Development of a Phosphoric Acid-Mediated Hyaluronic Acid Gel Sheet for Efficient Transdermal Delivery of Alendronate for Anti-Osteoporotic Therapy
Abstract
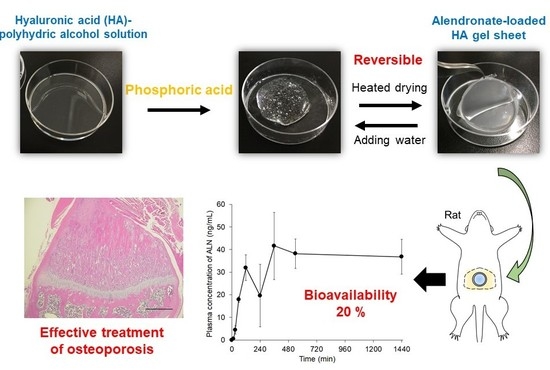
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Animals
2.3. Preparation of ALN-Loaded HA Gel Sheets
2.4. Effect of Phosphoric Acid and Water on HA Gelation
2.5. Skin Irritation After Application of HA Gel Sheets in Rats
2.6. In Vitro Permeation Study Using Rat and Human Skin
2.7. Pharmacokinetics of ALN in Rats
2.8. Effect of ALN-Loaded HA Gel Sheets on Plasma Calcium Levels
2.9. Therapeutic Potential of ALN-Loaded HA Gel Sheets for Treatment of Osteoporosis
2.10. Statistical Analysis
3. Results
3.1. HA Gelation
3.2. Effect of Phosphoric Acid on HA Gelation
3.3. Skin Irritation After Application of HA Gel Sheets to Rat Abdominal Skin
3.4. Skin Permeation of ALN After Application of ALN-Loaded HA Gel Sheets
3.5. Transdermal Absorption of ALN After Application of ALN-Loaded HA Gel Sheets
3.6. Effect of ALN-Loaded HA Gel Sheets on Plasma Calcium Levels in Rats
3.7. Therapeutic Potential of ALN-Loaded HA Gel Sheets for Treatment of Osteoporosis
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Eiken, P.; Vestergaard, P. Treatment of osteoporosis after alendronate or risedronate. Osteoporos. Int. 2016, 27, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Liberman, U.A.; Weiss, S.R.; Bröll, J.; Minne, H.W.; Quan, H.; Bell, N.H.; Rodriguez-Portales, J.; Downs, R.W.; Denqueker, J.; Favus, M.; et al. Effect of oral alendronate on bone mineral density and the incidence of fractures in postmenopausal osteoporosis. N. Engl. J. Med. 1995, 333, 1437–1443. [Google Scholar] [CrossRef] [PubMed]
- Fink, H.A.; MacDonald, R.; Forte, M.L.; Rosebush, C.E.; Ensrud, K.E.; Schousboe, J.T.; Nelson, V.A.; Ullman, K.; Bulter, M.; Olson, C.M.; et al. Long-term drug therapy and drug discontinuations and holidays for osteoporosis fracture prevention: A systematic review. Ann. Intern. Med. 2019, 171, 37–50. [Google Scholar] [CrossRef]
- Lin, J.H.; Duggan, D.E.; Chen, I.W.; Ellsworth, R.L. Physiological disposition of alendronate, a potent anti-osteolytic bisphosphonate, in laboratory animals. Drug Metab. Dispos. 1991, 19, 926–932. [Google Scholar] [PubMed]
- Lichtenberger, L.M.; Romero, J.J.; Gibson, G.W.; Blank, M.A. Effect of bisphosphonates on surface hydrophobicity and phosphatidylcholine concentration of rodent gastric mucosa. Dig. Dis. Sci. 2000, 45, 1792–1801. [Google Scholar] [CrossRef]
- Choi, A.; Gang, H.; Chun, I.; Gwak, H. The effects of fatty acids in propylene glycol on the percutaneous absorption of alendronate across the excised hairless mouse skin. Int. J. Pharm. 2008, 357, 126–131. [Google Scholar] [CrossRef]
- Choi, A.; Gang, H.; Whang, J.; Gwak, H. Pharmacokinetic characteristics of formulated alendronate transdermal delivery systems in rats and humans. Drug Deliv. 2010, 17, 249–254. [Google Scholar] [CrossRef]
