Plant-Mediated Zinc Oxide Nanoparticles: Advances in the New Millennium towards Understanding Their Therapeutic Role in Biomedical Applications
Abstract
:1. Introduction
2. Zinc Oxide Nanoparticles
3. Synthesis of Zinc Oxide Nanoparticles from Plants
4. Biomedical Applications of Plant-Mediated Zinc Oxide Nanoparticles
4.1. Antibacterial Activity
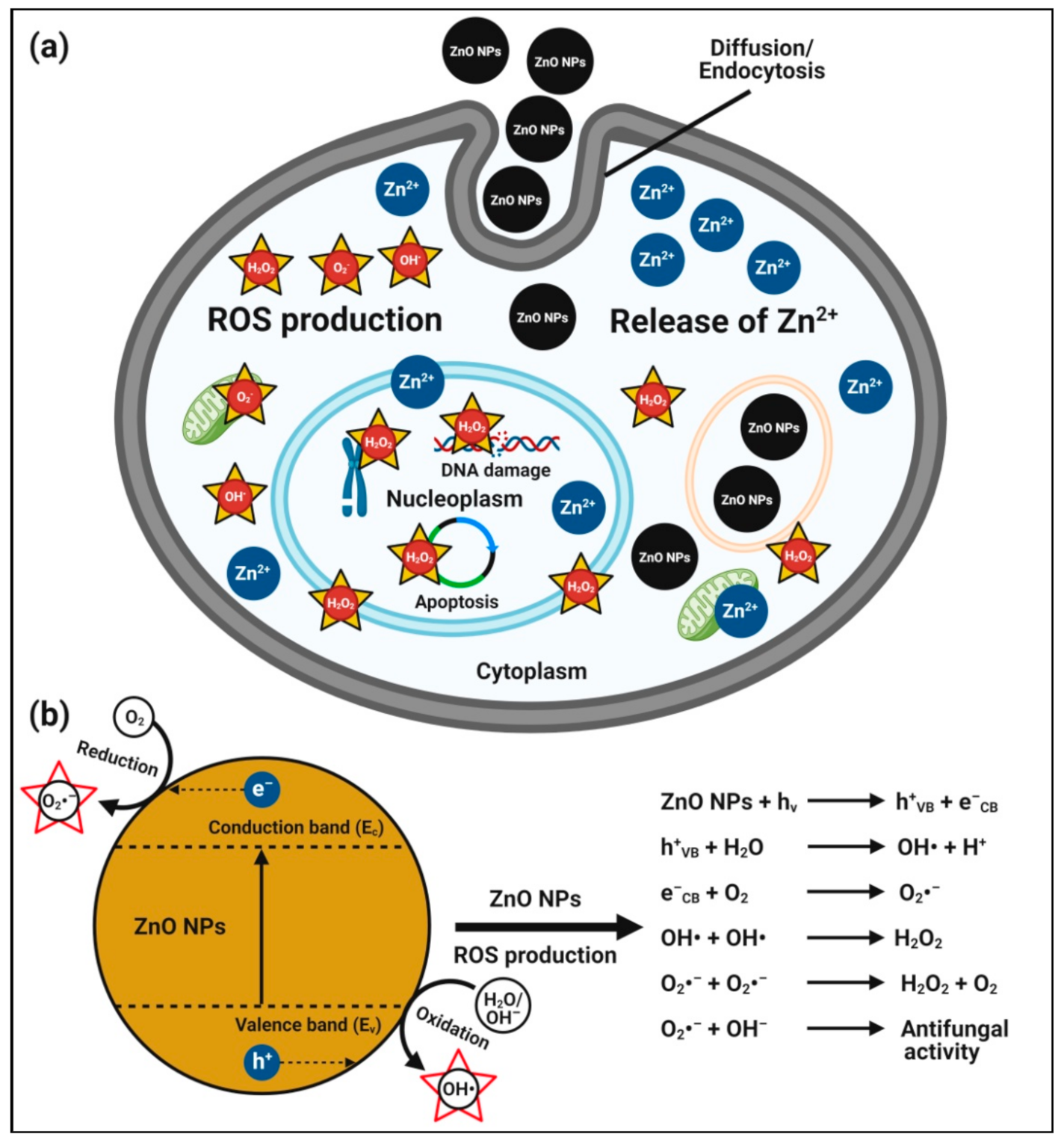
4.2. Antifungal Activity
4.3. Antioxidant Activity
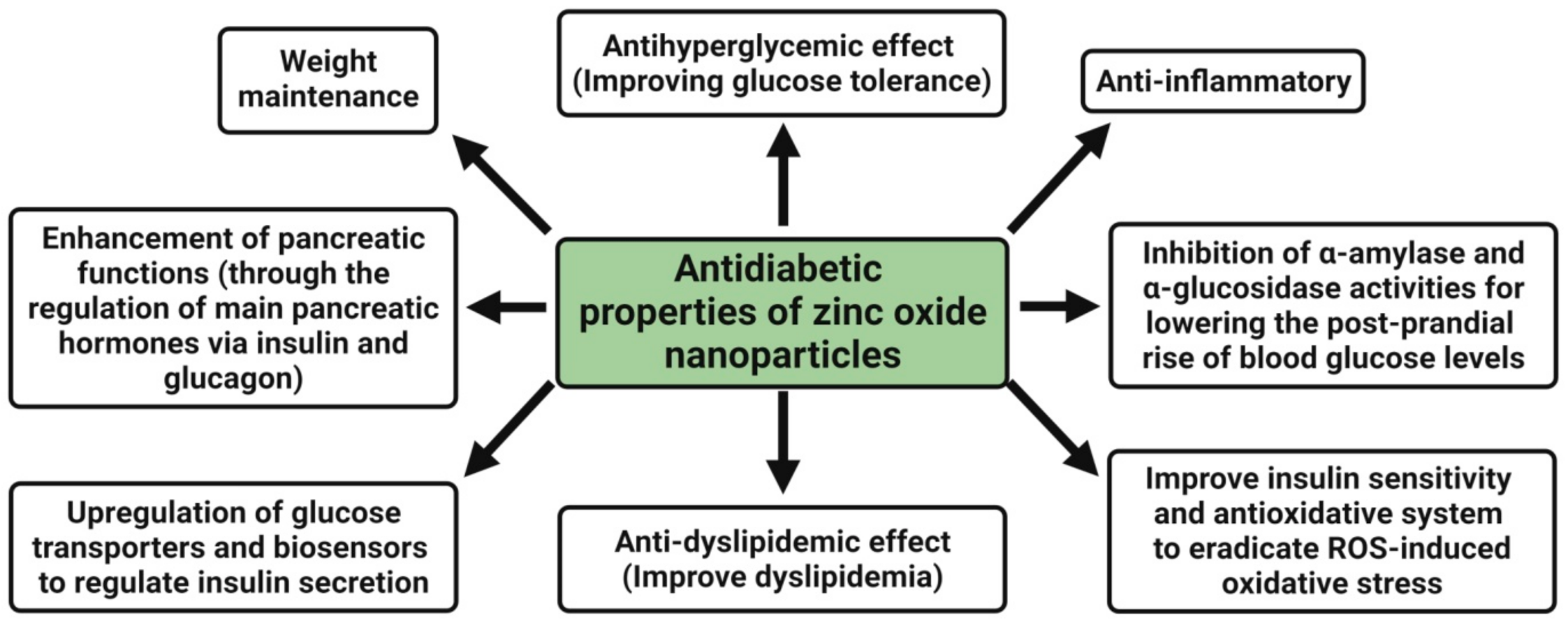
4.4. Antidiabetic Activity
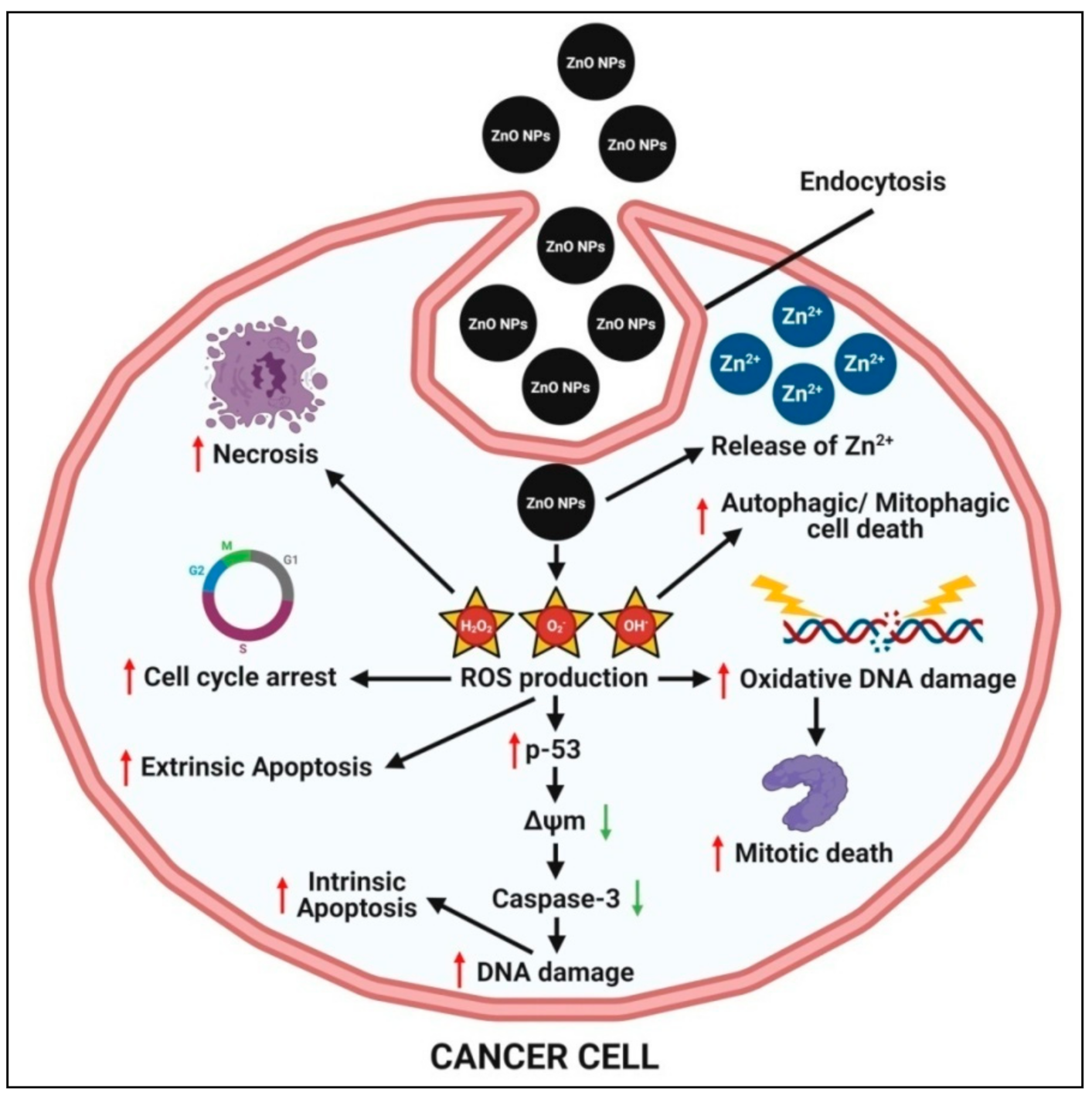
4.5. Anticancer Activity
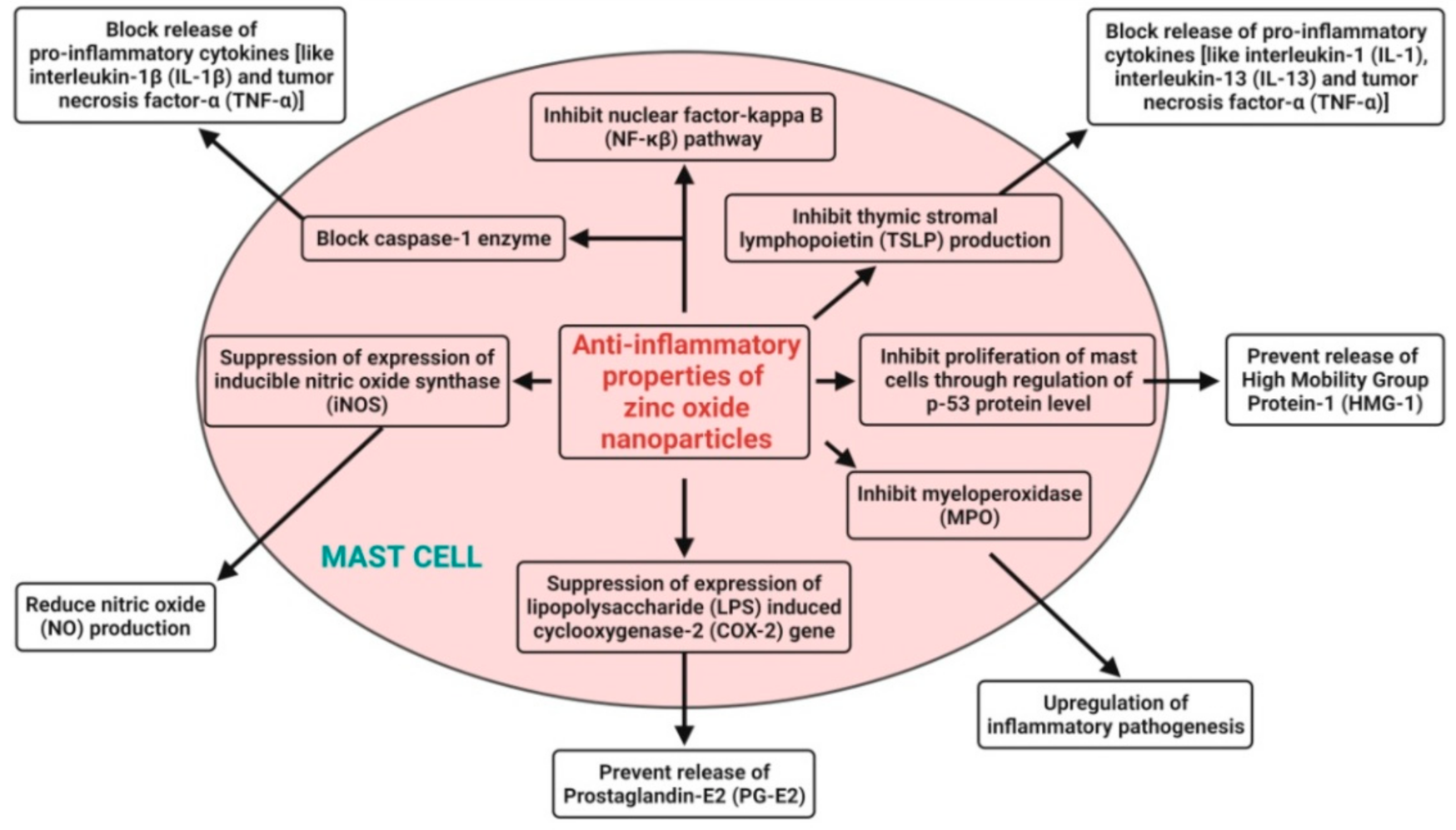
4.6. Anti-Inflammatory Activity
4.7. Photocatalytic Activity
4.8. Wound-Healing Activity
4.9. Targetted Drug Delivery System
4.10. Tissue Engineering and Regenerative Medicine
5. Future Perspective and Conclusion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Anandan, S.; Mahadevamurthy, M.; Ansari, M.A.; Alzohairy, M.A.; Alomary, M.N.; Farha Siraj, S.; Sarjan, H.N.; Mahendra, C.; Lakshmeesha, T.R.; Hemanth Kumar, N.K.; et al. Biosynthesized ZnO-NPs from Morus indica attenuates methylglyoxal-induced protein glycation and RBC damage: In-vitro, in-vivo and molecular docking study. Biomolecules 2019, 9, 882. [Google Scholar] [CrossRef] [Green Version]
- Ansari, M.A.; Murali, M.; Prasad, D.; Alzohairy, M.A.; Almatroudi, A.; Alomary, M.N.; Udayashankar, A.C.; Singh, S.B.; Asiri, S.M.M.; Ashwini, B.S.; et al. Cinnamomum verum bark extract mediated green synthesis of ZnO nanoparticles and their antibacterial potentiality. Biomolecules 2020, 10, 336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kavya, J.B.; Murali, M.; Manjula, S.; Basavaraj, G.L.; Prathibha, M.; Jayaramu, S.C.; Amruthesh, K.N. Genotoxic and antibacterial nature of biofabricated zinc oxide nanoparticles from Sida rhombifolia Linn. J. Drug Deliv. Sci. Technol. 2020, 60, 101982. [Google Scholar] [CrossRef]
- Murali, M.; Anandan, S.; Ansari, M.A.; Alzohairy, M.A.; Alomary, M.N.; Asiri, S.M.M.; Almatroudi, A.; Thrivveni, M.C.; Brijesh Singh, S.; Gowtham, H.G.; et al. Genotoxic and cytotoxic properties of Zinc oxide nanoparticles phyto-fabricated from the Obscure morning glory plant Ipomoea obscura (L.) Ker Gawl. Molecules 2021, 26, 891. [Google Scholar] [CrossRef] [PubMed]
- Murali, M.; Mahendra, C.; Nagabhushan; Rajashekar, N.; Sudarshana, M.S.; Raveesha, K.A.; Amruthesh, K.N. Antibacterial and antioxidant properties of biosynthesized zinc oxide nanoparticles from Ceropegia candelabrum L.—An endemic species. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2017, 179, 104–109. [Google Scholar] [CrossRef]
- Azizi, S.; Mohamad, R.; Bahadoran, A.; Bayat, S.; Rahim, R.A.; Ariff, A.; Saad, W.Z. Effect of annealing temperature on antimicrobial and structural properties of bio-synthesized zinc oxide nanoparticles using flower extract of Anchusa italic. J. Photochem. Photobiol. B Biol. 2016, 161, 441–449. [Google Scholar] [CrossRef]
- Padalia, H.; Chanda, S. Characterization, antifungal and cytotoxic evaluation of green synthesized zinc oxide nanoparticles using Ziziphus nummularia leaf extract. Artif. Cells Nanomed. Biotechnol. 2017, 45, 1751–1761. [Google Scholar] [CrossRef] [Green Version]
- Hemanth Kumar, N.K.; Andia, J.D.; Manjunatha, S.; Murali, M.; Amruthesh, K.N.; Jagannath, S. Antimitotic and DNA-binding potential of biosynthesized ZnO-NPs from leaf extract of Justicia wynaadensis (Nees) Heyne—A medicinal herb. Biocatal. Agric. Biotechnol. 2019, 18, 101024. [Google Scholar] [CrossRef]
- Prasad, K.S.; Prasad, S.K.; Ansari, M.A.; Alzohairy, M.A.; Alomary, M.N.; AlYahya, S.; Srinivasa, C.; Murali, M.; Ankegowda, V.M.; Shivamallu, C. Tumoricidal and bactericidal properties of ZnONPs synthesized using Cassia auriculata leaf extract. Biomolecules 2020, 10, 982. [Google Scholar] [CrossRef]
- Chunchegowda, U.A.; Shivaram, A.B.; Mahadevamurthy, M.; Ramachndrappa, L.T.; Lalitha, S.G.; Krishnappa, H.K.N.; Anandan, S.; Sudarshana, B.S.; Chanappa, E.G.; Ramachandrappa, N.S. Biosynthesis of Zinc oxide nanoparticles using leaf extract of Passiflora subpeltata: Characterization and antibacterial activity against Escherichia coli isolated from poultry faeces. J. Clust. Sci. 2020, 1–10. [Google Scholar] [CrossRef]
- Faisal, S.; Jan, H.; Shah, S.A.; Shah, S.; Khan, A.; Akbar, M.T.; Rizwan, M.; Jan, F.; Wajidullah; Akhtar, N.; et al. Green synthesis of Zinc oxide (ZnO) nanoparticles using aqueous fruit extracts of Myristica fragrans: Their characterizations and biological and environmental applications. ACS Omega 2021, 6, 9709–9722. [Google Scholar] [CrossRef]
- Stan, M.; Popa, A.; Toloman, D.; Silipas, T.D.; Vodnar, D.C. Antibacterial and antioxidant activities of ZnO nanoparticles synthesized using extracts of Allium sativum, Rosmarinus officinalis and Ocimum basilicum. Acta Metall. Sin. 2016, 29, 228–236. [Google Scholar] [CrossRef] [Green Version]
- Elumalai, K.; Velmurugan, S. Green synthesis, characterization and antimicrobial activities of zinc oxide nanoparticles from the leaf extract of Azadirachta indica (L.). Appl. Surf. Sci. 2015, 345, 329–336. [Google Scholar] [CrossRef]
- Hemanth Kumar, N.K.; Murali, M.; Satish, A.; Singh, S.B.; Gowtham, H.G.; Mahesh, H.M.; Lakshmeesha, T.R.; Amruthesh, K.N.; Jagannath, S. Bioactive and biocompatible nature of green synthesized zinc oxide nanoparticles from Simarouba glauca DC.: An endemic plant to Western Ghats, India. J. Clust. Sci. 2020, 31, 523–534. [Google Scholar] [CrossRef]
- Dobrucka, R.; Długaszewska, J. Biosynthesis and antibacterial activity of ZnO nanoparticles using Trifolium pratense flower extract. Saudi J. Biol. Sci. 2016, 23, 517–523. [Google Scholar] [CrossRef] [Green Version]
- Thatoi, P.; Kerry, R.G.; Gouda, S.; Das, G.; Pramanik, K.; Thatoi, H.; Patra, J.K. Photo-mediated green synthesis of silver and zinc oxide nanoparticles using aqueous extracts of two mangrove plant species, Heritiera fomes and Sonneratia apetala and investigation of their biomedical applications. J. Photochem. Photobiol. B Biol. 2016, 163, 311–318. [Google Scholar] [CrossRef]
- Nagajyothi, P.C.; Cha, S.J.; Yang, I.J.; Sreekanth, T.V.M.; Kim, K.J.; Shin, H.M. Antioxidant and anti-inflammatory activities of zinc oxide nanoparticles synthesized using Polygala tenuifolia root extract. J. Photochem. Photobiol. B Biol. 2015, 146, 10–17. [Google Scholar] [CrossRef]
- Wang, Z.L. Nanostructures of Zinc oxide. Mater. Today 2004, 7, 26–33. [Google Scholar] [CrossRef]
- Gao, Y.; Xu, D.; Ren, D.; Zeng, K.; Wu, X. Green synthesis of zinc oxide nanoparticles using Citrus sinensis peel extract and application to strawberry preservation: A comparison study. LWT 2020, 126, 109297. [Google Scholar] [CrossRef]
- Ali, S.G.; Ansari, M.A.; Jamal, Q.M.S.; Almatroudi, A.; Alzohairy, M.A.; Alomary, M.N.; Rehman, S.; Murali, M.; Jalal, M.; Khan, M.H.; et al. Butea monosperma seed extract mediated biosynthesis of ZnO NPs and their antibacterial, antibiofilm and anti-quorum sensing potentialities. Arab. J. Chem. 2021, 14, 103044. [Google Scholar] [CrossRef]
- Agarwal, H.; Shanmugam, V.K. Synthesis and optimization of zinc oxide nanoparticles using Kalanchoe pinnata towards the evaluation of its anti-inflammatory activity. J. Drug Deliv. Sci. Technol. 2019, 54, 101291. [Google Scholar] [CrossRef]
- Rehana, D.; Mahendiran, D.; Kumar, R.S.; Rahiman, A.K. In vitro antioxidant and antidiabetic activities of zinc oxide nanoparticles synthesized using different plant extracts. Bioprocess Biosyst. Eng. 2017, 40, 943–957. [Google Scholar] [CrossRef]
- Sanaeimehr, Z.; Javadi, I.; Namvar, F. Antiangiogenic and antiapoptotic effects of green-synthesized zinc oxide nanoparticles using Sargassum muticum algae extraction. Cancer Nanotechnol. 2018, 9, 1–16. [Google Scholar] [CrossRef]
- Yusof, H.M.; Mohamad, R.; Zaidan, U.H. Microbial synthesis of zinc oxide nanoparticles and their potential application as an antimicrobial agent and a feed supplement in animal industry: A review. J. Anim. Sci. Biotechnol. 2019, 10, 1–22. [Google Scholar]
