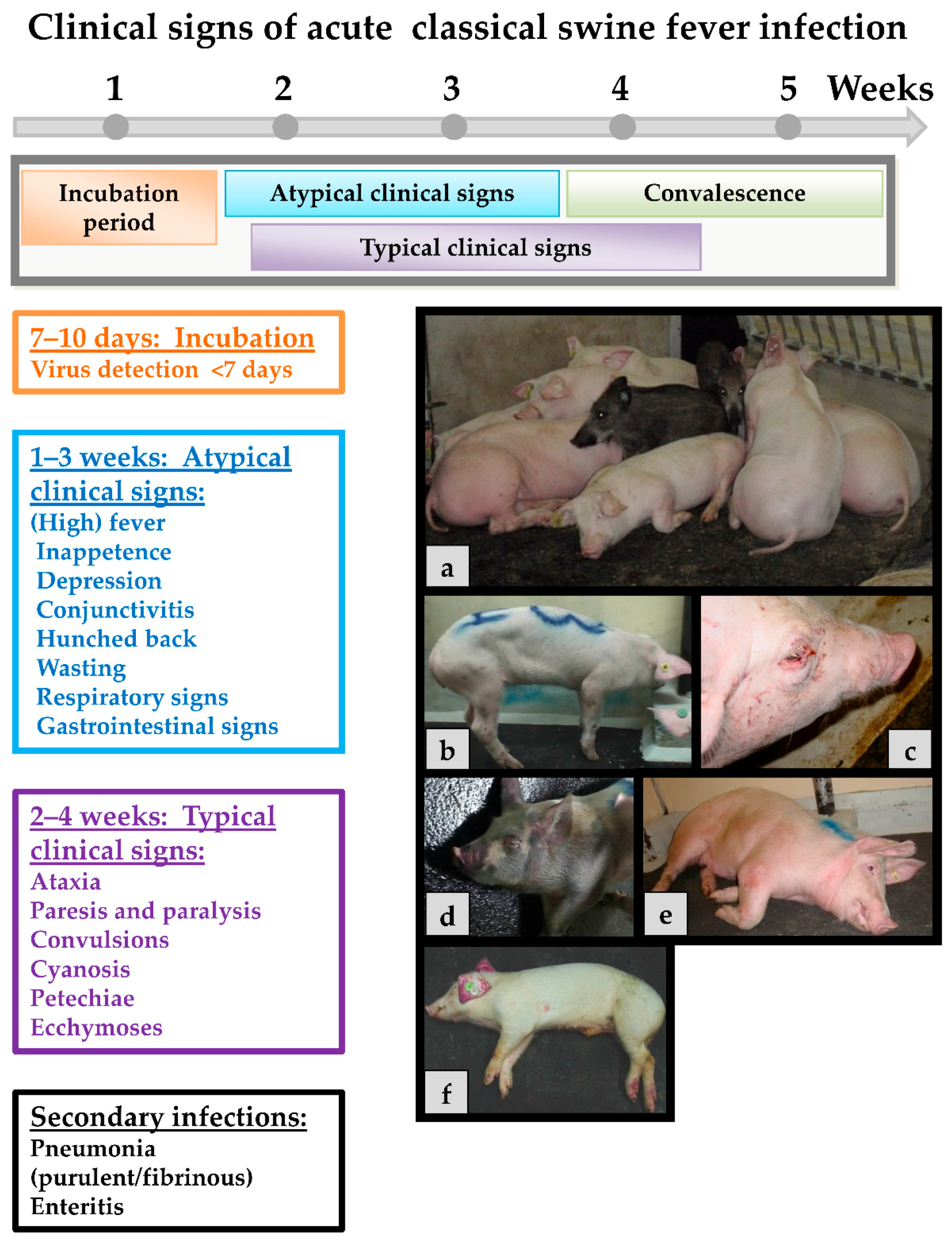
3. Clinical Signs and Pathomorphological Lesions
4. Pathogenesis and Immune Responses
5. Epidemiology
6. Diagnosis
7. Vaccination
Conflicts of Interest
References
- Edwards, S.; Fukusho, A.; Lefevre, P.C.; Lipowski, A.; Pejsak, Z.; Roehe, P.; Westergaard, J. Classical swine fever: The global situation. Vet. Microbiol. 2000, 73, 103–119. [Google Scholar] [CrossRef]
- Fritzemeier, J.; Teuffert, J.; Greiser-Wilke, I.; Staubach, C.; Schlüter, H.; Moennig, V. Epidemiology of classical swine fever in germany in the 1990s. Vet. Microbiol. 2000, 77, 29–41. [Google Scholar] [CrossRef]
- Rossi, S.; Staubach, C.; Blome, S.; Guberti, V.; Thulke, H.H.; Vos, A.; Koenen, F.; Le Potier, M.F. Controlling of csfv in european wild boar using oral vaccination: A review. Front. Microbiol. 2015, 6, 1141. [Google Scholar] [CrossRef] [PubMed]
- Rossi, S.; Pol, F.; Forot, B.; Masse-Provin, N.; Rigaux, S.; Bronner, A.; Le Potier, M.F. Preventive vaccination contributes to control classical swine fever in wild boar (Sus scrofa sp.). Vet. Microbiol. 2010, 142, 99–107. [Google Scholar] [CrossRef] [PubMed]
- von Rüden, S.; Staubach, C.; Kaden, V.; Hess, R.G.; Blicke, J.; Kühne, S.; Sonnenburg, J.; Fröhlich, A.; Teuffert, J.; Moennig, V. Retrospective analysis of the oral immunisation of wild boar populations against classical swine fever virus (csfv) in region Eifel of Rhineland-Palatinate. Vet. Microbiol. 2008, 132, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Kaden, V.; Heyne, H.; Kiupel, H.; Letz, W.; Kern, B.; Lemmer, U.; Gossger, K.; Rothe, A.; Böhme, H.; Tyrpe, P. Oral immunisation of wild boar against classical swine fever: Concluding analysis of the recent field trials in Germany. Berl. Munch. Tierarztl. Wochenschr. 2002, 115, 179–185. [Google Scholar] [PubMed]
- Blome, S.; Gabriel, C.; Staubach, C.; Leifer, I.; Strebelow, G.; Beer, M. Genetic differentiation of infected from vaccinated animals after implementation of an emergency vaccination strategy against classical swine fever in wild boar. Vet. Microbiol. 2011, 153, 373–376. [Google Scholar] [CrossRef] [PubMed]
- Hulst, M.M.; van Gennip, H.G.; Moormann, R.J. Passage of classical swine fever virus in cultured swine kidney cells selects virus variants that bind to heparan sulfate due to a single amino acid change in envelope protein Erns. J. Virol. 2000, 74, 9553–9561. [Google Scholar] [CrossRef] [PubMed]
- Weiland, E.; Ahl, R.; Stark, R.; Weiland, F.; Thiel, H.J. A second envelope glycoprotein mediates neutralization of a pestivirus, hog cholera virus. J. Virol. 1992, 66, 3677–3682. [Google Scholar] [PubMed]
- Iqbal, M.; Flick-Smith, H.; McCauley, J.W. Interactions of bovine viral diarrhoea virus glycoprotein Erns with cell surface glycosaminoglycans. J. Gen. Virol. 2000, 81, 451–459. [Google Scholar] [CrossRef] [PubMed]
- Hulst, M.M.; van Gennip, H.G.; Vlot, A.C.; Schooten, E.; de Smit, A.J.; Moormann, R.J. Interaction of classical swine fever virus with membrane-associated heparan sulfate: Role for virus replication in vivo and virulence. J. Virol. 2001, 75, 9585–9595. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Nie, Y.; Wang, P.; Ding, M.; Deng, H. Characterization of classical swine fever virus entry by using pseudotyped viruses: E1 and E2 are sufficient to mediate viral entry. Virology 2004, 330, 332–341. [Google Scholar] [CrossRef] [PubMed]
- Donis, R.O. Molecular biology of bovine viral diarrhea virus and its interactions with the host. Vet. Clin. N. Am. Food Anim. Pract. 1995, 11, 393–423. [Google Scholar] [CrossRef]
- Krey, T.; Thiel, H.J.; Rümenapf, T. Acid-resistant bovine pestivirus requires activation for ph-triggered fusion during entry. J. Virol. 2005, 79, 4191–4200. [Google Scholar] [CrossRef] [PubMed]
- Lecot, S.; Belouzard, S.; Dubuisson, J.; Rouille, Y. Bovine viral diarrhea virus entry is dependent on clathrin-mediated endocytosis. J. Virol. 2005, 79, 10826–10829. [Google Scholar] [CrossRef] [PubMed]
- Rijnbrand, R.; van der Straaten, T.; van Rijn, P.A.; Spaan, W.J.; Bredenbeek, P.J. Internal entry of ribosomes is directed by the 5′ noncoding region of classical swine fever virus and is dependent on the presence of an RNA pseudoknot upstream of the initiation codon. J. Virol. 1997, 71, 451–457. [Google Scholar] [PubMed]
- Pestova, T.V.; Hellen, C.U. Internal initiation of translation of bovine viral diarrhea virus RNA. Virology 1999, 258, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Poole, T.L.; Wang, C.; Popp, R.A.; Potgieter, L.N.; Siddiqui, A.; Collett, M.S. Pestivirus translation initiation occurs by internal ribosome entry. Virology 1995, 206, 750–754. [Google Scholar] [CrossRef]
- Rümenapf, T.; Unger, G.; Strauss, J.H.; Thiel, H.J. Processing of the envelope glycoproteins of pestiviruses. J. Virol. 1993, 67, 3288–3294. [Google Scholar] [PubMed]
- Stark, R.; Meyers, G.; Rümenapf, T.; Thiel, H.J. Processing of pestivirus polyprotein: Cleavage site between autoprotease and nucleocapsid protein of classical swine fever virus. J. Virol. 1993, 67, 7088–7095. [Google Scholar] [PubMed]
- Wiskerchen, M.; Belzer, S.K.; Collett, M.S. Pestivirus gene expression: The first protein product of the bovine viral diarrhea virus large open reading frame, p20, possesses proteolytic activity. J. Virol. 1991, 65, 4508–4514. [Google Scholar] [PubMed]
- Lackner, T.; Müller, A.; Pankraz, A.; Becher, P.; Thiel, H.J.; Gorbalenya, A.E.; Tautz, N. Temporal modulation of an autoprotease is crucial for replication and pathogenicity of an rna virus. J. Virol. 2004, 78, 10765–10775. [Google Scholar] [CrossRef] [PubMed]
- Lackner, T.; Thiel, H.J.; Tautz, N. Dissection of a viral autoprotease elucidates a function of a cellular chaperone in proteolysis. Proc. Natl. Acad. Sci. USA 2006, 103, 1510–1515. [Google Scholar] [CrossRef] [PubMed]
- Tautz, N.; Elbers, K.; Stoll, D.; Meyers, G.; Thiel, H.J. Serine protease of pestiviruses: Determination of cleavage sites. J. Virol. 1997, 71, 5415–5422. [Google Scholar] [PubMed]
- Gong, Y.; Trowbridge, R.; Macnaughton, T.B.; Westaway, E.G.; Shannon, A.D.; Gowans, E.J. Characterization of RNA synthesis during a one-step growth curve and of the replication mechanism of bovine viral diarrhoea virus. J. Gen. Virol. 1996, 77, 2729–2736. [Google Scholar] [CrossRef] [PubMed]
- Gray, E.W.; Nettleton, P.F. The ultrastructure of cell cultures infected with border disease and bovine virus diarrhoea viruses. J. Gen. Virol. 1987, 68, 2339–2346. [Google Scholar] [CrossRef] [PubMed]
- Ohmann, H.B. Electron microscopy of bovine virus diarrhoea virus. Rev. Sci. Tech. 1990, 9, 61–73. [Google Scholar] [CrossRef] [PubMed]
- Kramer, M.; Staubach, C.; Koenen, F.; Haegeman, A.; Pol, F.; Le Potier, M.F.; Greiser-Wilke, I. Scientific review on Classical Swine Fever. EFSA Support. Publ. 2009, 6. [Google Scholar] [CrossRef]
- Wijnker, J.J.; Depner, K.R.; Berends, B.R. Inactivation of classical swine fever virus in porcine casing preserved in salt. Int. J. Food Microbiol. 2008, 128, 411–413. [Google Scholar] [CrossRef] [PubMed]
- Farez, S.; Morley, R.S. Potential animal health hazards of pork and pork products. Rev. Sci. Tech. 1997, 16, 65–78. [Google Scholar] [CrossRef] [PubMed]
- Edwards, S. Survival and inactivation of classical swine fever virus. Vet. Microbiol. 2000, 73, 175–181. [Google Scholar] [CrossRef]
- Weesendorp, E.; Stegeman, A.; Loeffen, W.L. Survival of classical swine fever virus at various temperatures in faeces and urine derived from experimentally infected pigs. Vet. Microbiol. 2008, 132, 249–259. [Google Scholar] [CrossRef] [PubMed]
- Haas, B.; Ahl, R.; Böhm, R.; Strauch, D. Inactivation of viruses in liquid manure. Rev. Sci. Tech. 1995, 14, 435–445. [Google Scholar] [CrossRef] [PubMed]
- Botner, A.; Belsham, G.J. Virus survival in slurry: Analysis of the stability of foot-and-mouth disease, classical swine fever, bovine viral diarrhoea and swine influenza viruses. Vet. Microbiol. 2012, 157, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Turner, C.; Williams, S.M.; Cumby, T.R. The inactivation of foot and mouth disease, aujeszky's disease and classical swine fever viruses in pig slurry. J. Appl. Microbiol. 2000, 89, 760–767. [Google Scholar] [CrossRef] [PubMed]
- Gale, P. Risks to farm animals from pathogens in composted catering waste containing meat. Vet. Rec. 2004, 155, 77–82. [Google Scholar] [CrossRef] [PubMed]
