Mechanochemical Synthesis and Physicochemical Characterization of Previously Unreported Praziquantel Solvates with 2-Pyrrolidone and Acetic Acid
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Praziquantel Solvates
2.3. Characterization of Praziquantel Solvates
2.3.1. Thermal Analyses
2.3.2. FT-IR Spectroscopy
2.3.3. Powder X-ray Diffraction (PXRD)
2.3.4. Synchrotron X-ray Powder Diffraction and Solvate Structure Solution
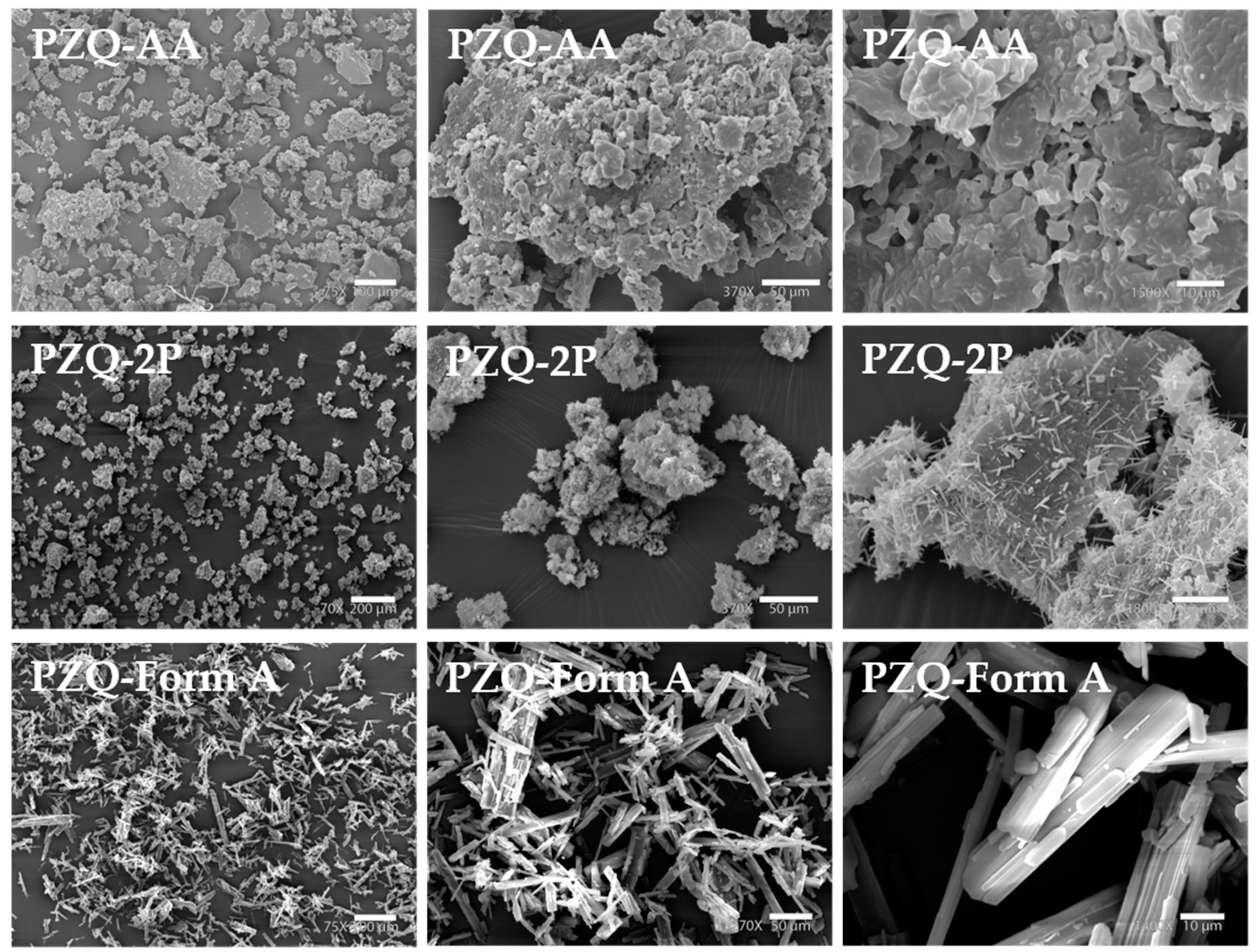
2.3.5. Scanning Electron Microscopy (SEM)
2.3.6. Solid-State NMR Measurements
2.3.7. Periodic DFT Geometry Optimization
2.3.8. GIPAW-DFT
2.3.9. Water Solubility and Intrinsic Dissolution Rate
2.3.10. Physical Stability
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Colley, D.G.; Bustinduy, A.L.; Secor, W.E.; King, C.H. Human Schistosomiasis. Lancet 2014, 383, 2253–2264. [Google Scholar] [CrossRef]
- Utzinger, J.; Keiser, J. Schistosomiasis and Soil-Transmitted Helminthiasis: Common Drugs for Treatment and Control. Expert Opin. Pharmacother. 2004, 5, 263–285. [Google Scholar] [CrossRef]
- Schistosomiasis. Available online: https://www.who.int/news-room/fact-sheets/detail/schistosomiasis (accessed on 11 September 2021).
