Characterization of a Reservoir-Style Implant for Sustained Release of Tenofovir Alafenamide (TAF) for HIV Pre-Exposure Prophylaxis (PrEP)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Implant Fabrication
2.2. Device Sterilization
2.3. In Vitro Release Studies
2.4. Stability Analysis of TAF Formulation
2.5. Characterization of PCL Extruded Tubes
2.5.1. Differential Scanning Calorimetry (DSC)
2.5.2. X-ray Diffraction (XRD)
2.5.3. Gel Permeation Chromatography (GPC)
2.5.4. Statistical Analysis
3. Results and Discussion
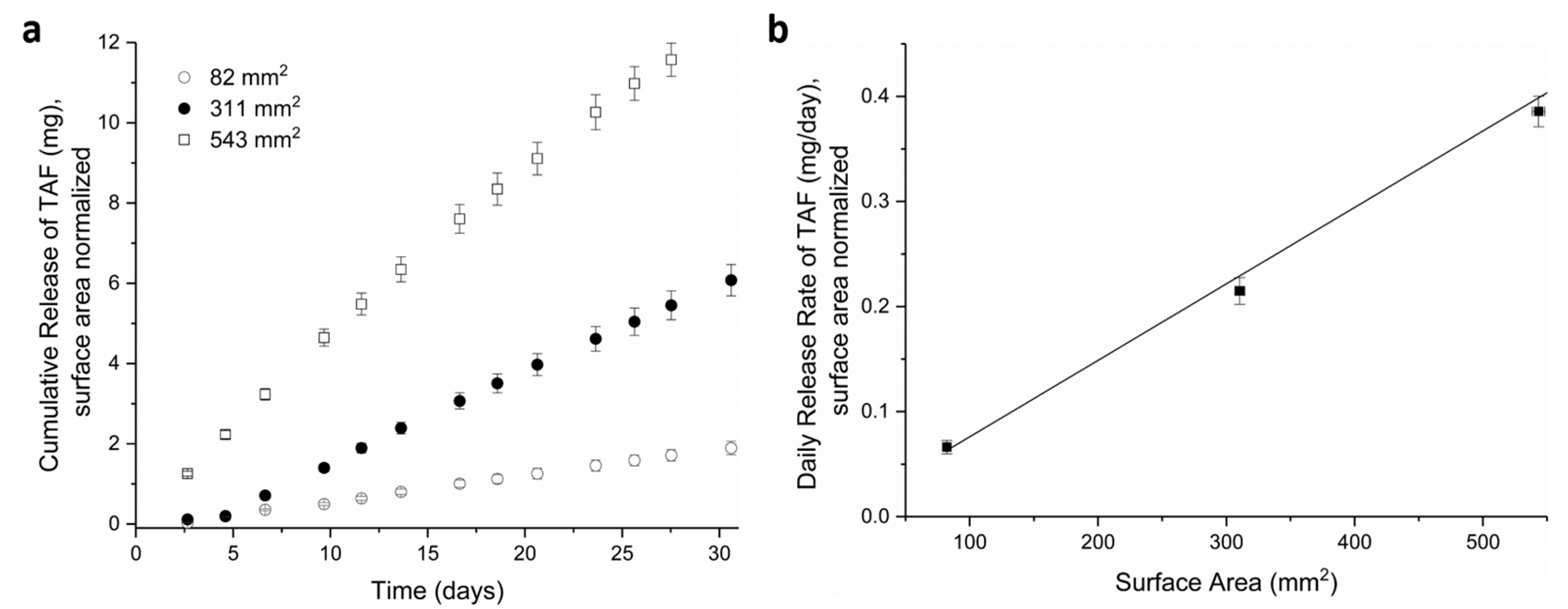
3.1. Tuning TAF Release Rates: Surface Area and Wall Thickness
3.2. Effects of PCL Properties on Implant Performance
3.3. Performance and Fabrication of a LA PCL Implant for Delivery of TAF
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Grant, R.M.; Lama, J.R.; Anderson, P.L.; McMahan, V.; Liu, A.Y.; Vargas, L.; Goicochea, P.; Casapía, M.; Guanira-Carranza, J.V.; Ramirez-Cardich, M.E.; et al. Preexposure Chemoprophylaxis for HIV prevention in men who have sex with men. N. Engl. J. Med. 2010, 363, 2587–2599. [Google Scholar] [CrossRef] [PubMed]
- Baeten, J.M.; Donnell, D.; Ndase, P.; Mugo, N.R.; Campbell, J.D.; Wangisi, J.; Tappero, J.W.; Bukusi, E.A.; Cohen, C.R.; Katabira, E.; et al. Antiretroviral prophylaxis for HIV prevention in heterosexual men and women. N. Engl. J. Med. 2012, 367, 399–410. [Google Scholar] [CrossRef] [PubMed]
- Molina, J.-M.; Capitant, C.; Spire, B.; Pialoux, G.; Cotte, L.; Charreau, I.; Tremblay, C.; Le Gall, J.-M.; Cua, E.; Pasquet, A.; et al. On-Demand preexposure prophylaxis in men at high risk for HIV-1 infection. N. Engl. J. Med. 2015, 373, 2237–2246. [Google Scholar] [CrossRef] [PubMed]
- McCormack, S.; Dunn, D.T.; Desai, M.; Dolling, D.I.; Gafos, M.; Gilson, R.; Sullivan, A.K.; Clarke, A.; Reeves, I.; Schembri, G.; et al. Pre-exposure prophylaxis to prevent the acquisition of HIV-1 infection (PROUD): Effectiveness results from the pilot phase of a pragmatic open-label randomised trial. Lancet 2016, 387, 53–60. [Google Scholar] [CrossRef]
- Cranston, R.D.; Lama, J.R.; Richardson, B.A.; Carballo-Diéguez, A.; Kunjara Na Ayudhya, R.P.; Liu, K.; Patterson, K.B.; Leu, C.-S.; Galaska, B.; Jacobson, C.E.; et al. MTN-017: A Rectal Phase 2 extended safety and acceptability study of tenofovir reduced-glycerin 1% gel. Clin. Infect. Dis. 2016, 64, 614–620. [Google Scholar] [CrossRef]
