The Impact of Improving Dermal Permeation on the Efficacy and Targeting of Liposome Nanoparticles as a Potential Treatment for Breast Cancer
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Box–Behnken Design
2.3. Preparation of Raloxifene-Loaded Deformable Liposome Formulations
2.4. In Vitro Characterization of RLDL Formulations
2.4.1. Entrapment Efficiency Determination
2.4.2. Zeta Potential and Particle Size Determination
2.4.3. Ex Vivo Drug Permeation and Skin Deposition Studies
2.5. Optimization of RLDL Formulations
2.6. In Vitro Characterization of the Optimum RLDL Formulation
2.6.1. Thermal Analysis Studies
2.6.2. STEM Measurements
2.6.3. In Vitro Drug Release Studies
2.6.4. Drug Release Kinetics
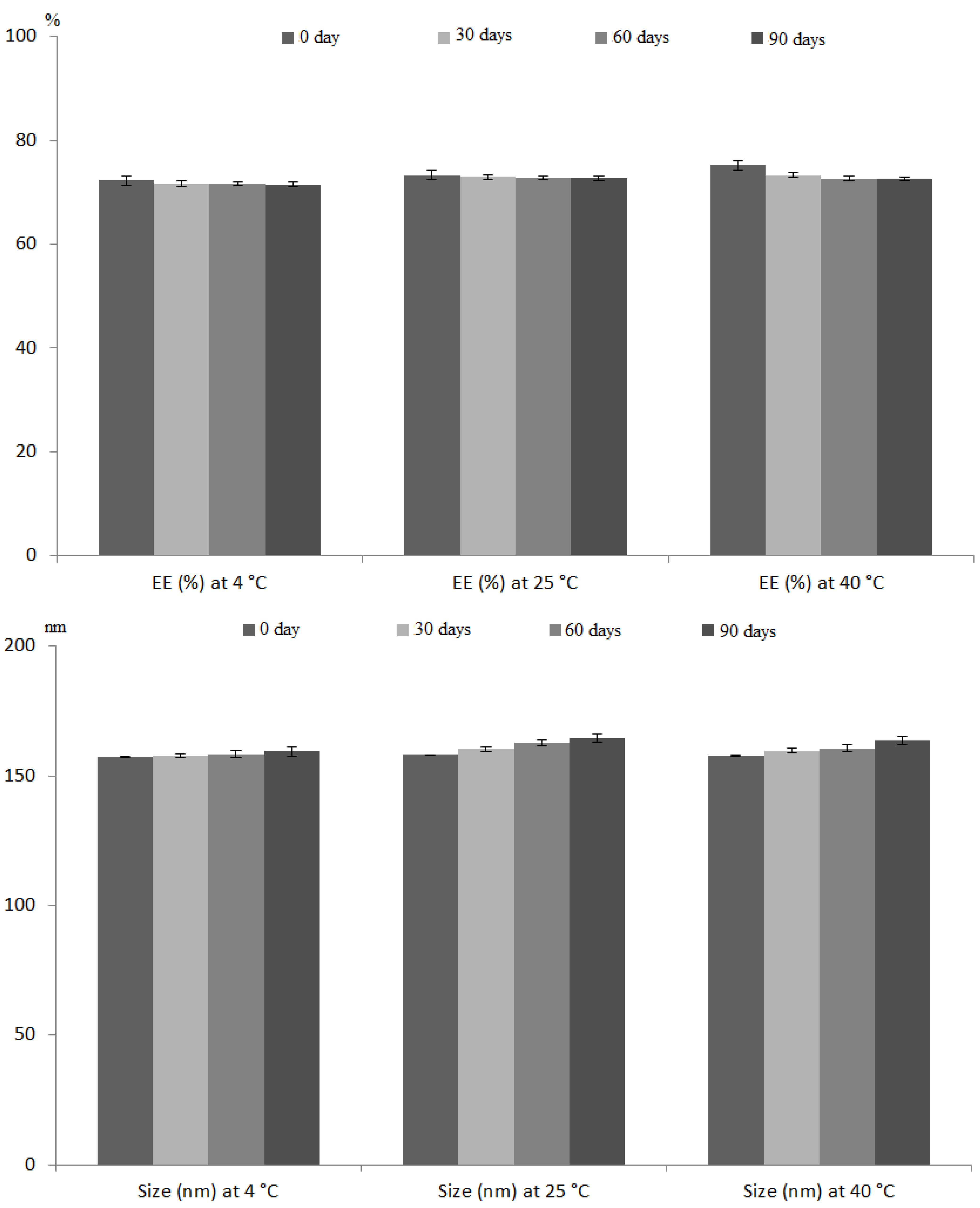
2.6.5. Stability Studies
2.7. Preparation and In Vitro Characterization of Optimum RLDL Formulation Gel
2.7.1. Preparation of Optimum RLDL Formulation Gel
2.7.2. In Vitro Characterization of Optimum RLDL Formulation Gel
2.8. In Vivo Antitumour Characterization of the Optimum RLDL Gel
2.8.1. Study Design
2.8.2. Animals
2.8.3. Antitumour Activity and Toxicity Determination
2.8.4. In Vivo Permeation and Bioavailability Studies
2.9. Statistical Analysis
3. Results and Discussion
3.1. Preparation of Raloxifene-Loaded Deformable Liposoms Formulations
3.2. In Vitro Characterization of RLDL Formultionse
3.2.1. Entrapment Efficiency Determination
3.2.2. Zeta Potential and Particle Size Determination
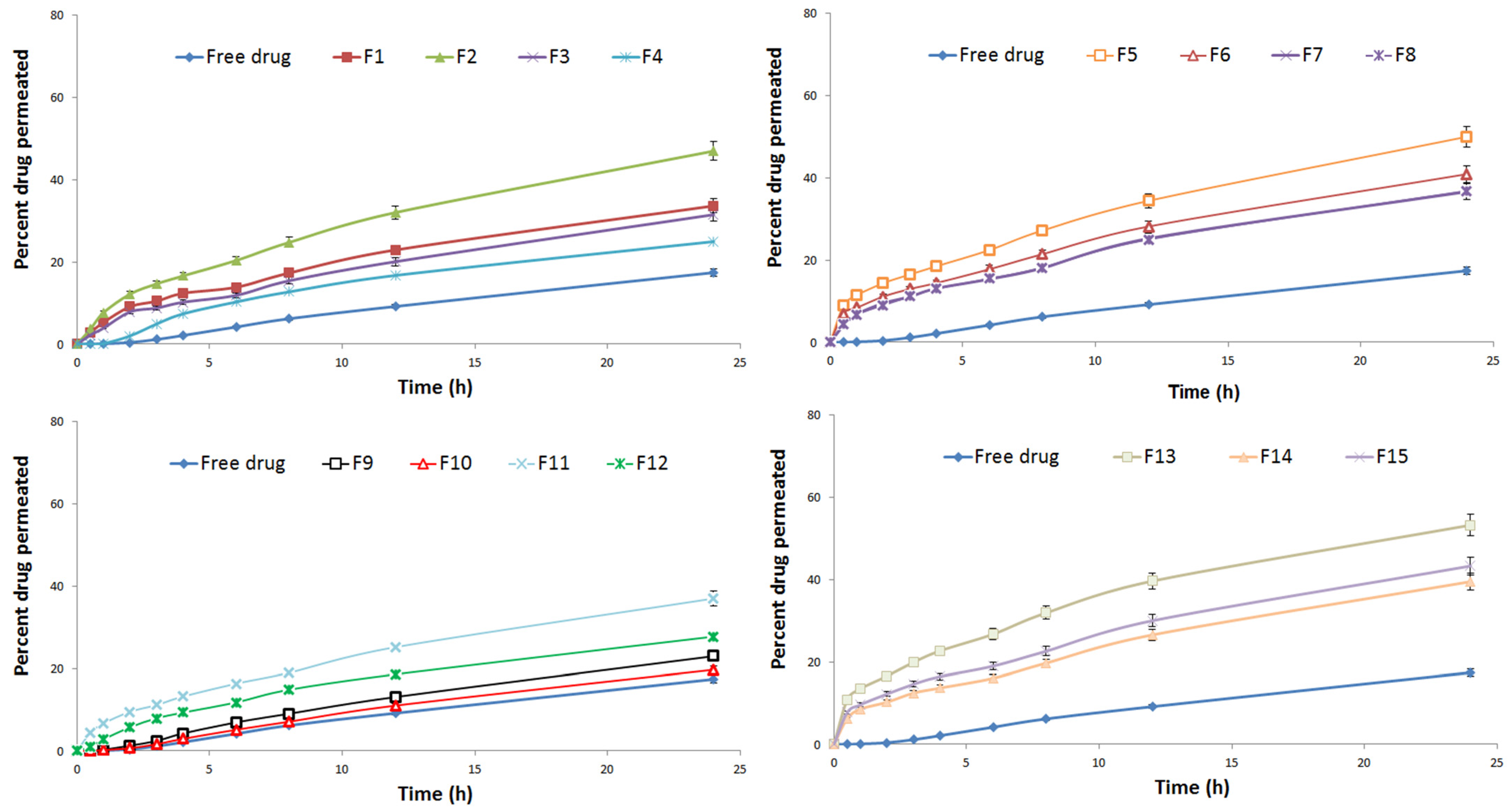
3.2.3. Ex Vivo Drug Permeation and Skin Deposition Studies
3.3. Optimization of RLDL Formulations
3.4. In Vitro Characterization of the Optimum RLDL Formulation
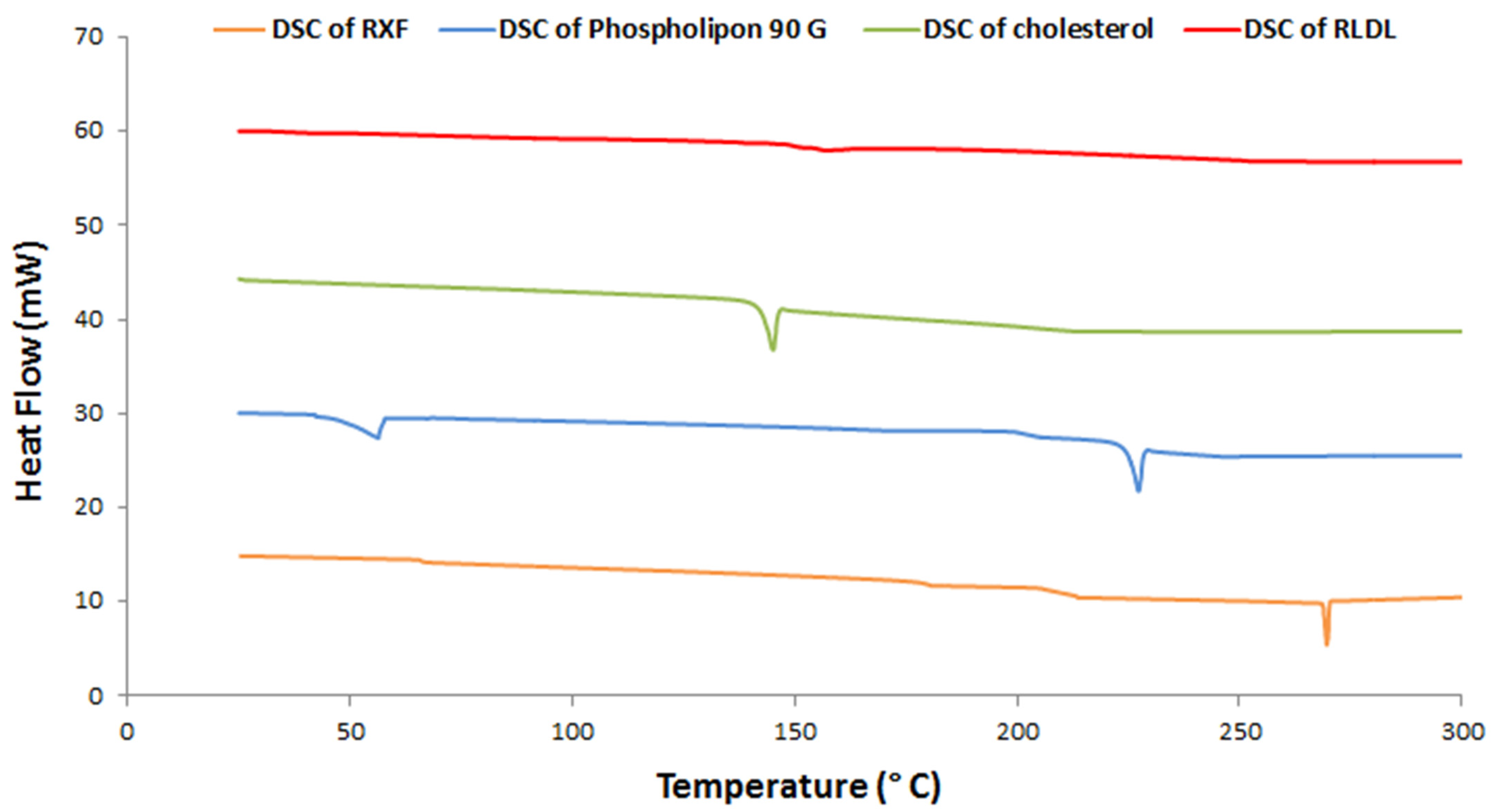
3.4.1. Thermal Analysis Studies
3.4.2. STEM Measurements
3.4.3. Stability Studies
3.4.4. In Vitro Drug Release Kinetic Studies
3.5. Preparation and In Vitro Characterization of RLDL Gel Formulations
3.6. In Vivo Antitumour Characterization of the Optimum RLDL Gel
3.6.1. Antitumour Activity and Toxicity Determination
