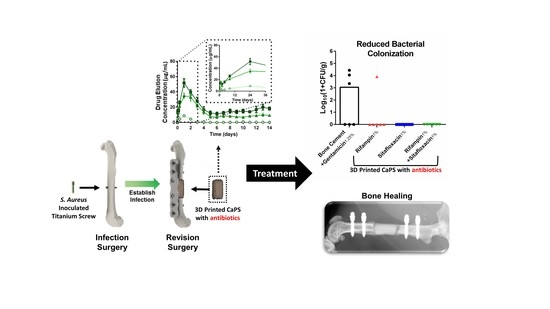
Calcium Phosphate Spacers for the Local Delivery of Sitafloxacin and Rifampin to Treat Orthopedic Infections: Efficacy and Proof of Concept in a Mouse Model of Single-Stage Revision of Device-Associated Osteomyelitis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fabrication of 3D Printed Antibiotic-Laden Calcium Phosphate Acaffolds (CaPS) and Poly (Methyl Methacrylate) PMMA Spacers
2.2. Characterization of Antibiotic Release and Bioactivity
2.3. Biomechanical Properties of 3D Printed Antibiotic-Laden Calcium Phosphate Scaffolds Coated with PLGA
2.4. Animal and Surgical Procedures
2.5. Radiographic Imaging and Quantification
2.6. Bioluminescent Imaging (BLI) of the Bacterial Burden
2.7. Serum C-Reactive Protein (CRP)
2.8. Scanning Electron Microscopy (SEM) of Harvest Titanium Screws
3. Results
3.1. Mechanical Properties of the 3D Printed CaPs
3.2. Rifampin and Sitafloxacin Release Kinetics from 3D Printed CaPS
3.3. In Vivo Efficacy 3D Printed Caps Incorporated with Rifampin and Sitafloxacin 3 Weeks After Post-Revision Surgery
3.4. In Vivo Efficacy of 3D Printed Caps with Incorporated Rifampin and Sitafloxacin 10 Weeks Post-Revision Surgery
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Darouiche, R.O. Treatment of infections associated with surgical implants. N. Engl. J. Med. 2004, 350, 1422–1429. [Google Scholar] [CrossRef] [PubMed]
- Momaya, A.M.; Hlavacek, J.; Etier, B.; Johannesmeyer, D.; Oladeji, L.O.; Niemeier, T.E.; Herrera, N.; Lowe, J.A. Risk factors for infection after operative fixation of Tibial plateau fractures. Injury 2016, 47, 1501–1505. [Google Scholar] [CrossRef] [PubMed]
- Ruffolo, M.R.; Gettys, F.K.; Montijo, H.E.; Seymour, R.B.; Karunakar, M.A. Complications of high-energy bicondylar tibial plateau fractures treated with dual plating through 2 incisions. J. Orthop. Trauma 2015, 29, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Colman, M.; Wright, A.; Gruen, G.; Siska, P.; Pape, H.-C.; Tarkin, I. Prolonged operative time increases infection rate in tibial plateau fractures. Injury 2013, 44, 249–252. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morris, B.J.; Unger, R.Z.; Archer, K.R.; Mathis, S.L.; Perdue, A.M.; Obremskey, W.T. Risk factors of infection after ORIF of bicondylar tibial plateau fractures. J. Orthop. Trauma 2013, 27, e196–e200. [Google Scholar] [CrossRef] [PubMed]
- Springer, B.D.; Cahue, S.; Etkin, C.D.; Lewallen, D.G.; McGrory, B.J. Infection burden in total hip and knee arthroplasties: An international registry-based perspective. Arthroplast. Today 2017, 3, 137–140. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Kang, C.I.; Lee, J.H.; Joung, M.; Moon, S.; Wi, Y.M.; Chung, D.R.; Ha, C.W.; Song, J.H.; Peck, K.R. Risk factors for treatment failure in patients with prosthetic joint infections. J. Hosp. Infect. 2010, 75, 273–276. [Google Scholar] [CrossRef]
- Marculescu, C.E.; Berbari, E.F.; Hanssen, A.D.; Steckelberg, J.M.; Harmsen, S.W.; Mandrekar, J.N.; Osmon, D.R. Outcome of prosthetic joint infections treated with debridement and retention of components. Clin. Infect. Dis. 2006, 42, 471–478. [Google Scholar] [CrossRef]
- Parvizi, J.; Pawasarat, I.M.; Azzam, K.A.; Joshi, A.; Hansen, E.N.; Bozic, K.J. Periprosthetic Joint Infection: The Economic Impact of Methicillin-Resistant Infections. The J. Arthroplast. 2010, 25 (Suppl. 6), 103–107. [Google Scholar] [CrossRef]