- Marrazzo, J.M.; Ramjee, G.; Richardson, B.A.; Gomez, K.; Mgodi, N.; Nair, G.; Palanee, T.; Nakabiito, C.; van der Straten, A.; Noguchi, L.; et al. Tenofovir-based preexposure prophylaxis for HIV infection among African women. N. Engl. J. Med. 2015, 372, 509–518. [Google Scholar] [CrossRef] [PubMed]
- Hosek, S.G.; Rudy, B.; Landovitz, R.; Kapogiannis, B.; Siberry, G.; Rutledge, B.; Liu, N.; Brothers, J.; Mulligan, K.; Zimet, G.; et al. An HIV preexposure prophylaxis demonstration project and safety study for young MSM. J. Acquir. Immune Defic. Syndr. 2017, 74, 21–29. [Google Scholar] [CrossRef]
- Hosek, S.G.; Landovitz, R.J.; Kapogiannis, B.; Siberry, G.K.; Rudy, B.; Rutledge, B.; Liu, N.; Harris, D.R.; Mulligan, K.; Zimet, G.; et al. Safety and feasibility of antiretroviral preexposure prophylaxis for adolescent men who have sex with men aged 15 to 17 years in the United States. JAMA Pediatrics 2017, 171, 1063–1071. [Google Scholar] [CrossRef]
- Morgan, E.; Ryan, D.T.; Newcomb, M.E.; Mustanski, B. High rate of discontinuation may diminish PrEP coverage among young men who have sex with men. AIDS Behav. 2018, 22, 3645–3648. [Google Scholar] [CrossRef]
- Deutsch, M.B.; Glidden, D.V.; Sevelius, J.; Keatley, J.; McMahan, V.; Guanira, J.; Kallas, E.G.; Chariyalertsak, S.; Grant, R.M.; For the iPrEx Study Team. HIV pre-exposure prophylaxis in transgender women: A subgroup analysis of the iPrEx trial. Lancet 2015, 2, e512–e519. [Google Scholar] [CrossRef]
- Van Damme, L.; Corneli, A.; Ahmed, K.; Agot, K.; Lombaard, J.; Kapiga, S.; Malahleha, M.; Owino, F.; Manongi, R.; Onyango, J.; et al. Preexposure prophylaxis for HIV infection among African women. N. Engl. J. Med. 2012, 367, 411–422. [Google Scholar] [CrossRef] [PubMed]
- HPTN. HPTN 083. A Phase 2b/3 Double Blind Safety and Efficacy Study of Injectable Cabotegravir Compared to Daily Oral Tenofovir Disoproxil Fumarate/Emtricitabine (TDF/FTC), for Pre-Exposure Prophylaxis in HIV-Uninfected Cisgender Men and Transgender Women who have Sex with Men. Available online: https://www.hptn.org/research/studies/hptn083 (accessed on 1 July 2019).