- Artois, M.; Depner, K.R.; Guberti, V.; Hars, J.; Rossi, S.; Rutili, D. Classical swine fever (hog cholera) in wild boar in Europe. Rev. Sci. Tech. 2002, 21, 287–303. [Google Scholar] [CrossRef] [PubMed]
- Harkness, J.W. Classical swine fever and its diagnosis: A current view. Vet. Rec. 1985, 116, 288–293. [Google Scholar] [CrossRef] [PubMed]
- Tessler, J.; Stewart, W.C.; Kresse, J.I. Stabilization of hog cholera virus by dimethyl sulfoxide. Can. J. Comp. Med. 1975, 39, 472–473. [Google Scholar] [PubMed]
- Depner, K.; Bauer, T.; Liess, B. Thermal and pH stability of pestiviruses. Rev. Sci. Tech. 1992, 11, 885–893. [Google Scholar] [CrossRef] [PubMed]
- Panina, G.F.; Civardi, A.; Cordioli, P.; Massirio, I.; Scatozza, F.; Baldini, P.; Palmia, F. Survival of hog cholera virus (HCV) in sausage meat products (italian salami). Int. J. Food Microbiol. 1992, 17, 19–25. [Google Scholar] [CrossRef]
- Mebus, C.; House, C.; Gonzalvo, F.R.; Pineda, J.; Tapiador, J.; Pire, J.; Bergada, J.; Yedloutschnig, R.; Sahu, S.; Becerra, V. Survival of foot-and-mouth disease, african swine fever, and hog cholera viruses in spanish serrano cured hams and Iberian cured hams, shoulders and loins. Food Microbiol. 1993, 10, 133–143. [Google Scholar] [CrossRef]
- Postel, A.; Schmeiser, S.; Perera, C.L.; Rodriguez, L.J.; Frias-Lepoureau, M.T.; Becher, P. Classical swine fever virus isolates from Cuba form a new subgenotype 1.4. Vet. Microbiol. 2013, 161, 334–338. [Google Scholar] [CrossRef] [PubMed]
- Cha, S.H.; Choi, E.J.; Park, J.H.; Yoon, S.R.; Kwon, J.H.; Yoon, K.J.; Song, J.Y. Phylogenetic characterization of classical swine fever viruses isolated in Korea between 1988 and 2003. Virus Res. 2007, 126, 256–261. [Google Scholar] [CrossRef] [PubMed]
- Blome, S.; Grotha, I.; Moennig, V.; Greiser-Wilke, I. Classical swine fever virus in South-Eastern Europe—Retrospective analysis of the disease situation and molecular epidemiology. Vet. Microbiol. 2010, 146, 276–284. [Google Scholar] [CrossRef] [PubMed]
- Chen, N.; Hu, H.; Zhang, Z.; Shuai, J.; Jiang, L.; Fang, W. Genetic diversity of the envelope glycoprotein E2 of classical swine fever virus: Recent isolates branched away from historical and vaccine strains. Vet. Microbiol. 2008, 127, 286–299. [Google Scholar] [CrossRef] [PubMed]
- Jiang, D.L.; Liu, G.H.; Gong, W.J.; Li, R.C.; Hu, Y.F.; Tu, C.; Yu, X.L. Complete genome sequences of classical swine fever virus isolates belonging to a new subgenotype, 2.1c, from Hunan province, China. Genome Announc. 2013, 28, e00080. [Google Scholar] [CrossRef] [PubMed]
- Vanderhallen, H.; Mittelholzer, C.; Hofmann, M.A.; Koenen, F. Classical swine fever virus is genetically stable in vitro and in vivo. Arch. Virol. 1999, 144, 1669–1677. [Google Scholar] [CrossRef] [PubMed]
- Greiser-Wilke, I.; Dreier, S.; Haas, L.; Zimmermann, B. Genetic typing of classical swine fever viruses—A review. Dtsch. Tierarztl. Wochenschr. 2006, 113, 134–138. [Google Scholar] [PubMed]
- Paton, D.J.; McGoldrick, A.; Greiser-Wilke, I.; Parchariyanon, S.; Song, J.Y.; Liou, P.P.; Stadejek, T.; Lowings, J.P.; Bjorklund, H.; Belak, S. Genetic typing of classical swine fever virus. Vet. Microbiol. 2000, 73, 137–157. [Google Scholar] [CrossRef]
- Postel, A.; Schmeiser, S.; Bernau, J.; Meindl-Boehmer, A.; Pridotkas, G.; Dirbakova, Z.; Mojzis, M.; Becher, P. Improved strategy for phylogenetic analysis of classical swine fever virus based on full-length E2 encoding sequences. Vet. Res. 2012, 43, 50. [Google Scholar] [CrossRef] [PubMed]
- Töpfer, A.; Höper, D.; Blome, S.; Beer, M.; Beerenwinkel, N.; Ruggli, N.; Leifer, I. Sequencing approach to analyze the role of quasispecies for classical swine fever. Virology 2013, 438, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Leifer, I.; Hoeper, D.; Blome, S.; Beer, M.; Ruggli, N. Clustering of classical swine fever virus isolates by codon pair bias. BMC Res. Notes 2011, 4, 521. [Google Scholar] [CrossRef] [PubMed]
- Goller, K.V.; Gabriel, C.; Dimna, M.L.; Potier, M.F.; Rossi, S.; Staubach, C.; Merboth, M.; Beer, M.; Blome, S. Evolution and molecular epidemiology of classical swine fever virus during a multi-annual outbreak amongst european wild boar. J. Gen. Virol. 2016, 97, 639–645. [Google Scholar] [CrossRef] [PubMed]
- Fahnoe, U.; Pedersen, A.G.; Risager, P.C.; Nielsen, J.; Belsham, G.J.; Höper, D.; Beer, M.; Rasmussen, T.B. Rescue of the highly virulent classical swine fever virus strain "Koslov" from cloned cDNA and first insights into genome variations relevant for virulence. Virology 2014, 468–470, 379–387. [Google Scholar] [CrossRef] [PubMed]
- Björklund, H.; Lowings, P.; Stadejek, T.; Vilcek, S.; Greiser-Wilke, I.; Paton, D.; Belak, S. Phylogenetic comparison and molecular epidemiology of classical swine fever virus. Virus Genes 1999, 19, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Greiser-Wilke, I.; Fritzemeier, J.; Koenen, F.; Vanderhallen, H.; Rutili, D.; De Mia, G.M.; Romero, L.; Rosell, R.; Sanchez-Vizcaino, J.M.; San Gabriel, A. Molecular epidemiology of a large classical swine fever epidemic in the European Union in 1997–1998. Vet. Microbiol. 2000, 77, 17–27. [Google Scholar] [CrossRef]
- Jemersic, L.; Greiser-Wilke, I.; Barlic-Maganja, D.; Lojkic, M.; Madic, J.; Terzic, S.; Grom, J. Genetic typing of recent classical swine fever virus isolates from Croatia. Vet. Microbiol. 2003, 96, 25–33. [Google Scholar] [CrossRef]
- Bartak, P.; Greiser-Wilke, I. Genetic typing of classical swine fever virus isolates from the territory of the Czech Republic. Vet. Microbiol. 2000, 77, 59–70. [Google Scholar] [CrossRef]
- Wonnemann, H.; Floegel-Niesmann, G.; Moennig, V.; Greiser-Wilke, I. Genetic typing of German isolates of classical swine fever virus. Dtsch. Tierarztl. Wochenschr. 2001, 108, 252–256. [Google Scholar] [PubMed]
- Biagetti, M.; Greiser-Wilke, I.; Rutili, D. Molecular epidemiology of classical swine fever in Italy. Vet. Microbiol. 2001, 83, 205–215. [Google Scholar] [CrossRef]
- Pol, F.; Rossi, S.; Mesplede, A.; Kuntz-Simon, G.; Le Potier, M.F. Two outbreaks of classical swine fever in wild boar in France. Vet. Rec. 2008, 162, 811–816. [Google Scholar] [CrossRef] [PubMed]
- Leifer, I.; Hoffmann, B.; Höper, D.; Bruun Rasmussen, T.; Blome, S.; Strebelow, G.; Höreth-Böntgen, D.; Staubach, C.; Beer, M. Molecular epidemiology of current classical swine fever virus isolates of wild boar in germany. J. Gen. Virol. 2010, 91, 2687–2697. [Google Scholar] [CrossRef] [PubMed]
- Simon, G.; Le Dimna, M.; Le Potier, M.F.; Pol, F. Molecular tracing of classical swine fever viruses isolated from wild boars and pigs in France from 2002 to 2011. Vet. Microbiol. 2013, 166, 631–638. [Google Scholar] [CrossRef] [PubMed]
- Moennig, V.; Floegel-Niesmann, G.; Greiser-Wilke, I. Clinical signs and epidemiology of classical swine fever: A review of new knowledge. Vet. J. 2003, 165, 11–20. [Google Scholar] [CrossRef]
- Beer, M.; Goller, K.V.; Staubach, C.; Blome, S. Genetic variability and distribution of classical swine fever virus. Anim. Health Res. Rev. 2015, 16, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Pereda, A.J.; Greiser-Wilke, I.; Schmitt, B.; Rincon, M.A.; Mogollon, J.D.; Sabogal, Z.Y.; Lora, A.M.; Sanguinetti, H.; Piccone, M.E. Phylogenetic analysis of classical swine fever virus (CSFV) field isolates from outbreaks in south and central America. Virus Res. 2005, 110, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Diaz de Arce, H.; Nunez, J.I.; Ganges, L.; Barreras, M.; Teresa Frias, M.; Sobrino, F. Molecular epidemiology of classical swine fever in Cuba. Virus Res. 1999, 64, 61–67. [Google Scholar] [CrossRef]
- de Arce, H.D.; Ganges, L.; Barrera, M.; Naranjo, D.; Sobrino, F.; Frias, M.T.; Nunez, J.I. Origin and evolution of viruses causing classical swine fever in Cuba. Virus Res. 2005, 112, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Sandvik, T.; Crooke, H.; Drew, T.W.; Blome, S.; Greiser-Wilke, I.; Moennig, V.; Gous, T.A.; Gers, S.; Kitching, J.A.; Buhrmann, G.; et al. Classical swine fever in South Africa after 87 years' absence. Vet. Rec. 2005, 157, 267. [Google Scholar] [CrossRef] [PubMed]
- David, D.; Edri, N.; Yakobson, B.A.; Bombarov, V.; King, R.; Davidson, I.; Pozzi, P.; Hadani, Y.; Bellaiche, M.; Schmeiser, S.; et al. Emergence of classical swine fever virus in Israel in 2009. Vet. J. 2011, 190, e146–e149. [Google Scholar] [CrossRef] [PubMed]