- WHO Model List of Essential Medicines. Available online: https://www.who.int/publications-detail-redirect/WHOMVPEMPIAU2019.06 (accessed on 11 September 2021).
- WHO Model List of Essential Medicines for Children—7th List. 2019. Available online: https://www.who.int/publications-detail-redirect/WHOMVPEMPIAU201907 (accessed on 11 September 2021).
- Espinosa-Lara, J.C.; Guzman-Villanueva, D.; Arenas-García, J.I.; Herrera-Ruiz, D.; Rivera-Islas, J.; Román-Bravo, P.; Morales-Rojas, H.; Höpfl, H. Cocrystals of Active Pharmaceutical Ingredients—Praziquantel in Combination with Oxalic, Malonic, Succinic, Maleic, Fumaric, Glutaric, Adipic, And Pimelic Acids. Cryst. Growth Des. 2013, 13, 169–185. [Google Scholar] [CrossRef]
- Meyer, T.; Sekljic, H.; Fuchs, S.; Bothe, H.; Schollmeyer, D.; Miculka, C. Taste, A New Incentive to Switch to (R)-Praziquantel in Schistosomiasis Treatment. PLoS Negl. Trop. Dis. 2009, 3, e357. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.; Wang, X.; Wang, J.-K.; Ching, C.B. Investigation of the Phase Diagrams of Chiral Praziquantel. Chirality 2006, 18, 259–264. [Google Scholar] [CrossRef]
- Zanolla, D.; Perissutti, B.; Passerini, N.; Chierotti, M.R.; Hasa, D.; Voinovich, D.; Gigli, L.; Demitri, N.; Geremia, S.; Keiser, J.; et al. A New Soluble and Bioactive Polymorph of Praziquantel. Eur. J. Pharm. Biopharm. 2018, 127, 19–28. [Google Scholar] [CrossRef]
- Zanolla, D.; Perissutti, B.; Vioglio, P.C.; Chierotti, M.R.; Gigli, L.; Demitri, N.; Passerini, N.; Albertini, B.; Franceschinis, E.; Keiser, J.; et al. Exploring Mechanochemical Parameters Using a DoE Approach: Crystal Structure Solution from Synchrotron XRPD and Characterization of a New Praziquantel Polymorph. Eur. J. Pharm. Sci. 2019, 140, 105084. [Google Scholar] [CrossRef]
- Zanolla, D.; Hasa, D.; Arhangelskis, M.; Schneider-Rauber, G.; Chierotti, M.R.; Keiser, J.; Voinovich, D.; Jones, W.; Perissutti, B. Mechanochemical Formation of Racemic Praziquantel Hemihydrate with Improved Biopharmaceutical Properties. Pharmaceutics 2020, 12, 289. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Griesser, U.J. The Importance of Solvates. In Polymorphism; John Wiley & Sons, Ltd: Hoboken, NJ, USA, 2006; pp. 211–233. ISBN 978-3-527-60788-4. [Google Scholar]
- Hasa, D.; Jones, W. Screening for New Pharmaceutical Solid Forms Using Mechanochemistry: A Practical Guide. Adv. Drug Deliv. Rev. 2017, 117, 147–161. [Google Scholar] [CrossRef]
- Aitipamula, S.; Banerjee, R.; Bansal, A.K.; Biradha, K.; Cheney, M.L.; Choudhury, A.R.; Desiraju, G.R.; Dikundwar, A.G.; Dubey, R.; Duggirala, N.; et al. Polymorphs, Salts, and Cocrystals: What’s in a Name? Cryst. Growth Des. 2012, 12, 2147–2152. [Google Scholar] [CrossRef]
- Trask, A.V.; Shan, N.; Motherwell, W.D.S.; Jones, W.; Feng, S.; Tan, R.B.H.; Carpenter, K.J. Selective Polymorph Transformation via Solvent-Drop Grinding. Chem. Commun. 2005, 880–882. [Google Scholar] [CrossRef] [PubMed]