- HPTN. HPTN 084. A Phase 3 Double Blind Safety and Efficacy Study of Long-Acting Injectable Cabotegravir Compared to Daily Oral TDF/FTC for Pre-Exposure Prophylaxis in HIV-Uninfected Women. Available online: https://www.hptn.org/research/studies/hptn084 (accessed on 1 July 2019).
- van der Straten, A.; Agot, K.; Ahmed, K.; Weinrib, R.; Browne, E.N.; Manenzhe, K.; Owino, F.; Schwartz, J.; Minnis, A.; on behalf of the TRIO Study Team. The Tablets, Ring, Injections as Options (TRIO) study: What young African women chose and used for future HIV and pregnancy prevention. J. Int. AIDS Soc. 2018, 21, e25094. [Google Scholar] [CrossRef] [PubMed]
- Landovitz, R.J.; Li, S.; Grinsztejn, B.; Dawood, H.; Liu, A.Y.; Magnus, M.; Hosseinipour, M.C.; Panchia, R.; Cottle, L.; Chau, G.; et al. Safety, tolerability, and pharmacokinetics of long-acting injectable cabotegravir in low-risk HIV-uninfected individuals: HPTN 077, a phase 2a randomized controlled trial. PLoS Med. 2018, 15, e1002690. [Google Scholar] [CrossRef] [PubMed]
- Markowitz, M.; Frank, I.; Grant, R.M.; Mayer, K.H.; Elion, R.; Goldstein, D.; Fisher, C.; Sobieszczyk, M.E.; Gallant, J.E.; Van Tieu, H.; et al. Safety and tolerability of long-acting cabotegravir injections in HIV-uninfected men (ECLAIR): A multicentre, double-blind, randomised, placebo-controlled, phase 2a trial. Lancet HIV 2017, 4, e331–e340. [Google Scholar] [CrossRef]
- Penrose, K.J.; Parikh, U.M.; Hamanishi, K.A.; Else, L.; Back, D.; Boffito, M.; Jackson, A.; Mellors, J.W. Selection of Rilpivirine-Resistant HIV-1 in a Seroconverter From the SSAT 040 Trial Who Received the 300-mg Dose of Long-Acting Rilpivirine (TMC278LA). J. Infect. Dis. 2016, 213, 1013–1017. [Google Scholar] [CrossRef] [PubMed]
- Kovarova, M.; Benhabbour, S.R.; Massud, I.; Spagnuolo, R.A.; Skinner, B.; Baker, C.E.; Sykes, C.; Mollan, K.R.; Kashuba, A.D.M.; García-Lerma, J.G.; et al. Ultra-long-acting removable drug delivery system for HIV treatment and prevention. Nat. Commun. 2018, 9, 4156. [Google Scholar] [CrossRef] [PubMed]
- Solorio, L.; Carlson, A.; Zhou, H.; Exner, A.A. Implantable drug delivery systems. In Engineering Polymer Systems for Improved Drug Delivery, 1st ed.; Bader, R.A., Putnam, D.A., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2014. [Google Scholar] [CrossRef]
- Yang, W.-W.; Pierstorff, E. Reservoir-based polymer drug delivery systems. J. Lab. Autom. 2012, 17, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Langer, R. Implantable controlled release systems. Pharmacol. Ther. 1983, 21, 35–51. [Google Scholar] [CrossRef]
- Highlights of Prescribing Information, Probuphine (Buprenorphine) Implant for Subdermal Administration. Available online: https://probuphine.com/prescribing-information/ (accessed on 1 July 2019).
- Highlights of Prescribing Information, Jadelle (Levonogestrel Implant). Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/020544s010lbl.pdf (accessed on 1 July 2019).
- “Norplant® II” Levonorgestrel Implants (Jadelle®), Supplement 003. Council, P. (Ed.) Vol. NDA 20-544. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2002/20544se2-003_jadelle_lbl.pdf (accessed on 1 July 2019).
