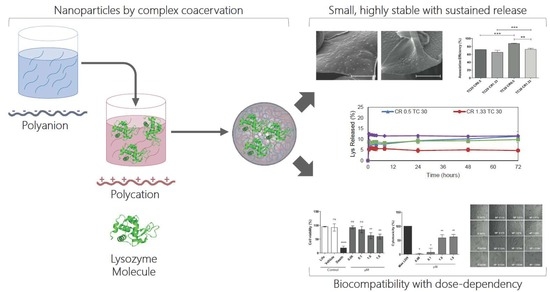
Development of Novel EE/Alginate Polyelectrolyte Complex Nanoparticles for Lysozyme Delivery: Physicochemical Properties and In Vitro Safety
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Nanoparticles
2.3. NP Physicochemical Characterization
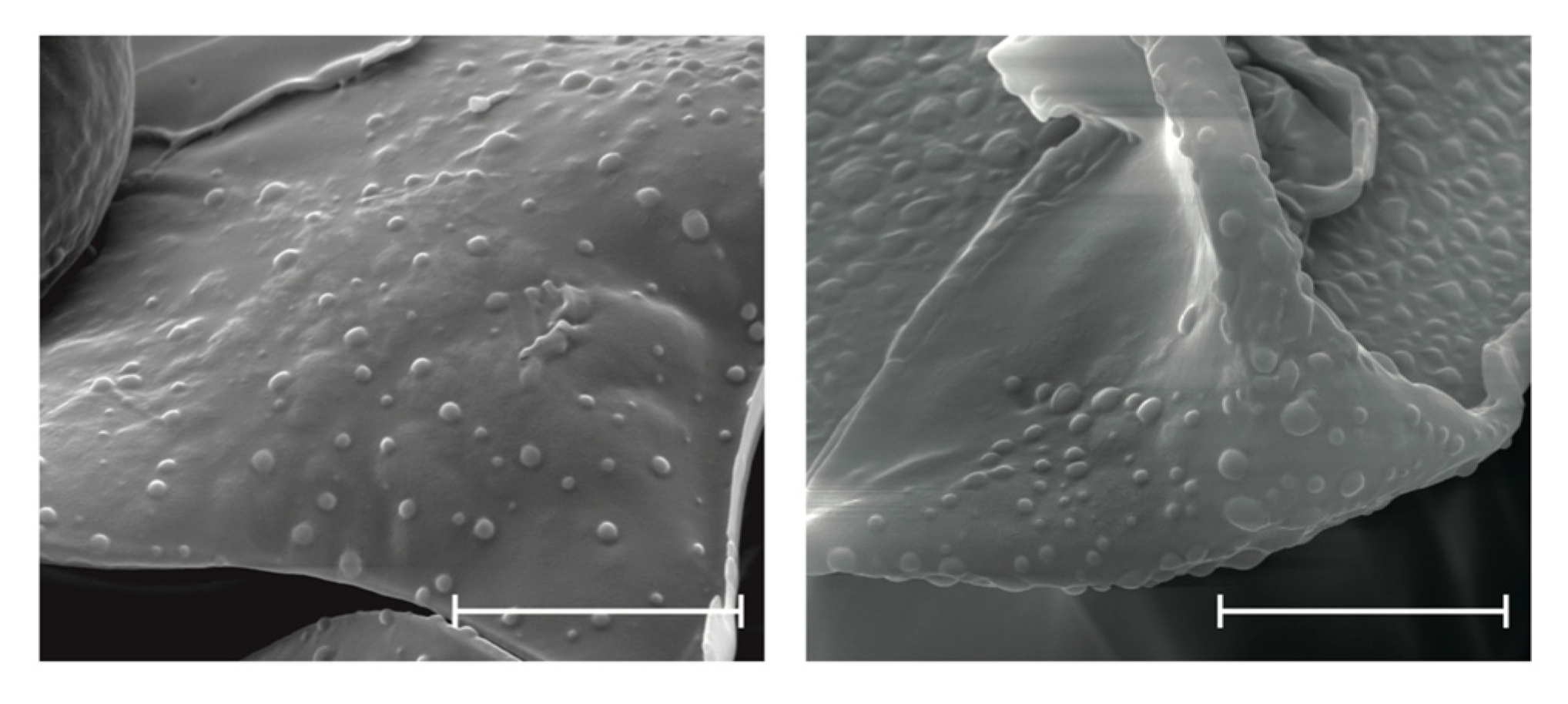
2.4. Scanning Electron Microscopy (SEM)
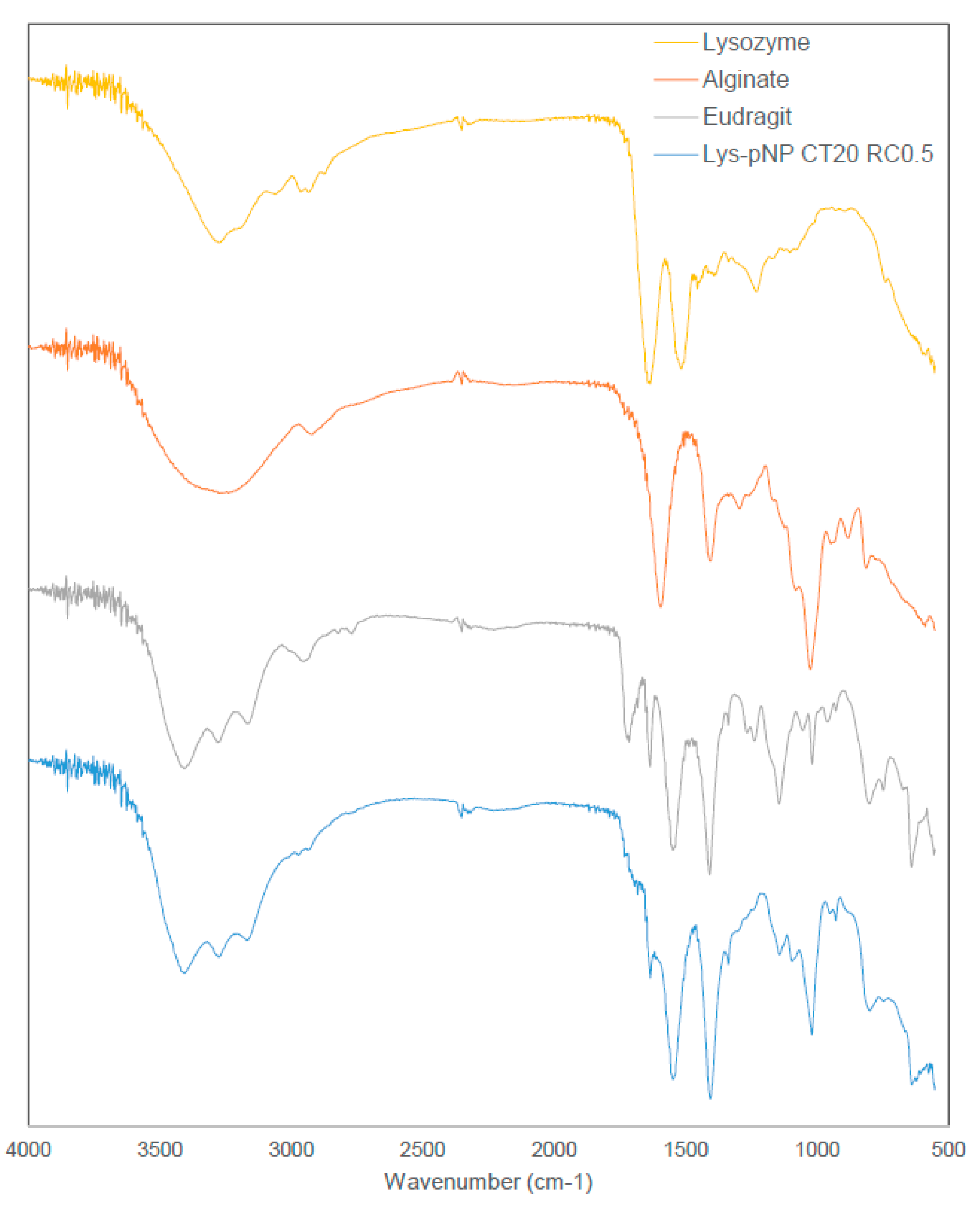
2.5. Fourier Transform Infrared Spectroscopy (FT-IR) Study
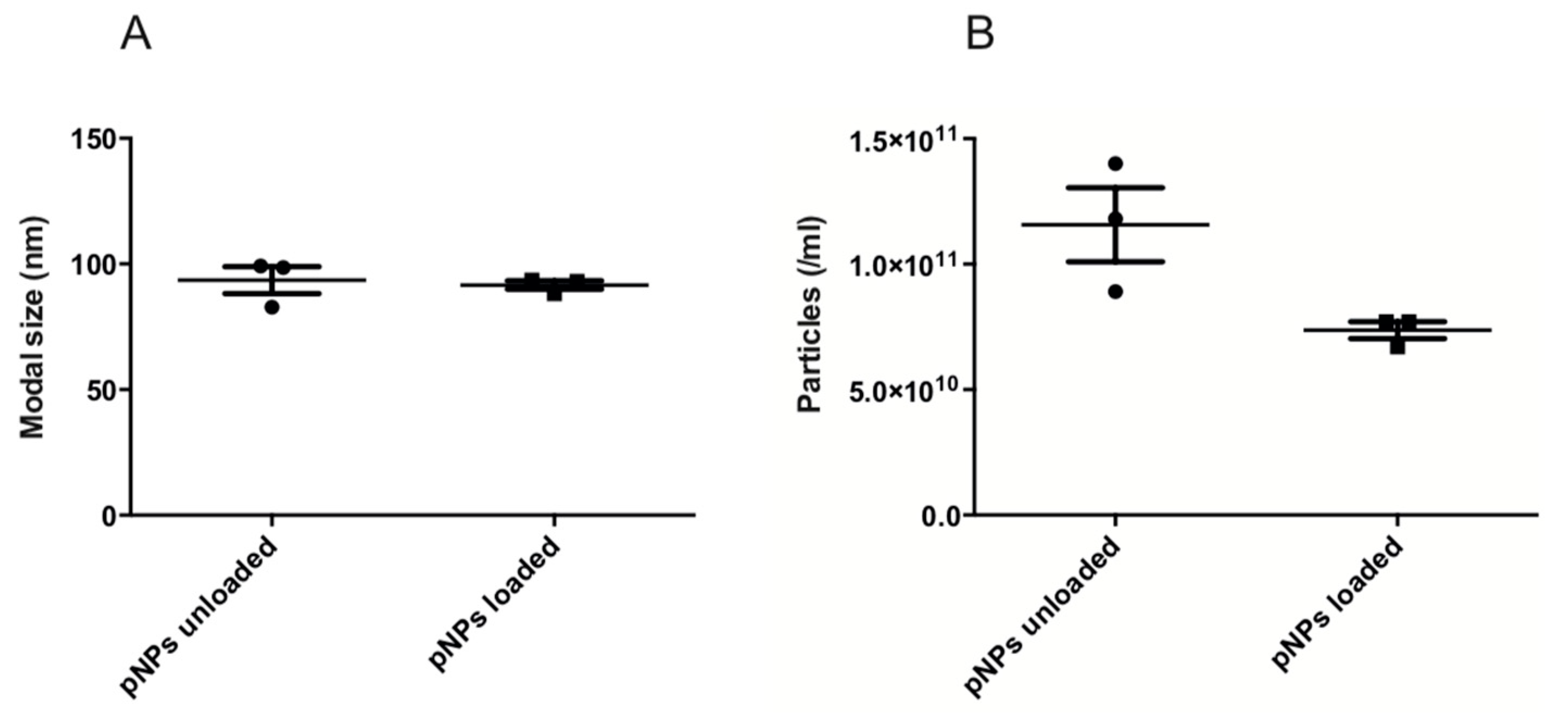
2.6. Nano Tracking Analysis
2.7. Lysozyme Association Efficiency
2.8. Stability of Unload and EE/Alginate Lys Loaded Nanoparticles
2.9. In Vitro Release Test
2.10. Cell Culturing and Cell Viability Assays
2.10.1. Models and Cell Culture
2.10.2. In Vitro Assays
MTS Assay
LDH Assay
2.11. Morphological Characterization of Interaction of the pNPs with HeLa Cell Lines
Cell Morphology
2.12. Statistical Analysis
3. Results
3.1. Nanoparticle Fabrication by Complex Coacervation and Physicochemical Characterization
3.2. Scanning Electron Microscopy (SEM)
3.3. Fourier Transform Infrared Spectroscopy (FT-IR) Study
3.4. Nano Tracking Analysis (NTA)
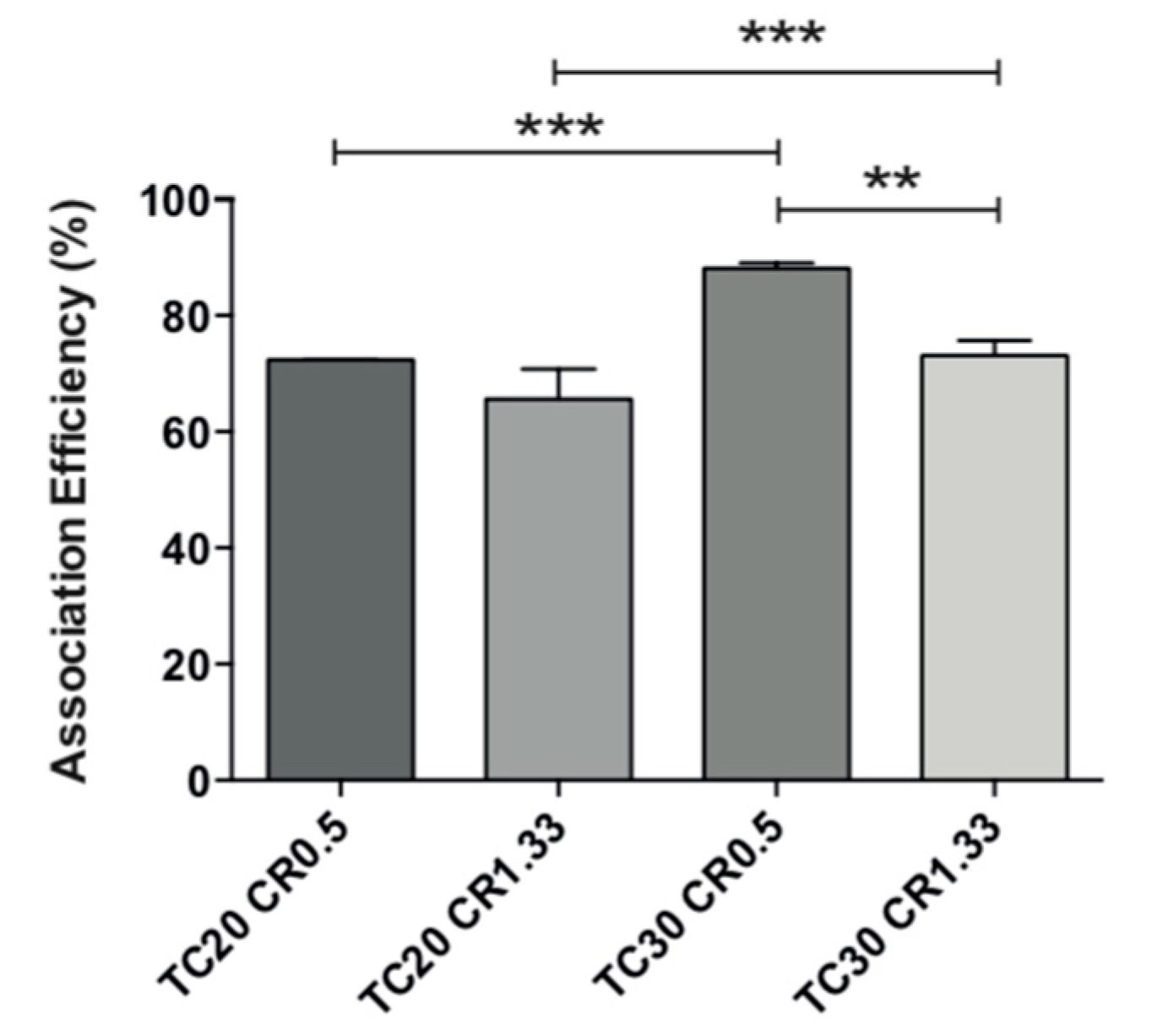
3.5. Lysozyme Association Efficiency
3.6. Stability of Lys Loaded EE/Alginate pNPs
3.7. In Vitro Release Test
3.8. In Vitro Assays
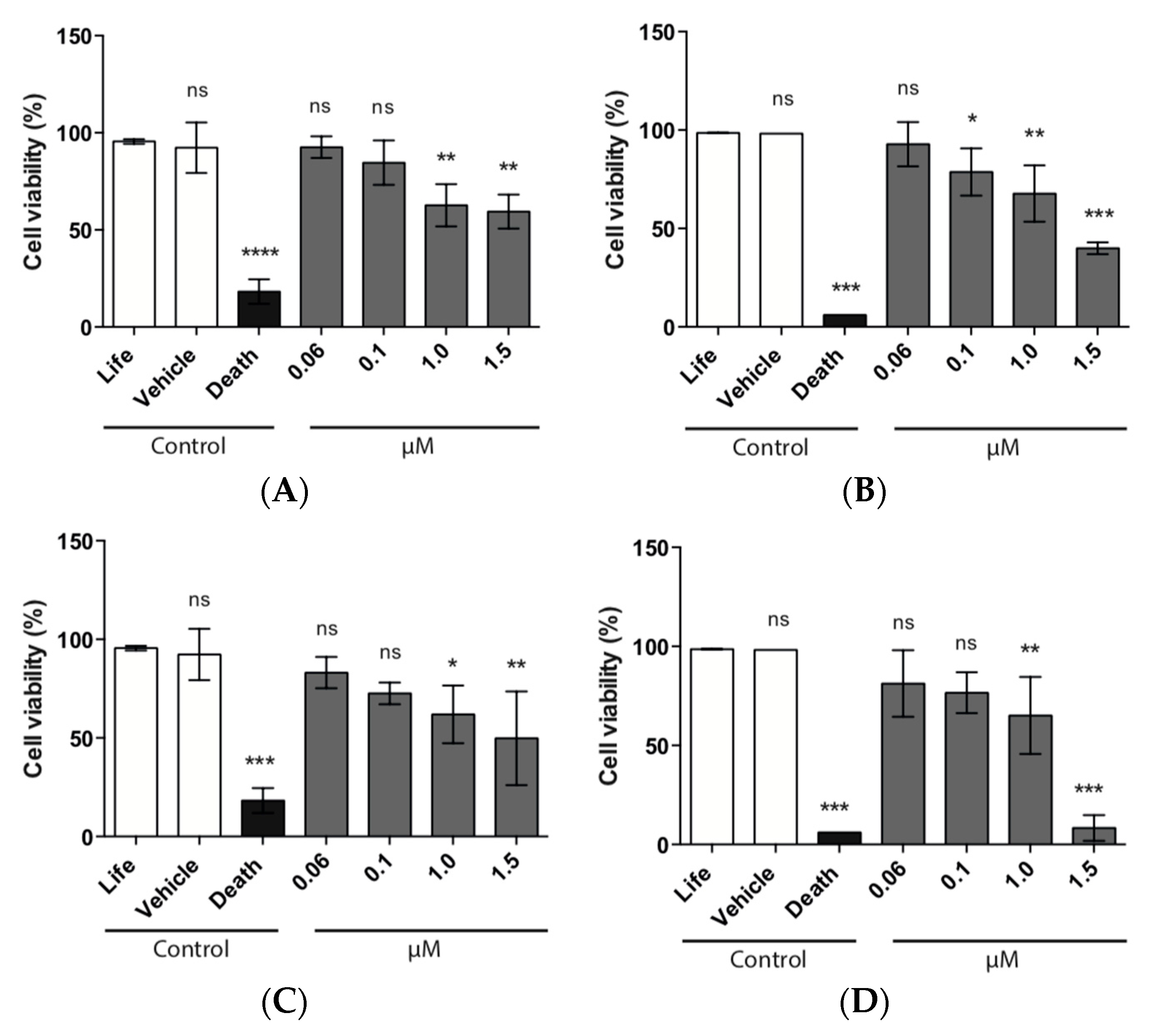
3.8.1. MTS Assay
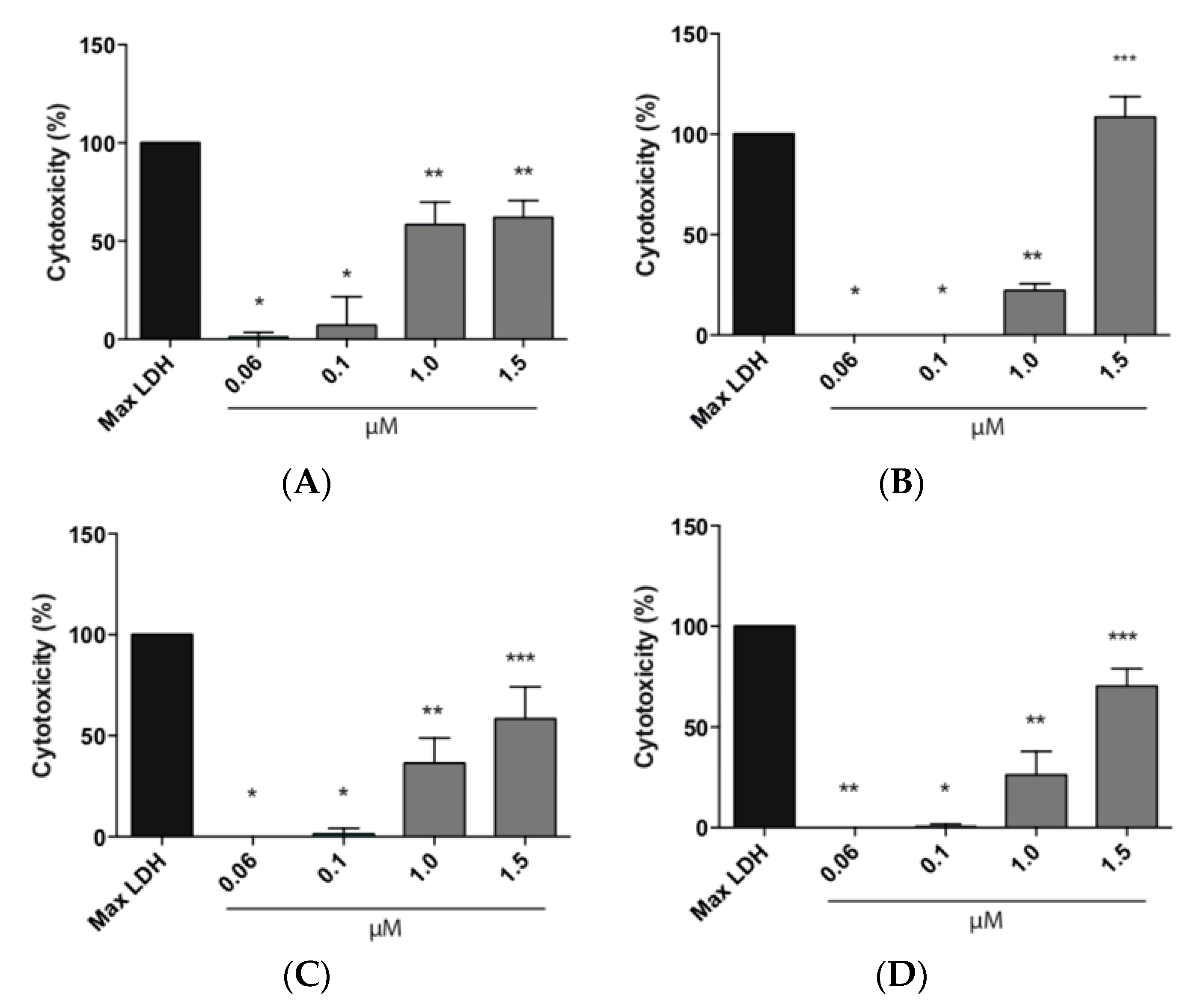
3.8.2. LDH Assay
3.8.3. Cell Morphology
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Li, X.; Yu, M.; Fan, W.; Gan, Y.; Hovgaard, L.; Yang, M. Orally active-targeted drug delivery systems for proteins and peptides. Expert Opin. Drug Deliv. 2014, 11, 1435–1447. [Google Scholar] [CrossRef] [PubMed]
- Pawar, V.K.; Meher, J.G.; Singh, Y.; Chaurasia, M.; Surendar Reddy, B.; Chourasia, M.K. Targeting gastrointestinal tract for amended delivery of protein/peptide therapeutics: Strategies and industrial perspectives. J. Control. Release 2014, 196, 168–183. [Google Scholar] [CrossRef] [PubMed]
- Lau, J.; Dunn, M. Therapeutic peptides: Historical perspectives, current development trends, and future directions. Bioorg. Med. Chem. 2018, 10, 2700–2707. [Google Scholar] [CrossRef] [PubMed]
- Ezan, E. Pharmacokinetic studies of protein drugs: Past, present and future. Adv. Drug Deliv. 2013, 65, 1065–1073. [Google Scholar] [CrossRef] [PubMed]
- Petrak, F.; Herpertz, S.; Stridde, E.; Pfützner, A. Psychological insulin resistance in type 2 diabetes patients regarding oral antidiabetes treatment, subcutaneous insulin injections or inhaled insulin. Diabetes Technol. Ther. 2013, 15, 703–711. [Google Scholar] [CrossRef] [PubMed]
