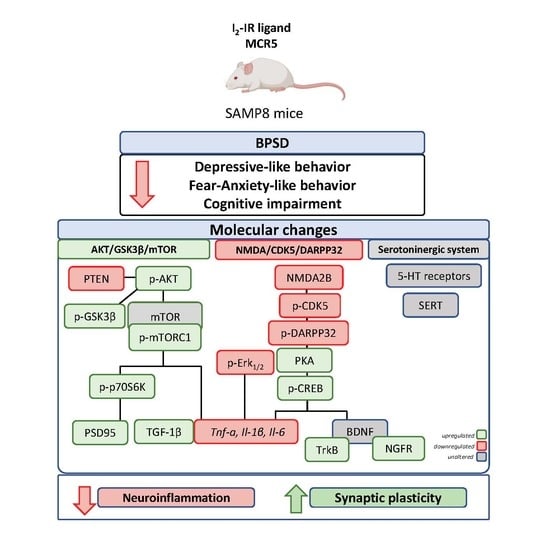
Amelioration of BPSD-Like Phenotype and Cognitive Decline in SAMP8 Mice Model Accompanied by Molecular Changes after Treatment with I2-Imidazoline Receptor Ligand MCR5
Abstract
:1. Introduction
2. Methods
2.1. Animals
2.2. Evaluation of Anxiety- and Depressive-Like Behaviour as Well as Cognitive Performance
2.2.1. Tail Suspension Test (TST)
2.2.2. Forced Swimming Test (FST)
2.2.3. Elevated Plus Maze (EPM)
2.2.4. Open Field Test (OFT)
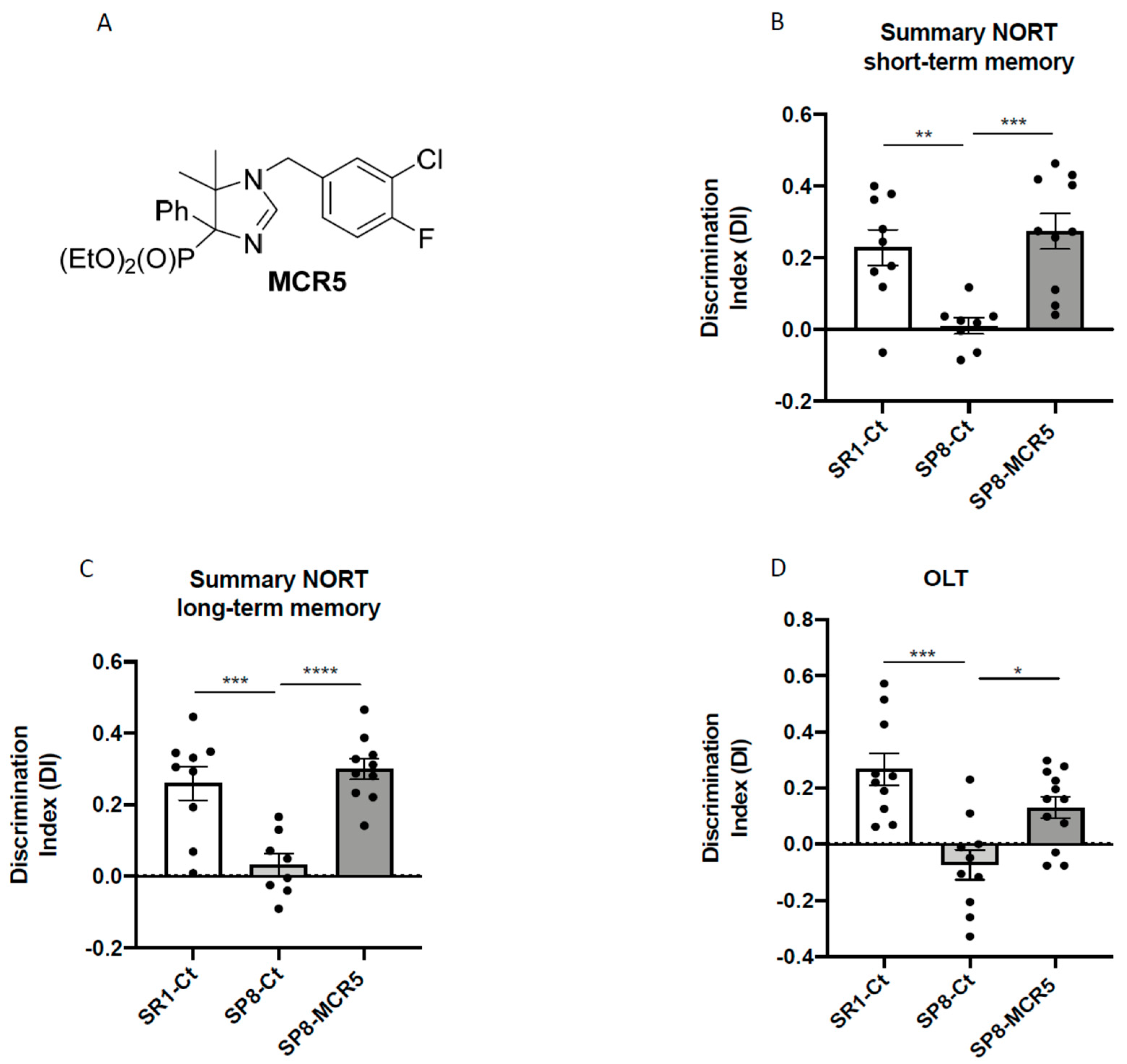
2.2.5. Novel Object Recognition Test (NORT)
2.2.6. Object Location Test (OLT)
2.3. Determination of Transporters, Receptors and Alterations in Molecular Pathways
2.3.1. In Vitro Pharmacology: Binding Assays
2.3.2. Tissue Preparation for Biochemical Analysis
2.3.3. Protein Levels Determination by Western Blot
2.3.4. Determination of ProBDNF and MBDNF Levels in the Hippocampus
2.3.5. RNA Extraction and Gene Expression Determination
2.4. Data Acquisition and Statistical Analysis
3. Results
3.1. I2-IR Ligand MCR5 Reduced Cognitive Loss in SAMP8 Male Mice
3.2. I2-IR Ligand MCR5 Improved Emotional Parameters Associated with Fear- Anxiety- and Depressive-Like Behaviours in SAMP8 Male Mice
3.3. Interaction of I2-IR Ligand MCR5 with Serotonin Receptors and Transporter
3.4. MCR5 Enhanced AKT/mTOR/GSK3β Pathways Promoting a Reduction of Pro-Inflammatory Cytokines in SAMP8 Male Mice
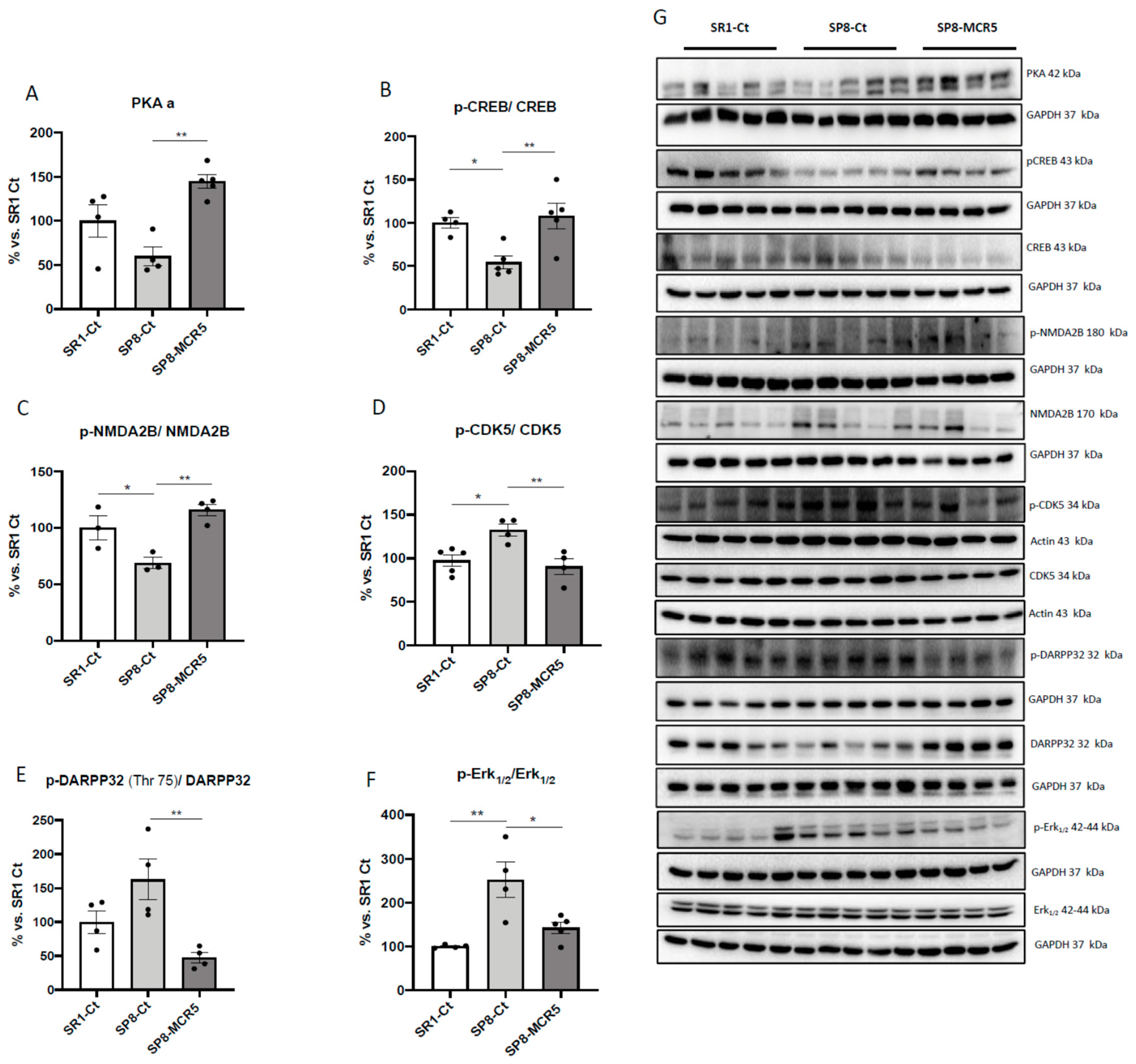
3.5. Changes in PKA/CREB and NMDAR/CDK5/DARPP32 Signalling Cascades after Treatment with I2-IR Ligand MCR5 in SAMP8 Male Mice
3.6. Effects of I2-IR Ligand MCR5 on BDNF/TrkB/NGFR(p75) Signalling Pathway after Treatment with MCR5
4. Discussion and Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Cummings, J.L.; Morstorf, T.; Zhong, K. Alzheimer’s Disease Drug-Development Pipeline: Few Candidates, Frequent Failures. Alzheimers. Res. Ther. 2014, 6, 37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cummings, J.; Lee, G.; Ritter, A.; Zhong, K. Alzheimer’s Disease Drug Development Pipeline: 2018. Alzheimer’s Dement. Transl. Res. Clin. Interv. 2018, 4, 195–214. [Google Scholar] [CrossRef]
- Griñan-Ferré, C.; Puigoriol-Illamola, D.; Palomera-ávalos, V.; Pérez-Cáceres, D.; Companys-Alemany, J.; Camins, A.; Ortuño-Sahagún, D.; Teresa Rodrigo, M.; Pallàs, M. Environmental Enrichment Modified Epigenetic Mechanisms in SAMP8 Mouse Hippocampus by Reducing Oxidative Stress and Inflammaging and Achieving Neuroprotection. Front. Aging Neurosci. 2016, 8. [Google Scholar] [CrossRef] [PubMed]