- Highlights of Prescribing Information, Implanon (Etonogestrel Implant). Available online: https://www.merck.com/product/usa/pi_circulars/i/implanon/implanon_pi.pdf (accessed on 1 July 2019).
- Highlights of Prescribing Information, Nexplanon (etonogestrel implants). Available online: https://www.merck.com/product/usa/pi_circulars/n/nexplanon/nexplanon_pi.pdf (accessed on 1 July 2019).
- Musch, D.C.; Martin, D.F.; Gordon, J.F.; Davis, M.D.; Kuppermann, B.D. Treatment of cytomegalovirus retinitis with a sustained-release ganciclovir implant. N. Engl. J. Med. 1997, 337, 83–90. [Google Scholar] [CrossRef]
- The Highlights of Prescribing Information Ozurdex (Dexamethasone Intravitreal Implant). Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2014/022315s009lbl.pdf (accessed on 1 July 2019).
- Haller, J.A.; Bandello, F.; Belfort, R.; Blumenkranz, M.S.; Gillies, M.; Heier, J.; Loewenstein, A.; Yoon, Y.-H.; Jacques, M.-L.; Jiao, J.; et al. Randomized, sham-controlled trial of dexamethasone intravitreal implant in patients with macular edema due to retinal vein occlusion. Ophthalmology 2010, 117, 1134–1146. [Google Scholar] [CrossRef]
- Jaffe, G.J.; Martin, D.; Callanan, D.; Pearson, P.A.; Levy, B.; Comstock, T. Fluocinolone Acetonide Implant (Retisert) for Noninfectious Posterior Uveitis: Thirty-Four–Week results of a multicenter randomized clinical study. Ophthalmology 2006, 113, 1020–1027. [Google Scholar] [CrossRef] [PubMed]
- Gunawardana, M.; Remedios-Chan, M.; Miller, C.S.; Fanter, R.; Yang, F.; Marzinke, M.A.; Hendrix, C.W.; Beliveau, M.; Moss, J.A.; Smith, T.J.; et al. Pharmacokinetics of long-acting tenofovir alafenamide (GS-7340) subdermal implant for HIV prophylaxis. Antimicrob. Agents Chemother. 2015, 59, 3913–3919. [Google Scholar] [CrossRef] [PubMed]
- Chua, C.Y.X.; Jain, P.; Ballerini, A.; Bruno, G.; Hood, R.L.; Gupte, M.; Gao, S.; Di Trani, N.; Susnjar, A.; Shelton, K.; et al. Transcutaneously refillable nanofluidic implant achieves sustained level of tenofovir diphosphate for HIV pre-exposure prophylaxis. J. Control. Release 2018, 286, 315–325. [Google Scholar] [CrossRef] [PubMed]
- Pipeline and Partners. Available online: https://www.intarcia.com/pipeline-technology/itca-650.html (accessed on 1 July 2019).
- Barrett, S.E.; Teller, R.S.; Forster, S.P.; Li, L.; Mackey, M.A.; Skomski, D.; Yang, Z.; Fillgrove, K.L.; Doto, G.J.; Wood, S.L.; et al. Extended-Duration MK-8591-Eluting Implant as a Candidate for HIV Treatment and Prevention. Antimicrob. Agents Chemother. 2018, 62, e01058-18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gatto, G.; Girouard, N.; Brand, R.M.; Johnson, L.; Marzinke, M.A.; Rowshan, S.; Engstrom, J.C.; McGowan, I.; Demkovich, Z.; Luecke, E.; et al. Pharmacokinetics of Tenofovir Alafenamide by Subcutaneous Implatn for HIV PrEP. In Proceedings of the Conference on Retrovirusus and Opportunistic Infections (CROI) 2018, Boston, MA, USA, 7 March 2018. [Google Scholar]
- New Multipurpose Device to Help Prevent HIV and Pregnancy—RTI International Awarded Project to Develop a Device to Help Women in Africa. Available online: https://www.rti.org/news/new-multipurpose-device-help-prevent-hiv-and-pregnancy (accessed on 1 July 2019).