- Tan, D.; Loots, L.; Friščić, T. Towards Medicinal Mechanochemistry: Evolution of Milling from Pharmaceutical Solid Form Screening to the Synthesis of Active Pharmaceutical Ingredients (APIs). Chem. Commun. 2016, 52, 7760–7781. [Google Scholar] [CrossRef] [PubMed]
- Friščić, T.; Halasz, I.; Strobridge, F.C.; Dinnebier, R.E.; Stein, R.S.; Fábián, L.; Curfs, C. A Rational Approach to Screen for Hydrated Forms of the Pharmaceutical Derivative Magnesium Naproxen Using Liquid-Assisted Grinding. CrystEngComm 2011, 13, 3125–3129. [Google Scholar] [CrossRef]
- Wang, P.; Li, G.; Chen, Y.; Chen, S.; James, S.L.; Yuan, W. Mechanochemical Interconversion between Discrete Complexes and Coordination Networks—Formal Hydration/Dehydration by LAG. CrystEngComm 2012, 14, 1994–1997. [Google Scholar] [CrossRef]
- Karki, S.; Friscić, T.; Jones, W.; Motherwell, W.D.S. Screening for Pharmaceutical Cocrystal Hydrates via Neat and Liquid-Assisted Grinding. Mol. Pharm. 2007, 4, 347–354. [Google Scholar] [CrossRef] [PubMed]
- Hasa, D.; Pastore, M.; Arhangelskis, M.; Gabriele, B.; Cruz-Cabeza, A.J.; Rauber, G.S.; Bond, A.D.; Jones, W. On the Kinetics of Solvate Formation through Mechanochemistry. CrystEngComm 2019, 21, 2097–2104. [Google Scholar] [CrossRef] [Green Version]
- Hasa, D.; Carlino, E.; Jones, W. Polymer-Assisted Grinding, a Versatile Method for Polymorph Control of Cocrystallization. Cryst. Growth Des. 2016, 16, 1772–1779. [Google Scholar] [CrossRef]
- Product Information Reports. Available online: https://www.usp-pqm.org/resources/product-information-reports (accessed on 11 September 2021).
- Friić, T.; Childs, S.L.; Rizvi, S.A.A.; Jones, W. The Role of Solvent in Mechanochemical and Sonochemical Cocrystal Formation: A Solubility-Based Approach for Predicting Cocrystallisation Outcome. CrystEngComm 2009, 11, 418–426. [Google Scholar] [CrossRef]
- Zanolla, D.; Perissutti, B.; Passerini, N.; Invernizzi, S.; Voinovich, D.; Bertoni, S.; Melegari, C.; Millotti, G.; Albertini, B. Milling and Comilling Praziquantel at Cryogenic and Room Temperatures: Assessment of the Process-Induced Effects on Drug Properties. J. Pharm. Biomed. Anal. 2018, 153, 82–89. [Google Scholar] [CrossRef]
- Lausi, A.; Polentarutti, M.; Onesti, S.; Plaisier, J.R.; Busetto, E.; Bais, G.; Barba, L.; Cassetta, A.; Campi, G.; Lamba, D.; et al. Status of the Crystallography Beamlines at Elettra. Eur. Phys. J. Plus 2015, 130, 43. [Google Scholar] [CrossRef]
- Hammersley, A.P.; Svensson, S.O.; Hanfland, M.; Fitch, A.N.; Hausermann, D. Two-Dimensional Detector Software: From Real Detector to Idealised Image or Two-Theta Scan. High Press. Res. 1996, 14, 235–248. [Google Scholar] [CrossRef]
- The FIT2D Home Page. Available online: https://www.esrf.fr/computing/scientific/FIT2D/ (accessed on 11 September 2021).