- Pillai, A.M.; Sivasankarapillai, V.S.; Rahdar, A.; Joseph, J.; Sadeghfar, F.; Ronaldo Anuf, A.; Rajesh, K.; Kyzas, G.Z. Green synthesis and characterization of zinc oxide nanoparticles with antibacterial and antifungal activity. J. Mol. Struct. 2020, 1211, 128107. [Google Scholar] [CrossRef]
- Umar, H.; Kavaz, D.; Rizaner, N. Biosynthesis of zinc oxide nanoparticles using Albizia lebbeck stem bark, and evaluation of its antimicrobial, antioxidant, and cytotoxic activities on human breast cancer cell lines. Int. J. Nanomedicine 2019, 14, 87–100. [Google Scholar] [CrossRef] [Green Version]
- Agarwal, H.; Menon, S.; Shanmugam, V.K. Functionalization of zinc oxide nanoparticles using Mucuna pruriens and its antibacterial activity. Surf. Interfaces 2020, 19, 100521. [Google Scholar] [CrossRef]
- Mahendra, C.; Chandra, M.N.; Murali, M.; Abhilash, M.R.; Singh, S.B.; Satish, S.; Sudarshana, M.S. Phyto-fabricated ZnO nanoparticles from Canthium dicoccum (L.) for Antimicrobial, Anti-tuberculosis and Antioxidant activity. Process Biochem. 2020, 89, 220–226. [Google Scholar] [CrossRef]
- Umamaheswari, A.; Prabu, S.L.; John, S.A.; Puratchikody, A. Green synthesis of zinc oxide nanoparticles using leaf extracts of Raphanus sativus var. longipinnatus and evaluation of their anticancer property in A549 cell lines. Biotechnol. Rep. 2021, 29, e00595. [Google Scholar] [CrossRef]
- Sharmila, G.; Muthukumaran, C.; Sandiya, K.; Santhiya, S.; Pradeep, R.S.; Kumar, N.M.; Suriyanarayanan, N.; Thirumarimurugan, M. Biosynthesis, characterization, and antibacterial activity of zinc oxide nanoparticles derived from Bauhinia tomentosa leaf extract. J. Nanostruct. Chem. 2018, 8, 293–299. [Google Scholar] [CrossRef] [Green Version]
- Quek, J.A.; Sin, J.C.; Lam, S.M.; Mohamed, A.R.; Zeng, H.H. Bioinspired green synthesis of ZnO structures with enhanced visible light photocatalytic activity. J. Mater. Sci. Mater. Electron. 2020, 31, 1144–1158. [Google Scholar] [CrossRef]
- Jamdagni, P.; Khatri, P.; Rana, J.S. Green synthesis of zinc oxide nanoparticles using flower extract of Nyctanthes arbor-tristis and their antifungal activity. J. King Saud Univ. Sci. 2018, 30, 168–175. [Google Scholar] [CrossRef] [Green Version]
- Vinotha, V.; Iswarya, A.; Thaya, R.; Govindarajan, M.; Alharbi, N.S.; Kadaikunnan, S.; Khaled, J.M.; Al-Anbr, M.N.; Vaseeharan, B. Synthesis of ZnO nanoparticles using insulin-rich leaf extract: Anti-diabetic, antibiofilm and anti-oxidant properties. J. Photochem. Photobiol. B Biol. 2019, 197, 111541. [Google Scholar] [CrossRef] [PubMed]
- Rajakumar, G.; Thiruvengadam, M.; Mydhili, G.; Gomathi, T.; Chung, I.M. Green approach for synthesis of zinc oxide nanoparticles from Andrographis paniculata leaf extract and evaluation of their antioxidant, anti-diabetic, and anti-inflammatory activities. Bioprocess Biosyst. Eng. 2018, 41, 21–30. [Google Scholar] [CrossRef]
- Bala, N.; Saha, S.; Chakraborty, M.; Maiti, M.; Das, S.; Basu, R.; Nandy, P. Green synthesis of zinc oxide nanoparticles using Hibiscus subdariffa leaf extract: Effect of temperature on synthesis, anti-bacterial activity and anti-diabetic activity. RSC Adv. 2015, 5, 4993–5003. [Google Scholar] [CrossRef]
- Madan, H.R.; Sharma, S.C.; Udayabhanu; Suresh, D.; Vidya, Y.S.; Nagabhushana, H.; Rajanaik, H.; Anantharaju, K.S.; Prashantha, S.C.; Sadananda Maiya, P. Facile green fabrication of nanostructure ZnO plates, bullets, flower, prismatic tip, closed pine cone: Their antibacterial, antioxidant, photoluminescent and photocatalytic properties. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2016, 152, 404–416. [Google Scholar] [CrossRef]
- Kołodziejczak-Radzimska, A.; Jesionowski, T. Zinc oxide—From synthesis to application: A review. Materials 2014, 7, 2833–2881. [Google Scholar] [CrossRef] [Green Version]
- Roopan, S.M.; Mathew, R.S.; Mahesh, S.S.; Titus, D.; Aggarwal, K.; Bhatia, N.; Damodharan, K.I.; Elumalai, K.; Samuel, J.J. Environmental friendly synthesis of zinc oxide nanoparticles and estimation of its larvicidal activity against Aedes aegypti. Int. J. Environ. Sci. Technol. 2019, 16, 8053–8060. [Google Scholar] [CrossRef]
- Khan, Z.U.H.; Sadiq, H.M.; Shah, N.S.; Khan, A.U.; Muhammad, N.; Hassan, S.U.; Tahir, K.; Safi, S.Z.; Khan, F.U.; Imran, M.; et al. Greener synthesis of zinc oxide nanoparticles using Trianthema portulacastrum extract and evaluation of its photocatalytic and biological applications. J. Photochem. Photobiol. B Biol. 2019, 192, 147–157. [Google Scholar] [CrossRef]
- Abdullah, F.H.; Abu Bakar, N.H.H.; Abu Bakar, M. Low temperature biosynthesis of crystalline zinc oxide nanoparticles from Musa acuminata peel extract for visible-light degradation of methylene blue. Optik 2020, 206, 164279. [Google Scholar] [CrossRef]
- Iravani, S. Green synthesis of metal nanoparticles using plants. Green Chem. 2011, 13, 2638–2650. [Google Scholar] [CrossRef]
- El Shafey, A.M. Green synthesis of metal and metal oxide nanoparticles from plant leaf extracts and their applications: A review. Green Process. Synth. 2020, 9, 304–339. [Google Scholar] [CrossRef]
- Singh, S.C.; Gopal, R. Drop shaped zinc oxide quantum dots and their self-assembly into dendritic nanostructures: Liquid assisted pulsed laser ablation and characterizations. Appl. Surf. Sci. 2012, 258, 2211–2218. [Google Scholar] [CrossRef]
- Gunalan, S.; Sivaraj, R.; Rajendran, V. Green synthesized ZnO nanoparticles against bacterial and fungal pathogens. Prog. Nat. Sci. Mater. Int. 2012, 22, 693–700. [Google Scholar] [CrossRef] [Green Version]
- Agarwal, H.; Menon, S.; Kumar, S.V.; Rajeshkumar, S. Mechanistic study on antibacterial action of zinc oxide nanoparticles synthesized using green route. Chem. Biol. Interact. 2018, 286, 60–70. [Google Scholar] [CrossRef]
- Das, D.; Nath, B.C.; Phukon, P.; Kalita, A.; Dolui, S.K. Synthesis of ZnO nanoparticles and evaluation of antioxidant and cytotoxic activity. Colloids Surf. B Biointerfaces 2013, 111, 556–560. [Google Scholar] [CrossRef]
- Nithya, K.; Kalyanasundharam, S. Effect of chemically synthesis compared to biosynthesized ZnO nanoparticles using aqueous extract of C. halicacabum and their antibacterial activity. OpenNano 2019, 4, 100024. [Google Scholar] [CrossRef]
- Happy, A.; Soumya, M.; Venkat Kumar, S.; Rajeshkumar, S.; Sheba, R.D.; Lakshmi, T.; Nallaswamy, V.D. Phyto-assisted synthesis of zinc oxide nanoparticles using Cassia alata and its antibacterial activity against Escherichia coli. Biochem. Biophys. Rep. 2019, 17, 208–211. [Google Scholar] [CrossRef]
- Marslin, G.; Siram, K.; Maqbool, Q.; Selvakesavan, R.K.; Kruszka, D.; Kachlicki, P.; Franklin, G. Secondary metabolites in the green synthesis of metallic nanoparticles. Materials 2018, 11, 940. [Google Scholar] [CrossRef] [Green Version]
- Basnet, P.; Chanu, T.I.; Samanta, D.; Chatterjee, S. A review on bio-synthesized zinc oxide nanoparticles using plant extracts as reductants and stabilizing agents. J. Photochem. Photobiol. B Biol. 2018, 183, 201–221. [Google Scholar] [CrossRef]
- Ramesh, M.; Anbuvannan, M.; Viruthagiri, G. Green synthesis of ZnO nanoparticles using Solanum nigrum leaf extract and their antibacterial activity. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2015, 136, 864–870. [Google Scholar] [CrossRef] [PubMed]
- Vimala, K.; Sundarraj, S.; Paulpandi, M.; Vengatesan, S.; Kannan, S. Green synthesized doxorubicin loaded zinc oxide nanoparticles regulates the Bax and Bcl-2 expression in breast and colon carcinoma. Process Biochem. 2014, 49, 160–172. [Google Scholar] [CrossRef]
- Anbuvannan, M.; Ramesh, M.; Viruthagiri, G.; Shanmugam, N.; Kannadasan, N. Synthesis, characterization and photocatalytic activity of ZnO nanoparticles prepared by biological method. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2015, 143, 304–308. [Google Scholar] [CrossRef]
- Anbuvannan, M.; Ramesh, M.; Viruthagiri, G.; Shanmugam, N.; Kannadasan, N. Anisochilus carnosus leaf extract mediated synthesis of zinc oxide nanoparticles for antibacterial and photocatalytic activities. Mater. Sci. Semicond. Process. 2015, 39, 621–628. [Google Scholar] [CrossRef]