- Pitt, C.G.; Chasalow, F.I.; Hibionada, Y.M.; Klimas, D.M.; Schindler, A. Aliphatic Polyesters. I. The Degradation of Poly(ε-caprolactone) In Vivo. J. Appl. Polym. Sci. 1981, 26, 3779–3787. [Google Scholar] [CrossRef]
- Woodruff, M.A.; Hutmacher, D.W. The return of a forgotten polymer—Polycaprolactone in the 21st century. Prog. Polym.Sci. 2010, 35, 1217–1256. [Google Scholar] [CrossRef]
- Dong, L.; Wang, S.-J.; Zhao, X.-R.; Zhu, Y.-F.; Yu, J.-K. 3D-Printed Poly(ε-caprolactone) Scaffold Integrated with Cell-laden Chitosan Hydrogels for Bone Tissue Engineering. Sci. Rep. 2017, 7, 13412. [Google Scholar] [CrossRef]
- Patrício, T.; Domingos, M.; Gloria, A.; Bártolo, P. Characterisation of PCL and PCL/PLA Scaffolds for Tissue Engineering. Procedia CIRP 2013, 5, 110–114. [Google Scholar] [CrossRef]
- Grossen, P.; Witzigmann, D.; Sieber, S.; Huwyler, J. PEG-PCL-based nanomedicines: A biodegradable drug delivery system and its application. J. Control. Release 2017, 260, 46–60. [Google Scholar] [CrossRef]
- Dash, T.K.; Konkimalla, V.B. Polymeric modification and its implication in drug delivery: Poly-epsilon-caprolactone (PCL) as a model polymer. Mol. Pharm. 2012, 9, 2365–2379. [Google Scholar] [CrossRef]
- Shrestha, D.; Wei, X.; Wu, W.-C.; Ling, J.-Q. Resilon: A methacrylate resin-based obturation system. J. Dental Sci. 2010, 5, 47–52. [Google Scholar] [CrossRef]
- Trott, A.T. Chapter 8—Instruments, Suture Materials, and Closure Choices. In Wounds and Lacerations, 4th ed.; Trott, A.T., Ed.; W.B. Saunders: Philadelphia, PA, USA, 2012; pp. 82–94. [Google Scholar] [CrossRef]
- Ory, S.J.; Hammond, C.B.; Yancy, S.G.; Wayne Hendren, R.; Pitt, C.G. The effect of a biodegradable contraceptive capsule (Capronor) containing levonorgestrel on gonadotropin, estrogen, and progesterone levels. Am. J. Obstet. Gynecol. 1983, 145, 600–605. [Google Scholar] [CrossRef]
- Gupta, B.; Geeta; Ray, A.R. Preparation of poly(ε-caprolactone)/poly(ε-caprolactone-co-lactide) (PCL/PLCL) blend filament by melt spinning. J. Appl. Polym. Sci. 2012, 123, 1944–1950. [Google Scholar] [CrossRef]
- Obregon, N.; Agubra, V.; Pokhrel, M.; Campos, H.; Flores, D.; De la Garza, D.; Mao, Y.; Macossay, J.; Alcoutlabi, M. Effect of Polymer Concentration, Rotational Speed, and Solvent Mixture on Fiber Formation Using Forcespinning®. Fibers 2016, 4, 20. [Google Scholar] [CrossRef]
- Su, H.-H.; Chen, H.-L.; Díaz, A.; Casas, M.T.; Puiggalí, J.; Hoskins, J.N.; Grayson, S.M.; Pérez, R.A.; Müller, A.J. New insights on the crystallization and melting of cyclic PCL chains on the basis of a modified Thomson–Gibbs equation. Polymer 2013, 54, 846–859. [Google Scholar] [CrossRef]
- Menczel, J.D.; Judovits, L.; Prime, R.B.; Bair, H.E.; Reading, M.; Swier, S. Chapter 2-Differential Scanning Calorimetry (DSC). In Thermal Analysis of Polymers: Fundamentals and Applications; Menczel, J.D., Prime, R.B., Eds.; Wiley: Hoboken, NJ, USA, 2009. [Google Scholar]
- Núñez, E. Crystallization in Constrained Polymer Structures: Approaching the Unsolved Problems in Polymer Crystallization. Ph.D. Thesis, KTH, Stockholm, Sweden, 2006. [Google Scholar]