3.6.2. In Vivo Permeation and Bioavailability Studies
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- Sun, Y.-S.; Zhao, Z.; Yang, Z.-N.; Xu, F.; Lu, H.-J.; Zhu, Z.-Y.; Shi, W.; Jiang, J.; Yao, P.-P.; Zhu, H.-P. Risk Factors and Preventions of Breast Cancer. Int. J. Biol. Sci. 2017, 13, 1387–1397. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harris, J.R.; Lippman, M.E.; Veronesi, U.; Willett, W. Breast cancer. N. Engl. J. Med. 1992, 327, 319–328. [Google Scholar] [CrossRef]
- Key, T.J.; Verkasalo, P.K.; Banks, E. Epidemiology of breast cancer. Lancet Oncol. 2001, 2, 133–140. [Google Scholar] [CrossRef]
- Kashanian, S.; Rafipour, R. A Review on Targeting Nanoparticles for Breast Cancer. Curr. Pharm. Biotechnol. 2019, 20, 1087–1107. [Google Scholar] [CrossRef]
- Yezhelyev, M.V.; Gao, X.; Xing, Y.; Al-Hajj, A.; Nie, S.; O’Regan, R.M. Emerging use of nanoparticles in diagnosis and treatment of breast cancer. Lancet Oncol. 2006, 7, 657–667. [Google Scholar] [CrossRef]
- Dianzani, C.; Zara, G.P.; Maina, G.; Pettazzoni, P.; Pizzimenti, S.; Rossi, F.; Gigliotti, C.L.; Ciamporcero, E.S.; Daga, M.; Barrera, G. Drug delivery nanoparticles in skin cancers. BioMed Res. Int. 2014, 2014, 895986. [Google Scholar] [CrossRef]
- Mezei, M.; Gulasekharam, V. Liposomes-a selective drug delivery system for the topical route of administration I. Lotion dosage form. Life Sci. 1980, 26, 1473–1477. [Google Scholar] [CrossRef]
- Elsayed, M.M.; Abdallah, O.Y.; Naggar, V.F.; Khalafallah, N.M. Deformable liposomes and ethosomes: Mechanism of enhanced skin delivery. Int. J. Pharm. 2006, 322, 60–66. [Google Scholar] [CrossRef]
- Zellmer, S.; Pfeil, W.; Lasch, J. Interaction of phosphatidylcholine liposomes with the human stratum corneum. Biochim. Biophys. Acta (BBA)-Biomembr. 1995, 1237, 176–182. [Google Scholar] [CrossRef] [Green Version]