- Vijayakumar, S.; Vinoj, G.; Malaikozhundan, B.; Shanthi, S.; Vaseeharan, B. Plectranthus amboinicus leaf extract mediated synthesis of zinc oxide nanoparticles and its control of methicillin resistant Staphylococcus aureus biofilm and blood sucking mosquito larvae. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2015, 137, 886–891. [Google Scholar] [CrossRef] [PubMed]
- Fowsiya, J.; Madhumitha, G.; Al-Dhabi, N.A.; Arasu, M.V. Photocatalytic degradation of Congo red using Carissa edulis extract capped zinc oxide nanoparticles. J. Photochem. Photobiol. B Biol. 2016, 162, 395–401. [Google Scholar] [CrossRef]
- Jafarirad, S.; Mehrabi, M.; Divband, B.; Kosari-Nasab, M. Biofabrication of zinc oxide nanoparticles using fruit extract of Rosa canina and their toxic potential against bacteria: A mechanistic approach. Mater. Sci. Eng. C 2016, 59, 296–302. [Google Scholar] [CrossRef]
- Karnan, T.; Selvakumar, S.A.S. Biosynthesis of ZnO nanoparticles using rambutan (Nephelium lappaceum L.) peel extract and their photocatalytic activity on methyl orange dye. J. Mol. Struct. 2016, 1125, 358–365. [Google Scholar] [CrossRef]
- Patil, B.N.; Taranath, T.C. Limonia acidissima L. leaf mediated synthesis of zinc oxide nanoparticles: A potent tool against Mycobacterium tuberculosis. Int. J. Mycobacteriol. 2016, 5, 197–204. [Google Scholar] [CrossRef] [Green Version]
- Sharma, S.C. ZnO nano-flowers from Carica papaya milk: Degradation of Alizarin Red-S dye and antibacterial activity against Pseudomonas aeruginosa and Staphylococcus aureus. Optik 2016, 127, 6498–6512. [Google Scholar] [CrossRef]
- Supraja, N.; Prasad, T.N.V.K.V.; Krishna, T.G.; David, E. Synthesis, characterization, and evaluation of the antimicrobial efficacy of Boswellia ovalifoliolata stem bark-extract-mediated zinc oxide nanoparticles. Appl. Nanosci. 2016, 6, 581–590. [Google Scholar] [CrossRef] [Green Version]
- Nava, O.J.; Luque, P.A.; Gómez-Gutiérrez, C.M.; Vilchis-Nestor, A.R.; Castro-Beltrán, A.; Mota-González, M.L.; Olivas, A. Influence of Camellia sinensis extract on Zinc oxide nanoparticle green synthesis. J. Mol. Struct. 2017, 1134, 121–125. [Google Scholar] [CrossRef]
- Bayrami, A.; Parvinroo, S.; Habibi-Yangjeh, A.; Pouran, S.R. Bio-extract-mediated ZnO nanoparticles: Microwave-assisted synthesis, characterization and antidiabetic activity evaluation. Artif. Cells Nanomed. Biotechnol. 2018, 46, 730–739. [Google Scholar] [CrossRef]
- Luque, P.A.; Soto-Robles, C.A.; Nava, O.; Gomez-Gutierrez, C.M.; Castro-Beltran, A.; Garrafa-Galvez, H.E.; Vilchis-Nestor, A.R.; Olivas, A. Green synthesis of zinc oxide nanoparticles using Citrus sinensis extract. J. Mater. Sci. Mater. Electron. 2018, 29, 9764–9770. [Google Scholar] [CrossRef]
- Rajeshkumar, S.; Kumar, S.V.; Ramaiah, A.; Agarwal, H.; Lakshmi, T.; Roopan, S.M. Biosynthesis of zinc oxide nanoparticles using Mangifera indica leaves and evaluation of their antioxidant and cytotoxic properties in lung cancer (A549) cells. Enzyme Microb. Technol. 2018, 117, 91–95. [Google Scholar] [CrossRef]
- Suresh, J.; Pradheesh, G.; Alexramani, V.; Sundrarajan, M.; Hong, S.I. Green synthesis and characterization of zinc oxide nanoparticle using insulin plant (Costus pictus D. Don) and investigation of its antimicrobial as well as anticancer activities. Adv. Nat. Sci. Nanosci. Nanotechnol. 2018, 9, 015008. [Google Scholar] [CrossRef]
- Ezealisiji, K.M.; Siwe-Noundou, X.; Maduelosi, B.; Nwachukwu, N.; Krause, R.W.M. Green synthesis of zinc oxide nanoparticles using Solanum torvum (L) leaf extract and evaluation of the toxicological profile of the ZnO nanoparticles–hydrogel composite in Wistar albino rats. Int. Nano Lett. 2019, 9, 99–107. [Google Scholar] [CrossRef] [Green Version]
- Shanavas, S.; Duraimurugan, J.; Kumar, G.S.; Ramesh, R.; Acevedo, R.; Anbarasan, P.M.; Maadeswaran, P. Ecofriendly green synthesis of ZnO nanostructures using Artabotrys hexapetalu and Bambusa vulgaris plant extract and investigation on their photocatalytic and antibacterial activity. Mater. Res. Express 2019, 6, 105098. [Google Scholar] [CrossRef]
- Ruddaraju, L.K.; Pammi, S.V.N.; Pallela, P.N.V.K.; Padavala, V.S.; Kolapalli, V.R.M. Antibiotic potentiation and anti-cancer competence through bio-mediated ZnO nanoparticles. Mater. Sci. Eng. C 2019, 103, 109756. [Google Scholar] [CrossRef]
- Tettey, C.O.; Shin, H.M. Evaluation of the antioxidant and cytotoxic activities of zinc oxide nanoparticles synthesized using Scutellaria baicalensis root. Sci. Afr. 2019, 6, e00157. [Google Scholar] [CrossRef]
- Sana, S.S.; Kumbhakar, D.V.; Pasha, A.; Pawar, S.C.; Grace, A.N.; Singh, R.P.; Nguyen, V.H.; Le, Q.V.; Peng, W. Crotalaria verrucosa leaf extract mediated synthesis of Zinc oxide nanoparticles: Assessment of antimicrobial and anticancer activity. Molecules 2020, 25, 4896. [Google Scholar] [CrossRef]
- Elumalai, K.; Velmurugan, S.; Ravi, S.; Kathiravan, V.; Adaikala Raj, G. Bio-approach: Plant mediated synthesis of ZnO nanoparticles and their catalytic reduction of methylene blue and antimicrobial activity. Adv. Powder Technol. 2015, 26, 1639–1651. [Google Scholar] [CrossRef]
- Fu, L.; Fu, Z. Plectranthus amboinicus leaf extract–assisted biosynthesis of ZnO nanoparticles and their photocatalytic activity. Ceram. Int. 2015, 41, 2492–2496. [Google Scholar] [CrossRef]
- Stan, M.; Popa, A.; Toloman, D.; Dehelean, A.; Lung, I.; Katona, G. Enhanced photocatalytic degradation properties of zinc oxide nanoparticles synthesized by using plant extracts. Mater. Sci. Semicond. Process. 2015, 39, 23–29. [Google Scholar] [CrossRef]
- Sundrarajan, M.; Ambika, S.; Bharathi, K. Plant-extract mediated synthesis of ZnO nanoparticles using Pongamia pinnata and their activity against pathogenic bacteria. Adv. Powder Technol. 2015, 26, 1294–1299. [Google Scholar] [CrossRef]
- Suresh, D.; Nethravathi, P.C.; Udayabhanu; Rajanaika, H.; Nagabhushana, H.; Sharma, S.C. Green synthesis of multifunctional zinc oxide (ZnO) nanoparticles using Cassia fistula plant extract and their photodegradative, antioxidant and antibacterial activities. Mater. Sci. Semicond. Process. 2015, 31, 446–454. [Google Scholar] [CrossRef]
- Suresh, D.; Shobharani, R.M.; Nethravathi, P.C.; Pavan Kumar, M.A.; Nagabhushana, H.; Sharma, S.C. Artocarpus gomezianus aided green synthesis of ZnO nanoparticles: Luminescence, photocatalytic and antioxidant properties. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2015, 141, 128–134. [Google Scholar] [CrossRef]
- Zheng, Y.; Fu, L.; Han, F.; Wang, A.; Cai, W.; Yu, J.; Yang, J.; Peng, F. Green biosynthesis and characterization of zinc oxide nanoparticles using Corymbia citriodora leaf extract and their photocatalytic activity. Green Chem. Lett. Rev. 2015, 8, 59–63. [Google Scholar] [CrossRef]
- Rana, N.; Chand, S.; Gathania, A.K. Green synthesis of zinc oxide nano-sized spherical particles using Terminalia chebula fruits extract for their photocatalytic applications. Int. Nano Lett. 2016, 6, 91–98. [Google Scholar] [CrossRef] [Green Version]
- Azizi, S.; Mohamad, R.; Shahri, M.M. Green microwave-assisted combustion synthesis of Zinc oxide nanoparticles with Citrullus colocynthis (L.) Schrad: Characterization and biomedical applications. Molecules 2017, 22, 301. [Google Scholar] [CrossRef] [Green Version]
- Dayakar, T.; Venkateswara Rao, K.; Bikshalu, K.; Rajendar, V.; Park, S.H. Novel synthesis and structural analysis of zinc oxide nanoparticles for the non enzymatic glucose biosensor. Mater. Sci. Eng. C 2017, 75, 1472–1479. [Google Scholar] [CrossRef] [PubMed]
- Mahendra, C.; Murali, M.; Manasa, G.; Ponnamma, P.; Abhilash, M.R.; Lakshmeesha, T.R.; Satish, A.; Amruthesh, K.N.; Sudarshana, M.S. Antibacterial and antimitotic potential of bio-fabricated zinc oxide nanoparticles of Cochlospermum religiosum (L.). Microb. Pathog. 2017, 110, 620–629. [Google Scholar] [CrossRef] [PubMed]
- Siripireddy, B.; Mandal, B.K. Facile green synthesis of zinc oxide nanoparticles by Eucalyptus globulus and their photocatalytic and antioxidant activity. Adv. Powder Technol. 2017, 28, 785–797. [Google Scholar] [CrossRef]