- Grammatico-Guillon, L.; Baron, S.; Gettner, S.; Lecuyer, A.I.; Gaborit, C.; Rosset, P.; Rusch, E.; Bernard, L. Bone and joint infections in hospitalized patients in France, 2008: Clinical and economic outcomes. J. Hosp. Infect. 2012, 82, 40–48. [Google Scholar] [CrossRef]
- National Joint Registry for England. Wales and Northern Ireland: 11th Annual Report; NJR Centre: Hemel Hempstead, UK, 2014. [Google Scholar]
- Osmon, D.R.; Berbari, E.F.; Berendt, A.R.; Lew, D.; Zimmerli, W.; Steckelberg, J.M.; Rao, N.; Hanssen, A.; Wilson, W.R. Diagnosis and Management of Prosthetic Joint Infection: Clinical Practice Guidelines by the Infectious Diseases Society of America. Clin. Infect. Dis. 2013, 56, e1–e25. [Google Scholar] [CrossRef]
- Brandt, C.M.; Sistrunk, W.W.; Duffy, M.C.; Hanssen, A.D.; Steckelberg, J.M.; Ilstrup, D.M.; Osmon, D.R. Staphylococcus aureus Prosthetic Joint Infection Treated with Debridement and Prosthesis Retention. Clin. Infect. Dis. 1997, 24, 914–919. [Google Scholar] [CrossRef] [PubMed]
- Del Pozo, J.L.; Patel, R. Infection Associated with Prosthetic Joints. N. Engl. J. Med. 2009, 361, 787–794. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Strange, S.; Whitehouse, M.R.; Beswick, A.D.; Board, T.; Burston, A.; Burston, B.; Carroll, F.E.; Dieppe, P.; Garfield, K.; Gooberman-Hill, R.; et al. One-stage or two-stage revision surgery for prosthetic hip joint infection—The INFORM trial: A study protocol for a randomised controlled trial. Trials 2016, 17, 90. [Google Scholar] [CrossRef] [PubMed]
- Mohapatra, N.; Jain, S. Antibiotic laden bone cement in chronic osteomyelitis. J. Orthop. Traumatol. Rehabil. 2017, 9, 74–77. [Google Scholar] [CrossRef]
- Metsemakers, W.J.; Kuehl, R.; Moriarty, T.F.; Richards, R.G.; Verhofstad, M.H.J.; Borens, O.; Kates, S.; Morgenstern, M. Infection after fracture fixation: Current surgical and microbiological concepts. Injury 2018, 49, 511–522. [Google Scholar] [CrossRef] [PubMed]
- Isefuku, S.; Joyner, C.J.; Simpson, A.H. Toxic effect of rifampicin on human osteoblast-like cells. J. Orthop. Res. 2001, 19, 950–954. [Google Scholar] [CrossRef] [Green Version]
- Blajchman, M.A.; Lowry, R.C.; Pettit, J.E.; Stradling, P. Rifampicin-induced immune thrombocytopenia. Br. Med. J. 1970, 3, 24–26. [Google Scholar] [CrossRef]
- Herrmann, M.; Vaudaux, P.E.; Pittet, D.; Auckenthaler, R.; Lew, P.D.; Perdreau, F.S.; Peters, G.; Waldvogel, F.A. Fibronectin, fibrinogen, and laminin act as mediators of adherence of clinical staphylococcal isolates to foreign material. J. Infect. Dis. 1988, 158, 693–701. [Google Scholar] [CrossRef]
- Brady, R.A.; Leid, J.G.; Calhoun, J.H.; Costerton, J.W.; Shirtliff, M.E. Osteomyelitis and the role of biofilms in chronic infection. FEMS Immunol. Med. Microbiol. 2008, 52, 13–22. [Google Scholar] [CrossRef] [Green Version]
- Lewis, K. Multidrug tolerance of biofilms and persister cells. In Bacterial Biofilms; Romeo, T., Ed.; Springer: Berlin/Heidelberg, Germany, 2008; pp. 107–131. [Google Scholar]
- Kostakioti, M.; Hadjifrangiskou, M.; Hultgren, S.J. Bacterial biofilms: Development, dispersal, and therapeutic strategies in the dawn of the postantibiotic era. Cold Spring Harb. Perspect. Med. 2013, 3, a010306. [Google Scholar] [CrossRef] [PubMed]