- Elmoslemany, R.M.; Abdallah, O.Y.; El-Khordagui, L.K.; Khalafallah, N.M. Propylene Glycol Liposomes as a Topical Delivery System for Miconazole Nitrate: Comparison with Conventional Liposomes. AAPS PharmSciTech 2012, 13, 723–731. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abdulbaqi, I.M.; Darwis, Y.; Khan, N.A.K.; Assi, R.A.; Khan, A.A. Ethosomal nanocarriers: The impact of constituents and formulation techniques on ethosomal properties, in vivo studies, and clinical trials. Int. J. Nanomed. 2016, 11, 2279–2304. [Google Scholar] [CrossRef] [Green Version]
- Calienni, M.N.; Febres-Molina, C.; Llovera, R.E.; Zevallos-Delgado, C.; Tuttolomondo, M.E.; Paolino, D.; Fresta, M.; Barazorda-Ccahuana, H.L.; Gómez, B.; Alonso, S.D.V.; et al. Nanoformulation for potential topical delivery of Vismodegib in skin cancer treatment. Int. J. Pharm. 2019, 565, 108–122. [Google Scholar] [CrossRef] [PubMed]
- Sabale, V.; Vora, S. Formulation and evaluation of microemulsion-based hydrogel for topical delivery. Int. J. Pharm. Investig. 2012, 2, 140–149. [Google Scholar] [CrossRef] [Green Version]
- Mandal, U.; Mahmood, S.; Taher, M. Experimental design and optimization of raloxifene hydrochloride loaded nanotransfersomes for transdermal application. Int. J. Nanomed. 2014, 9, 4331–4346. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smita, N.; Sanidhya, S.; Bhaskar, V. Formulation and evaluation of Raloxifene hydrochloride tablets with improved dissolution profile. Int. J. Adv. Pharm. 2016, 5, 127–139. [Google Scholar]
- Tefas, L.R.; Sylvester, B.; Tomuta, I.; Sesarman, A.; Licarete, E.; Banciu, M.; Porfire, A. Development of antiproliferative long-circulating liposomes co-encapsulating doxorubicin and curcumin, through the use of a quality-by-design approach. Drug Des. Dev. Ther. 2017, 11, 1605–1621. [Google Scholar] [CrossRef] [Green Version]
- Gamal, F.A.; Kharshoum, R.M.; Sayed, O.M.; El-Ela, F.I.A.; Salem, H.F. Control of basal cell carcinoma via positively charged ethosomes of Vismodegib: In vitro and in vivo studies. J. Drug Deliv. Sci. Technol. 2020, 56, 101556. [Google Scholar] [CrossRef]