- Cerejeira, J.; Lagarto, L.; Mukaetova-Ladinska, E. Behavioral and Psychological Symptoms of Dementia. Front. Neurol. 2012, 3, 73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reisberg, B.; Borenstein, J.; Salob, S.P.; Ferris, S.H. Behavioral Symptoms in Alzheimer’s Disease: Phenomenology and Treatment. J. Clin. Psychiatry 1987, 48 (Suppl. 5), 9–15. [Google Scholar]
- Kar, N. Behavioral and Psychological Symptoms of Dementia and Their Management. Indian J. Psychiatry 2009, 51 (Suppl. S1), S77. [Google Scholar]
- Kołaczkowski, M.; Mierzejewski, P.; Bienkowski, P.; Wesołowska, A.; Newman-Tancredi, A. Antipsychotic, Antidepressant, and Cognitive-Impairment Properties of Antipsychotics: Rat Profile and Implications for Behavioral and Psychological Symptoms of Dementia. Naunyn. Schmiedebergs. Arch. Pharmacol. 2014, 387, 545–557. [Google Scholar] [CrossRef] [Green Version]
- Leyhe, T.; Reynolds III, C.F.; Melcher, T.; Linnemann, C.; Klöppel, S.; Blennow, K.; Zetterberg, H.; Dubois, B.; Lista, S.; Hampel, H. A Common Challenge in Older Adults: Classification, Overlap, and Therapy of Depression and Dementia. Alzheimer’s Dement. 2017, 13, 59–71. [Google Scholar] [CrossRef] [Green Version]
- Mega, M.S.; Cummings, J.L.; Fiorello, T.; Gornbein, J. The Spectrum of Behavioral Changes in Alzheimer’s Disease. Neurology 1996, 46, 130–135. [Google Scholar] [CrossRef]
- Dillon, C.; Serrano, C.M.; Castro, D.; Leguizamón, P.P.; Heisecke, S.L.; Taragano, F.E. Behavioral Symptoms Related to Cognitive Impairment. Neuropsychiatr. Dis. Treat. 2013, 9, 1443. [Google Scholar] [CrossRef] [Green Version]
- Lanctôt, K.L.; Herrmann, N.; Mazzotta, P. Role of Serotonin in the Behavioral and Psychological Symptoms of Dementia. J. Neuropsychiatry Clin. Neurosci. 2001, 13, 5–21. [Google Scholar] [CrossRef] [PubMed]
- Lohoff, F.W. Overview of the Genetics of Major Depressive Disorder. Curr. Psychiatry Rep. 2010, 12, 539–546. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castrén, E.; Rantamäki, T. The Role of BDNF and Its Receptors in Depression and Antidepressant Drug Action: Reactivation of Developmental Plasticity. Dev. Neurobiol. 2010, 70, 289–297. [Google Scholar] [CrossRef] [PubMed]
- Serafini, G. Neuroplasticity and Major Depression, the Role of Modern Antidepressant Drugs. World J. Psychiatry 2012, 2, 49. [Google Scholar] [CrossRef] [PubMed]