- Garcia, L.G.; Lemaire, S.; Kahl, B.C.; Becker, K.; Proctor, R.A.; Denis, O.; Tulkens, P.M.; Van Bambeke, F. Antibiotic activity against small-colony variants of Staphylococcus aureus: Review of in vitro, animal and clinical data. J. Antimicrob. Chemother. 2013, 68, 1455–1464. [Google Scholar] [CrossRef] [PubMed]
- Proctor, R.A.; van Langevelde, P.; Kristjansson, M.; Maslow, J.N.; Arbeit, R.D. Persistent and relapsing infections associated with small-colony variants of Staphylococcus aureus. Clin. Infect. Dis. 1995, 20, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Tuchscherr, L.; Medina, E.; Hussain, M.; Volker, W.; Heitmann, V.; Niemann, S.; Holzinger, D.; Roth, J.; Proctor, R.A.; Becker, K.; et al. Staphylococcus aureus phenotype switching: An effective bacterial strategy to escape host immune response and establish a chronic infection. EMBO Mol. Med. 2011, 3, 129–141. [Google Scholar] [CrossRef] [PubMed]
- Tande, A.J.; Osmon, D.R.; Greenwood-Quaintance, K.E.; Mabry, T.M.; Hanssen, A.D.; Patel, R. Clinical Characteristics and Outcomes of Prosthetic Joint Infection Caused by Small Colony Variant Staphylococci. mBio 2014, 5, e01910-14. [Google Scholar] [CrossRef] [PubMed]
- Proctor, R.A. Microbial Pathogenic Factors: Small-Colony Variants. In Infections Associated with Indwelling Medical Devices, 3rd ed.; American Society of Microbiology: Washington, DC, USA, 2000. [Google Scholar]
- Gallie, W.E. First recurrence of osteomyelitis eighty years after infection. J. Bone Jt. Surg. Br. 1951, 33-b, 110–111. [Google Scholar] [CrossRef]
- Anagnostakos, K.; Wilmes, P.; Schmitt, E.; Kelm, J. Elution of gentamicin and vancomycin from polymethylmethacrylate beads and hip spacers in vivo. Acta Orthop. 2009, 80, 193–197. [Google Scholar] [CrossRef] [Green Version]
- Moojen, D.J.; Hentenaar, B.; Charles Vogely, H.; Verbout, A.J.; Castelein, R.M.; Dhert, W.J. In vitro release of antibiotics from commercial PMMA beads and articulating hip spacers. J. Arthroplast. 2008, 23, 1152–1156. [Google Scholar] [CrossRef]
- Jiranek, W.A.; Hanssen, A.D.; Greenwald, A.S. Antibiotic-Loaded Bone Cement for Infection Prophylaxis in Total Joint Replacement. JBJS 2006, 88, 2487–2500. [Google Scholar] [CrossRef]
- Lewis, G. Properties of antibiotic-loaded acrylic bone cements for use in cemented arthroplasties: A state-of-the-art review. J. Biomed. Mater. Res. Part B Appl. Biomater. 2009, 89B, 558–574. [Google Scholar] [CrossRef]
- Ma, D.; Shanks, R.M.Q.; Davis, C.M.; Craft, D.W.; Wood, T.K.; Hamlin, B.R.; Urish, K.L. Viable bacteria persist on antibiotic spacers following two-stage revision for periprosthetic joint infection. J. Orthop. Res. 2018, 36, 452–458. [Google Scholar] [CrossRef] [PubMed]
- Shiels, S.M.; Tennent, D.J.; Akers, K.S.; Wenke, J.C. Determining potential of PMMA as a depot for rifampin to treat recalcitrant orthopaedic infections. Injury 2017, 48, 2095–2100. [Google Scholar] [CrossRef] [PubMed]
- Inzana, J.A.; Trombetta, R.P.; Schwarz, E.M.; Kates, S.L.; Awad, H.A. 3D printed bioceramics for dual antibiotic delivery to treat implant-associated bone infection. Eur. Cell Mater. 2015, 30, 232–247. [Google Scholar] [CrossRef] [Green Version]
- Wu, S.; Liu, X.; Yeung, K.W.K.; Liu, C.; Yang, X. Biomimetic porous scaffolds for bone tissue engineering. Mater. Sci. Eng. R Rep. 2014, 80, 1–36. [Google Scholar] [CrossRef]