- Barman, N.N.; Bora, D.P.; Khatoon, E.; Mandal, S.; Rakshit, A.; Rajbongshi, G.; Depner, K.; Chakraborty, A.; Kumar, S. Classical swine fever in wild hog: Report of its prevalence in northeast India. Transbound. Emerg. Dis. 2014, 63, 540–547. [Google Scholar] [CrossRef] [PubMed]
- Roychoudhury, P.; Sarma, D.K.; Rajkhowa, S.; Munir, M.; Kuchipudi, S.V. Predominance of genotype 1.1 and emergence of genotype 2.2 classical swine fever viruses in north-eastern region of India. Transbound. Emerg. Dis. 2014, 61 (Suppl. 1), 69–77. [Google Scholar] [CrossRef] [PubMed]
- Patil, S.S.; Hemadri, D.; Shankar, B.P.; Raghavendra, A.G.; Veeresh, H.; Sindhoora, B.; Chandan, S.; Sreekala, K.; Gajendragad, M.R.; Prabhudas, K. Genetic typing of recent classical swine fever isolates from India. Vet. Microbiol. 2010, 141, 367–373. [Google Scholar] [CrossRef] [PubMed]
- Patil, S.S.; Hemadri, D.; Veeresh, H.; Sreekala, K.; Gajendragad, M.R.; Prabhudas, K. Phylogenetic analysis of NS5B gene of classical swine fever virus isolates indicates plausible Chinese origin of Indian subgroup 2.2 viruses. Virus Genes 2012, 44, 104–108. [Google Scholar] [CrossRef] [PubMed]
- Rajkhowa, T.K.; Hauhnar, L.; Lalrohlua, I.; Mohanarao, G.J. Emergence of 2.1. Subgenotype of classical swine fever virus in pig population of India in 2011. Vet. Q. 2014, 34, 224–228. [Google Scholar] [CrossRef] [PubMed]
- Desai, G.S.; Sharma, A.; Kataria, R.S.; Barman, N.N.; Tiwari, A.K. 5′ UTR-based phylogenetic analysis of classical swine fever virus isolates from India. Acta Virol. 2010, 54, 79–82. [Google Scholar] [CrossRef] [PubMed]
- Sarma, D.K.; Mishra, N.; Vilcek, S.; Rajukumar, K.; Behera, S.P.; Nema, R.K.; Dubey, P.; Dubey, S.C. Phylogenetic analysis of recent classical swine fever virus (CSFV) isolates from Assam, India. Comp. Immunol. Microbiol. Infect. Dis. 2011, 34, 11–15. [Google Scholar] [CrossRef] [PubMed]
- Nandi, S.; Muthuchelvan, D.; Ahuja, A.; Bisht, S.; Chander, V.; Pandey, A.B.; Singh, R.K. Prevalence of classical swine fever virus in India: A 6-year study (2004–2010). Transbound. Emerg. Dis. 2011, 58, 461–463. [Google Scholar] [CrossRef] [PubMed]
- Postel, A.; Jha, V.C.; Schmeiser, S.; Becher, P. First molecular identification and characterization of classical swine fever virus isolates from Nepal. Arch. Virol. 2013, 158, 207–210. [Google Scholar] [CrossRef] [PubMed]
- An, T.Q.; Peng, J.M.; Tian, Z.J.; Zhao, H.Y.; Li, N.; Liu, Y.M.; Chen, J.Z.; Leng, C.L.; Sun, Y.; Chang, D.; et al. Pseudorabies virus variant in Bartha-K61-vaccinated pigs, China, 2012. Emerg. Infect. Dis. 2013, 19, 1749–1755. [Google Scholar] [CrossRef] [PubMed]
- Afshar, A.; Dulac, G.C.; Dubuc, C.; Howard, T.H. Comparative evaluation of the fluorescent antibody test and microtiter immunoperoxidase assay for detection of bovine viral diarrhea virus from bull semen. Can. J. Vet. Res. 1991, 55, 91–93. [Google Scholar] [PubMed]
- Luo, T.R.; Liao, S.H.; Wu, X.S.; Feng, L.; Yuan, Z.X.; Li, H.; Liang, J.J.; Meng, X.M.; Zhang, H.Y. Phylogenetic analysis of the E2 gene of classical swine fever virus from the Guangxi province of southern China. Virus Genes 2011, 42, 347–354. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Li, S.; Sun, Y.; Qiu, H.J. Classical swine fever in China: A minireview. Vet. Microbiol. 2014, 172, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Pan, C.H.; Jong, M.H.; Huang, T.S.; Liu, H.F.; Lin, S.Y.; Lai, S.S. Phylogenetic analysis of classical swine fever virus in Taiwan. Arch. Virol. 2005, 150, 1101–1119. [Google Scholar] [CrossRef] [PubMed]
- Deng, M.C.; Huang, C.C.; Huang, T.S.; Chang, C.Y.; Lin, Y.J.; Chien, M.S.; Jong, M.H. Phylogenetic analysis of classical swine fever virus isolated from Taiwan. Vet. Microbiol. 2005, 106, 187–1893. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.J.; Chien, M.S.; Deng, M.C.; Huang, C.C. Complete sequence of a subgroup 3.4 strain of classical swine fever virus from Taiwan. Virus Genes 2007, 35, 737–744. [Google Scholar] [CrossRef] [PubMed]
- Blacksell, S.D.; Khounsy, S.; Boyle, D.B.; Gleeson, L.J.; Westbury, H.A.; Mackenzie, J.S. Genetic typing of classical swine fever viruses from Lao PDR by analysis of the 5' non-coding region. Virus Genes 2005, 31, 349–355. [Google Scholar] [CrossRef] [PubMed]
- Blacksell, S.D.; Khounsy, S.; Boyle, D.B.; Greiser-Wilke, I.; Gleeson, L.J.; Westbury, H.A.; Mackenzie, J.S. Phylogenetic analysis of the E2 gene of classical swine fever viruses from Lao PDR. Virus Res. 2004, 104, 87–92. [Google Scholar] [CrossRef] [PubMed]
- Sakoda, Y.; Ozawa, S.; Damrongwatanapokin, S.; Sato, M.; Ishikawa, K.; Fukusho, A. Genetic heterogeneity of porcine and ruminant pestiviruses mainly isolated in Japan. Vet. Microbiol. 1999, 65, 75–86. [Google Scholar] [CrossRef]
- Coronado, L.; Liniger, M.; Munoz-Gonzalez, S.; Postel, A.; Perez, L.J.; Perez-Simo, M.; Perera, C.L.; Frias-Lepoureau, M.T.; Rosell, R.; Grundhoff, A.; et al. Novel poly-uridine insertion in the 3' UTR and E2 amino acid substitutions in a low virulent classical swine fever virus. Vet. Microbiol. 2017, 201, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Lange, M.; Kramer-Schadt, S.; Blome, S.; Beer, M.; Thulke, H.H. Disease severity declines over time after a wild boar population has been affected by classical swine fever--legend or actual epidemiological process? Prev. Vet. Med. 2012, 106, 185–195. [Google Scholar] [CrossRef] [PubMed]
- Dreier, S.; Zimmermann, B.; Moennig, V.; Greiser-Wilke, I. A sequence database allowing automated genotyping of classical swine fever virus isolates. J. Virol. Methods 2007, 140, 95–99. [Google Scholar] [CrossRef] [PubMed]
- Greiser-Wilke, I.; Zimmermann, B.; Fritzemeier, J.; Floegel, G.; Moennig, V. Structure and presentation of a world wide web database of CSF virus isolates held at the EU reference laboratory. Vet. Microbiol. 2000, 73, 131–136. [Google Scholar] [CrossRef]
- Postel, A.; Schmeiser, S.; Zimmermann, B.; Becher, P. The European classical swine fever virus database: Blueprint for a pathogen-specific sequence database with integrated sequence analysis tools. Viruses 2016, 8, 302. [Google Scholar] [CrossRef] [PubMed]
- Mayer, D.; Hofmann, M.A.; Tratschin, J.D. Attenuation of classical swine fever virus by deletion of the viral Npro gene. Vaccine 2004, 22, 317–328. [Google Scholar] [CrossRef] [PubMed]
- Risatti, G.R.; Borca, M.V.; Kutish, G.F.; Lu, Z.; Holinka, L.G.; French, R.A.; Tulman, E.R.; Rock, D.L. The E2 glycoprotein of classical swine fever virus is a virulence determinant in swine. J. Virol. 2005, 79, 3787–3796. [Google Scholar] [CrossRef] [PubMed]
- Tews, B.A.; Schurmann, E.M.; Meyers, G. Mutation of cysteine 171 of pestivirus Erns RNase prevents homodimer formation and leads to attenuation of classical swine fever virus. J. Virol. 2009, 83, 4823–4834. [Google Scholar] [CrossRef] [PubMed]
- Meyers, G.; Saalmüller, A.; Büttner, M. Mutations abrogating the RNAse activity in glycoprotein Erns of the pestivirus classical swine fever virus lead to virus attenuation. J. Virol. 1999, 73, 10224–10235. [Google Scholar] [PubMed]
- Tamura, T.; Sakoda, Y.; Yoshino, F.; Nomura, T.; Yamamoto, N.; Sato, Y.; Okamatsu, M.; Ruggli, N.; Kida, H. Selection of classical swine fever virus with enhanced pathogenicity reveals synergistic virulence determinants in E2 and NS4B. J. Virol. 2012, 86, 8602–8613. [Google Scholar] [CrossRef] [PubMed]
- Risatti, G.R.; Holinka, L.G.; Fernandez Sainz, I.; Carrillo, C.; Kutish, G.F.; Lu, Z.; Zhu, J.; Rock, D.L.; Borca, M.V. Mutations in the carboxyl terminal region of E2 glycoprotein of classical swine fever virus are responsible for viral attenuation in swine. Virology 2007, 364, 371–382. [Google Scholar] [CrossRef] [PubMed]
- Risatti, G.R.; Holinka, L.G.; Fernandez Sainz, I.; Carrillo, C.; Lu, Z.; Borca, M.V. N-linked glycosylation status of classical swine fever virus strain brescia E2 glycoprotein influences virulence in swine. J. Virol. 2007, 81, 924–933. [Google Scholar] [CrossRef] [PubMed]
- Sainz, I.F.; Holinka, L.G.; Lu, Z.; Risatti, G.R.; Borca, M.V. Removal of a N-linked glycosylation site of classical swine fever virus strain Brescia Erns glycoprotein affects virulence in swine. Virology 2008, 370, 122–129. [Google Scholar] [CrossRef] [PubMed]