- Vangeli, E.; Bakhshi, S.; Baker, A.; Fisher, A.; Bucknor, D.; Mrowietz, U.; Oestoer, A.J.; Peyrin-Biroulet, L.; Lacerda, A.P.; Weinman, J. A systematic review of factors associated with nonadherence to treatment for immune-mediated inflammatory diseases. Adv. Ther. 2015, 32, 983–1028. [Google Scholar] [CrossRef] [PubMed]
- Yavuz, D.G.; Ozcan, S.; Deyneli, O. Adherence to insulin treatment in insulin-naïve type 2 diabetic patients initiated on different insulin regimens. Patient Prefer. Adher. 2015, 9, 1225–1231. [Google Scholar] [CrossRef] [PubMed]
- Bruno, B.J.; Miller, G.D.; Lim, C.S. Basics and recent advances in peptide and protein drug delivery. Ther. Deliv. 2013, 4, 1443–1467. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Richardson, P.; Murphy, W.L.; Mooney, D.J. Polymeric delivery of proteins and plasmid DNA for tissue engineering and gene therapy. Crit. Rev. Eukaryot. 2001, 11, 47–58. [Google Scholar] [CrossRef]
- Black, K.A.; Priftis, D.; Perry, S.L.; Yip, J.; Byun, W.Y.; Tirrell, M. Protein encapsulation vio polypeptide complex coacervation. ACS Macro Lett. 2014, 15, 1088–1091. [Google Scholar] [CrossRef]
- Siyawamwaya, M.; Choonara, Y.E.; Bijukumar, D.; Kumar, P.; Du Toit, L.C.; Pillay, V. A Review: Overview of Novel Polyelectrolyte Complexes as Prospective Drug Bioavailability Enhancers. Int. J. Polym. Mater. Polym. Biomater. 2015, 64, 955–968. [Google Scholar] [CrossRef]
- Luo, Y.; Wang, Q. Recent development of chitosan-based polyelectrolyte complexes with natural polysaccharides for drug delivery. Int. J. Biol. Macromol. 2014, 64, 353–367. [Google Scholar] [CrossRef] [PubMed]
- Thakker, S.P.; Rokhade, A.P.; Abbigerimath, S.S.; Iliger, S.R.; Kulkarni, V.H.; More, U.A.; Aminabhavi, T.M. Inter-polymer complex microspheres of chitosan and cellulose acetate phthalate for oral delivery of 5-fluorouracil. Polym. Bull. 2014, 71, 2113–2131. [Google Scholar] [CrossRef]
- Philipp, B.; Dautzenberg, H.; Linow, K.; Kotz, J. Polyelectrolyte complexes—Recent Developments and Open Problems. Prog. Polym. Sci. 1989, 14, 91–172. [Google Scholar] [CrossRef]
- Kabanov, V.A.; Zezin, A.B.; Rogacheva, V.B.; Grishina, N.V.; Goethals, E.J.; Vandevelde, M. Properties of Polyelectrolyte Complexes Containing Poly(N-Tert-Butylaziridine), Makromol. Chem.-Macromol. Chem. Phys. 1986, 187, 1151–1158. [Google Scholar] [CrossRef]