- Habraken, W.; Habibovic, P.; Epple, M.; Bohner, M. Calcium phosphates in biomedical applications: Materials for the future? Mater. Today 2016, 19, 69–87. [Google Scholar] [CrossRef]
- Inzana, J.A.; Olvera, D.; Fuller, S.M.; Kelly, J.P.; Graeve, O.A.; Schwarz, E.M.; Kates, S.L.; Awad, H.A. 3D printing of composite calcium phosphate and collagen scaffolds for bone regeneration. Biomaterials 2014, 35, 4026–4034. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanchez, C.J., Jr.; Shiels, S.M.; Tennent, D.J.; Hardy, S.K.; Murray, C.K.; Wenke, J.C. Rifamycin Derivatives Are Effective Against Staphylococcal Biofilms In Vitro and Elutable From PMMA. Clin. Orthop. Relat. Res. 2015, 473, 2874–2884. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trombetta, R.P.; Dunman, P.M.; Schwarz, E.M.; Kates, S.L.; Awad, H.A. A High-Throughput Screening Approach to Repurpose FDA-Approved Drugs for Bactericidal Applications against Staphylococcus aureus Small-Colony Variants. mSphere 2018, 3, e00422-18. [Google Scholar] [CrossRef]
- Trombetta, R.P.; de Mesy Bentley, K.L.; Schwarz, E.M.; Kates, S.L.; Awad, H.A. A murine femoral ostectomy model with hardware exchange to assess antibiotic-impregnated spacers for implant-associated osteomyelitis. Eur. Cell Mater. 2019. accepted. [Google Scholar]
- Reynolds, D.G.; Hock, C.; Shaikh, S.; Jacobson, J.; Zhang, X.; Rubery, P.T.; Beck, C.A.; O’Keefe, R.J.; Lerner, A.L.; Schwarz, E.M.; Awad, H.A. Micro-computed tomography prediction of biomechanical strength in murine structural bone grafts. J. Biomech. 2007, 40, 3178–3186. [Google Scholar] [CrossRef]
- Varrone, J.J.; de Mesy Bentley, K.L.; Bello-Irizarry, S.N.; Nishitani, K.; Mack, S.; Hunter, J.G.; Kates, S.L.; Daiss, J.L.; Schwarz, E.M. Passive Immunization with Anti-Glucosaminidase Monoclonal Antibodies Protects Mice from Implant-Associated Osteomyelitis by Mediating Opsonophagocytosis of Staphylococcus aureus Megaclusters. J. Orthop. Res. 2014, 32, 1389–1396. [Google Scholar] [CrossRef] [PubMed]
- Rathbone, C.R.; Cross, J.D.; Brown, K.V.; Murray, C.K.; Wenke, J.C. Effect of various concentrations of antibiotics on osteogenic cell viability and activity. J. Orthop. Res. 2011, 29, 1070–1074. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vergidis, P.; Rouse, M.S.; Euba, G.; Karau, M.J.; Schmidt, S.M.; Mandrekar, J.N.; Steckelberg, J.M.; Patel, R. Treatment with Linezolid or Vancomycin in Combination with Rifampin Is Effective in an Animal Model of Methicillin-Resistant Staphylococcus aureus Foreign Body Osteomyelitis. Antimicrob. Agents Chemother. 2011, 55, 1182–1186. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Bayer, A.; Cosgrove, S.E.; Daum, R.S.; Fridkin, S.K.; Gorwitz, R.J.; Kaplan, S.L.; Karchmer, A.W.; Levine, D.P.; Murray, B.E.; et al. Clinical practice guidelines by the infectious diseases society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children. Clin. Infect. Dis. 2011, 52, e18–e55. [Google Scholar] [CrossRef] [PubMed]
- Gogia, J.S.; Meehan, J.P.; Di Cesare, P.E.; Jamali, A.A. Local Antibiotic Therapy in Osteomyelitis. Semin. Plast. Surg. 2009, 23, 100–107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nelson, C.L. The current status of material used for depot delivery of drugs. Clin. Orthop. Relat. Res. 2004, 427, 72–78. [Google Scholar] [CrossRef]