- Tuba, F. Towards the Understanding of the molecular weight dependence of essential work of fracture in semi-crystalline polymers: A study on poly(ε-caprolactone). Express Polym. Lett. 2014, 8, 869–879. [Google Scholar] [CrossRef]
- Kosobrodova, E.; Kondyurin, A.; Chrzanowski, W.; Theodoropoulos, C.; Morganti, E.; Hutmacher, D.; Bilek, M.M.M. Effect of plasma immersion ion implantation on polycaprolactone with various molecular weights and crystallinity. J. Mater. Sci. Mater. Med. 2017, 29, 5. [Google Scholar] [CrossRef] [PubMed]
- Schlesinger, E.; Ciaccio, N.; Desai, T.A. Polycaprolactone thin-film drug delivery systems: Empirical and predictive models for device design. Mater. Sci. Eng. C Mater. Biol. Appl. 2015, 57, 232–239. [Google Scholar] [CrossRef] [Green Version]
- Lam, C.X.; Savalani, M.M.; Teoh, S.H.; Hutmacher, D.W. Dynamics of in vitro polymer degradation of polycaprolactone-based scaffolds: Accelerated versus simulated physiological conditions. Biomed. Mater. 2008, 3, 034108. [Google Scholar] [CrossRef]
- Schlesinger, E.; Johengen, D.; Luecke, E.; Rothrock, G.; McGowan, I.; van der Straten, A.; Desai, T. A Tunable, Biodegradable, Thin-Film Polymer Device as a Long-Acting Implant Delivering Tenofovir Alafenamide Fumarate for HIV Pre-exposure Prophylaxis. Pharm. Res. 2016, 33, 1649–1656. [Google Scholar] [CrossRef]
- Steiner, M.J.; Boler, T.; Obhai, G.; Hubacher, D. Assessment of a disposable trocar for insertion of contraceptive implants. Contraception 2010, 81, 140–142. [Google Scholar] [CrossRef] [PubMed]
- Gatto, G.J.; Brand, R.M.; Girouard, N.; Li, L.A.; Johnson, L.; Marzinke, M.A.; Krogstad, E.; Siegel, A.; Helms, E.; Demkovich, Z.; et al. Development of an End-user Informed Tenofovir Alafenamide (TAF) Implant for Long-acting (LA)-HIV Pre-exposure Prophylaxis (PrEP). In Proceedings of the HIV Research for Prevention (HIVR4P) 2018, Madrid, Spain, 25 October 2018. [Google Scholar]
- Wang, Y.; Rodriguez-Perez, M.A.; Reis, R.L.; Mano, J.F. Thermal and Thermomechanical Behaviour of Polycaprolactone and Starch/Polycaprolactone Blends for Biomedical Applications. Macromol. Mater. Eng. 2005, 290, 792–801. [Google Scholar] [CrossRef] [Green Version]
- Speranza, V.; Sorrentino, A.; De Santis, F.; Pantani, R. Characterization of the Polycaprolactone Melt Crystallization: Complementary Optical Microscopy, DSC, and AFM Studies. Sci. World J. 2014, 2014, 9. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Dorset, D.L. Crystal structure of poly(iε-caprolactone). Macromolecules 1990, 23, 4604–4607. [Google Scholar] [CrossRef]
- Lv, Q.; Wu, D.; Xie, H.; Peng, S.; Chen, Y.; Xu, C. Crystallization of poly(ε-caprolactone) in its immiscible blend with polylactide: Insight into the role of annealing histories. RSC Adv. 2016, 6, 37721–37730. [Google Scholar] [CrossRef]
- Scheler, S. The polymer free volume as a controlling factor for drug release from poly(lactide-co-glycolide) microspheres. J. Appl. Polym. Sci. 2014, 131. [Google Scholar] [CrossRef]
- Rajoli, R.; Demkovish, Z.; van der Straten, A.; Flexner, C.; Owen, A.; Siccardi, M. In Silico simulation of long-acting tenofovir alafenamide subcutaneous implant. In Proceedings of the CROI 2019, Seatle, WA, USA, 4–7 March 2019. [Google Scholar]