- Taghavi Fardood, S.; Ramazani, A.; Moradi, S.; Azimzadeh Asiabi, P. Green synthesis of zinc oxide nanoparticles using arabic gum and photocatalytic degradation of direct blue 129 dye under visible light. J. Mater. Sci. Mater. Electron. 2017, 28, 13596–13601. [Google Scholar] [CrossRef]
- Ali, J.; Irshad, R.; Li, B.; Tahir, K.; Ahmad, A.; Shakeel, M.; Khan, N.U.; Khan, Z.U.H. Synthesis and characterization of phytochemical fabricated zinc oxide nanoparticles with enhanced antibacterial and catalytic applications. J. Photochem. Photobiol. B Biol. 2018, 183, 349–356. [Google Scholar] [CrossRef]
- Aminuzzaman, M.; Ying, L.P.; Goh, W.S.; Watanabe, A. Green synthesis of zinc oxide nanoparticles using aqueous extract of Garcinia mangostana fruit pericarp and their photocatalytic activity. Bull. Mater. Sci. 2018, 41, 50. [Google Scholar] [CrossRef] [Green Version]
- Shao, F.; Yang, A.; Yu, D.M.; Wang, J.; Gong, X.; Tian, H.X. Bio-synthesis of Barleria gibsoni leaf extract mediated zinc oxide nanoparticles and their formulation gel for wound therapy in nursing care of infants and children. J. Photochem. Photobiol. B Biol. 2018, 189, 267–273. [Google Scholar] [CrossRef]
- Zhao, C.; Zhang, X.; Zheng, Y. Biosynthesis of polyphenols functionalized ZnO nanoparticles: Characterization and their effect on human pancreatic cancer cell line. J. Photochem. Photobiol. B Biol. 2018, 183, 142–146. [Google Scholar] [CrossRef]
- Asik, R.M.; Gowdhami, B.; Jaabir, M.S.M.; Archunan, G.; Suganthy, N. Anticancer potential of zinc oxide nanoparticles against cervical carcinoma cells synthesized via biogenic route using aqueous extract of Gracilaria edulis. Mater. Sci. Eng. C 2019, 103, 109840. [Google Scholar] [CrossRef]
- Hafeez, M.; Arshad, R.; Hameed, M.U.; Akram, B.; Ahmed, M.N.; Kazmi, S.A.; Ahmad, I.; Ali, S. Populus ciliata leaves extract mediated synthesis of zinc oxide nanoparticles and investigation of their anti-bacterial activities. Mater. Res. Express 2019, 6, 075064. [Google Scholar] [CrossRef]
- Rad, S.S.; Sani, A.M.; Mohseni, S. Biosynthesis, characterization and antimicrobial activities of zinc oxide nanoparticles from leaf extract of Mentha pulegium (L.). Microb. Pathog. 2019, 131, 239–245. [Google Scholar] [CrossRef] [PubMed]
- Chemingui, H.; Missaoui, T.; Mzali, J.C.; Yildiz, T.; Konyar, M.; Smiri, M.; Saidi, N.; Hafiane, A.; Yatmaz, H.C. Facile green synthesis of zinc oxide nanoparticles (ZnO NPs): Antibacterial and photocatalytic activities. Mater. Res. Express 2019, 6, 1050b4. [Google Scholar] [CrossRef]
- Majeed, S.; Danish, M.; Ismail, M.H.B.; Ansari, M.T.; Ibrahim, M.N.M. Anticancer and apoptotic activity of biologically synthesized zinc oxide nanoparticles against human colon cancer HCT-116 cell line- in vitro study. Sustain. Chem. Pharm. 2019, 14, 100179. [Google Scholar] [CrossRef]
- Ahmad, H.; Venugopal, K.; Rajagopal, K.; De Britto, S.; Nandini, B.; Pushpalatha, H.G.; Konappa, N.; Udayashankar, A.C.; Geetha, N.; Jogaiah, S. Green synthesis and characterization of Zinc oxide nanoparticles using Eucalyptus globules and their fungicidal ability against pathogenic fungi of Apple orchards. Biomolecules 2020, 10, 425. [Google Scholar] [CrossRef] [Green Version]
- Akbarian, M.; Mahjoub, S.; Elahi, S.M.; Zabihi, E.; Tashakkorian, H. Green synthesis, formulation and biological evaluation of a novel ZnO nanocarrier loaded with paclitaxel as drug delivery system on MCF-7 cell line. Colloids Surf. B Biointerfaces 2020, 186, 110686. [Google Scholar] [CrossRef]
- Golmohammadi, M.; Honarmand, M.; Ghanbari, S. A green approach to synthesis of ZnO nanoparticles using jujube fruit extract and their application in photocatalytic degradation of organic dyes. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2020, 229, 117961. [Google Scholar] [CrossRef]
- Jayappa, M.D.; Ramaiah, C.K.; Kumar, M.A.P.; Suresh, D.; Prabhu, A.; Devasya, R.P.; Sheikh, S. Green synthesis of zinc oxide nanoparticles from the leaf, stem and in vitro grown callus of Mussaenda frondosa L.: Characterization and their applications. Appl. Nanosci. 2020, 10, 3057–3074. [Google Scholar] [CrossRef]
- Mallikarjunaswamy, C.; Lakshmi Ranganatha, V.; Ramu, R.; Udayabhanu; Nagaraju, G. Facile microwave-assisted green synthesis of ZnO nanoparticles: Application to photodegradation, antibacterial and antioxidant. J. Mater. Sci. Mater. Electron. 2020, 31, 1004–1021. [Google Scholar] [CrossRef]
- Selim, Y.A.; Azb, M.A.; Ragab, I.; Abd El-Azim, M.H.M. Green synthesis of Zinc oxide nanoparticles using aqueous extract of Deverra tortuosa and their cytotoxic activities. Sci. Rep. 2020, 10, 3445. [Google Scholar] [CrossRef] [Green Version]
- Santhoshkumar, J.; Kumar, S.V.; Rajeshkumar, S. Synthesis of zinc oxide nanoparticles using plant leaf extract against urinary tract infection pathogen. Resour. Technol. 2017, 3, 459–465. [Google Scholar] [CrossRef]
- Mahdizadeh, R.; Homayouni-Tabrizi, M.; Neamati, A.; Seyedi, S.M.R.; Tavakkol Afshari, H.S. Green synthesized-zinc oxide nanoparticles, the strong apoptosis inducer as an exclusive antitumor agent in murine breast tumor model and human breast cancer cell lines (MCF7). J. Cell. Biochem. 2019, 120, 17984–17993. [Google Scholar] [CrossRef]
- Mohammad, R.K.S.G.; Homayouni Tabrizi, M.; Ardalan, T.; Yadamani, S.; Safavi, E. Green synthesis of zinc oxide nanoparticles and evaluation of anti-angiogenesis, anti-inflammatory and cytotoxicity properties. J. Biosci. 2019, 44, 30. [Google Scholar] [CrossRef]
- Vijayakumar, S.; Mahadevan, S.; Arulmozhi, P.; Sriram, S.; Praseetha, P.K. Green synthesis of zinc oxide nanoparticles using Atalantia monophylla leaf extracts: Characterization and antimicrobial analysis. Mater. Sci. Semicond. Process. 2018, 82, 39–45. [Google Scholar] [CrossRef]
- Cheng, J.; Wang, X.; Qiu, L.; Li, Y.; Marraiki, N.; Elgorban, A.M.; Xue, L. Green synthesized zinc oxide nanoparticles regulates the apoptotic expression in bone cancer cells MG-63 cells. J. Photochem. Photobiol. B Biol. 2020, 202, 111644. [Google Scholar] [CrossRef]
- Jevapatarakul, D.; T-Thienprasert, J.; Payungporn, S.; Chavalit, T.; Khamwut, A.; T-Thienprasert, N.P. Utilization of Cratoxylum formosum crude extract for synthesis of ZnO nanosheets: Characterization, biological activities and effects on gene expression of nonmelanoma skin cancer cell. Biomed. Pharmacother. 2020, 130, 110552. [Google Scholar] [CrossRef]
- Rafique, M.; Tahir, R.; Gillani, S.S.A.; Tahir, M.B.; Shakil, M.; Iqbal, T.; Abdellahi, M.O. Plant-mediated green synthesis of zinc oxide nanoparticles from Syzygium cumini for seed germination and wastewater purification. Int. J. Environ. Anal. Chem. 2020, 1–16. [Google Scholar] [CrossRef]
- Rahimi Kalateh Shah Mohammad, G.; Karimi, E.; Oskoueian, E.; Homayouni-Tabrizi, M. Anticancer properties of green-synthesised zinc oxide nanoparticles using Hyssopus officinalis extract on prostate carcinoma cells and its effects on testicular damage and spermatogenesis in Balb/C mice. Andrologia 2020, 52, e13450. [Google Scholar] [CrossRef]
- Ullah, S.; Ahmad, A.; Ri, H.; Khan, A.U.; Khan, U.A.; Yuan, Q. Green synthesis of catalytic Zinc oxide nano-flowers and their bacterial infection therapy. Appl. Organomet. Chem. 2020, 34, e5298. [Google Scholar] [CrossRef]
- Banumathi, B.; Malaikozhundan, B.; Vaseeharan, B. In vitro acaricidal activity of ethnoveterinary plants and green synthesis of zinc oxide nanoparticles against Rhipicephalus (Boophilus) microplus. Vet. Parasitol. 2016, 216, 93–100. [Google Scholar] [CrossRef]
- Fatimah, I.; Pradita, R.Y.; Nurfalinda, A. Plant extract mediated of ZnO nanoparticles by using ethanol extract of Mimosa pudica leaves and Coffee powder. Procedia Eng. 2016, 148, 43–48. [Google Scholar] [CrossRef] [Green Version]
- Malaikozhundan, B.; Vaseeharan, B.; Vijayakumar, S.; Pandiselvi, K.; Kalanjiam, M.A.R.; Murugan, K.; Benelli, G. Biological therapeutics of Pongamia pinnata coated zinc oxide nanoparticles against clinically important pathogenic bacteria, fungi and MCF-7 breast cancer cells. Microb. Pathog. 2017, 104, 268–277. [Google Scholar] [CrossRef]