- Gamal, A.; Saeed, H.; Sayed, O.M.; Kharshoum, R.M.; Salem, H.F. Proniosomal Microcarriers: Impact of Constituents on the Physicochemical Properties of Proniosomes as a New Approach to Enhance Inhalation Efficiency of Dry Powder Inhalers. AAPS PharmSciTech 2020, 21, 156. [Google Scholar] [CrossRef]
- Salem, H.F.; Kharshoum, R.M.; Abou-Taleb, H.A.; AbouTaleb, H.A.; AbouElhassan, K.M. Progesterone-loaded nanosized transethosomes for vaginal permeation enhancement: Formulation, statistical optimization, and clinical evaluation in anovulatory polycystic ovary syndrome. J. Liposome Res. 2019, 29, 183–194. [Google Scholar] [CrossRef]
- Shen, L.-N.; Zhang, Y.-T.; Wang, Q.; Xu, L.; Feng, N.-P. Enhanced in vitro and in vivo skin deposition of apigenin delivered using ethosomes. Int. J. Pharm. 2014, 460, 280–288. [Google Scholar] [CrossRef]
- Salem, H.F.; Kharshoum, R.M.; El-Ela, F.I.A.; Amr Gamal, F.; Abdellatif, K.R.A. Evaluation and optimization of pH-responsive niosomes as a carrier for efficient treatment of breast cancer. Drug Deliv. Transl. Res. 2018, 8, 633–644. [Google Scholar] [CrossRef] [PubMed]
- Salem, H.F.; Kharshoum, R.M.; El-Ela, F.I.A.; Amr Gamal, F.; Abdellatif, K.R.A. Treatment of breast cancer with engineered novel pH-sensitive Triaryl-(Z)-olefin niosomes con-taining hydrogel: An in vitro and in vivo study. J. Liposome Res. 2020, 30, 126–135. [Google Scholar] [CrossRef]
- Salem, H.F.; Kharshoum, R.M.; Sayed, O.M.; Hakim, L.F.A. Formulation design and optimization of novel soft glycerosomes for enhanced topical delivery of celecoxib and cupferron by Box–Behnken statistical design. Drug Dev. Ind. Pharm. 2018, 44, 1871–1884. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Huo, M.; Zhou, J.; Zou, A.; Li, W.; Yao, C.; Xie, S. DDSolver: An Add-In Program for Modeling and Comparison of Drug Dissolution Profiles. AAPS J. 2010, 12, 263–271. [Google Scholar] [CrossRef] [Green Version]
- Zuo, J.; Gao, Y.; Bou-Chacra, N.; Löbenberg, R. Evaluation of the DDSolver Software Applications. BioMed Res. Int. 2014, 2014, 204925. [Google Scholar] [CrossRef] [Green Version]