- Tang, F.; Pan, Z.; Zhang, C. The selection pressure analysis of classical swine fever virus envelope protein genes Erns and E2. Virus Res. 2008, 131, 132–135. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Wang, Q.; Feng, Q.; Liu, Y.; Teng, J.; Yu, A.C.; Chen, J. Correlation of the virulence of CSFV with evolutionary patterns of E2 glycoprotein. Front. Biosci. (Elite Ed.) 2010, 2, 204–220. [Google Scholar] [PubMed]
- Ishikawa, K.; Nagai, H.; Katayama, K.; Tsutsui, M.; Tanabayashi, K.; Takeuchi, K.; Hishiyama, M.; Saitoh, A.; Takagi, M.; Gotoh, K.; et al. Comparison of the entire nucleotide and deduced amino acid sequences of the attenuated hog cholera vaccine strain GPE- and the wild-type parental strain ALD. Arch. Virol. 1995, 140, 1385–1391. [Google Scholar] [CrossRef] [PubMed]
- Jenckel, M.; Blome, S.; Beer, M.; Höper, D. Quasispecies composition and diversity do not reveal any predictors for chronic classical swine fever virus infection. Arch. Virol. 2017, 162, 775–786. [Google Scholar] [CrossRef] [PubMed]
- Petrov, A.; Blohm, U.; Beer, M.; Pietschmann, J.; Blome, S. Comparative analyses of host responses upon infection with moderately virulent classical swine fever virus in domestic pigs and wild boar. Virol. J. 2014, 11, 134. [Google Scholar] [CrossRef] [PubMed]
- Bunzenthal, C. Determination of the virulence of classical swine fever virus isolates [Bestimmung der Virulenz von Virusisolaten der Klassischen Schweinepest]. Ph.D. Thesis, University of Veterinary Medicine Hannover, Foundation, Hannover, Germany, 2003. [Google Scholar]
- Floegel-Niesmann, G.; Blome, S.; Gerss-Dülmer, H.; Bunzenthal, C.; Moennig, V. Virulence of classical swine fever virus isolates from Europe and other areas during 1996 until 2007. Vet. Microbiol. 2009, 139, 165–169. [Google Scholar] [CrossRef] [PubMed]
- Floegel-Niesmann, G.; Bunzenthal, C.; Fischer, S.; Moennig, V. Virulence of recent and former classical swine fever virus isolates evaluated by their clinical and pathological signs. J. Vet. Med. B Infect. Dis. Vet. Public Health 2003, 50, 214–220. [Google Scholar] [CrossRef] [PubMed]
- Tarradas, J.; de la Torre, M.E.; Rosell, R.; Perez, L.J.; Pujols, J.; Munoz, M.; Munoz, I.; Munoz, S.; Abad, X.; Domingo, M.; et al. The impact of CSFV on the immune response to control infection. Virus Res. 2014, 185, 82–91. [Google Scholar] [CrossRef] [PubMed]
- Depner, K.R.; Müller, A.; Gruber, A.; Rodriguez, A.; Bickhardt, K.; Liess, B. Classical swine fever in wild boar (Sus scrofa)—Experimental infections and viral persistence. Dtsch. Tierarztl. Wochenschr. 1995, 102, 381–384. [Google Scholar] [PubMed]
- Kaden, V.; Steyer, H.; Schnabel, J.; Bruer, W. Classical swine fever (CSF) in wild boar: The role of the transplacental infection in the perpetuation of CSF. J. Vet. Med. B Infect. Dis. Vet. Public Health 2005, 52, 161–164. [Google Scholar] [CrossRef] [PubMed]
- Kern, B.; Depner, K.R.; Letz, W.; Rott, M.; Thalheim, S.; Nitschke, B.; Plagemann, R.; Liess, B. Incidence of classical swine fever (CSF) in wild boar in a densely populated area indicating CSF virus persistence as a mechanism for virus perpetuation. Zentralbl. Veterinarmed. B 1999, 46, 63–67. [Google Scholar] [CrossRef] [PubMed]
- von Benten, K.; Trautwein, G.; Richter-Reichhelm, H.B.; Liess, B.; Frey, H.R. Experimental transplacental transmission of hog cholera virus in pigs. III. Histopathological findings in the fetus. Zentralbl. Veterinarmed. B 1980, 27, 714–724. [Google Scholar] [CrossRef] [PubMed]
- Stewart, W.C.; Carbrey, E.A.; Kresse, J.I. Transplacental hog cholera infection in susceptible sows. Am. J. Vet. Res. 1973, 34, 637–640. [Google Scholar] [PubMed]
- Stewart, W.C.; Carbrey, E.A.; Kresse, J.I. Transplacental hog cholera infection in immune sows. Am. J. Vet. Res. 1972, 33, 791–798. [Google Scholar] [PubMed]
- Richter-Reichhelm, H.B.; Trautwein, G.; von Benten, K.; Liess, B.; Frey, H.R. Experimental transplacental transmission of hog cholera virus in pigs. II. Immunopathological findings in the fetus. Zentralbl. Veterinarmed. B 1980, 27, 243–252. [Google Scholar] [CrossRef] [PubMed]
- Overby, E.; Eskildsen, M. Transplacental Infection in Susceptible Gilts after Inoculation with: I. Lapinized Swine Fever Vaccine, II. Bovine Viral Diarrhoea Virus Strains; Commission of the European Communities, DG Scientific and Technical Information and Information Management: Luxembourg, 1977; Volume EUR 5904. [Google Scholar]
- Meyer, H.; Liess, B.; Frey, H.R.; Hermanns, W.; Trautwein, G. Experimental transplacental transmission of hog cholera virus in pigs. IV. Virological and serological studies in newborn piglets. Zentralbl. Veterinarmed. B 1981, 28, 659–668. [Google Scholar] [CrossRef] [PubMed]
- Hermanns, W.; Trautwein, G.; Meyer, H.; Liess, B. Experimental transplacental transmission of hog cholera virus in pigs. V. Immunopathological findings in newborn pigs. Zentralbl. Veterinarmed. B 1981, 28, 669–683. [Google Scholar] [CrossRef] [PubMed]
- Frey, H.R.; Liess, B.; Richter-Reichhelm, H.B.; von Benten, K.; Trautwein, G. Experimental transplacental transmission of hog cholera virus in pigs. I. Virological and serological studies. Zentralbl. Veterinarmed. B 1980, 27, 154–164. [Google Scholar] [CrossRef] [PubMed]
- Cabezon, O.; Colom-Cadena, A.; Munoz-Gonzalez, S.; Perez-Simo, M.; Bohorquez, J.A.; Rosell, R.; Marco, I.; Domingo, M.; Lavin, S.; Ganges, L. Post-natal persistent infection with classical swine fever virus in wild boar: A strategy for viral maintenance? Transbound. Emerg. Dis. 2017, 64, 651–655. [Google Scholar] [CrossRef] [PubMed]
- Munoz-Gonzalez, S.; Perez-Simo, M.; Munoz, M.; Bohorquez, J.A.; Rosell, R.; Summerfield, A.; Domingo, M.; Ruggli, N.; Ganges, L. Efficacy of a live attenuated vaccine in classical swine fever virus postnatally persistently infected pigs. Vet. Res. 2015, 46, 78. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Villamandos, J.C.; Garcia de Leaniz, I.; Nunez, A.; Salguero, F.J.; Ruiz-Villamor, E.; Romero-Trevejo, J.L.; Sanchez-Cordon, P.J. Neuropathologic study of experimental classical swine fever. Vet. Pathol. 2006, 43, 530–540. [Google Scholar] [CrossRef] [PubMed]
- Van Oirschot, J.T. Classial Swine Fever (Hog Cholera), 8th ed.; Straw, B.E., D’Allaire, S., Mengeling, W.L., Taylor, D.J., Eds.; Iowa State University Press: Ames, IA, USA, 1999; pp. 159–172. [Google Scholar]
- Depner, K.R.; Lange, E.; Pontrakulpipat, S.; Fichtner, D. Does porcine reproductive and respiratory syndrome virus potentiate classical swine fever virus infection in weaner pigs? Zentralbl. Veterinarmed. B 1999, 46, 485–491. [Google Scholar] [CrossRef] [PubMed]
- Kaden, V.; Ziegler, U.; Lange, E.; Dedek, J. Classical swine fever virus: Clinical, virological, serological and hematological findings after infection of domestic pigs and wild boars with the field isolate "Spante" originating from wild boar. Berl. Munch. Tierarztl. Wochenschr. 2000, 113, 412–416. [Google Scholar] [PubMed]
- Gomez-Villamandos, J.C.; Carrasco, L.; Bautista, M.J.; Sierra, M.A.; Quezada, M.; Hervas, J.; Chacon Mde, L.; Ruiz-Villamor, E.; Salguero, F.J.; Sonchez-Cordon, P.J.; et al. African swine fever and classical swine fever: A review of the pathogenesis. Dtsch. Tierarztl. Wochenschr. 2003, 110, 165–169. [Google Scholar] [PubMed]
- Lange, A.; Blome, S.; Moennig, V.; Greiser-Wilke, I. Pathogenesis of classical swine fever—Similarities to viral haemorrhagic fevers: A review. Berl. Munch. Tierarztl. Wochenschr. 2011, 124, 36–47. [Google Scholar] [PubMed]
- Liess, B. Pathogenesis and epidemiology of hog cholera. Ann. Rech. Vet. 1987, 18, 139–145. [Google Scholar] [PubMed]
- Dunne, H.W. Hog Cholera, 3rd ed.; Dunne, H.W., Ed.; The Iowa State University Press: Ames, IA, USA, 1970; Vol. Diseases of Swine; pp. 177–239. [Google Scholar]
- Ressang, A.A. Studies on the pathogenesis of hog cholera. II. Virus distribution in tissue and the morphology of the immune response. Zentralbl. Veterinarmed. B 1973, 20, 272–288. [Google Scholar] [CrossRef] [PubMed]
- Ressang, A.A. Studies on the pathogenesis of hog cholera. I. Demonstration of hog cholera virus subsequent to oral exposure. Zentralbl. Veterinarmed. B 1973, 20, 256–271. [Google Scholar] [CrossRef] [PubMed]