- Sathishkumar, G.; Rajkuberan, C.; Manikandan, K.; Prabukumar, S.; DanielJohn, J.; Sivaramakrishnan, S. Facile biosynthesis of antimicrobial zinc oxide (ZnO) nanoflakes using leaf extract of Couroupita guianensis Aubl. Mater. Lett. 2017, 188, 383–386. [Google Scholar] [CrossRef]
- Gupta, M.; Tomar, R.S.; Kaushik, S.; Mishra, R.K.; Sharma, D. Effective antimicrobial activity of green ZnO nano particles of Catharanthus roseus. Front. Microbiol. 2018, 9, 2030. [Google Scholar] [CrossRef]
- Khatami, M.; Varma, R.S.; Zafarnia, N.; Yaghoobi, H.; Sarani, M.; Kumar, V.G. Applications of green synthesized Ag, ZnO and Ag/ZnO nanoparticles for making clinical antimicrobial wound-healing bandages. Sustain. Chem. Pharm. 2018, 10, 9–15. [Google Scholar] [CrossRef]
- Mehr, E.S.; Sorbiun, M.; Ramazani, A.; Fardood, S.T. Plant-mediated synthesis of zinc oxide and copper oxide nanoparticles by using Ferulago angulata (schlecht) boiss extract and comparison of their photocatalytic degradation of Rhodamine B (RhB) under visible light irradiation. J. Mater. Sci. Mater. Electron. 2018, 29, 1333–1340. [Google Scholar] [CrossRef]
- Ramanarayanan, R.; Bhabhina, N.M.; Dharsana, M.V.; Nivedita, C.V.; Sindhu, S. Green synthesis of zinc oxide nanoparticles using extract of Averrhoa bilimbi (L) and their photoelectrode applications. Mater. Today Proc. 2018, 5, 16472–16477. [Google Scholar] [CrossRef]
- Safawo, T.; Sandeep, B.V.; Pola, S.; Tadesse, A. Synthesis and characterization of zinc oxide nanoparticles using tuber extract of anchote (Coccinia abyssinica (Lam.) Cong.) for antimicrobial and antioxidant activity assessment. OpenNano 2018, 3, 56–63. [Google Scholar] [CrossRef]
- Chandra, H.; Patel, D.; Kumari, P.; Jangwan, J.S.; Yadav, S. Phyto-mediated synthesis of zinc oxide nanoparticles of Berberisaristata: Characterization, antioxidant activity and antibacterial activity with special reference to urinary tract pathogens. Mater. Sci. Eng. C 2019, 102, 212–220. [Google Scholar] [CrossRef] [PubMed]
- Darvishi, E.; Kahrizi, D.; Arkan, E. Comparison of different properties of zinc oxide nanoparticles synthesized by the green (using Juglans regia L. leaf extract) and chemical methods. J. Mol. Liq. 2019, 286, 110831. [Google Scholar] [CrossRef]
- Hu, D.; Si, W.; Qin, W.; Jiao, J.; Li, X.; Gu, X.; Hao, Y. Cucurbita pepo leaf extract induced synthesis of zinc oxide nanoparticles, characterization for the treatment of femoral fracture. J. Photochem. Photobiol. B Biol. 2019, 195, 12–16. [Google Scholar] [CrossRef] [PubMed]
- Hussain, A.; Oves, M.; Alajmi, M.F.; Hussain, I.; Amir, S.; Ahmed, J.; Rehman, M.T.; El-Seedi, H.R.; Ali, I. Biogenesis of ZnO nanoparticles using Pandanus odorifer leaf extract: Anticancer and antimicrobial activities. RSC Adv. 2019, 9, 15357–15369. [Google Scholar] [CrossRef] [Green Version]
- Kahsay, M.H.; Tadesse, A.; RamaDevi, D.; Belachew, N.; Basavaiah, K. Green synthesis of zinc oxide nanostructures and investigation of their photocatalytic and bactericidal applications. RSC Adv. 2019, 9, 36967–36981. [Google Scholar] [CrossRef]
- Prasad, A.R.; Garvasis, J.; Oruvil, S.K.; Joseph, A. Bio-inspired green synthesis of zinc oxide nanoparticles using Abelmoschusesculentus mucilage and selective degradation of cationic dye pollutants. J. Phys. Chem. Solids 2019, 127, 265–274. [Google Scholar] [CrossRef]
- Alamdari, S.; Ghamsari, M.S.; Lee, C.; Han, W.; Park, H.H.; Tafreshi, M.J.; Afarideh, H.; Ara, M.H.M. Preparation and characterization of Zinc oxide nanoparticles using leaf extract of Sambucus ebulus. Appl. Sci. 2020, 10, 3620. [Google Scholar] [CrossRef]
- Liu, H.; Kang, P.; Liu, Y.; An, Y.; Hu, Y.; Jin, X.; Cao, X.; Qi, Y.; Ramesh, T.; Wang, X. Zinc oxide nanoparticles synthesised from the Vernonia amygdalina shows the anti-inflammatory and antinociceptive activities in the mice model. Artif. Cells Nanomed. Biotechnol. 2020, 48, 1068–1078. [Google Scholar] [CrossRef]
- Naseer, M.; Aslam, U.; Khalid, B.; Chen, B. Green route to synthesize Zinc oxide Nanoparticles using leaf extracts of Cassia fistula and Melia azadarach and their antibacterial potential. Sci. Rep. 2020, 10, 9055. [Google Scholar] [CrossRef]
- Sharma, S.; Kumar, K.; Thakur, N.; Chauhan, S.; Chauhan, M.S. The effect of shape and size of ZnO nanoparticles on their antimicrobial and photocatalytic activities: A green approach. Bull. Mater. Sci. 2020, 43, 20. [Google Scholar] [CrossRef]
- Vinayagam, R.; Selvaraj, R.; Arivalagan, P.; Varadavenkatesan, T. Synthesis, characterization and photocatalytic dye degradation capability of Calliandra haematocephala-mediated zinc oxide nanoflowers. J. Photochem. Photobiol. B Biol. 2020, 203, 111760. [Google Scholar] [CrossRef]
- Zhang, H.; Liang, Z.; Zhang, J.; Wang, W.P.; Zhang, H.; Lu, Q. Zinc oxide nanoparticle synthesized from Euphorbia fischeriana root inhibits the cancer cell growth through modulation of apoptotic signaling pathways in lung cancer cells. Arab. J. Chem. 2020, 13, 6174–6183. [Google Scholar] [CrossRef]
- Vinayagam, R.; Pai, S.; Varadavenkatesan, T.; Pugazhendhi, A.; Selvaraj, R. Characterization and photocatalytic activity of ZnO nanoflowers synthesized using Bridelia retusa leaf extract. Appl. Nanosci. 2021, 1–10. [Google Scholar] [CrossRef]
- Ali, K.; Dwivedi, S.; Azam, A.; Saquib, Q.; Al-Said, M.S.; Alkhedhairy, A.A.; Musarrat, J. Aloe vera extract functionalized zinc oxide nanoparticles as nanoantibiotics against multi-drug resistant clinical bacterial isolates. J. Colloid Interface Sci. 2016, 472, 145–156. [Google Scholar] [CrossRef]
- Sharmila, G.; Thirumarimurugan, M.; Muthukumaran, C. Green synthesis of ZnO nanoparticles using Tecoma castanifolia leaf extract: Characterization and evaluation of its antioxidant, bactericidal and anticancer activities. Microchem. J. 2019, 145, 578–587. [Google Scholar] [CrossRef]
- Wang, D.; Liu, H.; Ma, Y.; Qu, J.; Guan, J.; Lu, N.; Lu, Y.; Yuan, X. Recycling of hyper-accumulator: Synthesis of ZnO nanoparticles and photocatalytic degradation for dichlorophenol. J. Alloys Compd. 2016, 680, 500–505. [Google Scholar] [CrossRef]
- Saemi, R.; Taghavi, E.; Jafarizadeh-Malmiri, H.; Anarjan, N. Fabrication of green ZnO nanoparticles using walnut leaf extract to develop an antibacterial film based on polyethylene–starch–ZnO NPs. Green Process. Synthesis 2021, 10, 112–124. [Google Scholar] [CrossRef]
- Sun, Q.; Li, J.; Le, T. Zinc oxide nanoparticle as a novel class of antifungal agents: Current advances and future perspectives. J. Agric. Food Chem. 2018, 66, 11209–11220. [Google Scholar] [CrossRef]
- Sharma, D.; Sabela, M.I.; Kanchi, S.; Mdluli, P.S.; Singh, G.; Stenström, T.A.; Bisetty, K. Biosynthesis of ZnO nanoparticles using Jacaranda mimosifolia flowers extract: Synergistic antibacterial activity and molecular simulated facet specific adsorption studies. J. Photochem. Photobiol. B Biol. 2016, 162, 199–207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vijayakumar, S.; Vaseeharan, B.; Sudhakaran, R.; Jeyakandan, J.; Ramasamy, P.; Sonawane, A.; Padhi, A.; Velusamy, P.; Anbu, P.; Faggio, C. Bioinspired Zinc oxide nanoparticles using Lycopersicon esculentum for antimicrobial and anticancer applications. J. Clust. Sci. 2019, 30, 1465–1479. [Google Scholar] [CrossRef]
- Tang, K.S. The current and future perspectives of zinc oxide nanoparticles in the treatment of diabetes mellitus. Life Sci. 2019, 239, 117011. [Google Scholar] [CrossRef]
- Tian, W.; Wang, C.; Li, D.; Hou, H. Novel anthraquinone compounds as anticancer agents and their potential mechanism. Future Med. Chem. 2020, 12, 627–644. [Google Scholar] [CrossRef]
- Agarwal, H.; Nakara, A.; Shanmugam, V.K. Anti-inflammatory mechanism of various metal and metal oxide nanoparticles synthesized using plant extracts: A review. Biomed. Pharmacother. 2019, 109, 2561–2572. [Google Scholar] [CrossRef]
- Moghaddas, S.M.T.H.; Elahi, B.; Javanbakht, V. Biosynthesis of pure zinc oxide nanoparticles using Quince seed mucilage for photocatalytic dye degradation. J. Alloy. Compd. 2020, 821, 153519. [Google Scholar] [CrossRef]