- Kazemzadeh-Narbat, M.; Reid, M.; Brooks, M.S.L.; Ghanem, A. Chitosan nanoparticles as adenosine carriers. J. Microencapsul. 2015, 32, 460–466. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Lee, S.; Kim, J.H.; Park, K.; Kim, K.; Kwon, I.C. Polymeric nanomedicine for cancer therapy. Prog. Polym. Sci. 2008, 33, 113–137. [Google Scholar] [CrossRef]
- Motwani, S.K.; Chopra, S.; Talegaonkar, S.; Kohli, K.; Ahmad, F.J.; Khar, R.K. Chitosan-sodium alginate nanoparticles as submicroscopic reservoirs for ocular delivery: Formulation, optimisation and in vitro characterisation. Eur. J. Pharm. Biopharm. 2008, 68, 513–525. [Google Scholar] [CrossRef] [PubMed]
- Bulmer, C.; Margaritis, A.; Xenocostas, A. Encapsulation and Controlled Release of Recombinant Human Erythropoietin from Chitosan-Carrageenan Nanoparticles. Curr. Drug Deliv. 2012, 9, 527–537. [Google Scholar] [CrossRef] [PubMed]
- Prusty, A.K.; Sahu, S.K. Development and Evaluation of Insulin Incorporated Nanoparticles for Oral Administration. ISRN Nanotechnol. 2013, 2013, 591751. [Google Scholar] [CrossRef]
- Hartig, S.M.; Greene, R.R.; Dikov, M.M.; Prokop, A.; Davidson, J.M. Multifunctional nanoparticulate polyelectrolyte complexes. Pharm. Res. 2007, 24, 2353–2369. [Google Scholar] [CrossRef] [PubMed]
- Krone, V.; Magerstadt, M.; Walch, A.; Groner, A.; Hoffmann, D. Pharmacological Composition Containing Polyelectrolyte Complexes in Microparticulate form and at Least on Active Agent. U.S. Patent 5,700,459, 23 December 1997. [Google Scholar]
- Schmitt, C.; Sanchez, C.; Despond, S.; Renard, D.; Thomas, F.; Hardy, J. Effect of protein aggregates on the complex coacervation between b -lactoglobulin and acacia gum at pH 4. Food Hydrocoll. 2000, 14, 403–413. [Google Scholar] [CrossRef]
- Saha, A.K.; Ray, S.D. Effect of cross-linked biodegradable polymers on sustained release of sodium diclofenac-loaded microspheres. Braz. J. Pharm. Sci. 2013, 49, 873–888. [Google Scholar] [CrossRef] [Green Version]
- Rasente, R.Y.; Imperiale, J.C.; Lázaro-Martínez, J.M.; Gualco, L.; Oberkersch, R.; Sosnik, A.; Calabrese, G.C. Dermatan sulfate/chitosan polyelectrolyte complex with potential application in the treatment and diagnosis of vascular disease. Carbohydr. Polym. 2016, 144, 362–370. [Google Scholar] [CrossRef] [PubMed]
- Jelvehgari, M.; Zakeri-Milani, P.; Siahi-Shadbad, M.R.; Loveymi, B.D.; Nokhodchi, A.; Azari, Z.; Valizadeh, H. Development of pH-sensitive Insulin Nanoparticles using Eudragit L100-55 and Chitosan with Different Molecular Weights. AAPS PharmSciTech 2010, 11, 1237–1242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moustafine, R.I.; Salachova, A.R.; Frolova, E.S.; Kemenova, V.A.; Van den Mooter, G. Interpolyelectrolyte complexes of Eudragit E PO with sodium alginate as potential carriers for colonic drug delivery: Monitoring of structural transformation and composition changes during swellability and release evaluating. Drug Dev. Ind. Pharm. 2009, 35, 1439–1451. [Google Scholar] [CrossRef] [PubMed]