- McLaren, A.C.; Nugent, M.; Economopoulos, K.; Kaul, H.; Vernon, B.L.; McLemore, R. Hand-mixed and Premixed Antibiotic-loaded Bone Cement Have Similar Homogeneity. Clin. Orthop. Relat. Res. 2009, 467, 1693–1698. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van de Belt, H.; Neut, D.; Schenk, W.; van Horn, J.R.; van der Mei, H.C.; Busscher, H.J. Gentamicin release from polymethylmethacrylate bone cements and Staphylococcus aureus biofilm formation. Acta Orthop. Scand. 2000, 71, 625–629. [Google Scholar] [CrossRef]
- Penner, M.J.; Masri, B.A.; Duncan, C.P. Elution characteristics of vancomycin and tobramycin combined in acrylic bone-cement. J. Arthroplast. 1996, 11, 939–944. [Google Scholar] [CrossRef]
- Penner, M.J.; Duncan, C.P.; Masri, B.A. The in vitro elution characteristics of antibiotic-loaded CMW and Palacos-R bone cements. J. Arthroplast. 1999, 14, 209–214. [Google Scholar] [CrossRef]
- Mariconda, M.; Ascione, T.; Balato, G.; Rotondo, R.; Smeraglia, F.; Costa, G.G.; Conte, M. Sonication of antibiotic-loaded cement spacers in a two-stage revision protocol for infected joint arthroplasty. BMC Musculoskelet. Disord. 2013, 14, 193. [Google Scholar] [CrossRef] [PubMed]
- Schmolders, J.; Hischebeth, G.T.; Friedrich, M.J.; Randau, T.M.; Wimmer, M.D.; Kohlhof, H.; Molitor, E.; Gravius, S. Evidence of MRSE on a gentamicin and vancomycin impregnated polymethyl-methacrylate (PMMA) bone cement spacer after two-stage exchange arthroplasty due to periprosthetic joint infection of the knee. BMC Infect. Dis. 2014, 14, 144. [Google Scholar] [CrossRef] [PubMed]
- Wehrli, W. Rifampin: Mechanisms of action and resistance. Rev. Infect. Dis. 1983, 5 (Suppl. 3), S407–S411. [Google Scholar] [CrossRef] [PubMed]
- Beeching, N.J.; Thomas, M.G.; Roberts, S.; Lang, S.D. Comparative in-vitro activity of antibiotics incorporated in acrylic bone cement. J. Antimicrob. Chemother. 1986, 17, 173–184. [Google Scholar] [CrossRef] [PubMed]
- Akasaka, T.; Kurosaka, S.; Uchida, Y.; Tanaka, M.; Sato, K.; Hayakawa, I. Antibacterial Activities and Inhibitory Effects of Sitafloxacin (DU-6859a) and Its Optical Isomers against Type II Topoisomerases. Antimicrob. Agents Chemother. 1998, 42, 1284–1287. [Google Scholar] [CrossRef] [PubMed]
- Makadia, H.K.; Siegel, S.J. Poly Lactic-co-Glycolic Acid (PLGA) as Biodegradable Controlled Drug Delivery Carrier. Polymers 2011, 3, 1377–1397. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hutmacher, D.W. Scaffolds in tissue engineering bone and cartilage. Biomaterials 2000, 21, 2529–2543. [Google Scholar] [CrossRef]
- Kaigler, D.; Wang, Z.; Horger, K.; Mooney, D.J.; Krebsbach, P.H. VEGF Scaffolds Enhance Angiogenesis and Bone Regeneration in Irradiated Osseous Defects. J. Bone Miner. Res. 2009, 21, 735–744. [Google Scholar] [CrossRef]
- Bertoldi, C.; Zaffe, D.; Consolo, U. Polylactide/polyglycolide copolymer in bone defect healing in humans. Biomaterials 2008, 29, 1817–1823. [Google Scholar] [CrossRef]
- Ruhe, P.Q.; Hedberg-Dirk, E.L.; Padron, N.T.; Spauwen, P.H.; Jansen, J.A.; Mikos, A.G. Porous poly(DL-lactic-co-glycolic acid)/calcium phosphate cement composite for reconstruction of bone defects. Tissue Eng. 2006, 12, 789–800. [Google Scholar] [CrossRef]
- Kang, S.-W.; Yang, H.S.; Seo, S.-W.; Han, D.K.; Kim, B.-S. Apatite-coated poly(lactic-co-glycolic acid) microspheres as an injectable scaffold for bone tissue engineering. J. Biomed. Mater. Res. Part A 2008, 85A, 747–756. [Google Scholar] [CrossRef] [PubMed]