- Bauhofer, O.; Summerfield, A.; McCullough, K.C.; Ruggli, N. Role of double-stranded RNA and Npro of classical swine fever virus in the activation of monocyte-derived dendritic cells. Virology 2005, 343, 93–105. [Google Scholar] [CrossRef] [PubMed]
- Carrasco, C.P.; Rigden, R.C.; Vincent, I.E.; Balmelli, C.; Ceppi, M.; Bauhofer, O.; Tache, V.; Hjertner, B.; McNeilly, F.; van Gennip, H.G.; et al. Interaction of classical swine fever virus with dendritic cells. J. Gen. Virol. 2004, 85, 1633–1641. [Google Scholar] [CrossRef] [PubMed]
- Fiebach, A.R.; Guzylack-Piriou, L.; Python, S.; Summerfield, A.; Ruggli, N. Classical swine fever virus Npro limits type I interferon induction in plasmacytoid dendritic cells by interacting with interferon regulatory factor 7. J. Virol. 2011, 85, 8002–8011. [Google Scholar] [CrossRef] [PubMed]
- Jamin, A.; Gorin, S.; Cariolet, R.; Le Potier, M.F.; Kuntz-Simon, G. Classical swine fever virus induces activation of plasmacytoid and conventional dendritic cells in tonsil, blood, and spleen of infected pigs. Vet. Res. 2008, 39, 7. [Google Scholar] [CrossRef] [PubMed]
- Summerfield, A.; Ruggli, N. Immune responses against classical swine fever virus: Between ignorance and lunacy. Front. Vet. Sci. 2015, 2, 10. [Google Scholar] [CrossRef] [PubMed]
- Summerfield, A.; Alves, M.; Ruggli, N.; de Bruin, M.G.; McCullough, K.C. High IFN-α responses associated with depletion of lymphocytes and natural IFN-producing cells during classical swine fever. J. Interferon Cytokine Res. 2006, 26, 248–255. [Google Scholar] [CrossRef] [PubMed]
- Renson, P.; Blanchard, Y.; Le Dimna, M.; Felix, H.; Cariolet, R.; Jestin, A.; Le Potier, M.F. Acute induction of cell death-related IFN stimulated genes (ISG) differentiates highly from moderately virulent CSFV strains. Vet. Res. 2010, 41, 7. [Google Scholar] [CrossRef] [PubMed]
- Pauly, T.; König, M.; Thiel, H.J.; Saalmüller, A. Infection with classical swine fever virus: Effects on phenotype and immune responsiveness of porcine T lymphocytes. J. Gen. Virol. 1998, 79, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Summerfield, A.; Knoetig, S.M.; Tschudin, R.; McCullough, K.C. Pathogenesis of granulocytopenia and bone marrow atrophy during classical swine fever involves apoptosis and necrosis of uninfected cells. Virology 2000, 272, 50–60. [Google Scholar] [CrossRef] [PubMed]
- Susa, M.; König, M.; Saalmüller, A.; Reddehase, M.J.; Thiel, H.J. Pathogenesis of classical swine fever: B-lymphocyte deficiency caused by hog cholera virus. J. Virol. 1992, 66, 1171–1175. [Google Scholar] [PubMed]
- Trautwein, G. Classical swine fever and related infections. In Pathology and Pathogenesis of the Disease; Martinus Nijhoff: Boston, MA, USA, 1988; pp. 27–54. [Google Scholar]
- Bautista, M.J.; Ruiz-Villamor, E.; Salguero, F.J.; Sanchez-Cordon, P.J.; Carrasco, L.; Gomez-Villamandos, J.C. Early platelet aggregation as a cause of thrombocytopenia in classical swine fever. Vet. Pathol. 2002, 39, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Heene, D.; Hoffmann-Fezer, G.; Müller-Berghaus, G.; Hoffmann, R.; Weiss, E.; Lasch, H.G. Coagulation disorders in acute hog cholera. Beitr. Pathol. 1971, 144, 259–271. [Google Scholar] [PubMed]
- Weiss, E.; Teredesai, A.; Hoffmann, R.; Hoffmann-Fezer, G. Volume distribution and ultrastructure of platelets in acute hog cholera. Thromb. Diath. Haemorrh. 1973, 30, 371–380. [Google Scholar] [PubMed]
- Blome, S.; Meindl-Böhmer, A.; Nowak, G.; Moennig, V. Disseminated intravascular coagulation does not play a major role in the pathogenesis of classical swine fever. Vet. Microbiol. 2013, 162, 360–368. [Google Scholar] [CrossRef] [PubMed]
- Bensaude, E.; Turner, J.L.; Wakeley, P.R.; Sweetman, D.A.; Pardieu, C.; Drew, T.W.; Wileman, T.; Powell, P.P. Classical swine fever virus induces proinflammatory cytokines and tissue factor expression and inhibits apoptosis and interferon synthesis during the establishment of long-term infection of porcine vascular endothelial cells. J. Gen. Virol. 2004, 85, 1029–1037. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Villamandos, J.C.; Ruiz-Villamor, E.; Bautista, M.J.; Quezada, M.; Sanchez, C.P.; Salguero, F.J.; Sierra, M.A. Pathogenesis of classical swine fever: Renal haemorrhages and erythrodiapedesis. J. Comp. Pathol. 2000, 123, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, R.; Hoffmann-Fezer, G.; Kimeto, B.; Weiss, E. Microthrombi as morphological evidence of consumption coagulopathy in acute hog cholera. Zentralbl. Veterinarmed. B 1971, 18, 710–718. [Google Scholar] [CrossRef] [PubMed]
- Hulst, M.M.; Westra, D.F.; Wensvoort, G.; Moormann, R.J. Glycoprotein E1 of hog cholera virus expressed in insect cells protects swine from hog cholera. J. Virol. 1993, 67, 5435–5442. [Google Scholar] [PubMed]
- Rümenapf, T.; Stark, R.; Meyers, G.; Thiel, H.J. Structural proteins of hog cholera virus expressed by vaccinia virus: Further characterization and induction of protective immunity. J. Virol. 1991, 65, 589–597. [Google Scholar] [PubMed]
- König, M.; Lengsfeld, T.; Pauly, T.; Stark, R.; Thiel, H.J. Classical swine fever virus: Independent induction of protective immunity by two structural glycoproteins. J. Virol. 1995, 69, 6479–6486. [Google Scholar] [PubMed]
- Paton, D.J.; Ibata, G.; Edwards, S.; Wensvoort, G. An ELISA detecting antibody to conserved pestivirus epitopes. J. Virol. Methods 1991, 31, 315–324. [Google Scholar] [CrossRef]
- Graham, S.P.; Everett, H.E.; Haines, F.J.; Johns, H.L.; Sosan, O.A.; Salguero, F.J.; Clifford, D.J.; Steinbach, F.; Drew, T.W.; Crooke, H.R. Challenge of pigs with classical swine fever viruses after C-strain vaccination reveals remarkably rapid protection and insights into early immunity. PLoS ONE 2012, 7, e29310. [Google Scholar] [CrossRef] [PubMed]
- Renson, P.; Le Dimna, M.; Keranflech, A.; Cariolet, R.; Koenen, F.; Le Potier, M.F. CP7_E2alf oral vaccination confers partial protection against early classical swine fever virus challenge and interferes with pathogeny-related cytokine responses. Vet. Res. 2013, 44, 9. [Google Scholar] [CrossRef] [PubMed]
- van Oirschot, J.T. Vaccinology of classical swine fever: From lab to field. Vet. Microbiol. 2003, 96, 367–384. [Google Scholar] [CrossRef] [PubMed]
- Graham, S.P.; Haines, F.J.; Johns, H.L.; Sosan, O.; La Rocca, S.A.; Lamp, B.; Rümenapf, T.; Everett, H.E.; Crooke, H.R. Characterisation of vaccine-induced, broadly cross-reactive IFN-γ secreting T cell responses that correlate with rapid protection against classical swine fever virus. Vaccine 2012, 30, 2742–2748. [Google Scholar] [CrossRef] [PubMed]
- Tarradas, J.; Argilaguet, J.M.; Rosell, R.; Nofrarias, M.; Crisci, E.; Cordoba, L.; Perez-Martin, E.; Diaz, I.; Rodriguez, F.; Domingo, M.; et al. Interferon-γ induction correlates with protection by DNA vaccine expressing E2 glycoprotein against classical swine fever virus infection in domestic pigs. Vet. Microbiol. 2010, 142, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Ganges, L.; Barrera, M.; Nunez, J.I.; Blanco, I.; Frias, M.T.; Rodriguez, F.; Sobrino, F. A DNA vaccine expressing the E2 protein of classical swine fever virus elicits T cell responses that can prime for rapid antibody production and confer total protection upon viral challenge. Vaccine 2005, 23, 3741–3752. [Google Scholar] [CrossRef] [PubMed]
- Blacksell, S.D.; Khounsy, S.; Van Aken, D.; Gleeson, L.J.; Westbury, H.A. Comparative susceptibility of indigenous and improved pig breeds to classical swine fever virus infection: Practical and epidemiological implications in a subsistence-based, developing country setting. Trop. Anim. Health Prod. 2006, 38, 467–474. [Google Scholar] [CrossRef] [PubMed]
- Everett, H.; Crooke, H.; Gurrala, R.; Dwarka, R.; Kim, J.; Botha, B.; Lubisi, A.; Pardini, A.; Gers, S.; Vosloo, W.; et al. Experimental infection of common warthogs (Phacochoerus africanus) and bushpigs (Potamochoerus larvatus) with classical swine fever virus. I: Susceptibility and transmission. Transbound. Emerg. Dis. 2011. [Google Scholar] [CrossRef] [PubMed]
- Terpstra, C. Epizootiology of swine fever. Vet. Q. 1987, 9 (Suppl. 1), 50S–60S. [Google Scholar] [CrossRef] [PubMed]
- Weesendorp, E.; Landman, W.J.; Stegeman, A.; Loeffen, W.L. Detection and quantification of classical swine fever virus in air samples originating from infected pigs and experimentally produced aerosols. Vet. Microbiol. 2008, 127, 50–62. [Google Scholar] [CrossRef] [PubMed]