- Akash MS, H.; Rehman, K.; Li, N.; Gao, J.Q.; Sun, H.; Chen, S. Sustained delivery of IL-1Ra from pluronic F127-based thermosensitive gel prolongs its thera-peutic potentials. Pharm. Res. 2012, 29, 3475–3485. [Google Scholar] [CrossRef]
- Nasr, M.; Mansour, S.; Mortada, N.; Elshamy, A.A. Vesicular aceclofenac systems: A comparative study between liposomes and niosomes. J. Microencapsul. 2008, 25, 499–512. [Google Scholar] [CrossRef] [PubMed]
- Dias, M.F.; de Figueiredo BC, P.; Teixeira-Neto, J.; Guerra MC, A.; Fialho, S.L.; Cunha, A.S. In vivo evaluation of antitumoral and antiangiogenic effect of imiquimod-loaded polymeric na-noparticles. Biomed. Pharmacother. 2018, 103, 1107–1114. [Google Scholar] [CrossRef]
- Aziz RL, A.; Abdel-Wahab, A.; El-Ela FI, A.; Hassan NE, H.Y.; El-Nahass, E.S.; Ibrahim, M.A.; Khalil, A.T.A. Dose-dependent ameliorative effects of quercetin and l-Carnitine against atrazine-induced re-productive toxicity in adult male Albino rats. Biomed. Pharmacother. 2018, 102, 855–864. [Google Scholar] [CrossRef]
- Trontelj, J.; Vovk, T.; Bogataj, M.; Mrhar, A. HPLC analysis of raloxifene hydrochloride and its application to drug quality control studies. Pharmacol. Res. 2005, 52, 334–339. [Google Scholar] [CrossRef]
- Ammar, H.O.; Haider, M.; Ibrahim, M.; El Hoffy, N.M. In vitro and in vivo investigation for optimization of niosomal ability for sustainment and bioa-vailability enhancement of diltiazem after nasal administration. Drug Deliv. 2017, 24, 414–421. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Praça, F.S.G.; Medina, W.S.G.; Eloy, J.O.; Petrilli, R.; Campos, P.M.; Ascenso, A.; Bentley, M.V.L. Evaluation of critical parameters for in vitro skin permeation and penetration studies using animal skin models. Eur. J. Pharm. Sci. 2018, 111, 121–132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abd, E.; Gomes, J.; Sales, C.C.; Yousef, S.; Forouz, F.; Telaprolu, K.C.; Roberts, M.S.; Grice, J.E.; Lopes, P.S.; Leite-Silva, V.R.; et