- Sonavane, G.S.; Devarajan, P.V. Preparation of alginate nanoparticles using Eudragit E100 as a new complexing agent: Development, in-vitro, and in-vivo evaluation. J. Biomed. Nanotechnol. 2007, 3, 160–169. [Google Scholar] [CrossRef]
- Yusif, R.M.; Abu Hashim, I.I.; El-Dahan, M.S. Some variables affecting the characteristics of Eudragit E-sodium alginate polyelectrolyte complex as a tablet matrix for diltiazem hydrochloride. Acta Pharm. 2014, 64, 89–104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gürsoy, A.; Kalkan, F.; Okar, I. Preparation and tableting of dipyridamole alginate-Eudragit microspheres. J. Microencapsul. 1998, 15, 621–628. [Google Scholar] [CrossRef] [PubMed]
- Laurienzo, P.; Malinconico, M.; Mattia, G.; Russo, R.; Rotonda, M.L.; Quaglia, F.; Capitani, D.; Mannina, L. Novel alginate-acrylic polymers as a platform for drug delivery. J. Biomed. Mater. Res. 2006, 78, 523–531. [Google Scholar] [CrossRef] [PubMed]
- Moustafine, R.I.; Kemenova, V.A.; Van den Mooter, G. Characteristics of interpolyelectrolyte complexes of Eudragit E 100 with sodium alginate. Int. J. Pharm. 2005, 294, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Draget, K.I.; Taylor, C. Chemical, physical and biological properties of alginates and their biomedical implications. Food Hydrocoll. 2011, 25, 251–256. [Google Scholar] [CrossRef]
- Downs, E.C.; Robertson, N.E.; Riss, T.L.; Plunkett, M.L. Calcium alginate beads as a slow-release system for delivering angiogenic molecules In Vivo and In Vitro. J. Cell. Physiol. 1992, 152, 422–429. [Google Scholar] [CrossRef] [PubMed]
- Rajaonarivony, M.; Vauthier, C.; Couarraze, G.; Puisieux, F.; Couvreur, P. Development of a new drug carrier made from Alginate. J. Pharm. Sci. 1993, 912–917. [Google Scholar] [CrossRef]
- Tang, J.; Xu, N.; Ji, H.; Liu, H.; Wang, Z.; Wu, L. Eudragit nanoparticles containing genistein: Formulation, development, and bioavailability assessment. Int. J. Nanomed. 2011, 6, 2429–2435. [Google Scholar]
- Chang, R.K.; Peng, Y.; Trivedi, N.; Shukla, A.J. Polymethacrylate. In Handbook of Pharmaceutical Excipients; Rowe, R.C., Sheskey, P.J., Quinn, M.E., Eds.; Pharmaceutical Press and American Pharmacists Association: Washington, DC, USA, 2009; pp. 525–533. [Google Scholar]