- Altomare, A.; Cuocci, C.; Giacovazzo, C.; Moliterni, A.; Rizzi, R.; Corriero, N.; Falcicchio, A. EXPO2013: A kit of tools for phasing crystal structures from powder data. J. Appl. Cryst. 2013, 46, 1231–1235. [Google Scholar] [CrossRef]
- Espinosa-Lara, J.C.; Guzman-Villanueva, D.; Arenas-Garcia, J.I.; Herrera-Ruiz, D.; Rivera-Islas, J.; Roman-Bravo, P.; Morales-Rojas, H.; Hopfl, H. CCDC 896766: Experimental Crystal Structure Determination; The Cambridge Crystallographic Data Centre (CCDC): Cambridge, UK, 2013. [Google Scholar]
- BIOVIA Materials Studio—BIOVIA—Dassault Systèmes®. Available online: https://www.3ds.com/products-services/biovia/products/molecular-modeling-simulation/biovia-materials-studio/ (accessed on 11 September 2021).
- Clark, S.J.; Segall, M.D.; Pickard, C.J.; Hasnip, P.J.; Probert, M.I.J.; Refson, K.; Payne, M.C. First Principles Methods Using CASTEP. Z. Krist. Cryst. Mater. 2005, 220, 567–570. [Google Scholar] [CrossRef] [Green Version]
- Björkman, T. CIF2Cell: Generating Geometries for Electronic Structure Programs. Comput. Phys. Commun. 2011, 182, 1183–1186. [Google Scholar] [CrossRef]
- Vela, A.; Pacheco-Kato, J.C.; Gázquez, J.L.; del Campo, J.M.; Trickey, S.B. Improved Constraint Satisfaction in a Simple Generalized Gradient Approximation Exchange Functional. J. Chem. Phys. 2012, 136, 144115. [Google Scholar] [CrossRef]
- Tkatchenko, A.; DiStasio, R.A.; Car, R.; Scheffler, M. Accurate and Efficient Method for Many-Body van Der Waals Interactions. Phys. Rev. Lett. 2012, 108, 236402. [Google Scholar] [CrossRef]
- Ambrosetti, A.; Reilly, A.M.; DiStasio, R.A.; Tkatchenko, A. Long-Range Correlation Energy Calculated from Coupled Atomic Response Functions. J. Chem. Phys. 2014, 140, 18A508. [Google Scholar] [CrossRef] [Green Version]
- Reilly, A.M.; Tkatchenko, A. Van Der Waals Dispersion Interactions in Molecular Materials: Beyond Pairwise Additivity. Chem. Sci. 2015, 6, 3289–3301. [Google Scholar] [CrossRef] [Green Version]
- van de Streek, J.; Neumann, M.A. Validation of Molecular Crystal Structures from Powder Diffraction Data with Dispersion-Corrected Density Functional Theory (DFT-D). Acta Crystallogr. Sect. B Struct. Sci. Cryst. Eng. Mater. 2014, 70, 1020–1032. [Google Scholar] [CrossRef]
- Pickard, C.J.; Mauri, F. All-Electron Magnetic Response with Pseudopotentials: NMR Chemical Shifts. Phys. Rev. B 2001, 63, 245101. [Google Scholar] [CrossRef] [Green Version]
- Handbook of Organic Solvents. Available online: https://www.routledge.com/Handbook-of-Organic-Solvents/Lide/p/book/9780849389306 (accessed on 1 March 2021).