- Weesendorp, E.; Stegeman, A.; Loeffen, W.L. Quantification of classical swine fever virus in aerosols originating from pigs infected with strains of high, moderate or low virulence. Vet. Microbiol. 2009, 135, 222–230. [Google Scholar] [CrossRef] [PubMed]
- Laevens, H.; Koenen, F.; Deluyker, H.; de Kruif, A. Experimental infection of slaughter pigs with classical swine fever virus: Transmission of the virus, course of the disease and antibody response. Vet. Rec. 1999, 145, 243–248. [Google Scholar] [CrossRef] [PubMed]
- de Smit, A.J.; Bouma, A.; Terpstra, C.; van Oirschot, J.T. Transmission of classical swine fever virus by artificial insemination. Vet. Microbiol. 1999, 67, 239–249. [Google Scholar] [CrossRef]
- Floegel, G.; Wehrend, A.; Depner, K.R.; Fritzemeier, J.; Waberski, D.; Moennig, V. Detection of classical swine fever virus in semen of infected boars. Vet. Microbiol. 2000, 77, 109–116. [Google Scholar] [CrossRef]
- Moennig, V.; Greiser-Wilke, I. Classical swine fever virus. In Encyclopedia of Virology; Mahy, B.W.J., van Regenmortel, M.H.V., Eds.; Elsevier: Amsterdam, The Netherlands, 2008; pp. 525–532. [Google Scholar]
- Pasick, J. Classical swine fever. In Foreign Animal Diseases, 7th ed.; Brown, C., Torres, A., Eds.; Committee on Foreign and Emerging Diseases of the United States Animal Health Association, Boca Publications Group: Boca Raton, FL, USA, 2008; pp. 197–205. [Google Scholar]
- Hoffmann, B.; Beer, M.; Reid, S.M.; Mertens, P.; Oura, C.A.; van Rijn, P.A.; Slomka, M.J.; Banks, J.; Brown, I.H.; Alexander, D.J.; et al. A review of RT-PCR technologies used in veterinary virology and disease control: Sensitive and specific diagnosis of five livestock diseases notifiable to the World Organisation for Animal Health. Vet. Microbiol. 2009, 139, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, B.; Beer, M.; Schelp, C.; Schirrmeier, H.; Depner, K. Validation of a real-time RT-PCR assay for sensitive and specific detection of classical swine fever. J. Virol. Methods 2005, 130, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, B.; Blome, S.; Bonilauri, P.; Fernandez-Pinero, J.; Greiser-Wilke, I.; Haegeman, A.; Isaksson, M.; Koenen, F.; Leblanc, N.; Leifer, I.; et al. Classical swine fever virus detection: Results of a real-time reverse transcription polymerase chain reaction ring trial conducted in the framework of the European network of excellence for epizootic disease diagnosis and control. J. Vet. Diagn. Investig. 2011, 23, 999–1004. [Google Scholar] [CrossRef] [PubMed]
- Le Dimna, M.; Vrancken, R.; Koenen, F.; Bougeard, S.; Mesplede, A.; Hutet, E.; Kuntz-Simon, G.; Le Potier, M.F. Validation of two commercial real-time RT-PCR kits for rapid and specific diagnosis of classical swine fever virus. J. Virol. Methods 2008, 147, 136–142. [Google Scholar] [CrossRef] [PubMed]
- Le Potier, M.F.; Le Dimna, M.; Kuntz-Simon, G.; Bougeard, S.; Mesplede, A. Validation of a real-time RT-PCR assay for rapid and specific diagnosis of classical swine fever virus. Dev. Biol. (Basel) 2006, 126, 179–186; discusssion 326–327. [Google Scholar] [PubMed]
- Leifer, I.; Blome, S.; Beer, M.; Hoffmann, B. Development of a highly sensitive real-time RT-PCR protocol for the detection of classical swine fever virus independent of the 5′ untranslated region. J. Virol. Methods 2011, 171, 314–317. [Google Scholar] [CrossRef] [PubMed]
- McGoldrick, A.; Lowings, J.P.; Ibata, G.; Sands, J.J.; Belak, S.; Paton, D.J. A novel approach to the detection of classical swine fever virus by RT-PCR with a fluorogenic probe (TaqMan). J. Virol. Methods 1998, 72, 125–135. [Google Scholar] [CrossRef]
- Paton, D.J.; McGoldrick, A.; Belak, S.; Mittelholzer, C.; Koenen, F.; Vanderhallen, H.; Biagetti, M.; De Mia, G.M.; Stadejek, T.; Hofmann, M.A.; et al. Classical swine fever virus: A ring test to evaluate RT-PCR detection methods. Vet. Microbiol. 2000, 73, 159–174. [Google Scholar] [CrossRef]
- Paton, D.J.; McGoldrick, A.; Bensaude, E.; Belak, S.; Mittelholzer, C.; Koenen, F.; Vanderhallen, H.; Greiser-Wilke, I.; Scheibner, H.; Stadejek, T.; et al. Classical swine fever virus: A second ring test to evaluate RT-PCR detection methods. Vet. Microbiol. 2000, 77, 71–81. [Google Scholar] [CrossRef]
- Belak, S. Experiences of an oie collaborating centre in molecular diagnosis of transboundary animal diseases: A review. Dev. Biol. (Basel) 2007, 128, 103–112. [Google Scholar] [PubMed]
- Liu, L.; Luo, Y.; Accensi, F.; Ganges, L.; Rodriguez, F.; Shan, H.; Stahl, K.; Qiu, H.J.; Belak, S. Pre-clinical evaluation of a real-time PCR assay on a portable instrument as a possible field diagnostic tool: Experiences from the testing of clinical samples for African and classical swine fever viruses. Transbound. Emerg. Dis. 2016. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.T.; Zhang, J.; Ma, L.N.; Ma, Y.P.; Ding, Y.Z.; Liu, X.T.; Chen, L.; Ma, L.Q.; Zhang, Y.G.; Liu, Y.S. Rapid pre-clinical detection of classical swine fever by reverse transcription loop-mediated isothermal amplification. Mol. Cell. Probes 2009, 23, 71–74. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Fan, X.Z.; Wang, Q.; Xu, L.; Zhao, Q.Z.; Zhou, Y.C.; Liu, J.; Tang, B.; Zou, X.Q. A novel RT-LAMP assay for rapid and simple detection of classical swine fever virus. Virol. Sin. 2010, 25, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Chowdry, V.K.; Luo, Y.; Widen, F.; Qiu, H.J.; Shan, H.; Belak, S.; Liu, L. Development of a loop-mediated isothermal amplification assay combined with a lateral flow dipstick for rapid and simple detection of classical swine fever virus in the field. J. Virol. Methods 2014, 197, 14–18. [Google Scholar] [CrossRef] [PubMed]
- Yin, S.; Shang, Y.; Zhou, G.; Tian, H.; Liu, Y.; Cai, X.; Liu, X. Development and evaluation of rapid detection of classical swine fever virus by reverse transcription loop-mediated isothermal amplification (RT-LAMP). J. Biotechnol. 2010, 146, 147–150. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.J.; Han, Q.Y.; Sun, Y.; Belak, S.; Liu, L.; Qiu, H.J. Development of a loop-mediated isothermal amplification for visual detection of the HCLV vaccine against classical swine fever in China. J. Virol. Methods 2011, 171, 200–205. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.J.; Sun, Y.; Liu, L.; Belak, S.; Qiu, H.J. Validation of a loop-mediated isothermal amplification assay for visualised detection of wild-type classical swine fever virus. J. Virol. Methods 2010, 167, 74–78. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Xia, H.; Belak, S.; Widen, F. Development of a primer-probe energy transfer real-time PCR assay for improved detection of classical swine fever virus. J. Virol. Methods 2009, 160, 69–73. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.J.; Xia, H.; Everett, H.; Sosan, O.; Crooke, H.; Belak, S.; Widen, F.; Qiu, H.J.; Liu, L. Evaluation of a primer-probe energy transfer real-time PCR assay for detection of classical swine fever virus. J. Virol. Methods 2010, 168, 259–261. [Google Scholar] [CrossRef] [PubMed]
- Lung, O.; Pasick, J.; Fisher, M.; Buchanan, C.; Erickson, A.; Ambagala, A. Insulated isothermal reverse transcriptase PCR (iiRT-PCR) for rapid and sensitive detection of classical swine fever virus. Transbound. Emerg. Dis. 2016, 63, e395–402. [Google Scholar] [CrossRef] [PubMed]
- Turner, L.W.; Brown, L.N.; Carbrey, E.A.; Mengeling, W.L.; Perella, D.H.; Solorzano, R.F. Recommended minimum standards for the isolation and identification of hog cholera by the fluorescent antibody-cell culture technique. Proc. Annu. Meet. U. S. Anim. Health Assoc. 1968, 72, 444–447. [Google Scholar] [PubMed]
- De Smit, A.J.; Eble, P.L.; de Kluijver, E.P.; Bloemraad, M.; Bouma, A. Laboratory experience during the classical swine fever virus epizootic in the Netherlands in 1997–1998. Vet. Microbiol. 2000, 73, 197–208. [Google Scholar] [CrossRef]
- Blome, S.; Meindl-Böhmer, A.; Loeffen, W.; Thuer, B.; Moennig, V. Assessment of classical swine fever diagnostics and vaccine performance. Rev. Sci. Tech. 2006, 25, 1025–1038. [Google Scholar] [CrossRef] [PubMed]
- Greiser-Wilke, I.; Blome, S.; Moennig, V. Diagnostic methods for detection of classical swine fever virus--status quo and new developments. Vaccine 2007, 25, 5524–5530. [Google Scholar] [CrossRef] [PubMed]