- Golla, V.M.; Kurmi, M.; Shaik, K.; Singh, S. Stability behaviour of antiretroviral drugs and their combinations. 4: Characterization of degradation products of tenofovir alafenamide fumarate and comparison of its degradation and stability behaviour with tenofovir disoproxil fumarate. J. Pharm. Biomed. Anal. 2016, 131, 146–155. [Google Scholar] [CrossRef]
- Navarro, R.; Burillo, G.; Adem, E.; Marcos-Fernández, A. Effect of Ionizing Radiation on the Chemical Structure and the Physical Properties of Polycaprolactones of Different Molecular Weight. Polymers 2018, 10, 397. [Google Scholar] [CrossRef]
- Cooke, S.L.; Whittington, A.R. Influence of therapeutic radiation on polycaprolactone and polyurethane biomaterials. Mater. Sci. Eng. C 2016, 60, 78–83. [Google Scholar] [CrossRef]
- Krogstad, E.A.; Montgomery, E.T.; Atujuna, M.; Minnis, A.M.; O’Rourke, S.; Ahmed, K.; Bekker, L.-G.; van der Straten, A. Design of an Implant for Long-Acting HIV Pre-Exposure Prophylaxis: Input from South African Health Care Providers. AIDS Patient Care STDs 2019, 33, 157–166. [Google Scholar] [CrossRef]
- Krogstad, E.A.; Atujuna, M.; Montgomery, E.T.; Minnis, A.; Ndwayana, S.; Malapane, T.; Shapley-Quinn, M.K.; Manenzhe, K.; Bekker, L.-G.; van der Straten, A. Perspectives of South African youth in the development of an implant for HIV prevention. J. Int. AIDS Soc. 2018, 21, e25170. [Google Scholar] [CrossRef] [PubMed] [Green Version]





| PCL Type | Wall Thickness (µm) | Tm (°C) | % Crystallinity | Crystallite Size (nm) |
|---|---|---|---|---|
| PC-12 | 70 | 59.4 ± 0.1 | 56 ± 1.0 | 27 ± 0.2 |
| 100 | 59.4 ± 0.1 | 53 ± 2.0 | 27 ± 0.4 | |
| 200 | 59.7 ± 0.4 | 56 ± 1.0 | 27 ± 1.2 | |
| Sigma-PCL | 70 | 60.7 ± 0.1 | 53 ± 0.3 | 31 ± 0.2 |
| 100 | 61.1 ± 0.2 | 52 ± 1.2 | 32 ± 0.6 | |
| 200 | 61.3 ± 0.1 | 53 ± 0.1 | 33 ± 0.3 |
| PCL Type | Crystallite Size (nm) | |
|---|---|---|
| L110 | L200 | |
| PC-12 | 13.2 | 10.2 |
| Sigma-PCL | 14.2 | 10.8 |
| PCL Type | Wall Thickness (µm) | Release Rates of TAF (mg/Day) | |
|---|---|---|---|
| Non-Irradiated | Gamma Irradiated | ||
| Sigma-PCL | 70 | 0.62 ± 0.09 | 0.54 ± 0.06 |
| 100 | 0.29 ± 0.05 | 0.32 ± 0.03 | |
| PC-12 | 70 | 0.37 ± 0.05 | 0.30 ± 0.03 |
| 100 | 0.20 ± 0.03 | 0.20 ± 0.02 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Johnson, L.M.; Krovi, S.A.; Li, L.; Girouard, N.; Demkovich, Z.R.; Myers, D.; Creelman, B.; van der Straten, A. Characterization of a Reservoir-Style Implant for Sustained Release of Tenofovir Alafenamide (TAF) for HIV Pre-Exposure Prophylaxis (PrEP). Pharmaceutics 2019, 11, 315. https://doi.org/10.3390/pharmaceutics11070315
Johnson LM, Krovi SA, Li L, Girouard N, Demkovich ZR, Myers D, Creelman B, van der Straten A. Characterization of a Reservoir-Style Implant for Sustained Release of Tenofovir Alafenamide (TAF) for HIV Pre-Exposure Prophylaxis (PrEP). Pharmaceutics. 2019; 11(7):315. https://doi.org/10.3390/pharmaceutics11070315
Chicago/Turabian StyleJohnson, Leah M., Sai Archana Krovi, Linying Li, Natalie Girouard, Zach R. Demkovich, Daniel Myers, Ben Creelman, and Ariane van der Straten. 2019. "Characterization of a Reservoir-Style Implant for Sustained Release of Tenofovir Alafenamide (TAF) for HIV Pre-Exposure Prophylaxis (PrEP)" Pharmaceutics 11, no. 7: 315. https://doi.org/10.3390/pharmaceutics11070315