- Castilho, M.; Rodrigues, J.; Pires, I.; Gouveia, B.; Pereira, M.; Moseke, C.; Groll, J.; Ewald, A.; Vorndran, E. Fabrication of individual alginate-TCP scaffolds for bone tissue engineering by means of powder printing. Biofabrication 2015, 7, 015004. [Google Scholar] [CrossRef] [PubMed]
- Suwanprateeb, J.; Sanngam, R.; Suvannapruk, W.; Panyathanmaporn, T. Mechanical and in vitro performance of apatite–wollastonite glass ceramic reinforced hydroxyapatite composite fabricated by 3D-printing. J. Mater. Sci. Mater. Med. 2009, 20, 1281. [Google Scholar] [CrossRef] [PubMed]
- Klammert, U.; Gbureck, U.; Vorndran, E.; Rodiger, J.; Meyer-Marcotty, P.; Kubler, A.C. 3D powder printed calcium phosphate implants for reconstruction of cranial and maxillofacial defects. J. Cranio-Maxillofac. Surg. 2010, 38, 565–570. [Google Scholar] [CrossRef] [PubMed]
- Vella, J.B.; Trombetta, R.P.; Hoffman, M.D.; Inzana, J.; Awad, H.; Benoit, D.S.W. Three dimensional printed calcium phosphate and poly(caprolactone) composites with improved mechanical properties and preserved microstructure. J. Biomed. Mater. Res. A 2018, 106, 663–672. [Google Scholar] [CrossRef] [PubMed]
- Roohani-Esfahani, S.-I.; Newman, P.; Zreiqat, H. Design and Fabrication of 3D printed Scaffolds with a Mechanical Strength Comparable to Cortical Bone to Repair Large Bone Defects. Sci. Rep. 2016, 6, 19468. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suwanprateeb, J.; Sanngam, R.; Suwanpreuk, W. Fabrication of bioactive hydroxyapatite/bis-GMA based composite via three dimensional printing. J. Mater. Sci. Mater. Med. 2008, 19, 2637–2645. [Google Scholar] [CrossRef] [PubMed]
- Gullberg, E.; Cao, S.; Berg, O.G.; Ilbäck, C.; Sandegren, L.; Hughes, D.; Andersson, D.I. Selection of resistant bacteria at very low antibiotic concentrations. PLoS Pathog. 2011, 7, e1002158. [Google Scholar] [CrossRef]
- Inzana, J.A.; Schwarz, E.M.; Kates, S.L.; Awad, H.A. A novel murine model of established Staphylococcal bone infection in the presence of a fracture fixation plate to study therapies utilizing antibiotic-laden spacers after revision surgery. Bone 2015, 72, 128–136. [Google Scholar] [CrossRef] [Green Version]
- Yokogawa, N.; Ishikawa, M.; Nishitani, K.; Beck, C.A.; Tsuchiya, H.; Mesfin, A.; Kates, S.L.; Daiss, J.L.; Xie, C.; Schwarz, E.M. Immunotherapy synergizes with debridement and antibiotic therapy in a murine 1-stage exchange model of MRSA implant-associated osteomyelitis. J. Orthop. Res. 2018, 36, 1590–1598. [Google Scholar] [CrossRef]
- Kunutsor, S.K.; Whitehouse, M.R.; Blom, A.W.; Beswick, A.D.; Team, I. Re-Infection Outcomes following One- and Two-Stage Surgical Revision of Infected Hip Prosthesis: A Systematic Review and Meta-Analysis. PLoS ONE 2015, 10, e0139166. [Google Scholar] [CrossRef] [PubMed]
- Westrich, G.H.; Walcott-Sapp, S.; Bornstein, L.J.; Bostrom, M.P.; Windsor, R.E.; Brause, B.D. Modern Treatment of Infected Total Knee Arthroplasty with a 2-Stage Reimplantation Protocol. J. Arthroplast. 2010, 25, 1015–1021.e2. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Sotelo, J.; Berry, D.J.; Hanssen, A.D.; Cabanela, M.E. Midterm to Long-term Followup of Staged Reimplantation for Infected Hip Arthroplasty. Clin. Orthop. Relat. Res. 2009, 467, 219–224. [Google Scholar] [CrossRef] [PubMed]