- Floegel-Niesmann, G. Marker vaccines and companion diagnostic tests for classical swine fever. Dev. Biol. (Basel) 2003, 114, 185–191. [Google Scholar] [PubMed]
- Floegel-Niesmann, G. Classical swine fever (CSF) marker vaccine. Trial III. Evaluation of discriminatory ELISAs. Vet. Microbiol. 2001, 83, 121–136. [Google Scholar] [CrossRef]
- Meyer, D.; Fritsche, S.; Luo, Y.; Engemann, C.; Blome, S.; Beyerbach, M.; Chang, C.Y.; Qiu, H.J.; Becher, P.; Postel, A. The double-antigen ELISA concept for early detection of Erns -specific classical swine fever virus antibodies and application as an accompanying test for differentiation of infected from marker vaccinated animals. Transbound. Emerg. Dis. 2017. [Google Scholar] [CrossRef] [PubMed]
- Aebischer, A.; Müller, M.; Hofmann, M.A. Two newly developed Erns-based ELISAs allow the differentiation of classical swine fever virus-infected from marker-vaccinated animals and the discrimination of pestivirus antibodies. Vet. Microbiol. 2013, 161, 274–285. [Google Scholar] [CrossRef] [PubMed]
- Xia, H.; Harimoorthy, R.; Vijayaraghavan, B.; Blome, S.; Widen, F.; Beer, M.; Belak, S.; Liu, L. Differentiation of classical swine fever virus infection from CP7_E2alf marker vaccination by a multiplex microsphere immunoassay. Clin. Vaccine Immunol. 2015, 22, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.L.; Pang, V.F.; Pan, C.H.; Chen, T.H.; Jong, M.H.; Huang, T.S.; Jeng, C.R. Development of a reverse transcription multiplex real-time PCR for the detection and genotyping of classical swine fever virus. J. Virol. Methods 2009, 160, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Leifer, I.; Depner, K.; Blome, S.; Le Potier, M.F.; Le Dimna, M.; Beer, M.; Hoffmann, B. Differentiation of C-strain "Riems" or CP7_E2alf vaccinated animals from animals infected by classical swine fever virus field strains using real-time RT-PCR. J. Virol. Methods 2009, 158, 114–122. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhao, J.J.; Li, N.; Shi, Z.; Cheng, D.; Zhu, Q.H.; Tu, C.; Tong, G.Z.; Qiu, H.J. A multiplex nested RT-PCR for the detection and differentiation of wild-type viruses from C-strain vaccine of classical swine fever virus. J. Virol. Methods 2007, 143, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Hoffmann, B.; Baule, C.; Beer, M.; Belak, S.; Widen, F. Two real-time RT-PCR assays of classical swine fever virus, developed for the genetic differentiation of naturally infected from vaccinated wild boars. J. Virol. Methods 2009, 159, 131–133. [Google Scholar] [CrossRef] [PubMed]
- Widen, F.; Everett, H.; Blome, S.; Fernandez Pinero, J.; Uttenthal, A.; Cortey, M.; von Rosen, T.; Tignon, M.; Liu, L. Comparison of two real-time RT-PCR assays for differentiation of C-strain vaccinated from classical swine fever infected pigs and wild boars. Res. Vet. Sci. 2014, 97, 455–457. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.J.; Cheng, D.; Li, N.; Sun, Y.; Shi, Z.; Zhu, Q.H.; Tu, C.; Tong, G.Z.; Qiu, H.J. Evaluation of a multiplex real-time RT-PCR for quantitative and differential detection of wild-type viruses and C-strain vaccine of classical swine fever virus. Vet. Microbiol. 2008, 126, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Dietze, K.; Tucakov, A.; Engel, T.; Wirtz, S.; Depner, K.; Globig, A.; Kammerer, R.; Mouchantat, S. Rope-based oral fluid sampling for early detection of classical swine fever in domestic pigs at group level. BMC Vet. Res. 2017, 13, 5. [Google Scholar] [CrossRef] [PubMed]
- Michaud, V.; Gil, P.; Kwiatek, O.; Prome, S.; Dixon, L.; Romero, L.; Le Potier, M.F.; Arias, M.; Couacy-Hymann, E.; Roger, F.; et al. Long-term storage at tropical temperature of dried-blood filter papers for detection and genotyping of RNA and DNA viruses by direct PCR. J. Virol. Methods 2007, 146, 257–265. [Google Scholar] [CrossRef] [PubMed]
- Mouchantat, S.; Globig, A.; Böhle, W.; Petrov, A.; Strebelow, H.G.; Mettenleiter, T.C.; Depner, K. Novel rope-based sampling of classical swine fever shedding in a group of wild boar showing low contagiosity upon experimental infection with a classical swine fever field strain of genotype 2.3. Vet. Microbiol. 2014, 170, 425–429. [Google Scholar] [CrossRef] [PubMed]
- Prickett, J.R.; Zimmerman, J.J. The development of oral fluid-based diagnostics and applications in veterinary medicine. Anim. Health Res. Rev. 2010, 11, 207–216. [Google Scholar] [CrossRef] [PubMed]
- Greiser-Wilke, I.; Moennig, V. Vaccination against classical swine fever virus: Limitations and new strategies. Anim. Health Res. Rev. 2004, 5, 223–226. [Google Scholar] [CrossRef] [PubMed]
- World Animal Health Information Database (WAHIS) Interface. Available online: http://www.oie.int/wahis_2/public/wahid.php/Diseasecontrol/measures (accessed 19 April 2017).
- Kaden, V.; Lange, E.; Fischer, U.; Strebelow, G. Oral immunisation of wild boar against classical swine fever: Evaluation of the first field study in Germany. Vet. Microbiol. 2000, 73, 239–252. [Google Scholar] [CrossRef]
- Kaden, V.; Lange, E.; Küster, H.; Müller, T.; Lange, B. An update on safety studies on the attenuated "RIEMSER Schweinepestoralvakzine" for vaccination of wild boar against classical swine fever. Vet. Microbiol. 2010, 143, 133–138. [Google Scholar] [CrossRef] [PubMed]
- Milicevic, V.; Dietze, K.; Plavsic, B.; Tikvicki, M.; Pinto, J.; Depner, K. Oral vaccination of backyard pigs against classical swine fever. Vet. Microbiol. 2012, 225, 167–171. [Google Scholar] [CrossRef] [PubMed]
- Dietze, K.; Milicevic, V.; Depner, K. Prospects of improved classical swine fever control in backyard pigs through oral vaccination. Berl. Munch. Tierarztl. Wochenschr. 2013, 126, 476–480. [Google Scholar] [PubMed]
- Monger, V.R.; Stegeman, J.A.; Dukpa, K.; Gurung, R.B.; Loeffen, W.L. Evaluation of oral bait vaccine efficacy against classical swine fever in village backyard pig farms in Bhutan. Transbound. Emerg. Dis. 2016, 63, e211–e218. [Google Scholar] [CrossRef] [PubMed]
- Dahle, J.; Liess, B. Assessment of safety and protective value of a cell culture modified strain "C" vaccine of hog cholera/classical swine fever virus. Berl. Munch. Tierarztl. Wochenschr. 1995, 108, 20–25. [Google Scholar] [PubMed]
- Kaden, V.; Riebe, B. Classical swine fever (CSF): A historical review of research and vaccine production on the Isle of Riems. Berl. Munch. Tierarztl. Wochenschr. 2001, 114, 246–251. [Google Scholar] [PubMed]
- Terpstra, C.; Woortmeyer, R.; Barteling, S.J. Development and properties of a cell culture produced vaccine for hog cholera based on the Chinese strain. Dtsch. Tierarztl. Wochenschr. 1990, 97, 77–79. [Google Scholar] [PubMed]
- Ferrari, M. A tissue culture vaccine with lapinized Chinese (LC) strain of hog cholera virus (HCV). Comp. Immunol. Microbiol. Infect. Dis. 1992, 15, 221–228. [Google Scholar] [CrossRef]
- Blome, S.; Gabriel, C.; Beer, M. Possibilities and limitations in veterinary vaccine development using the example of classical swine fever. Berl. Munch. Tierarztl. Wochenschr. 2013, 126, 481–490. [Google Scholar] [PubMed]
- Ahrens, U.; Kaden, V.; Drexler, C.; Visser, N. Efficacy of the classical swine fever (CSF) marker vaccine Porcilis Pesti in pregnant sows. Vet. Microbiol. 2000, 77, 83–97. [Google Scholar] [CrossRef]
- Bouma, A.; De Smit, A.J.; De Jong, M.C.; De Kluijver, E.P.; Moormann, R.J. Determination of the onset of the herd-immunity induced by the E2 sub-unit vaccine against classical swine fever virus. Vaccine 2000, 18, 1374–1381. [Google Scholar] [CrossRef]
- Bouma, A.; de Smit, A.J.; de Kluijver, E.P.; Terpstra, C.; Moormann, R.J. Efficacy and stability of a subunit vaccine based on glycoprotein E2 of classical swine fever virus. Vet. Microbiol. 1999, 66, 101–114. [Google Scholar] [CrossRef]
- de Smit, A.J.; Bouma, A.; de Kluijver, E.P.; Terpstra, C.; Moormann, R.J. Duration of the protection of an E2 subunit marker vaccine against classical swine fever after a single vaccination. Vet. Microbiol. 2001, 78, 307–317. [Google Scholar] [CrossRef]
- de Smit, A.J.; Bouma, A.; de Kluijver, E.P.; Terpstra, C.; Moormann, R.J. Prevention of transplacental transmission of moderate-virulent classical swine fever virus after single or double vaccination with an E2 subunit vaccine. Vet. Q. 2000, 22, 150–153. [Google Scholar] [CrossRef] [PubMed]