- Boche, M.; Pokharkar, V. Positive effect of alendronate on bone turnover in ovariectomised rats’ osteoporosis: Comparison of transdermal lipid-based delivery with conventional oral administration. Drug Deliv. Transl. Res. 2018, 8, 1078–1089. [Google Scholar] [CrossRef]
- Kusamori, K.; Katsumi, H.; Abe, M.; Ueda, A.; Sakai, R.; Hayashi, R.; Hirai, Y.; Quan, Y.S.; Kamiyama, F.; Sakane, T.; et al. Development of a novel transdermal patch of alendronate, a nitrogen-containing bisphosphonate, for the treatment of osteoporosis. J. Bone Miner. Res. 2010, 25, 2306–2315. [Google Scholar] [CrossRef]
- Katsumi, H.; Liu, S.; Tanaka, Y.; Hitomi, K.; Hayashi, R.; Hirai, Y.; Kusamori, K.; Quan, Y.S.; Kamiyama, F.; Sakane, T.; et al. Development of a novel self-dissolving microneedle array of alendronate, a nitrogen-containing bisphosphonate: Evaluation of transdermal absorption, safety, and pharmacological effects after application in rats. J. Pharm. Sci. 2012, 101, 3230–3238. [Google Scholar] [CrossRef]
- Katsumi, H.; Tanaka, Y.; Hitomi, K.; Liu, S.; Quan, Y.S.; Kamiyama, F.; Sakane, T.; Yamamoto, A. Efficient transdermal delivery of alendronate, a nitrogen-containing bisphosphonate, using tip-loaded self-dissolving microneedle arrays for the treatment of osteoporosis. Pharmaceutics 2017, 9, 29. [Google Scholar] [CrossRef] [PubMed]
- Brown, M.; Jones, S. Hyaluronic acid: A unique topical vehicle for the localized delivery of drugs to the skin. J. Eur. Acad. Dermatol. Venereol. 2005, 19, 308–318. [Google Scholar] [CrossRef] [PubMed]
- Makvandi, P.; Ali, G.W.; Sala, F.D.; Abdel-Fattah, W.I.; Borzacchiello, A. Biosynthesis and Characterization of Antibacterial Thermosensitive Hydrogels Based on Corn Silk Extract, Hyaluronic Acid and Nanosilver for Potential Wound Healing. Carbohydr. Polym. 2019, 223, 115023. [Google Scholar] [CrossRef] [PubMed]
- Makvandi, P.; Ali, G.W.; Sala, F.D.; Abdel-Fattah, W.I.; Borzacchiello, A. Hyaluronic acid/corn silk extract based injectable nanocomposite: A biomimetic antibacterial scaffold for bone tissue regeneration. Mat. Sci. Eng. C 2020, 107, 110195. [Google Scholar] [CrossRef]
- Fisher, S.A.; Anandakumaran, P.N.; Owen, S.C.; Shoichet, M.S. Tuning the Microenvironment: Click-Crosslinked Hyaluronic Acid-Based Hydrogels Provide a Platform for Studying Breast Cancer Cell Invasion. Adv. Funct. Mater. 2015, 25, 7163–7172. [Google Scholar] [CrossRef]
- Xu, K.; Narayanan, K.; Lee, F.; Bae, K.H.; Gao, S.; Kurisawa, M. Enzyme-mediated hyaluronic acid-tyramine hydrogels for the propagation of human embryonic stem cells in 3D. Acta Biomater. 2015, 24, 159–171. [Google Scholar] [CrossRef]
- Bermejo-Velasco, D.; Azémar, A.; Oommen, O.P.; Hilborn, J.; Varghese, O.P. Modulating Thiol p Ka Promotes Disulfide Formation at Physiological pH: An Elegant Strategy to Design Disulfide Cross-Linked Hyaluronic Acid Hydrogels. Biomacromolecules 2019, 20, 1412–1420. [Google Scholar] [CrossRef]
- Hennink, W.E.; van Nostrum, C.F. Novel crosslinking methods to design hydrogels. Adv. Drug Deliv. Rev. 2002, 54, 13–36. [Google Scholar] [CrossRef]