- Attama, A.A.; Ezeamama, U.F. Systematic delivery of chloroquine and promethazine using pH-sensitive polymers. Drug Deliv. J. Deliv. Target. Ther. Agents 2005, 12, 103–107. [Google Scholar] [CrossRef] [PubMed]
- Ito, Y.; Kwon, O.H.; Ueda, M.; Tanaka, A.; Imanishi, Y. Gene-engineered hydrophobilization to alter the bactericidal activity of lysozyme. J. Bioact. Compat. Polym. 2000, 15, 376–395. [Google Scholar] [CrossRef]
- Mocanu, G.; Mihai, D.; Legros, M.; Picton, L.; Lecerf, D. New Polysaccharide-based Microparticles Crosslinked with Siloxane: Interactions with Biologically Active Substances. J. Bioact. Compat. Polym. 2008, 23, 82–94. [Google Scholar] [CrossRef]
- Garripelli, V.K.; Jo, S. Nanocomposite thermogel for controlled release of small proteins. J. Bioact. Compat. Polym. 2012, 27, 198–209. [Google Scholar] [CrossRef]
- Shi, P.; Luo, S.; Voit, B.; Appelhans, D.; Zan, X. A Facile and Efficient Strategy to Encapsulate the Model Basic Protein Lysozyme into the Porous CaCO3. J. Mater. Chem. B 2018, 6, 4205–4215. [Google Scholar] [CrossRef]
- Aghili, Z.; Taheri, S.; Zeinabad, H.A.; Pishkar, L.; Saboury, A.A.; Rahimi, A.; Falahati, M. Investigating the Interaction of Fe Nanoparticles with Lysozyme by Biophysical and Molecular Docking Studies. PLoS ONE 2016, 11, e0164878. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.S.; Yang, S.M.; Hsin, A.; Chang, Y.K. Dye-Affinity Nanofibrous Membrane for Adsorption of Lysozyme: Preparation and Performance Evaluation. Food Technol. Biotechnol. 2018, 56, 40–50. [Google Scholar] [CrossRef] [PubMed]
- Bera, H.; Kandukuri, S.G.; Nayak, A.K.; Boddupalli, S. Alginate-sterculia Gum Gel-Coated Oil Entrapped Alginate Beads for Gastroretentive Risperidone Delivery. Carbohydr. Polym. 2015, 120, 74–84. [Google Scholar] [CrossRef] [PubMed]
- Moustafine, R.I.; Bukhovets, A.V.; Sitenkov, A.Y.; Kemenova, V.A.; Rombaut, P.; Van den Mooter, G. Eudragit E PO as a Complementary Material for Designing Oral Drug Delivery Systems with Controlled Release Properties: Comparative Evaluation of New Interpolyelectrolyte Complexes with Countercharged Eudragit L100 Copolymers. Mol. Pharm. 2013, 10, 2630–2641. [Google Scholar] [CrossRef] [PubMed]
- Obeidat, W.M.; Abuznait, A.H.; Sallam, A.S. Sustained Release Tablets Containing Soluble Polymethacrylates: Comparison with Tableted Polymethacrylate IPEC Polymers. AAPS PharmSciTech 2010, 11, 54–63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yingsukwattana, K.; Puttipipatkhachorn, S.; Ruktanonchai, U.; Sarisuta, N. Enhanced permeability across Caco-2 cell monolayers by specific mannosylating ligand of buserelin acetate proliposomes. J. Liposome Res. 2016, 26, 69–79. [Google Scholar] [CrossRef] [PubMed]