- Dewulf, J.; Laevens, H.; Koenen, F.; Vanderhallen, H.; Mintiens, K.; Deluyker, H.; de Kruif, A. An experimental infection with classical swine fever in E2 sub-unit marker-vaccine vaccinated and in non-vaccinated pigs. Vaccine 2000, 19, 475–482. [Google Scholar] [CrossRef]
- Klinkenberg, D.; Moormann, R.J.; de Smit, A.J.; Bouma, A.; de Jong, M.C. Influence of maternal antibodies on efficacy of a subunit vaccine: Transmission of classical swine fever virus between pigs vaccinated at 2 weeks of age. Vaccine 2002, 20, 3005–3013. [Google Scholar] [CrossRef]
- Lipowski, A.; Drexler, C.; Pejsak, Z. Safety and efficacy of a classical swine fever subunit vaccine in pregnant sows and their offspring. Vet. Microbiol. 2000, 77, 99–108. [Google Scholar] [CrossRef]
- Moormann, R.J.; Bouma, A.; Kramps, J.A.; Terpstra, C.; De Smit, H.J. Development of a classical swine fever subunit marker vaccine and companion diagnostic test. Vet. Microbiol. 2000, 73, 209–219. [Google Scholar] [CrossRef]
- van Aarle, P. Suitability of an E2 subunit vaccine of classical swine fever in combination with the Erns-marker-test for eradication through vaccination. Dev. Biol. (Basel) 2003, 114, 193–200. [Google Scholar] [PubMed]
- van Oirschot, J.T. DIVA vaccines that reduce virus transmission. J. Biotechnol. 1999, 73, 195–205. [Google Scholar] [CrossRef]
- van Oirschot, J.T. Emergency vaccination against classical swine fever. Dev. Biol. (Basel) 2003, 114, 259–267. [Google Scholar] [PubMed]
- Depner, K.R.; Bouma, A.; Koenen, F.; Klinkenberg, D.; Lange, E.; de Smit, H.; Vanderhallen, H. Classical swine fever (CSF) marker vaccine. Trial II. Challenge study in pregnant sows. Vet. Microbiol. 2001, 83, 107–120. [Google Scholar] [CrossRef]
- Beer, M.; Reimann, I.; Hoffmann, B.; Depner, K. Novel marker vaccines against classical swine fever. Vaccine 2007, 25, 5665–5670. [Google Scholar] [CrossRef] [PubMed]
- Blome, S.; Moss, C.; Reimann, I.; König, P.; Beer, M. Classical swine fever vaccines-state-of-the-art. Vet. Microbiol. 2017, 201–204. [Google Scholar] [CrossRef] [PubMed]
- Blome, S.; Aebischer, A.; Lange, E.; Hofmann, M.; Leifer, I.; Loeffen, W.; Koenen, F.; Beer, M. Comparative evaluation of live marker vaccine candidates "CP7_E2alf" and "flc11" along with C-strain "Riems" after oral vaccination. Vet. Microbiol. 2012, 158, 42–59. [Google Scholar] [CrossRef] [PubMed]
- Blome, S.; Gabriel, C.; Schmeiser, S.; Meyer, D.; Meindl-Böhmer, A.; Koenen, F.; Beer, M. Efficacy of marker vaccine candidate CP7_E2alf against challenge with classical swine fever virus isolates of different genotypes. Vet. Microbiol. 2014, 169, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Dräger, C.; Petrov, A.; Beer, M.; Teifke, J.P.; Blome, S. Classical swine fever virus marker vaccine strain CP7_E2alf: Shedding and dissemination studies in boars. Vaccine 2015, 33, 3100–3103. [Google Scholar] [CrossRef] [PubMed]
- Dräger, C.; Schröder, C.; König, P.; Tegtmeyer, B.; Beer, M.; Blome, S. Efficacy of Suvaxyn CSF marker (CP7_E2alf) in the presence of pre-existing pestiviral antibodies against bovine viral diarrhea virus type 1. Vaccine 2016, in press. [Google Scholar]
- Eble, P.L.; Geurts, Y.; Quak, S.; Moonen-Leusen, H.W.; Blome, S.; Hofmann, M.A.; Koenen, F.; Beer, M.; Loeffen, W.L. Efficacy of chimeric pestivirus vaccine candidates against classical swine fever: Protection and DIVA characteristics. Vet. Microbiol. 2012, 162, 437–446. [Google Scholar] [CrossRef] [PubMed]
- Eble, P.L.; Quak, S.; Geurts, Y.; Moonen-Leusen, H.W.; Loeffen, W.L. Efficacy of csf vaccine CP7_E2alf in piglets with maternally derived antibodies. Vet. Microbiol. 2014, 174, 27–38. [Google Scholar] [CrossRef] [PubMed]
- Feliziani, F.; Blome, S.; Petrini, S.; Giammarioli, M.; Iscaro, C.; Severi, G.; Convito, L.; Pietschmann, J.; Beer, M.; De Mia, G.M. First assessment of classical swine fever marker vaccine candidate CP7_E2alf for oral immunization of wild boar under field conditions. Vaccine 2014, 32, 2050–2055. [Google Scholar] [CrossRef] [PubMed]
- Gabriel, C.; Blome, S.; Urniza, A.; Juanola, S.; Koenen, F.; Beer, M. Towards licensing of CP7_E2alf as marker vaccine against classical swine fever-duration of immunity. Vaccine 2012, 30, 2928–2936. [Google Scholar] [CrossRef] [PubMed]
- Goller, K.V.; Dräger, C.; Höper, D.; Beer, M.; Blome, S. Classical swine fever virus marker vaccine strain CP7_E2alf: Genetic stability in vitro and in vivo. Arch. Virol. 2015, 160, 3121–3125. [Google Scholar] [CrossRef] [PubMed]
- König, P.; Blome, S.; Gabriel, C.; Reimann, I.; Beer, M. Innocuousness and safety of classical swine fever marker vaccine candidate CP7_E2alf in non-target and target species. Vaccine 2011, 30, 5–8. [Google Scholar] [CrossRef] [PubMed]
- König, P.; Hoffmann, B.; Depner, K.R.; Reimann, I.; Teifke, J.P.; Beer, M. Detection of classical swine fever vaccine virus in blood and tissue samples of pigs vaccinated either with a conventional C-strain vaccine or a modified live marker vaccine. Vet. Microbiol. 2007, 120, 343–351. [Google Scholar] [CrossRef] [PubMed]
- König, P.; Lange, E.; Reimann, I.; Beer, M. CP7_E2alf: A safe and efficient marker vaccine strain for oral immunisation of wild boar against classical swine fever virus (CSFV). Vaccine 2007, 25, 3391–3399. [Google Scholar] [CrossRef] [PubMed]
- Leifer, I.; Lange, E.; Reimann, I.; Blome, S.; Juanola, S.; Duran, J.P.; Beer, M. Modified live marker vaccine candidate CP7_E2alf provides early onset of protection against lethal challenge infection with classical swine fever virus after both intramuscular and oral immunization. Vaccine 2009, 27, 6522–6529. [Google Scholar] [CrossRef] [PubMed]
- Levai, R.; Barna, T.; Fabian, K.; Blome, S.; Belak, K.; Balint, A.; Koenen, F.; Kulcsar, G.; Farsang, A. Pre-registration efficacy study of a novel marker vaccine against classical swine fever on maternally derived antibody negative (MDA-) target animals. Biologicals 2015, 14, 1045–1056. [Google Scholar] [CrossRef] [PubMed]
- Rangelova, D.; Nielsen, J.; Strandbygaard, B.; Koenen, F.; Blome, S.; Uttenthal, A. Efficacy of marker vaccine candidate CP7_E2alf in piglets with maternally derived C-strain antibodies. Vaccine 2012, 30, 6376–6381. [Google Scholar] [CrossRef] [PubMed]
- Reimann, I.; Depner, K.; Trapp, S.; Beer, M. An avirulent chimeric Pestivirus with altered cell tropism protects pigs against lethal infection with classical swine fever virus. Virology 2004, 322, 143–157. [Google Scholar] [CrossRef] [PubMed]
- Renson, P.; Le Dimna, M.; Gabriel, C.; Levai, R.; Blome, S.; Kulcsar, G.; Koenen, F.; Le Potier, M.F. Cytokine and immunoglobulin isotype profiles during CP7_E2alf vaccination against a challenge with the highly virulent Koslov strain of classical swine fever virus. Res. Vet. Sci. 2014, 96, 389–395. [Google Scholar] [CrossRef] [PubMed]
- Farsang, A.; Levai, R.; Barna, T.; Fabian, K.; Blome, S.; Belak, K.; Balint, A.; Koenen, F.; Kulcsar, G. Pre-registration efficacy study of a novel marker vaccine against classical swine fever on maternally derived antibody positive (MDA+) target animals. Biologicals 2017, 45, 85–92. [Google Scholar] [CrossRef] [PubMed]
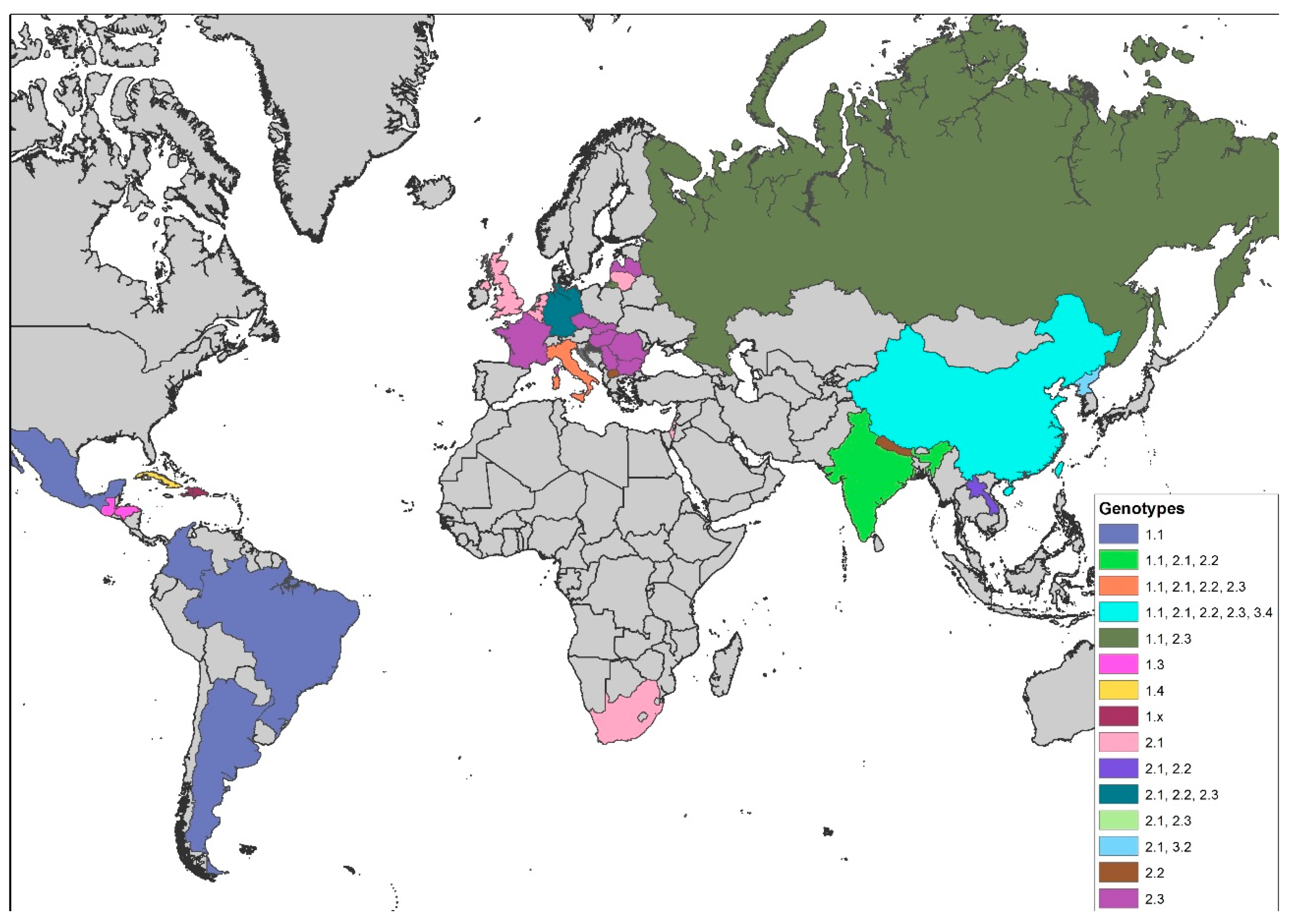


| Country | Last reported CSF outbreak |
|---|---|
| Albania | no reports |
| Armenia | 2006 |
| Azerbaijan | no reports |
| Belarus | no reports |
| Bosnia and Herzegovina | 2007 |
| Bulgaria (wb) | 2009 wb |
| China | 2015 |
| Colombia | 2016 |
| Cuba | 2016 |
| Dominican Republic | 2016 |
| Ecuador | 2016 |
| Macedonia | 2008 |
| Georgia | no reports |
| Hong Kong | 2005 |
| Madagascar | 2016 |
| Moldova | (no reports) |
| Mongolia | 2016 |
| Myanmar | 2015 |
| Peru | 2016 |
| Philippines | 2016 |
| Russia | 2016 |
| Ukraine | 2015 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Blome, S.; Staubach, C.; Henke, J.; Carlson, J.; Beer, M. Classical Swine Fever—An Updated Review. Viruses 2017, 9, 86. https://doi.org/10.3390/v9040086
Blome S, Staubach C, Henke J, Carlson J, Beer M. Classical Swine Fever—An Updated Review. Viruses. 2017; 9(4):86. https://doi.org/10.3390/v9040086
Chicago/Turabian StyleBlome, Sandra, Christoph Staubach, Julia Henke, Jolene Carlson, and Martin Beer. 2017. "Classical Swine Fever—An Updated Review" Viruses 9, no. 4: 86. https://doi.org/10.3390/v9040086