- Autry, A.E.; Adachi, M.; Nosyreva, E.; Na, E.S.; Los, M.F.; Cheng, P.; Kavalali, E.T.; Monteggia, L.M. NMDA Receptor Blockade at Rest Triggers Rapid Behavioural Antidepressant Responses. Nature 2011, 475, 91–95. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- García-Sevilla, J.A.; Escriba, P.V.; Sastre, M.; Walzer, C.; Busquets, X.; Jaquet, G.; Reis, D.J.; Guimon, J. Immunodetection and Quantitation of Imidazoline Receptor Proteins in Platelets of Patients with Major Depression and in Brains of Suicide Victims. Arch. Gen. Psychiatry 1996, 53, 803–810. [Google Scholar] [CrossRef]
- Gargalidis-Moudanos, C.; Pizzinat, N.; Javoy-Agid, F.; Remaury, A.; Parini, A. I2-Imidazoline Binding Sites and Monoamine Oxidase Activity in Human Postmortem Brain from Patients with Parkinson’s Disease. Neurochem. Int. 1997, 30, 31–36. [Google Scholar] [CrossRef]
- Ruiz, J.; Martin, I.; Callado, L.F.; Meana, J.J.; Barturen, F.; Garcia-Sevilla, J.A. Non-Adrenoceptor [3H] Idazoxan Binding Sites (I2-Imidazoline Sites) Are Increased in Postmortem Brain from Patients with Alzheimer’s Disease. Neurosci. Lett. 1993, 160, 109–112. [Google Scholar] [CrossRef]
- Parini, A.; Moudanos, C.G.; Pizzinat, N.; Lanier, S.M. The Elusive Family of Imidazoline Binding Sites. Trends Pharmacol. Sci. 1996, 17, 13–16. [Google Scholar] [CrossRef]
- Finn, D.P.; Martí, O.; Harbuz, M.S.; Vallès, A.; Belda, X.; Márquez, C.; Jessop, D.S.; Lalies, M.D.; Armario, A.; Nutt, D.J. Behavioral, Neuroendocrine and Neurochemical Effects of the Imidazoline I 2 Receptor Selective Ligand BU224 in Naive Rats and Rats Exposed to the Stress of the Forced Swim Test. Psychopharmacology 2003, 167, 195–202. [Google Scholar] [CrossRef]
- Tonello, R.; Villarinho, J.G.; da Silva Sant’Anna, G.; Tamiozzo, L.; Machado, P.; Trevisan, G.; Martins, M.A.P.; Ferreira, J.; Rubin, M.A. The Potential Antidepressant-like Effect of Imidazoline I2 Ligand 2-BFI in Mice. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2012, 37, 15–21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, G.; Regunathan, S.; Barrow, C.J.; Eshraghi, J.; Cooper, R.; Reis, D.J. Agmatine: An Endogenous Clonidine-Displacing Substance in the Brain. Science (80-) 1994, 263, 966–969. [Google Scholar] [CrossRef] [PubMed]
- Aricioglu, F.; Altunbas, H. Is Agmatine an Endogenous Anxiolytic/Antidepressant Agent? Ann. N. Y. Acad. Sci. 2003, 1009, 136–140. [Google Scholar] [CrossRef] [PubMed]