- Shiels, S.M.; Tennent, D.J.; Lofgren, A.L.; Wenke, J.C. Topical rifampin powder for orthopaedic trauma part II: Topical rifampin allows for spontaneous bone healing in sterile and contaminated wounds. J. Orthop. Res. 2018, 36, 3142–3150. [Google Scholar] [CrossRef] [PubMed]
- Huddleston, P.M.; Steckelberg, J.M.; Hanssen, A.D.; Rouse, M.S.; Bolander, M.E.; Patel, R. Ciprofloxacin inhibition of experimental fracture healing. J. Bone Jt. Surg. Am. 2000, 82, 161–173. [Google Scholar] [CrossRef]
- Perry, A.C.; Prpa, B.; Rouse, M.S.; Piper, K.E.; Hanssen, A.D.; Steckelberg, J.M.; Patel, R. Levofloxacin and trovafloxacin inhibition of experimental fracture-healing. Clin. Orthop. Relat. Res. 2003, 414, 95–100. [Google Scholar] [CrossRef] [PubMed]
- He, F.; Chen, Y.; Li, J.; Lin, B.; Ouyang, Y.; Yu, B.; Xia, Y.; Yu, B.; Ye, J. Improving bone repair of femoral and radial defects in rabbit by incorporating PRP into PLGA/CPC composite scaffold with unidirectional pore structure. J. Biomed. Mater. Res. Part A 2015, 103, 1312–1324. [Google Scholar] [CrossRef] [PubMed]
- Goshima, K.; Nakase, J.; Xu, Q.; Matsumoto, K.; Tsuchiya, H. Repair of segmental bone defects in rabbit tibia promoted by a complex of β-tricalcium phosphate and hepatocyte growth factor. J. Orthop. Sci. 2012, 17, 639–648. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hollinger, J.O.; Onikepe, A.O.; MacKrell, J.; Einhorn, T.; Bradica, G.; Lynch, S.; Hart, C.E. Accelerated fracture healing in the geriatric, osteoporotic rat with recombinant human platelet-derived growth factor-bb and an injectable beta-tricalcium phosphate/collagen matrix. J. Orthop. Res. 2008, 26, 83–90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Street, J.; Bao, M.; deGuzman, L.; Bunting, S.; Peale, F.V.; Ferrara, N.; Steinmetz, H.; Hoeffel, J.; Cleland, J.L.; Daugherty, A.; et al. Vascular endothelial growth factor stimulates bone repair by promoting angiogenesis and bone turnover. Proc. Natl. Acad. Sci. USA 2002, 99, 9656–9661. [Google Scholar] [CrossRef] [Green Version]
- Mendes, D., Jr.; Domingues, J.A.; Hausen, M.A.; Cattani, S.M.M.; Aragones, A.; Oliveira, A.L.R.; Inácio, R.F.; Barbo, M.L.P.; Duek, E.A.R. Study of Mesenchymal Stem Cells Cultured on a Poly(Lactic-co-Glycolic Acid) Scaffold Containing Simvastatin for Bone Healing. J. Appl. Biomater. Funct. Mater. 2017, 15, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Shuqiang, M.; Kunzheng, W.; Xiaoqiang, D.; Wei, W.; Mingyu, Z.; Daocheng, W. Osteogenic growth peptide incorporated into PLGA scaffolds accelerates healing of segmental long bone defects in rabbits. J. Plast. Reconstr. Aesthet. Surg. 2008, 61, 1558–1560. [Google Scholar] [CrossRef] [PubMed]
- Namikawa, T.; Terai, H.; Suzuki, E.; Hoshino, M.; Toyoda, H.; Nakamura, H.; Miyamoto, S.; Takahashi, N.; Ninomiya, T.; Takaoka, K. Experimental Spinal Fusion with Recombinant Human Bone Morphogenetic Protein-2 Delivered by a Synthetic Polymer and β-Tricalcium Phosphate in a Rabbit Model. Spine 2005, 30, 1717–1722. [Google Scholar] [CrossRef] [PubMed]
- Minier, K.; Touré, A.; Fusellier, M.; Fellah, B.; Bouvy, B.; Weiss, P.; Gauthier, O. BMP-2 delivered from a self-cross-linkable CaP/hydrogel construct promotes bone regeneration in a critical-size segmental defect model of non-union in dogs. Vet. Comp. Orthop. Traumatol. 2014, 27, 411–421. [Google Scholar] [PubMed]