- Kiran Kumar, A.B.V.; Saila, E.S.; Narang, P.; Aishwarya, M.; Raina, R.; Gautam, M.; Shankar, E.G. Biofunctionalization and biological synthesis of the ZnO nanoparticles: The effect of Raphanus sativus (white radish) root extract on antimicrobial activity against MDR strain for wound healing applications. Inorg. Chem. Commun. 2019, 100, 101–106. [Google Scholar] [CrossRef]
- Gao, Y.; Han, Y.; Cui, M.; Tey, H.L.; Wang, L.; Xu, C. ZnO nanoparticles as an antimicrobial tissue adhesive for skin wound closure. J. Mater. Chem. B 2017, 5, 4535–4541. [Google Scholar] [CrossRef]
- Lin, C.C.; Lee, M.H.; Chi, M.H.; Chen, C.J.; Lin, H.Y. Preparation of Zinc oxide nanoparticles containing spray and barrier films for potential photoprotection on wound healing. ACS Omega 2019, 4, 1801–1809. [Google Scholar] [CrossRef]
- Nosrati, H.; Khodaei, M.; Banitalebi-Dehkordi, M.; Alizadeh, M.; Asadpour, S.; Sharifi, E.; Ai, J.; Soleimannejad, M. Preparation and characterization of poly (ethylene oxide)/zinc oxide nanofibrous scaffold for chronic wound healing applications. Polim. Med. 2020, 50, 41–51. [Google Scholar] [CrossRef]
- Bagheri, M.; Validi, M.; Gholipour, A.; Makvandi, P.; Sharifi, E. Chitosan nanofiber biocomposites for potential wound healing applications: Antioxidant activity with synergic antibacterial effect. Bioeng. Transl. Med. 2021, e10254. [Google Scholar] [CrossRef]
- Bazak, R.; Houri, M.; Achy, S.E.; Hussein, W.; Refaat, T. Passive targeting of nanoparticles to cancer: A comprehensive review of the literature. Mol. Clin. Oncol. 2014, 2, 904–908. [Google Scholar] [CrossRef] [Green Version]
- Ghazali, N.A.B.; Mani, M.P.; Jaganathan, S.K. Green-synthesized Zinc oxide nanoparticles decorated nanofibrous polyurethane mesh loaded with virgin coconut oil for tissue engineering application. Curr. Nanosci. 2018, 14, 280–289. [Google Scholar] [CrossRef]
- Laurenti, M.; Cauda, V. ZnO nanostructures for tissue engineering applications. Nanomaterials 2017, 7, 374. [Google Scholar] [CrossRef] [Green Version]
- Medina-Cruz, D.; Mostafavi, E.; Vernet-Crua, A.; Cheng, J.; Shah, V.; Cholula-Diaz, J.L.; Guisbiers, G.; Tao, J.; García-Martín, J.M.; Webster, T.J. Green nanotechnology-based drug delivery systems for osteogenic disorders. Expert Opin. Drug Deliv. 2020, 17, 341–356. [Google Scholar] [CrossRef]
- Cruz, D.M.; Mostafavi, E.; Vernet-Crua, A.; Barabadi, H.; Shah, V.; Cholula-Díaz, J.-L.; Guisbiers, G.; Webster, T.J. Green nanotechnology-based zinc oxide (ZnO) nanomaterials for biomedical applications: A review. J. Phys. Mater. 2020, 3, 034005. [Google Scholar] [CrossRef]
- Shubha, P.; Likhith Gowda, M.; Namratha, K.; Manjunatha, H.B.; Byrappa, K. In vitro and in vivo evaluation of green-hydrothermal synthesized ZnO nanoparticles. J. Drug Deliv. Sci. Technol. 2019, 49, 692–699. [Google Scholar] [CrossRef]
- Shafique, S.; Jabeen, N.; Ahmad, K.S.; Irum, S.; Anwaar, S.; Ahmad, N.; Alam, S.; Ilyas, M.; Khan, T.F.; Hussain, S.Z. Green fabricated zinc oxide nanoformulated media enhanced callus induction and regeneration dynamics of Panicum virgatum L. PLoS ONE 2020, 15, e0230464. [Google Scholar] [CrossRef]




| Plant Name | Plant Part Used | Size (nm) | Shape/ Morphology | Applications | Reference |
|---|---|---|---|---|---|
| ZINC NITRATE | |||||
| Solanum nigrum | Leaf | 29 | Quasi-spherical | Antibacterial | Ramesh et al. [51] |
| Borassus flabellifer | Fruit | 55 | Rod like | Drug delivery | Vimala et al. [52] |
| Phyllanthus niruri | Leaf | 25 | Quasi-spherical | Photocatalytic | Anbuvannan et al. [53] |
| Anisochilus carnosus | Leaf | 30–40 | Quasi-spherical | Antibacterial and Photocatalytic | Anbuvannan et al. [54] |
| Hibiscus subdariffa | Leaf | 12–46 | Spherical | Antibacterial and Anti-diabetic | Bala et al. [35] |
| Plectranthus amboinicus | Leaf | 20–50 | Spherical and hexagonal | Antibacterial, Antibiofilm and Larvicidal | Vijayakumar et al. [55] |
| Carissa edulis | Fruit | 50–55 | Flower shape | Photocatalytic | Fowsiya et al. [56] |
| Rosa canina | Fruit | <50 | Spherical | Antibacterial, Antioxidant and Anticancer | Jafarirad et al. [57] |
| Nephelium lappaceum | Peel | 25–40 | Spherical | Photocatalytic | Karnan and Selvakumar [58] |
| Limonia acidissima | Leaf | 12–53 | Spherical | Antibacterial (TB) | Patil and Taranath [59] |
| Carica papaya | Milk latex | 11–26 | Hexagonal | Photocatalytic and Antibacterial | Sharma [60] |
| Boswellia ovalifoliolata | Bark | 20 | Spherical | Antimicrobial | Supraja et al. [61] |
| Camellia sinensis | Leaf | - | Hexagonal | Photocatalytic | Nava et al. [62] |
| Ceropegia candelabrum | Leaf | 12–35 | Hexagonal wurtzite | Antibacterial and Antioxidant | Murali et al. [5] |
| Ziziphus nummularia | Leaf | 12–26 | Spherical and irregular | Antifungal and Anticancer | Padalia and Chanda [7] |
| Vaccinium arctostaphylos | Fruit | 13 | Spherical | Anti-diabetic | Bayrami et al. [63] |
| Citrus sinensis | Peel | 12–24 | Hexagonal prisms and oval spheres; highly irregular sponge-like | Photocatalytic | Luque et al. [64] |
| Mangifera indica | Leaf | 45–60 | Nearly spherical and hexagonal quartzite | Antioxidant and Anticancer | Rajeshkumar et al. [65] |
| Costus pictus | Leaf | 40 | Hexagonal, rod-shaped and spherical | Antimicrobial and Anticancer | Suresh et al. [66] |
| Solanum torvum | Leaf | 28 | Spherical | Toxicological effect in Wistar albino rats | Ezealisiji et al. [67] |
| Artabotrys hexapetalu | Leaf | 20–30 | Spherical and rod-like | Antibacterial and Photocatalytic | Shanavas et al. [68] |
| Bambusa vulgaris | |||||
| Annona squamosa | Leaf | 20–50 | Hexagonal and quasi hexagonal plate like | Antibacterial and Anticancer | Ruddaraju et al. [69] |
| Scutellaria baicalensis | Root | 33–99 | Spherical | Antioxidant and Anticancer | Tettey and Shin [70] |
| Albizia lebbeck | Bark | 66 | Irregular spherical | Antibacterial, Antioxidant, Cytotoxic and Antiproliferative | Umar et al. [26] |
| Citrus sinensis | Peel | 33 | Hexagonal | Antibacterial, Antifungal and Anticancer | Gao et al. [19] |
| Beta vulgaris | Plant | 20 | Spherical | Antibacterial and Antifungal | Pillai et al. [25] |
| Cinnamomum tamala | 30 | Rod | |||
| Cinnamomum verum | 46 | Spherical | |||
| Brassica oleracea var. italica | 47 | Spherical | |||
| Crotalaria verrucosa | Leaf | 16–38 | Hexagonal | Antibacterial and Anticancer | Sana et al. [71] |
| ZINC NITRATE HEXAHYDRATE | |||||
| Azadirachta indica | Leaf | 40 | Spherical | Antimicrobial | Elumalai and Velmurugan [13] |
| Vitex trifolia | Leaf | 30 | Nearly spherical and hexagonal | Antimicrobial and Photocatalytic | Elumalai et al. [72] |
| Plectranthus amboinicus | Leaf | 88 | Rod shape | Photocatalytic | Fu and Fu [73] |
| Polygala tenuifolia | Root | 33–73 | Spherical | Antioxidant and Anti-inflammatory | Nagajyothi et al. [17] |
| Allium sativum and A. cepa | Bulbs | 14–70 | Spherical | Photocatalytic activity | Stan et al. [74] |
| Petroselinum crispum | Leaf | ||||
| Pongamia pinnata | Leaf | 100 | Spherical, nanorod and hexagonal | Antibacterial | Sundrarajan et al. [75] |
| Cassia fistula | Leaf | ~5–15 | Sponge like irregular | Photocatalytic, Antioxidant and Antibacterial | Suresh et al. [76] |
| Artocarpus gomezianus | Fruit | 11.53 | Spherical | Photocatalytic and Antioxidant | Suresh et al. [77] |
| Corymbia citriodora | Leaf | 64 | Polyhedron | Photocatalytic | Zheng et al. [78] |
| Azadirachta indica | Leaf | 10–30 | Hexagonal | Antibacterial, Antioxidant and Photocatalytic | Madan et al. [36] |
| Terminalia chebula | Fruit | 12 | Roughly spherical | Photocatalytic | Rana et al. [79] |
| Citrullus colocynthis | Fruit | 85–100 | Flower | Antibacterial, Antioxidant and Anticancer | Azizi et al. [80] |
| Seed | 20–35 | Hexagonal | |||
| Pulp | 30–80 | Irregular polygons | |||
| Ocimum tenuiflorum | Leaf | 10–20 | Spherical | Non-enzymatic glucose sensor | Dayakar et al. [81] |
| Cochlospermum religiosum | Leaf | ∼76 | Hexagonal | Antibacterial and Antimitotic | Mahendra et al. [82] |
| Azadirachta indica, Hibiscus rosa-sinensis, Murraya koenigii, Moringa oleifera and Tamarindus indica | Leaf | 27–54 | Spherical | Antioxidant and Anti-diabetic | Rehana et al. [22] |