- Head, G.A.; Mayorov, D.N. Imidazoline Receptors, Novel Agents and Therapeutic Potential. Cardiovasc. Hematol. Agents Med. Chem. (Formerly Curr. Med. Chem. Hematol. Agents) 2006, 4, 17–32. [Google Scholar] [CrossRef]
- Abas, S.; Estarellas, C.; Luque, F.J.; Escolano, C. Easy Access to (2-Imidazolin-4-Yl) Phosphonates by a Microwave Assisted Multicomponent Reaction. Tetrahedron 2015, 71, 2872–2881. [Google Scholar] [CrossRef]
- Abás, S.; Erdozain, A.M.; Keller, B.; Rodríguez-Arévalo, S.; Callado, L.F.; García-Sevilla, J.A.; Escolano, C. Neuroprotective Effects of a Structurally New Family of High Affinity Imidazoline I2 Receptor Ligands. ACS Chem. Neurosci. 2017, 8, 737–742. [Google Scholar] [CrossRef]
- Griñán-Ferré, C.; Vasilopoulou, F.; Abás, S.; Rodríguez-Arévalo, S.; Bagán, A.; Sureda, F.X.; Pérez, B.; Callado, L.F.; García-Sevilla, J.A.; García-Fuster, M.J.; et al. Behavioral and Cognitive Improvement Induced by Novel Imidazoline I2 Receptor Ligands in Female SAMP8 Mice. Neurotherapeutics 2019, 16. [Google Scholar] [CrossRef] [Green Version]
- Butterfield, D.A.; Poon, H.F. The Senescence-Accelerated Prone Mouse (SAMP8): A Model of Age-Related Cognitive Decline with Relevance to Alterations of the Gene Expression and Protein Abnormalities in Alzheimer’s Disease. Exp. Gerontol. 2005, 40, 774–783. [Google Scholar] [CrossRef]
- Miyamoto, M. Characteristics of Age-Related Behavioral Changes in Senescence-Accelerated Mouse SAMP8 and SAMP10. Exp. Gerontol. 1997, 32, 139–148. [Google Scholar] [CrossRef]
- Pallàs, M. Senescence-Accelerated Mice P8: A Tool to Study Brain Aging and Alzheimer’s Disease in a Mouse Model. ISRN Cell Biol. 2012, 2012, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Pérez-Cáceres, D.; Rodrigo, M.T.; Pubill, D.; Camins, A.; Camarasa, J.; Escubedo, E.; Pallàs, M. Depression-like Behavior Is Dependent on Age in Male SAMP8 Mice. Biogerontology 2013, 14, 165–176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Steru, L.; Chermat, R.; Thierry, B.; Simon, P. The Tail Suspension Test: A New Method for Screening Antidepressants in Mice. Psychopharmacology 1985, 85, 367–370. [Google Scholar] [CrossRef] [PubMed]
- Can, A.; Dao, D.T.; Terrillion, C.E.; Piantadosi, S.C.; Bhat, S.; Gould, T.D. The Tail Suspension Test. JoVE (J. Vis. Exp). 2012, 59, e3769. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Porsolt, R.D.; Le Pichon, M.; Jalfre, M. Depression: A New Animal Model Sensitive to Antidepressant Treatments. Nature 1977, 266, 730–732. [Google Scholar] [CrossRef] [PubMed]