- Chen, Y.; Xu, J.; Huang, Z.; Yu, M.; Zhang, Y.; Chen, H.; Ma, Z.; Liao, H.; Hu, J. An Innovative Approach for Enhancing Bone Defect Healing Using PLGA Scaffolds Seeded with Extracorporeal-shock-wave-treated Bone Marrow Mesenchymal Stem Cells (BMSCs). Sci. Rep. 2017, 7, 44130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martinez-Vazquez, F.J.; Cabanas, M.V.; Paris, J.L.; Lozano, D.; Vallet-Regi, M. Fabrication of novel Si-doped hydroxyapatite/gelatine scaffolds by rapid prototyping for drug delivery and bone regeneration. Acta Biomater. 2015, 15, 200–209. [Google Scholar] [CrossRef] [Green Version]
- Hawkshead, J.J., 3rd; Patel, N.B.; Steele, R.W.; Heinrich, S.D. Comparative severity of pediatric osteomyelitis attributable to methicillin-resistant versus methicillin-sensitive Staphylococcus aureus. J. Pediatr. Orthop. 2009, 29, 85–90. [Google Scholar] [CrossRef]
- Cosgrove, S.E.; Sakoulas, G.; Perencevich, E.N.; Schwaber, M.J.; Karchmer, A.W.; Carmeli, Y. Comparison of mortality associated with methicillin-resistant and methicillin-susceptible Staphylococcus aureus bacteremia: A meta-analysis. Clin. Infect. Dis. 2003, 36, 53–59. [Google Scholar] [CrossRef]
- Govender, S.; Csimma, C.; Genant, H.K.; Valentin-Opran, A.; Amit, Y.; Arbel, R.; Aro, H.; Atar, D.; Bishay, M.; Börner, M.G.; et al. Recombinant Human Bone Morphogenetic Protein-2 for Treatment of Open Tibial Fractures: A Prospective, Controlled, Randomized Study of Four Hundred and Fifty Patients. JBJS 2002, 84, 2123–2134. [Google Scholar] [CrossRef]
- Kraus, K.H.; Kirker-Head, C. Mesenchymal Stem Cells and Bone Regeneration. Vet. Surg. 2006, 35, 232–242. [Google Scholar] [CrossRef]
- Bostrom, M.P.G.; Seigerman, D.A. The Clinical Use of Allografts, Demineralized Bone Matrices, Synthetic Bone Graft Substitutes and Osteoinductive Growth Factors: A Survey Study. HSS J. 2005, 1, 9–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]







© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trombetta, R.P.; Ninomiya, M.J.; El-Atawneh, I.M.; Knapp, E.K.; de Mesy Bentley, K.L.; Dunman, P.M.; Schwarz, E.M.; Kates, S.L.; Awad, H.A. Calcium Phosphate Spacers for the Local Delivery of Sitafloxacin and Rifampin to Treat Orthopedic Infections: Efficacy and Proof of Concept in a Mouse Model of Single-Stage Revision of Device-Associated Osteomyelitis. Pharmaceutics 2019, 11, 94. https://doi.org/10.3390/pharmaceutics11020094
Trombetta RP, Ninomiya MJ, El-Atawneh IM, Knapp EK, de Mesy Bentley KL, Dunman PM, Schwarz EM, Kates SL, Awad HA. Calcium Phosphate Spacers for the Local Delivery of Sitafloxacin and Rifampin to Treat Orthopedic Infections: Efficacy and Proof of Concept in a Mouse Model of Single-Stage Revision of Device-Associated Osteomyelitis. Pharmaceutics. 2019; 11(2):94. https://doi.org/10.3390/pharmaceutics11020094
Chicago/Turabian StyleTrombetta, Ryan P., Mark J. Ninomiya, Ihab M. El-Atawneh, Emma K. Knapp, Karen L. de Mesy Bentley, Paul M. Dunman, Edward M. Schwarz, Stephen L. Kates, and Hani A. Awad. 2019. "Calcium Phosphate Spacers for the Local Delivery of Sitafloxacin and Rifampin to Treat Orthopedic Infections: Efficacy and Proof of Concept in a Mouse Model of Single-Stage Revision of Device-Associated Osteomyelitis" Pharmaceutics 11, no. 2: 94. https://doi.org/10.3390/pharmaceutics11020094