| Eucalyptus globulus | Leaf | 11.6 | Spherical | Antioxidant and Photocatalytic | Siripireddy and Mandal [83] |
| Acacia senegal | Arabic gum | 10 | Spherical | Photocatalytic | Taghavi Fardood et al. [84] |
| Conyza canadensis | Leaf | – | Irregular | Antibacterial and Photocatalytic | Ali et al. [85] |
| Garcinia mangostana | Fruit pericarp | 21 | Spherical | Photocatalytic | Aminuzzaman et al. [86] |
| Andrographis paniculata | Leaf | 57 | Spherical, oval and hexagonal | Antioxidant, Anti-diabetic and Anti-inflammatory | Rajakumar et al. [34] |
| Barleria gibsoni | Leaf | 50 | Hexagonal (Wurtzite) | Wound healing | Shao et al. [87] |
| Anacardium occidentale | Leaf | 33 | Hexagonal | Anticancer | Zhao et al. [88] |
| Gracilaria edulis | Aqueous | 20–50 | Hexagonal (Wurtzite) rod | Anticancer | Asik et al. [89] |
| Populus ciliata | Leaf | 60–70 | Sphere like | Antibacterial | Hafeez et al. [90] |
| Mentha pulegium | Leaf | 40 | Quasi- spherical | Antimicrobial | Rad et al. [91] |
| Laurus nobilis | Leaf | 20–30 | Spherical and hexagonal | Antibacterial and Photocatalytic | Chemingui et al. [92] |
| Justicia wynaadensis | Leaf | ∼39 | Hexagonal | Antimitotic and DNA-binding activities | Hemanth Kumar et al. [8] |
| Artocarpus heterophyllus | Leaf | 12–24 | Spherical | Anticancer | Majeed et al. [93] |
| Eucalyptus globules | Leaf | 52–70 | Spherical or globular | Antifungal | Ahmad et al. [94] |
| Camellia sinensis | Leaf | 11 | Sphere | Drug delivery | Akbarian et al. [95] |
| Cinnamomum verum | Bark | ~45 | Hexagonal wurtzite | Antibacterial | Ansari et al. [2] |
| Ziziphus jujuba | Fruit | 29 | Spherical | Photocatalytic | Golmohammadi et al. [96] |
| Mussaenda frondosa | Leaf | 8–15 | Hexagonal | Antibacterial, Antioxidant, Antidiabetic, Anticancer, Anti-inflammatory and Photocatalytic | Jayappa et al. [97] |
| Stem | 9–12 | Spherical | |||
| Leaf-derived callus | 5–7 | ||||
| Aegle marmelos | Juice | ~20 | Hexagonal | Antibacterial, Antioxidant and Photocatalytic | Mallikarjunaswamy et al. [98] |
| Zea mays | Husk | 300–550 | Flower-like | Antibacterial and Antioxidant | Quek et al. [31] |
| Artocarpus heterophyllus | Peel | 380–900 | Cauliflower-like | ||
| Punica granatum | Peel | 260–500 | Nanoflowers | ||
| Deverra tortuosa | Plant | 9–31 | Hexagonal | Anticancer | Selim et al. [99] |
| ZINC ACETATE | |||||
| Passiflora caerulea | Leaf | 70 | Spherical | Antibacterial | Santhoshkumar et al. [100] |
| Cucumis melo inodorus | Rough shell | 25–40 | Crystals with pseudo spherical | Anticancer | Mahdizadeh et al. [101] |
| Hyssops officinalis | Plant | 20–40 | Pseudo spherical | Anti-angiogenesis, Anti-inflammatory and Anticancer | Rahimi Kalateh Shah Mohammad et al. [102] |
| Syzgium cumini | Seed | 50–60 | Spherical | Larvicidal | Roopan et al. [38] |
| Lycopersicon esculentum | Leaf | 10–50 | Hexagonal wurtzite | Antimicrobial and Anticancer | Vijayakumar et al. [103] |
| Costus igneus | Leaf | 25–40 | Hexagonal | Antibacterial, Antioxidant and Antidiabetic | Vinotha et al. [33] |
| Rehmanniae radix | Plant | 10–12 | Rod shape | Anticancer | Cheng et al. [104] |
| ∼200 | Spherical | ||||
| Cratoxylum formosum | Leaf | ∼500 | Nanosheets | Antibacterial and Anticancer | Jevapatarakul et al. [105] |
| Syzygium cumini | Leaf | 64–78 | Spherical | Photocatalytic | Rafique et al. [106] |
| Hyssopus officinalis | Leaf | 20–40 | Pseudo spherical | Anticancer activity | Rahimi Kalateh Shah Mohammad et al. [107] |
| Thlaspi arvense | Plant | 70–90 | Flower | Antibacterial and Photocatalytic | Ullah et al. [108] |
| Raphanus sativus | Leaf | 66 | Spherical | Anticancer | Umamaheshwari et al. [29] |
| ZINC ACETATE DIHYDRATE | |||||
| Anchusa italica | Flower | ~8–14 | Hexagonal | Antimicrobial and Cytotoxicity | Azizi et al. [6] |
| Lobelia leschenaultiana | Leaf | 20–65 | Spherical and hexagonal | Acaricidal | Banumathi et al. [109] |
| Mimosa pudica | Leaf | 27 | Wurtzite and hexagonal | Photocatalytic | Fatimah et al. [110] |
| Coffea arabioca | Seed | 46 | Wurtzite and hexagonal | Photocatalytic | |
| Pongamia pinnata | Seed | 30–40 | Spherical | Anticancer and Antibiofilm | Malaikozhundan et al. [111] |
| Couroupita guianensis | Leaf | – | Hexagonal | Antibacterial | Sathishkumar et al. [112] |
| Catharanthus roseus | Leaf | 50–92 | Hexagonal wurtzite | Antibacterial | Gupta et al. [113] |
| Nyctanthes arbor-tristis | Flower | 12–32 | – | Antifungal | Jamdagni et al. [32] |
| Coffea arabica | Seeds | 26 | Spherical | Wound-healing | Khatami et al. [114] |
| Ferulago angulata | Boiss | 32–36 | Spheroid | Photocatalytic | Mehr et al. [115] |
| Averrhoa bilimbi | Fruit | 37 | Spherical | Photoelectrode | Ramanarayanan et al. [116] |
| Coccinia abyssinica | Tuber | 10.4 | Hexagonal | Antibacterial and Antioxidant | Safawo et al. [117] |
| Atalantia monophylla | Leaf | 30 | Spherical and hexagonal | Antimicrobial | Vijayakumar et al. [103] |
| Kalanchoe pinnata | Leaf | 24 | Hexagonal and spherical | Antioxidant, Anticancer and Anti-inflammatory | Agarwal and Shanmugam [21] |
| Berberis aristata | Leaf | 20–40 | Needle like | Antibacterial and Antioxidant | Chandra et al. [118] |
| Juglans regia | Leaf | 45–65 | Spherical | Antibacterial and Anticancer | Darvishi et al. [119] |
| 95–150 | Flower | ||||
| Cucurbita pepo | Leaf | 8 | Spherical | Cytotoxicity | Hu et al. [120] |
| Pandanus odorifer | Leaf | 90 | Spherical | Antibacterial and Anticancer | Hussain et al. [121] |
| Dolichos lablab | Leaf | 29 | Hexagonal wurtzite | Bactericidal and Photocatalytic | Kahsay et al. [122] |
| Abelmoschus esculentus | Okra mucilage | 29–70 | Spherical, elongated, and rod-like | Photocatalytic | Prasad et al. [123] |
| Musa acuminata | Peel | 30−80 | Triangular-like | Photocatalytic | Abdullah et al. [40] |
| Mucuna pruriens | Seed | 60 | Flower and spherical | Antibacterial | Agarwal et al. [27] |
| Sambucus ebulus | Leaf | 25−30 | Spherical | Antibacterial, Antioxidant and Photocatalytic | Alamdari et al. [124] |
| Vernonia amygdalina | Leaf | 20–40 | Cylindrical | Anti-inflammatory | Liu et al. [125] |
| Cassia fistula and Melia azadarach | Leaf | 3–68 | Spherical | Antibacterial | Naseer et al. [126] |
| Aloe vera | Leaf | ∼65 | Hexagonal | Antibacterial and Photocatalytic | Sharma et al. [127] |
| 60–180 | Spherical | ||||
| 40–45 | Cuboidal and Rod | ||||
| Calliandra haematocephala | Leaf | 19 | Flower | Photocatalytic | Vinayagam et al. [128] |
| Euphorbia fischeriana | Root | 30 | Spherical | Anticancer | Zhang et al. [129] |
| Myristica fragrans | Fruit | 43–83 | Spherical or elliptical | Antibacterial, Antiparasitic, Antioxidant, Antidiabetic, Anticancer and Photocatalytic | Faisal et al. [11] |
| Bridelia retusa | Leaf | 11 | Flower-shape | Photocatalytic | Vinayagam et al. [130] |
| ZINC SULPHATE | |||||
| Aloe barbadensis | Leaf | 8–18 | Spherical, oval and hexagonal | Antibacterial | Ali et al. [131] |
| Bauhinia tomentosa | Leaf | 22–94 | Hexagonal | Antibacterial | Sharmila et al. [30] |
| Trianthema portulacastrum | Plant | 25–90 | Spherical | Antibacterial, Antifungal, Antioxidant, Anticancer and Photocatalytic | Khan et al. [39] |
| OTHERS | |||||
| Tecoma castanifolia | Leaf | 70–75 | Spherical | Antibacterial, Antioxidant, and Anticancer | Sharmila et al. [132] |
| Trifolium pratense | Flower | 60–70 | Agglomerated | Antibacterial | Dobrucka and Długaszewska [15] |
| Jacaranda mimosifolia | Flower | 2–4 | Spherical | Antibacterial | Sharma et al. [60] |
| Heritiera fomes and Sonneratia apetala | Bark and leaf | 40–50 | – | Antibacterial, Antioxidant, Anti-diabetic and Anti-inflammatory | Thatoi et al. [16] |
| Sedum alfredii | Shoots | 100 | Columnar in shape | Photocatalytic | Wang et al. [133] |
| Juglans regia | Leaf | – | – | Antifungal | Saemi et al. [134] |
| Plants | – | – | – | Antifungal | Sun et al. [135] |
| Plant Name | Plant Part Used | Pathogen Name | Minimum Inhibitory Concentration (MIC) * (mg·mL−1) | Results | Reference |
|---|---|---|---|---|---|
| ANTIBACTERIAL ACTIVITY | |||||