- Malzert, A.; Boury, F.; Renard, D.; Robert, P.; Proust, J.E.; Benoít, J.O. Influence of some formulation parameters on lysozyme adsorption and on its stability in solution. Int. J. Pharm. 2002, 242, 405–409. [Google Scholar] [CrossRef]
- Van De Weert, M.; Van Dijkhuizen-Radersma, R.; Bezemer, J.M.; Hennink, W.E.; Crommelin, D.J. Reversible aggregation of lysozyme in a biodegradable amphiphilic multiblock copolymer. Eur. J. Pharm. Biopharm. 2002, 54, 89–93. [Google Scholar] [CrossRef]
- Berne, B.J.; Pecora, R. Dynamic Light Scattering: With Applications to Chemistry, Biology, and Physics; Courier Dover Publications: Mineola, NY, USA, 2000. [Google Scholar]
- Applications of Nanoparticle Tracking Analysis (NTA) in Nanoparticle Research. Available online: http://www.nanosight.co.uk (accessed on 17 December 2018).
- Aksungur, P.; Demirbilek, M.; Denkbaş, E.B.; Vandervoort, J.; Ludwig, A.; Ünlü, N. Development and characterization of Cyclosporine A loaded nanoparticles for ocular drug delivery: Cellular toxicity, uptake, and kinetic studies. J. Control. Release 2011, 151, 286–294. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharjee, S. DLS and zeta potential—What they are and what they are not? J. Control. Release 2016, 235, 337–351. [Google Scholar] [CrossRef] [PubMed]
- Patel, V.R.; Agrawal, Y.K. Nanosuspension: An approach to enhance solubility of drugs. J. Adv. Pharm. Technol. Res. 2011, 2, 81. [Google Scholar] [PubMed] [Green Version]
- Tziveleka, L.A.; Pippa, N.; Georgantea, P.; Ioannou, E.; Demetzos, C.; Roussis, V. Marine Sulfated Polysaccharides as Versatile Polyelectrolytes for the Development of Drug Delivery Nanoplatforms: Complexation of Ulvan with Lysozyme. Int. J. Biol. Macromol. 2018, 118, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Smeller, L.; Meersman, F.; Heremans, K. Refolding Studies Using Pressure: The Folding Landscape of Lysozyme in the Pressure–temperature Plane. Biochim. Biophys. Acta 2006, 1764, 497–505. [Google Scholar] [CrossRef] [PubMed]
- Lad, M.D.; Ledger, V.M.; Briggs, B.; Green, R.J.; Frazier, R.A. Analysis of the SDS−Lysozyme Binding Isotherm. Langmuir 2003, 19, 5098–5103. [Google Scholar] [CrossRef]
- Filipe, V.; Hawe, A.; Jiskoot, W. Critical evaluation of nanoparticle tracking analysis (NTA) by NanoSight for the measurement of nanoparticles and protein aggregates. Pharm. Res. 2010, 27, 796–810. [Google Scholar] [CrossRef] [PubMed]