- Marcus, Y. The Properties of Organic Liquids That Are Relevant to Their Use as Solvating Solvents. Chem. Soc. Rev. 1993, 22, 409–416. [Google Scholar] [CrossRef]
- Gu, C.-H.; Li, H.; Gandhi, R.B.; Raghavan, K. Grouping Solvents by Statistical Analysis of Solvent Property Parameters: Implication to Polymorph Screening. Int. J. Pharm. 2004, 283, 117–125. [Google Scholar] [CrossRef]
- Lee, S.H.; Lee, S.B. The Hildebrand Solubility Parameters, Cohesive Energy Densities and Internal Energies of 1-Alkyl-3-Methylimidazolium-Based Room Temperature Ionic Liquids. Chem. Commun. 2005, 3469. [Google Scholar] [CrossRef] [Green Version]
- Berziņš, A.; Skarbulis, E.; Actiņš, A. Structural Characterization and Rationalization of Formation, Stability, and Transformations of Benperidol Solvates. Cryst. Growth Des. 2015, 15, 2337–2351. [Google Scholar] [CrossRef]
- Gumbert, S.D.; Körbitzer, M.; Alig, E.; Schmidt, M.U.; Chierotti, M.R.; Gobetto, R.; Li, X.; van de Streek, J. Crystal Structure and Tautomerism of Pigment Yellow 138 Determined by X-Ray Powder Diffraction and Solid-State NMR. Dye. Pigment. 2016, 131, 364–372. [Google Scholar] [CrossRef]
- Többens, D.M.; Glinneman, J.; Chierotti, M.R.; van de Streek, J.; Sheptyakov, D. On the High-Temperature Phase of Barbituric Acid. CrystEngComm 2012, 14, 3046–3055. [Google Scholar] [CrossRef]
- Chierotti, M.R.; Ferrero, L.; Garino, N.; Gobetto, R.; Pellegrino, L.; Braga, D.; Grepioni, F.; Maini, L. The Richest Collection of Tautomeric Polymorphs: The Case of 2-Thiobarbituric Acid. Chem. Eur. J. 2010, 16, 4347–4358. [Google Scholar] [CrossRef]
- Othman, A.; Evans, J.S.O.; Evans, I.R.; Harris, R.K.; Hodgkinson, P. Structural Study of Polymorphs and Solvates of Finasteride. J. Pharm. Sci. 2007, 96, 1380–1397. [Google Scholar] [CrossRef]
- Rossi, F.; Cerreia Vioglio, P.; Bordignon, S.; Giorgio, V.; Nervi, C.; Priola, E.; Gobetto, R.; Yazawa, K.; Chierotti, M.R. Unraveling the Hydrogen Bond Network in a Theophylline–Pyridoxine Salt Cocrystal by a Combined X-Ray Diffraction, Solid-State NMR, and Computational Approach. Cryst. Growth Des. 2018, 18, 2225–2233. [Google Scholar] [CrossRef]
- Bordignon, S.; Cerreia Vioglio, P.; Priola, E.; Voinovich, D.; Gobetto, R.; Nishiyama, Y.; Chierotti, M.R. Engineering Codrug Solid Forms: Mechanochemical Synthesis of an Indomethacin–Caffeine System. Cryst. Growth Des. 2017, 17, 5744–5752. [Google Scholar] [CrossRef]
- Bērziņš, A.; Hodgkinson, P. Solid-State NMR and Computational Investigation of Solvent Molecule Arrangement and Dynamics in Isostructural Solvates of Droperidol. Solid State Nucl. Magn. Reson. 2015, 65, 12–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cerreia Vioglio, P.; Chierotti, M.R.; Gobetto, R. Pharmaceutical Aspects of Salt and Cocrystal Forms of APIs and Characterization Challenges. Adv. Drug Deliv. Rev. 2017, 117, 86–110. [Google Scholar] [CrossRef]
- LeBlanc, L.M.; Dale, S.G.; Taylor, C.R.; Becke, A.D.; Day, G.M.; Johnson, E.R. Pervasive Delocalisation Error Causes Spurious Proton Transfer in Organic Acid–Base Co-Crystals. Angew. Chem. Int. Ed. 2018, 57, 14906–14910. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.; Zhang, X.; Wang, M.; Ma, Y.; Tang, W. Uncovering the Effect of Solvents on Solid-Liquid Phase Equilibrium of Praziquantel. J. Mol. Liq. 2020, 297, 111917. [Google Scholar] [CrossRef]