- Lister, R.G. The Use of a Plus-Maze to Measure Anxiety in the Mouse. Psychopharmacology 1987, 92, 180–185. [Google Scholar] [CrossRef] [PubMed]
- Cohen, H.; Matar, M.A.; Joseph, Z. Animal Models of Post-traumatic Stress Disorder. Curr. Protoc. Neurosci. 2013, 64, 9–45. [Google Scholar] [CrossRef] [PubMed]
- Archer, J. Tests for Emotionality in Rats and Mice: A Review. Anim. Behav. 1973, 21, 205–235. [Google Scholar] [CrossRef]
- Ennaceur, A.; Delacour, J. A New One-Trial Test for Neurobiological Studies of Memory in Rats. 1: Behavioral Data. Behav. Brain Res. 1988, 31, 47–59. [Google Scholar] [CrossRef]
- Griñán-Ferré, C.; Marsal-García, L.; Bellver-Sanchis, A.; Kondengaden, S.M.; Turga, R.C.; Vázquez, S.; Pallàs, M. Pharmacological Inhibition of G9a/GLP Restores Cognition and Reduces Oxidative Stress, Neuroinflammation and ß-Amyloid Plaques in an Early-Onset Alzheimer’s Disease Mouse Model. Aging 2019, 11. [Google Scholar] [CrossRef]
- GARCÍA-SEVILLA, J.A.; Escriba, P.V.; Guimon, J. Imidazoline Receptors and Human Brain Disorders A. Ann. N. Y. Acad. Sci. 1999, 881, 392–409. [Google Scholar] [CrossRef]
- Li, J.-X.; Zhang, Y. Imidazoline I2 Receptors: Target for New Analgesics? Eur. J. Pharmacol. 2011, 658, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Abás, S.; Rodríguez-Arévalo, S.; Bagán, A.; Griñán-Ferré, C.; Vasilopoulou, F.; Brocos-Mosquera, I.; Muguruza, C.; Pérez, B.; Molins, E.; Luque, F.J. Bicyclic α-Iminophosphonates as High Affinity Imidazoline I2 Receptor Ligands for Alzheimer’s Disease. J. Med. Chem. 2020, 63, 3610–3633. [Google Scholar] [CrossRef] [PubMed]
- Kitagishi, Y.; Kobayashi, M.; Kikuta, K.; Matsuda, S. Roles of PI3K/AKT/GSK3/MTOR Pathway in Cell Signaling of Mental Illnesses. Depress. Res. Treat. 2012, 2012, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Hashioka, S.; Klegeris, A.; Monji, A.; Kato, T.; Sawada, M.; McGeer, P.L.; Kanba, S. Antidepressants Inhibit Interferon-γ-Induced Microglial Production of IL-6 and Nitric Oxide. Exp. Neurol. 2007, 206, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Knezevic, D.; Verhoeff, N.P.L.G.; Hafizi, S.; Strafella, A.P.; Graff-Guerrero, A.; Rajji, T.; Pollock, B.G.; Houle, S.; Rusjan, P.M.; Mizrahi, R. Imaging Microglial Activation and Amyloid Burden in Amnestic Mild Cognitive Impairment. J. Cereb. Blood Flow Metab. 2018, 38, 1885–1895. [Google Scholar] [CrossRef]