- Presas, E.; McCartney, F.; Sultan, E.; Hunger, C.; Nellen, S.; Alvarez, C.V.; Werner, U.; Bazile, D.; Brayden, D.J.; O’Driscoll, C.M. Physicochemical, pharmacokinetic and pharmacodynamic analyses of amphiphilic cyclodextrin-based nanoparticles designed to enhance intestinal delivery of insulin. J. Control. Release 2018, 286, 402–414. [Google Scholar] [CrossRef] [PubMed]
- Fuenzalida, J.P.; Nareddy, P.; Moreno-Villoslada, I.; Moerschbacher, B.; Swamy, M.; Goycoolea, F. Lysozyme–alginate nanocomplex: Effect of alginate composition. Nanotechnology 2013, 3, 331–334. [Google Scholar]
- Susanto, E.; Rosyidi, D.; Radiati, L.E.; Manab, A. Improved antibacterial spectrum of hen egg white lysozyme with thermal modified. RRBS 2014, 8, 437–442. [Google Scholar]
- Agnihotri, S.A.; Mallikarjuna, N.N.; Aminabhavi, T.M. Recent advances on chitosan-based micro- and nanoparticles in drug delivery. J. Control. Release 2004, 100, 5–28. [Google Scholar] [CrossRef] [PubMed]
- Zare Mirakabadi, A.; Moradhaseli, S. Comparative cytotoxic evaluation of free and sodium alginate nanoparticle-encapsulated ICD-85 on primary lamb kidney cells. Iran. J. Cancer Prev. 2013, 6, 151–159. [Google Scholar]
- Pissuwan, D.; Boyer, C.; Gunasekaran, K.; Davis, T.P.; Bulmus, V. In vitro cytotoxicity of RAFT polymers. Biomacromolecules 2010, 11, 412–420. [Google Scholar] [CrossRef] [PubMed]
- Kaba, S.I.; Egorova, E.M. In vitro studies of the toxic effects of silver nanoparticles on HeLa and U937 cells. Nanotechnol. Sci. Appl. 2015, 8, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Shao, X.R.; Wei, X.Q.; Song, X.; Hao, L.Y.; Cai, X.X.; Zhang, Z.R.; Peng, Q.; Lin, Y.F. Independent effect of polymeric nanoparticle zeta potential/surface charge, on their cytotoxicity and affinity to cells. Cell Prolif. 2015, 48, 465–474. [Google Scholar] [CrossRef] [PubMed]
- Schaeublin, N.M.; Braydich-Stolle, L.K.; Schrand, A.M.; Miller, J.M.; Hutchison, J.; Schlager, J.J.; Hussain, S.M. Surface charge of gold nanoparticles mediates mechanism of toxicity. Nanoscale 2011, 3, 410–420. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Huang, N.; Li, H.; Jin, Q.; Ji, J. Surface and size effects on Cell interaction of gold nanoparticles with both phagocytic and nonphagocytic cells. Langmuir 2013, 29, 9138–9148. [Google Scholar] [CrossRef] [PubMed]
- Fratoddi, I.; Venditti, I.; Cametti, C.; Russo, M.V. The puzzle of toxicity of gold nanoparticles. The case-study of HeLa cells. Toxicol. Res. 2015, 4, 796–800. [Google Scholar] [CrossRef]