al. Deformable liposomes as enhancer of caffeine penetration through human skin in a Franz diffusion cell test. Int. J. Cosmet. Sci. 2020, 43, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Schreier, H.; Bouwstra, J. Liposomes and niosomes as topical drug carriers: Dermal and transdermal drug delivery. J. Control. Release 1994, 30, 1–15. [Google Scholar] [CrossRef]
- Vemuri, S.; Rhodes, C. Preparation and characterization of liposomes as therapeutic delivery systems: A review. Pharm. Acta Helv. 1995, 70, 95–111. [Google Scholar] [CrossRef]
- Bangham, A.D.; Hill, M.W.; Miller, N.G.A. Preparation and Use of Liposomes as Models of Biological Membranes. In Methods in Membrane Biology; Springer: Berlin/Heidelberg, Germany, 1974; pp. 1–68. [Google Scholar]
- Franklin, R.K.; Marcus, S.A.; Talaat, A.M.; KuKanich, B.K.; Sullivan, R.; Krugner-Higby, L.A.; Heath, T.D. A novel loading method for doxycycline liposomes for intracellular drug delivery: Characteri-zation of in vitro and in vivo release kinetics and efficacy in a J774A. 1 cell line model of Mycobacterium smegmatis infection. Drug Metab. Dispos. 2015, 43, 1236–1245. [Google Scholar] [CrossRef] [Green Version]
- Akhtar, N.; Pathak, K. Cavamax W7 Composite Ethosomal Gel of Clotrimazole for Improved Topical Delivery: Development and Comparison with Ethosomal Gel. AAPS PharmSciTech 2012, 13, 344–355. [Google Scholar] [CrossRef] [Green Version]
- Nasseri, B. Effect of cholesterol and temperature on the elastic properties of niosomal membranes. Int. J. Pharm. 2005, 300, 95–101. [Google Scholar] [CrossRef]
- El-Ela, F.I.A.; Hussein, K.H.; El-Banna, H.A.; Gamal, A.; Rouby, S.; Menshawy, A.M.; EL-Shimaa, E.N.; Anwar, S.; Zeinhom, M.M.; Salem, H.F.; et al. Treatment of Brucellosis in Guinea Pigs via a Combination of Engineered Novel pH-Responsive Curcumin Niosome Hydrogel and Doxycycline-Loaded Chitosan–Sodium Alginate Nanoparticles: An In Vitro and In Vivo Study. AAPS PharmSciTech 2020, 21, 326. [Google Scholar] [CrossRef]