- Xu, K.; Qian, M.; Leng, J.; Bai, J.; Li, Q.; Liu, Z.; Zhong, S.; Zhao, S. Direct Salinization of Trelagliptin from Solid Forms by Mechanochemistry and Its Mechanism of Salt Formation. CrystEngComm 2020, 22, 8256–8265. [Google Scholar] [CrossRef]
- Ahmadi, S.; Mondal, P.K.; Mirmehrabi, M.; Rohani, S. Desolvation of Dasatinib Methanolate: An Improved Anhydrous Polymorph. CrystEngComm 2021, 23, 4272–4283. [Google Scholar] [CrossRef]
- Braga, D.; Giaffreda, S.L.; Grepioni, F.; Pettersen, A.; Maini, L.; Curzi, M.; Polito, M. Mechanochemical Preparation of Molecular and Supramolecular Organometallic Materials and Coordination Networks. Dalton Trans. 2006, 1249–1263. [Google Scholar] [CrossRef]
- Arhangelskis, M.; Bučar, D.-K.; Bordignon, S.; Chierotti, M.R.; Stratford, S.A.; Voinovich, D.; Jones, W.; Hasa, D. Mechanochemical Reactivity Inhibited, Prohibited and Reversed by Liquid Additives: Examples from Crystal-Form Screens. Chem. Sci. 2021, 12, 3264–3269. [Google Scholar] [CrossRef]
- Sha, J.; Gong, Y.; Cao, Z.; Huang, Z.; Hu, X.; Wan, Y.; Sun, R.; He, H.; Jiang, G.; Li, Y.; et al. Solid-Liquid Phase Equilibrium of Praziquantel in Eleven Pure Solvents: Determination, Model Correlation, Solvent Effect, Molecular Simulation and Thermodynamic Analysis. J. Chem. Thermodyn. 2020, 154, 106327. [Google Scholar] [CrossRef]
- El-Subbagh, H.I.; Al-Badr, A.A. Praziquantel. In Analytical Profiles of Drug Substances and Excipients; Brittain, H.G., Ed.; Analytical Profiles of Drug Substances and Excipients; Academic Press: Cambridge, MA, USA, 1998; Volume 25, pp. 463–500. [Google Scholar]
- Zhang, X.; Zhou, L.; Wang, C.; Li, Y.; Wu, Y.; Zhang, M.; Yin, Q. Insight into the Role of Hydrogen Bonding in the Molecular Self-Assembly Process of Sulfamethazine Solvates. Cryst. Growth Des. 2017, 17, 6151–6157. [Google Scholar] [CrossRef]
- Bourne, S.A.; Caira, M.R.; Nassimbeni, L.R.; Shabalala, I. X-ray Structural Studies and Physicochemical Characterization of the I-Butanol, I-Pentanol, and 1,4-Dioxane Solvates of Succinylsulfathiazole. J. Pharm. Sci. 1994, 83, 887–892. [Google Scholar] [CrossRef] [PubMed]
- Braun, D.E.; Gelbrich, T.; Griesser, U.J. Experimental and Computational Approaches to Produce and Characterise Isostructural Solvates. CrystEngComm 2019, 21, 5533–5545. [Google Scholar] [CrossRef]
- Sridhar, B.; Nanubolu, J.B.; Ravikumar, K.; Karthik, G.; Reddy, B.V.S. Three Isostructural Solvates of a Tetra hydro furochromenone Derivative. Acta Crystallogr. Sect. C Struct. Chem. 2017, 73, 407–413. [Google Scholar] [CrossRef] [PubMed]







| Solvent | Boiling Point (°C) [39] | H donor Ability (α) [40] | H bond Acceptor Ability (β) [40] | Polarizability [40] | Cohesive Energy Density (J mol/mL) [41] | Starting PZQ Solid Form | Observed Solid Form |
|---|---|---|---|---|---|---|---|
| acetic acid | 118 | 112 | 45 | 64 | 370.80 | A | AA |
| B | AA | ||||||
| acetonitrile | 81.6 | 19 | 40 | 75 | 522.95 | A | A |
| 1,4-dioxane | 101.3 | 0 | 37 | 55 | 372.17 | A | A |
| ethanol | 78.3 | 86 | 75 | 54 | 618.87 | A | A |
| B | A | ||||||