- Larrañeta, E.; Henry, M.; Irwin, N.J.; Trotter, J.; Perminova, A.A.; Donnelly, R.F. Synthesis and characterization of hyaluronic acid hydrogels crosslinked using a solvent-free process for potential biomedical applications. Carbohydr. Polym. 2018, 181, 1194–1205. [Google Scholar] [CrossRef]
- Montanari, E.; D’Arrigo, G.; Di Meo, C.; Virga, A.; Coviello, T.; Passariello, C.; Matricardi, P. Chasing bacteria within the cells using levofloxacin-loaded hyaluronic acid nanohydrogels. Eur. J. Pharm. Biopharm. 2014, 87, 518–523. [Google Scholar] [CrossRef]
- Draize, J.H.; Woodard, G.; Calvery, H.O. Methods for the study of irritation and toxicity of substances applied topically to the skin and mucos membranes. J. Pharmacol. Exp. Ther. 1944, 82, 377–390. [Google Scholar]
- OECD Guidelines for the Testing of Chemicals, Section 4. Test No. 404: Acute Dermal Irritation/Corrosion; OECD iLibrary: Paris, French, 2015; Available online: https://www.oecd-ilibrary.org/environment/test-no-404-acute-dermal-irritation-corrosion_9789264242678-en (accessed on 27 October 2019).
- Wong, J.A.; Renton, K.W.; Crocker, J.F.S.; O’Regan, P.A.; Acott, P.D. Determination of pamidronate in human whole blood and urine by reversed-phase HPLC with fluorescence detection. Biomed. Chromatogr. 2004, 18, 98–101. [Google Scholar] [CrossRef] [PubMed]
- Yamaoka, K.; Tanigawara, Y.; Nakagawa, T.; UNO, T. A pharmacokinetic analysis program (multi) for microcomputer. J. Pharm.-Dyn. 1981, 4, 879–885. [Google Scholar] [CrossRef] [PubMed]
- Yamaoka, K.; Nakagawa, T.; Uno, T. Statistical moments in pharmacokinetics. J. Pharmacokinet. Biopharm. 1978, 6, 547–558. [Google Scholar] [CrossRef]
- Xiang, A.; Kanematsu, M.; Mitamura, M.; Kikkawa, H.; Asano, S.; Kinoshita, M. Analysis of Change Patterns of Microcomputed Tomography 3-Dimensional Bone Parameters as a High-Throughput Tool to Evaluate Antiosteoporotic Effects of Agents at an Early Stage of Ovariectomy-Induced Osteoporosis in Mice. Investig. Radiol. 2006, 41, 704–712. [Google Scholar] [CrossRef]
- Ahmed, E.M. Hydrogel: Preparation, characterization, and applications: A review. J. Adv. Res. 2015, 6, 105–121. [Google Scholar] [CrossRef]
- Jung, E.C.; Maibach, H.I. Animal models for percutaneous absorption. In Health Risk Assessment Dermal and Inhalation Exposure and Absorption of Toxicants, 1st ed.; Wang, R.G.M., Knaak, J.B., Maibach, H.I., Eds.; CRC Press: Boca Raton, FL, USA, 2017; pp. 89–103. [Google Scholar] [CrossRef]
- Barber, E.; Teetsel, N.M.; Kolberg, K.F.; Guest, D. A comparative study of the rates of in Vitro percutaneous absorption of eight chemicals using rat and human skin. Toxicol. Sci. 1992, 19, 493–497. [Google Scholar] [CrossRef]
- Schmook, F.P.; Meingassner, J.G.; Billich, A. Comparison of human skin or epidermis models with human and animal skin in in-vitro percutaneous absorption. Int. J. Pharm. 2001, 215, 51–56. [Google Scholar] [CrossRef]
- Hughes, M.F.; Edwards, B.C. In vitro dermal absorption of pyrethroid pesticides in human and rat skin. Toxicol. Appl. Pharmacol. 2010, 246, 29–37. [Google Scholar] [CrossRef]