- Gamal, A.; Sayed, O.; El-Ela, F.I.A.; Kharshoum, R.M.; Salem, H.F. Treatment of Basal Cell Carcinoma Via Binary Ethosomes of Vismodegib: In Vitro and In Vivo Studies. AAPS PharmSciTech 2020, 21, 51. [Google Scholar] [CrossRef]
- Shaker, S.; Gardouh, A.R.M.; Ghorab, M.M. Factors affecting liposomes particle size prepared by ethanol injection method. Res. Pharm. Sci. 2017, 12, 346–352. [Google Scholar] [CrossRef]
- Hoare, T.R.; Kohane, D.S. Hydrogels in drug delivery: Progress and challenges. Polymer 2008, 49, 1993–2007. [Google Scholar] [CrossRef] [Green Version]
- Jain, S.; Patel, N.; Madan, P.; Lin, S. Formulation and rheological evaluation of ethosome-loaded carbopol hydrogel for transdermal application. Drug Dev. Ind. Pharm. 2016, 42, 1315–1324. [Google Scholar] [CrossRef]
- Poorahmary Kermany, B. Carbopol Hydrogels for Topical Administration: Treatment of Wounds. Master’s Thesis, Universitetet i Tromsø, Tromsø, Norway, 2010. [Google Scholar]
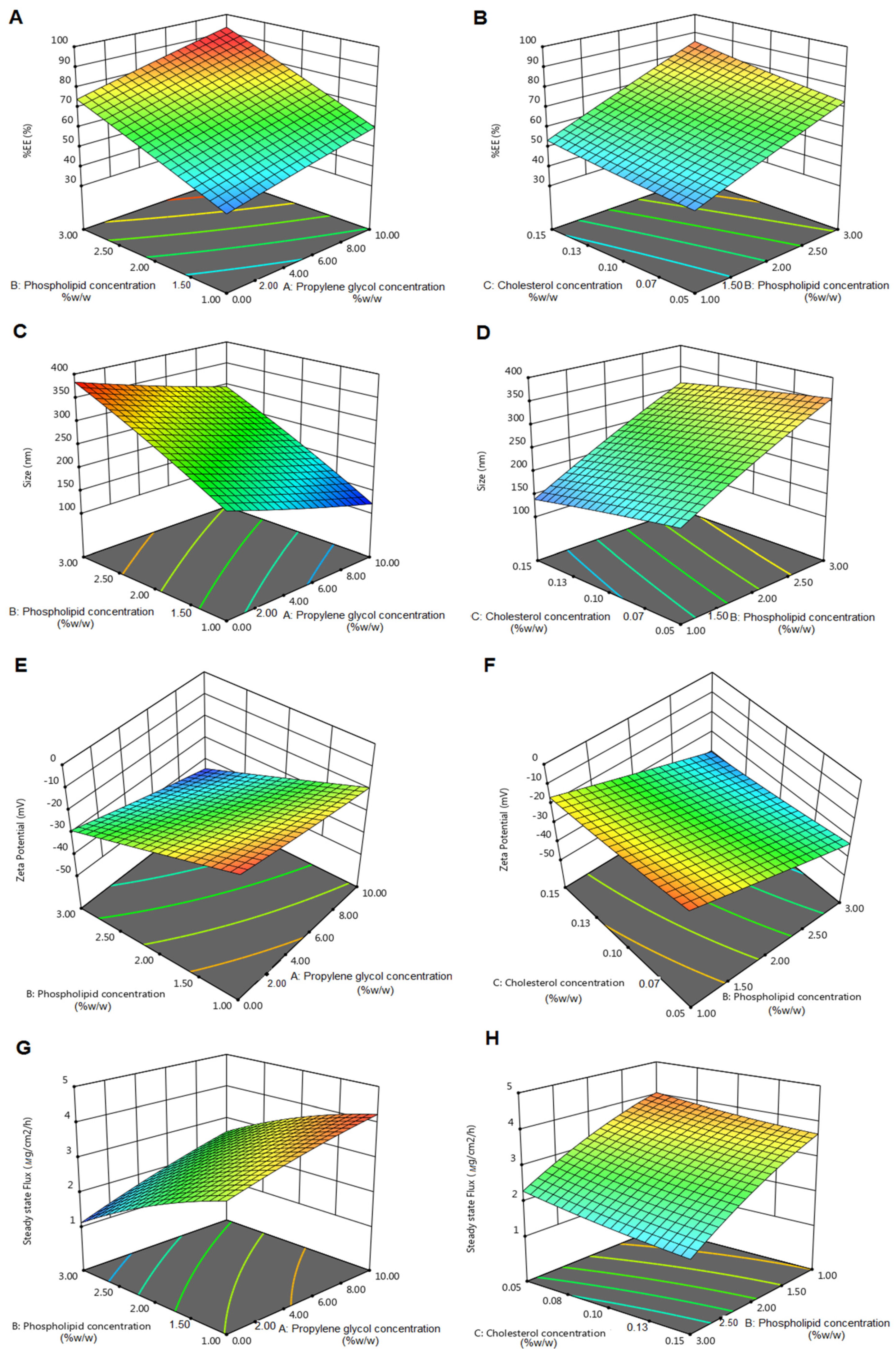


| Formulation Code | (X1) % w/v | (X2) % w/v | (X3) % w/v | Y1 (%) (Mean ± SD) | Y2 (nm) (Mean ± SD) | Y3 (mV) (Mean ± SD) | Y4 (µg/cm2/h) (Mean ± SD) |
|---|---|---|---|---|---|---|---|
| F1 | 0 | 2 | 0.05 | 52.32 ± 0.84 | 329.80 ± 4.51 | −14.17 ± 0.31 | 2.72 ± 0.01 |
| F2 | 10 | 2 | 0.05 | 66.39 ± 0.83 | 242.80 ± 5.07 | −27.10 ± 0.56 | 3.87 ± 0.02 |
| F3 | 10 | 3 | 0.1 | 87.53 ± 1.10 | 295.33 ± 2.60 | −44.30 ± 0.20 | 2.54 ± 0.03 |
| F4 | 0 | 2 | 0.15 | 61.35 ± 0.96 | 283.50 ± 2.89 | −20.63 ± 0.38 | 2.05 ± 0.02 |
| F5 | 5 | 1 | 0.05 | 46.32 ± 0.84 | 192.43 ± 2.52 | −9.73 ± 0.59 | 4.00 ± 0.03 |
| F6 | 10 | 2 | 0.15 | 78.50 ± 0.88 | 181.83 ± 2.51 | −35.03 ± 0.40 | 3.44 ± 0.03 |
| F7 | 5 | 2 | 0.1 | 64.19 ± 0.53 | 258.93 ± 6.62 | −25.50 ± 0.36 | 3.06 ± 0.03 |
| F8 | 5 | 2 | 0.1 | 64.41 ± 0.56 | 257.87 ± 5.24 | −25.23 ± 0.51 | 3.04 ± 0.03 |
| F9 | 5 | 3 | 0.15 | 85.05 ± 0.82 | 315.53 ± 6.24 | −39.67 ± 0.51 | 1.72 ± 0.05 |