| ethyl acetate | 77.1 | 0 | 45 | 55 | 300.64 | A | A |
| B | A | ||||||
| methanol | 64.7 | 98 | 66 | 60 | 808.26 | A | A |
| nitromethane | 101.0 | 22 | 06 | 85 | 587.22 | A | A |
| 2-pyrrolidone | 245.0 | 36 | 77 | 85 | 789.61 [42] | A | 2P |
| B | 2P |
| Phase | PZQ-AA | PZQ-2P |
|---|---|---|
| Empirical Formula | C19H24N2O2·(C2H4O2) | C19H24N2O2·(C4H7NO) |
| Formula weight | 372.46 | 397.51 |
| Crystal system | triclinic | Triclinic |
| Space group | P-1 | P-1 |
| a (Å) | 6.0762 (2) | 6.1839 (2) |
| b (Å) | 11.6197 (4) | 12.2615 (3) |
| c (Å) | 14.5619 (8) | 14.5539 (5) |
| Volume (Å3) | 997.16 | 1058.04 |
| α (°) | 97.292 (3) | 93.823 (2) |
| β (°) | 98.851 (3) | 100.064 (2) |
| γ (°) | 96.987 (2) | 101.693(1) |
| Density (g·cm−3) | 1.240 | 1.248 |
| Z | 2 | 2 |
| R-Bragg factor (%) | 3.32 | 2.58 |
| wR factor (%) | 4.70 | 4.04 |
| Goodness of fit | 4.65 | 3.79 |
| Atom | Group | PZQ-AA | PZQ-2P | ||
|---|---|---|---|---|---|
| δiso(exp)/ppm | δiso(calc)/ppm * | δiso(exp)/ppm | δiso(calc)/ppm * | ||
| 7’ | C=O | 172.1 | 172.68 | 172.9 | 173.22 |
| 4 | C=O | 166.8 | 167.92 | 165.7 | 166.66 |
| 11a | Cq | 133.1 | 135.96 | 133.4 | 135.75 |
| 7a | Cq | 134.8 | 138.03 | 133.4 | 137.14 |
| 8 | CH(ar.) | 129.1 | 132.30 | 128.1 | 131.17 |
| 11 | CH(ar.) | 124.7 | 126.84 | 124.6 | 126.89 |
| 10 | CH(ar.) | 126.7 | 128.66 | 128.1 | 130.88 |
| 9 | CH(ar.) | 126.7 | 128.91 | 127.4 | 130.10 |
| 11b | CH(aliph.) | 53.7 | 53.42 | 54.0 | 54.2 |
| 3 | CH2 | 46.4 | 45.51 | 47.2 | 46.3 |
| 1 | CH2 | 43.3 | 41.07 | 44.3 | 42.80 |
| 1’ | CH(aliph.) | 39.8 | 39.06 | 39.7 | 39.63 |
| 6 | CH2 | 37.2 | 34.71 | 37.6 | 34.84 |
| 6’ | CH2 | 28.9 | 27.68 | 28.9 | 27.22 |
| 2’ | CH2 | 28.9 | 27.38 | 28.9 | 28.41 |
| 7 | CH2 | 26.4 | 25.30 | 27.8 | 26.22 |
| 4’ | CH2 | 27.0 | 25.61 | 25.8 | 25.05 |
| 3’ | CH2 | 26.4 | 25.46 | 25.8 | 24.37 |
| 5’ | CH2 | 25.0 | 23.60 | 25.8 | 24.58 |
| AA ** | COOH | 172.1 | 177.13 | ||
| AA | CH3 | 19.7 | 18.03 | ||
| 2P | CH2 | 19.4 | 19.09 | ||
| 2P | CH2 | 28.9 | 28.16 | ||
| 2P | CH2 | 40.4 | 41.48 | ||
| 2P | CONH | 177.5 | 178.94 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zanolla, D.; Gigli, L.; Hasa, D.; Chierotti, M.R.; Arhangelskis, M.; Demitri, N.; Jones, W.; Voinovich, D.; Perissutti, B. Mechanochemical Synthesis and Physicochemical Characterization of Previously Unreported Praziquantel Solvates with 2-Pyrrolidone and Acetic Acid. Pharmaceutics 2021, 13, 1606. https://doi.org/10.3390/pharmaceutics13101606
Zanolla D, Gigli L, Hasa D, Chierotti MR, Arhangelskis M, Demitri N, Jones W, Voinovich D, Perissutti B. Mechanochemical Synthesis and Physicochemical Characterization of Previously Unreported Praziquantel Solvates with 2-Pyrrolidone and Acetic Acid. Pharmaceutics. 2021; 13(10):1606. https://doi.org/10.3390/pharmaceutics13101606
Chicago/Turabian StyleZanolla, Debora, Lara Gigli, Dritan Hasa, Michele R. Chierotti, Mihails Arhangelskis, Nicola Demitri, William Jones, Dario Voinovich, and Beatrice Perissutti. 2021. "Mechanochemical Synthesis and Physicochemical Characterization of Previously Unreported Praziquantel Solvates with 2-Pyrrolidone and Acetic Acid" Pharmaceutics 13, no. 10: 1606. https://doi.org/10.3390/pharmaceutics13101606