- Haidinger, M.; Poglitsch, M.; Geyeregger, R.; Kasturi, S.; Zeyda, M.; Zlabinger, G.J.; Pulendran, B.; Hörl, W.H.; Säemann, M.D.; Weichhart, T. A Versatile Role of Mammalian Target of Rapamycin in Human Dendritic Cell Function and Differentiation. J. Immunol. 2010, 185, 3919–3931. [Google Scholar] [CrossRef]
- Duman, R.S.; Voleti, B. Signaling Pathways Underlying the Pathophysiology and Treatment of Depression: Novel Mechanisms for Rapid-Acting Agents. Trends Neurosci. 2012, 35, 47–56. [Google Scholar] [CrossRef] [Green Version]
- Caraci, F.; Battaglia, G.; Busceti, C.; Biagioni, F.; Mastroiacovo, F.; Bosco, P.; Drago, F.; Nicoletti, F.; Sortino, M.A.; Copani, A. TGF-Β1 Protects against Aβ-Neurotoxicity via the Phosphatidylinositol-3-Kinase Pathway. Neurobiol. Dis. 2008, 30, 234–242. [Google Scholar] [CrossRef]
- Caraci, F.; Spampinato, S.F.; Morgese, M.G.; Tascedda, F.; Salluzzo, M.G.; Giambirtone, M.C.; Caruso, G.; Munafò, A.; Torrisi, S.A.; Leggio, G.M. Neurobiological Links between Depression and AD: The Role of TGF-Β1 Signaling as a New Pharmacological Target. Pharmacol. Res. 2018, 130, 374–384. [Google Scholar] [CrossRef]
- Derynck, R.; Zhang, Y.E. Smad-Dependent and Smad-Independent Pathways in TGF-β Family Signalling. Nature 2003, 425, 577–584. [Google Scholar] [CrossRef]
- Dudek, H.; Datta, S.R.; Franke, T.F.; Birnbaum, M.J.; Yao, R.; Cooper, G.M.; Segal, R.A.; Kaplan, D.R.; Greenberg, M.E. Regulation of Neuronal Survival by the Serine-Threonine Protein Kinase Akt. Science (80-) 1997, 275, 661–665. [Google Scholar] [CrossRef] [PubMed]
- David, D.J.; Samuels, B.A.; Rainer, Q.; Wang, J.-W.; Marsteller, D.; Mendez, I.; Drew, M.; Craig, D.A.; Guiard, B.P.; Guilloux, J.-P. Neurogenesis-Dependent and-Independent Effects of Fluoxetine in an Animal Model of Anxiety/Depression. Neuron 2009, 62, 479–493. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Canudas, A.M.; Gutierrez-Cuesta, J.; Rodríguez, M.I.; Acuña-Castroviejo, D.; Sureda, F.X.; Camins, A.; Pallàs, M. Hyperphosphorylation of Microtubule-Associated Protein Tau in Senescence-Accelerated Mouse (SAM). Mech. Ageing Dev. 2005, 126, 1300–1304. [Google Scholar] [CrossRef] [PubMed]
- Casadesús, G.; Gutierrez-Cuesta, J.; Lee, H.; Jiménez, A.; Tajes, M.; Ortuño-Sahagún, D.; Camins, A.; Smith, M.A.; Pallàs, M. Neuronal Cell Cycle Re-Entry Markers Are Altered in the Senescence Accelerated Mouse P8 (SAMP8). J. Alzheimer’s Dis. 2012, 30, 573–583. [Google Scholar] [CrossRef]