- Blechinger, J.; Bauer, A.T.; Torrano, A.A.; Gorzelanny, C.; Bräuchle, C.; Schneider, S.W. Uptake kinetics and nanotoxicity of silica nanoparticles are cell type dependent. Small 2013, 9, 3970–3980. [Google Scholar] [CrossRef] [PubMed]
- Teeguarden, J.G.; Hinderliter, P.M.; Orr, G.; Thrall, B.D.; Pounds, J.G. Particokinetics in vitro: Dosimetry considerations for in vitro nanoparticle toxicity assessments. Toxicol. Sci. 2007, 95, 300–312. [Google Scholar] [CrossRef] [PubMed]



| Formulation | Size (nm) | PDI | ZP (mV) | ||||
|---|---|---|---|---|---|---|---|
| TC | CR | EE/Alginate Unloaded | EE/Alginate lyz Loaded | EE/Alginate Unloaded | EE/Alginate lyz Loaded | EE/Alginate Unloaded | EE/Alginate lyz Loaded |
| 10 | 0.5 | 110.4 ± 1.6 | 112.1±1.1 | 0.100 ± 0.002 | 0.224 ± 0.016 | −40.1 ± 0.1 | −50.4 ± 5.0 |
| 10 | 1.33 | 130.3 ± 18.7 | 161.3 ± 67.8 | 0.144 ± 0.075 | 0.321 ± 0.035 | 39.3 ± 1.2 | 50.6 ± 3.7 |
| 10 | 10 | 122.7 ± 1.7 | 121.1 ± 1.8 | 0.114 ± 0.027 | 0.160 ± 0.009 | 44.0 ± 3.1 | 25.5 ± 1.2 |
| 20 | 0.5 | 171.8 ± 4.7 | 181.5 ± 1.1 | 0.153 ± 0.130 | 0.193 ± 0.016 | −37.3 ± 1.2 | −30.6 ± 0.4 |
| 20 | 1.33 | 132.2 ± 0.6 | 118.5 ± 0.0 | 0.150 ± 0.004 | 0.145 ± 0.001 | 27.6 ± 3.6 | 35.3 ± 0.0 |
| 20 | 10 | 94.1 ± 11.7 | 133.9 ± 1.2 | 0.233 ± 0.046 | 0.212 ± 0.006 | 44.7 ± 4.7 | 43.9 ± 0.2 |
| 30 | 0.5 | 154.5 ± 5.7 | 158.8 ± 1.1 | 0.176 ± 0.019 | 0.191 ± 0.044 | −42.4 ± 1.2 | −40.0 ± 1.0 |
| 30 | 1.33 | 106.8 ± 2.5 | 155.1 ± 9.4 | 0.221 ± 0.012 | 0.154 ± 0.016 | 38.1 ± 1.6 | 38.1 ± 0.9 |
| 30 | 10 | 150.4 ± 3.2 | 158.8 ± 3.4 | 0.181 ± 0.10 | 0.262 ± 0.001 | 38.5 ± 0.8 | 33.7 ± 2.0 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sepúlveda-Rivas, S.; Fritz, H.F.; Valenzuela, C.; Santiviago, C.A.; Morales, J.O. Development of Novel EE/Alginate Polyelectrolyte Complex Nanoparticles for Lysozyme Delivery: Physicochemical Properties and In Vitro Safety. Pharmaceutics 2019, 11, 103. https://doi.org/10.3390/pharmaceutics11030103
Sepúlveda-Rivas S, Fritz HF, Valenzuela C, Santiviago CA, Morales JO. Development of Novel EE/Alginate Polyelectrolyte Complex Nanoparticles for Lysozyme Delivery: Physicochemical Properties and In Vitro Safety. Pharmaceutics. 2019; 11(3):103. https://doi.org/10.3390/pharmaceutics11030103
Chicago/Turabian StyleSepúlveda-Rivas, Sabrina, Hans F. Fritz, Camila Valenzuela, Carlos A. Santiviago, and Javier O. Morales. 2019. "Development of Novel EE/Alginate Polyelectrolyte Complex Nanoparticles for Lysozyme Delivery: Physicochemical Properties and In Vitro Safety" Pharmaceutics 11, no. 3: 103. https://doi.org/10.3390/pharmaceutics11030103