| F10 | 0 | 3 | 0.1 | 69.41 ± 0.94 | 382.87 ± 2.48 | −28.67 ± 0.4 | 1.08 ± 0.04 |
| F11 | 5 | 2 | 0.1 | 64.24 ± 0.6 | 257.10 ± 7.49 | −25.23 ± 0.35 | 3.02 ± 0.01 |
| F12 | 5 | 3 | 0.05 | 73.27 ± 0.91 | 355.27 ± 4.9 | −32.17 ± 0.45 | 2.32 ± 0.03 |
| F13 | 10 | 1 | 0.1 | 56.31 ± 0.93 | 122.13 ± 2.63 | −18.50 ± 0.40 | 4.27 ± 0.02 |
| F14 | 0 | 1 | 0.1 | 41.31 ± 0.95 | 221.90 ± 3.78 | −7.80 ± 0.36 | 3.27 ± 0.02 |
| F15 | 5 | 1 | 0.15 | 53.38 ± 0.69 | 140.77 ± 2.08 | −16.23 ± 0.31 | 3.63 ± 0.04 |
| Parameter | Value | |
|---|---|---|
| Symbol | Name | |
| f1 | Difference factor | 40.5738 ± 3.21 |
| f2 | Similarity factor | 34.1217 ± 2.65 |
| f1cp | Difference factor modified by Costa. P | 48.7473 ± 4.02 |
| D | Sum of squared mean differences | 4306.495 ± 14.43 |
| D1 | Mean distance | 16.9071 ± 2.10 |
| D2 | Mean squared distance | 20.7520 ± 2.14 |
| Res1 | Rescigno index 1 | 0.24452 ± 0.03 |
| Res2 | Rescigno index 2 | 0.2448 ± 0.05 |
| Sd | Difference in similarity | 0.2503 ± 0.05 |
| DAUC | Difference of area under the profiles | −636.824 ± 9.46 |
| DABC | Area between the profiles | 426.730 ± 5.17 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salem, H.F.; Gamal, A.; Saeed, H.; Tulbah, A.S. The Impact of Improving Dermal Permeation on the Efficacy and Targeting of Liposome Nanoparticles as a Potential Treatment for Breast Cancer. Pharmaceutics 2021, 13, 1633. https://doi.org/10.3390/pharmaceutics13101633
Salem HF, Gamal A, Saeed H, Tulbah AS. The Impact of Improving Dermal Permeation on the Efficacy and Targeting of Liposome Nanoparticles as a Potential Treatment for Breast Cancer. Pharmaceutics. 2021; 13(10):1633. https://doi.org/10.3390/pharmaceutics13101633
Chicago/Turabian StyleSalem, Heba F., Amr Gamal, Haitham Saeed, and Alaa S. Tulbah. 2021. "The Impact of Improving Dermal Permeation on the Efficacy and Targeting of Liposome Nanoparticles as a Potential Treatment for Breast Cancer" Pharmaceutics 13, no. 10: 1633. https://doi.org/10.3390/pharmaceutics13101633