- Li, X.; Polter, A. Glycogen Synthase Kinase-3 Is an Intermediate Modulator of Serotonin Neurotransmission. Front. Mol. Neurosci. 2011, 4, 31. [Google Scholar]
- Young, W. Review of Lithium Effects on Brain and Blood. Cell Transplant. 2009, 18, 951–975. [Google Scholar] [CrossRef]
- Seo, M.K.; Choi, C.M.; McIntyre, R.S.; Cho, H.Y.; Lee, C.H.; Mansur, R.B.; Lee, Y.; Lee, J.-H.; Kim, Y.H.; Park, S.W. Effects of Escitalopram and Paroxetine on MTORC1 Signaling in the Rat Hippocampus under Chronic Restraint Stress. BMC Neurosci. 2017, 18, 39. [Google Scholar] [CrossRef]
- Li, N.; Lee, B.; Liu, R.-J.; Banasr, M.; Dwyer, J.M.; Iwata, M.; Li, X.-Y.; Aghajanian, G.; Duman, R.S. MTOR-Dependent Synapse Formation Underlies the Rapid Antidepressant Effects of NMDA Antagonists. Science (80-) 2010, 329, 959–964. [Google Scholar] [CrossRef] [Green Version]
- Fienberg, A.A.; Hiroi, N.; Mermelstein, P.G.; Song, W.-J.; Snyder, G.L.; Nishi, A.; Cheramy, A.; O’callaghan, J.P.; Miller, D.B.; Cole, D.G. DARPP-32: Regulator of the Efficacy of Dopaminergic Neurotransmission. Science (80-) 1998, 281, 838–842. [Google Scholar] [CrossRef] [Green Version]
- Nishi, A.; Shuto, T. Potential for Targeting Dopamine/DARPP-32 Signaling in Neuropsychiatric and Neurodegenerative Disorders. Expert Opin. Ther. Targets 2017, 21, 259–272. [Google Scholar] [CrossRef]
- Nishi, A.; Bibb, J.A.; Matsuyama, S.; Hamada, M.; Higashi, H.; Nairn, A.C.; Greengard, P. Regulation of DARPP-32 Dephosphorylation at PKA-and Cdk5-sites by NMDA and AMPA Receptors: Distinct Roles of Calcineurin and Protein Phosphatase-2A. J. Neurochem. 2002, 81, 832–841. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Kuang, S.; Li, H.; Ran, D.; Yang, J. CAMP/PKA-CREB-BDNF Signaling Pathway in Hippocampus Mediates Cyclooxygenase 2-Induced Learning/Memory Deficits of Rats Subjected to Chronic Unpredictable Mild Stress. Oncotarget 2017, 8, 35558. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krishnan, V.; Han, M.-H.; Graham, D.L.; Berton, O.; Renthal, W.; Russo, S.J.; LaPlant, Q.; Graham, A.; Lutter, M.; Lagace, D.C. Molecular Adaptations Underlying Susceptibility and Resistance to Social Defeat in Brain Reward Regions. Cell 2007, 131, 391–404. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patki, G.; Solanki, N.; Atrooz, F.; Allam, F.; Salim, S. Depression, Anxiety-like Behavior and Memory Impairment Are Associated with Increased Oxidative Stress and Inflammation in a Rat Model of Social Stress. Brain Res. 2013, 1539, 73–86. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shin, J.A.; Lee, K.-E.; Kim, H.-S.; Park, E.-M. Acute Resveratrol Treatment Modulates Multiple Signaling Pathways in the Ischemic Brain. Neurochem. Res. 2012, 37, 2686–2696. [Google Scholar] [CrossRef] [PubMed]
- Greenberg, M.E.; Xu, B.; Lu, B.; Hempstead, B.L. New Insights in the Biology of BDNF Synthesis and Release: Implications in CNS Function. J. Neurosci. 2009, 29, 12764–12767. [Google Scholar] [CrossRef] [Green Version]
- Minichiello, L. TrkB Signalling Pathways in LTP and Learning. Nat. Rev. Neurosci. 2009, 10, 850–860. [Google Scholar] [CrossRef]
- Chao, M.V.; Bothwell, M.A.; Ross, A.H.; Koprowski, H.; Lanahan, A.A.; Buck, C.R.; Sehgal, A. Gene Transfer and Molecular Cloning of the Human NGF Receptor. Science (80-) 1986, 232, 518–521. [Google Scholar] [CrossRef]
- Sandhya, V.K.; Raju, R.; Verma, R.; Advani, J.; Sharma, R.; Radhakrishnan, A.; Nanjappa, V.; Narayana, J.; Somani, B.L.; Mukherjee, K.K. A Network Map of BDNF/TRKB and BDNF/P75NTR Signaling System. J. Cell Commun. Signal. 2013, 7, 301–307. [Google Scholar] [CrossRef] [Green Version]





© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vasilopoulou, F.; Bagan, A.; Rodriguez-Arevalo, S.; Escolano, C.; Griñán-Ferré, C.; Pallàs, M. Amelioration of BPSD-Like Phenotype and Cognitive Decline in SAMP8 Mice Model Accompanied by Molecular Changes after Treatment with I2-Imidazoline Receptor Ligand MCR5. Pharmaceutics 2020, 12, 475. https://doi.org/10.3390/pharmaceutics12050475
Vasilopoulou F, Bagan A, Rodriguez-Arevalo S, Escolano C, Griñán-Ferré C, Pallàs M. Amelioration of BPSD-Like Phenotype and Cognitive Decline in SAMP8 Mice Model Accompanied by Molecular Changes after Treatment with I2-Imidazoline Receptor Ligand MCR5. Pharmaceutics. 2020; 12(5):475. https://doi.org/10.3390/pharmaceutics12050475
Chicago/Turabian StyleVasilopoulou, Foteini, Andrea Bagan, Sergio Rodriguez-Arevalo, Carmen Escolano, Christian Griñán-Ferré, and Mercè Pallàs. 2020. "Amelioration of BPSD-Like Phenotype and Cognitive Decline in SAMP8 Mice Model Accompanied by Molecular Changes after Treatment with I2-Imidazoline Receptor Ligand MCR5" Pharmaceutics 12, no. 5: 475. https://doi.org/10.3390/pharmaceutics12050475