- Porras, A.G.; Holland, S.D.; Gertz, B.J. Pharmacokinetics of alendronate. Clin. Pharm. 1999, 36, 315–328. [Google Scholar] [CrossRef]
- Mazières, B.; Rouanet, S.; Velicy, J.; Scarsi, C.; Reiner, V. Topical ketoprofen patch (100 mg) for the treatment of ankle sprain: A randomized, double-blind, placebo-controlled study. Am. J. Sports Med. 2005, 33, 515–523. [Google Scholar] [CrossRef] [PubMed]
- Katsumi, H.; Nakatani, M.; Sano, J.; Abe, M.; Kusamori, K.; Kurihara, M.; Shiota, R.; Takashima, M.; Fujita, T.; Sakane, T.; et al. Absorption and safety of alendronate, a nitrogen-containing bisphosphonate; after intrapulmonary administration in rats. Int. J. Pharm. 2010, 400, 124–130. [Google Scholar] [CrossRef] [PubMed]
- Sato, M.; Grasser, W.; Endo, N.; Akins, R.; Simmons, H.; Thompson, D.D.; Golub, E.; Rodan, G.A. Bisphosphonate action: Alendronate localization in rat bone and effects on osteoclast ultrastructure. J. Clin. Investig. 1991, 88, 2095–2105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sahni, M.; Guenther, H.L.; Fleisch, H.; Collin, P.; Martin, T.J. Bisphosphonates act on rat bone resorption through the mediation of osteoblasts. J. Clin. Investig. 1993, 91, 2004–2011. [Google Scholar] [CrossRef] [Green Version]
- Azuma, Y.; Sato, H.; Oue, Y.; Okabe, K.; Ohta, T.; Tsuchimoto, M.; Kiyoki, M. Alendronate distributed on bone surfaces inhibits osteoclastic bone resorption in vitro and in experimental hypercalcemia models. Bone 1995, 16, 235–245. [Google Scholar] [CrossRef]






| Viscosity (Pa·s) | ||
|---|---|---|
| Before heated drying | After heated drying | |
| Phosphoric acid (+) | 7.90 ± 0.93 | out of range |
| Phosphoric acid (−) | 1.45 ± 0.06 | 50.7 ± 2.38 |
| Dose (mg/kg) | Tmax (min) | Cmax (ng/mL) | AUC (μg·min/mL) | BA (%) | |
|---|---|---|---|---|---|
| Intravenous injection | 1 | - | - | 105 ± 12 | - |
| ALN-loaded HA gel sheet | 2.5 | 720 ± 360 | 53 ± 10 | 50 ± 6.5 | 20 ± 2.6 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Naito, C.; Katsumi, H.; Yoneto, K.; Omura, M.; Nishidono, M.; Kamei, S.; Mizoguchi, A.; Tamba, A.; Tanaka, A.; Morishita, M.; et al. Development of a Phosphoric Acid-Mediated Hyaluronic Acid Gel Sheet for Efficient Transdermal Delivery of Alendronate for Anti-Osteoporotic Therapy. Pharmaceutics 2019, 11, 643. https://doi.org/10.3390/pharmaceutics11120643
Naito C, Katsumi H, Yoneto K, Omura M, Nishidono M, Kamei S, Mizoguchi A, Tamba A, Tanaka A, Morishita M, et al. Development of a Phosphoric Acid-Mediated Hyaluronic Acid Gel Sheet for Efficient Transdermal Delivery of Alendronate for Anti-Osteoporotic Therapy. Pharmaceutics. 2019; 11(12):643. https://doi.org/10.3390/pharmaceutics11120643
Chicago/Turabian StyleNaito, Chihiro, Hidemasa Katsumi, Kunio Yoneto, Mao Omura, Mayuko Nishidono, Sachi Kamei, Akiya Mizoguchi, Ayaka Tamba, Akiko Tanaka, Masaki Morishita, and et al. 2019. "Development of a Phosphoric Acid-Mediated Hyaluronic Acid Gel Sheet for Efficient Transdermal Delivery of Alendronate for Anti-Osteoporotic Therapy" Pharmaceutics 11, no. 12: 643. https://doi.org/10.3390/pharmaceutics11120643