| Anisochilus carnosus | Leaf | Salmonella paratyphi, Vibrio cholerae, Staphylococcus aureus and Escherichia coli | - | Showed antibacterial activity towards various human pathogens | Anbuvannan et al. [54] |
| Hibiscus subdariffa | Leaf | Escherichia coli and Staphylococcus aureus | 0.05 | Exerted better bactericidal property on S. aureus and E. coli | Bala et al. [35] |
| Azadirachta indica | Leaf | Staphylococcus aureus, Pseudomonas aeruginosa, B. subtilis, Proteus mirabilis, E. coli | 0.006–0.05 | Showed significant inhibition against bacterial strains in a dose-dependent manner | Elumalai and Velmurugan [13] |
| Vitex trifolia | Leaf | Staphylococcus aureus, Bacillus subtilis, Pseudomonas aeruginosa, Proteus mirabilis and Escherichia coli | 0.006–0.05 | Showed outstanding antibacterial activity against Gram positive and Gram negative bacteria | Elumalai et al. [72] |
| Pongamia pinnata | Leaf | Staphylococcus aureus and Escherichia coli | 0.1 | Superior antibacterial activity against Gram positive and Gram negative bacteria | Sundrarajan et al. [75] |
| Cassia fistula | Leaf | Klebsiella aerogenes, Escherichia coli, Pseudomonas desmolyticum and Staphylococcus aureus | 0.5–0.1 | Showed an excellent bactericidal activity against pathogenic bacteria | Suresh et al. [76] |
| Plectranthus amboinicus | Leaf | Staphylococcus aureus | ≤0.01 | Controlled the growth of methicillin-resistant S. aureus biofilm | Vijayakumar et al. [55] |
| Aloe barbadensis | Leaf | Escherichia coli, Pseudomonas aeruginosa and Staphylococcus aureus | 2.2–2.4 | Significant antibacterial activity against extended spectrum β-lactamases (ESBL) positive E. coli, P. aeruginosa, and methicillin resistant S. aureus (MRSA) clinical isolates | Ali et al. [131] |
| Anchusa italica | Flower | Bacillus megaterium, Stapphylococcus aureus, Escherichia coli and Salmonella typhimurium | 0.016–0.032 | Showed antimicrobial activity against Gram positive and Gram negative bacteria decreased with increasing the heat treating temperature | Azizi et al. [6] |
| Trifolium pratense | Flower | Escherichia coli, Pseudomonas aeruginosa, and Staphylococcus aureus | – | Exhibited high activity against standard and clinical strain of Gram-positive and Gram-negative bacteria | Dobrucka and Długaszewska [15] |
| Rosa canina | Fruit | Listeria monocytogenes, Staphylococcus aureus and Escherichia coli | 0.5–1 | Relatively good antibacterial activity against Gram positive and Gram negative bacteria | Jafarirad et al. [57] |
| Azadirachta indica | Leaf | Klebsiella aerogenes and Staphylococcus aureus | 0.1–1 | Showed significant antibacterial activity against K. aerogenes and S. aureus | Madan et al. [36] |
| Limonia acidissima | Leaf | Mycobacterium tuberculosis | 0.0125 | Control the growth of M. tuberculosis | Patil and Taranath [59] |
| Carica papaya | Milk | Pseudomonas aeruginosa, Staphylococcus aureus, Klebsiella aerogenes and Pseudomonas desmolyticum | 0.2–0.4 | Showed significant antibacterial activity against bacterial stains | Sharma [60] |
| Jacaranda mimosifolia | Flower | Escherichia coli and Enterococcus faecium | 0.1 | Enhanced antibacterial activity against pathogenic strains | Sharma et al. [136] |
| Boswellia ovalifoliolata | Bark | Sphingobacterium thalpophilum, Uncultured organism clone, Ochrobactrum sp., Uncultured Achromobacter sp., Uncultured bacterium clone, Sphingobacterium sp., Acinetobacter sp., Uncultured soil bacterium, Ochrobactrum sp., Uncultured bacterium | - | Showed good antibacterial activity at 170 ppm compared to 50 and 100 ppm | Supraja et al. [61] |
| Heritiera fomes | Bark and Leaf | Shigella flexneri | 0.1 | Displayed positive inhibition activity against S. flexneri | Thatoi et al. [16] |
| Sonneratia apetala | |||||
| Citrullus colocynthis | Fruit, Seed and Pulp | Bacillus subtilis, Methicillin-resistant Staphylococcus aureus, Pseudomonas aeruginosa and Escherichia coli | – | Inhibited the growth of medically significant pathogenic Gram positive and Gram negative bacteria | Azizi et al. [80] |
| Cochlospermum religiosum | Leaf | Bacillus subtilis, Staphylococcus aureus, Pseudomonas aeruginosa and Escherichia coli | 0.004–0.312 | Showed significant inhibition against Gram positive and Gram negative bacteria | Mahendra et al. [82] |
| Pongamia pinnata | Seed | Bacillus licheniformis, Pseudomonas aeruginosa, Vibrio parahaemolyticus | 0.025 | Effectively inhibited Gram positive and Gram negative bacteria growth | Malaikozhundan et al. [111] |
| Ceropegia candelabrum | Leaf | Staphylococcus aureus, Bacillus subtilis, Escherichia coli, Salmonella typhi | 0.1 | Showed significant inhibition against Gram positive and Gram negative bacterial pathogens | Murali et al. [5] |
| Passiflora caerulea | Leaf | Klebsiella sp., Streptococcus sp., Enterococcus sp., and Escherichia coli | - | Showed very good inhibition of urinary tract infection causing microbes | Santhoshkumar et al. [100] |
| Couroupita guianensis | Leaf | Bacillus cereus, Klebsiella pneumoniae, Escherichia coli, Micrococcus luteus, Salmonella typhi,and Vibrio cholerae | 0.005 | Exhibited excellent dose dependent bactericidal effect against human pathogens | Sathishkumar et al. [112] |
| Conyza canadensis | Leaf | Escherichia coli and Staphylococcus aureus | 0.055–0.094 | Exhibited strong antibacterial activity | Ali et al. [85] |
| Catharanthus roseus | Leaf | Staphylococcus aureus, Streptococcus pyogenes, Bacillus cereus, Pseudomonas aeruginosa, Proteus mirabilis and Escherichia coli | 1.5 | Displayed good antibacterial activity against pathogenic bacteria | Gupta et al. [113] |
| Coccinia abyssinica | Tuber | Bacillus coagulans, Staphylococcus aureus, Shigella dysenteriae, Salmonella typhimurium and Sphingomonas paucimobilis | 0.001–0.005 | Showed effective growth inhibition activity against Gram negative and Gram positive bacteria | Safawo et al. [117] |
| Bauhinia tomentosa | Leaf | Bacillus subtilis, Staphylococcus aureus, Escherichia coli and Pseudomonas aeruginosa | – | Exhibited better antibacterial activity against Gram negative bacteria than Gram positive bacteria | Sharmila et al. [30] |
| Costus pictus | Leaf | Staphylococcus aureus, Bacillus subtilis, Escherichia coli, Salmonella paratyphi | 0.1 | Exhibited strong antimicrobial behavior against bacterial species | Suresh et al. [66] |
| Atalantia monophylla | Leaf | Bacillus subtilis, Bacillus cereus, Staphylococcus aureus, Escherichia coli, Pseudomonas aeruginosa and Klebsiella pnemoniae | – | Showed antimicrobial potential against pathogenic bacteria | Vijayakumar et al. [103] |
| Berberis aristata | Leaf | Escherichia coli, Staphylococcus aureus, Klebsiella pneumoniae, Bacillus subtilis, Bacillus cereus and Serratia marcescens | 0.064–0.256 | Displayed antibacterial activity against urinary tract infection causing pathogens | Chandra et al. [118] |
| Laurus nobilis | Leaf | Escherichia coli | 1.2 | Proved as an effective antibacterial agent against E. coli | Chemingui et al. [92] |
| Juglans regia | Leaf | Escherichia coli, Pseudomonas aeruginosa and Acinetobacter baumannii | 0.2 | Exerted bactericidal property on resistant strains | Darvishi et al. [119] |
| Populus ciliata | Leaf | Escherichia coli, Pseudomonas aeruginosa, Klebsiella pneumonia, Staphylococcus aureus and Streptococcus pyogene | – | Showed significant antibacterial potential on test pathogens | Hafeez et al. [90] |
| Pandanus odorifer | Leaf | Bacillus subtilis, Escherichia coli | 0.05 | Showed significant antibacterial potential on test pathogens | Hussain et al. [121] |
| Dolichos lablab | Leaf | Bacillus pumilus and Sphingomonas paucimobilis | 5 | Showed a bactericidal activity for pathogenic Gram positive and Gram negative bacteria | Kahsay et al. [122] |
| Trianthema portulacastrum | Plant | Staphylococcus aureus and Escherichia coli | – | Showed significant antibacterial property | Khan et al. [39] |
| Mentha pulegium | Leaf | Staphylococcus aureus and Escherichia coli | 0.2 | Exhibited significant antimicrobial potential on some food-borne pathogens | Rad et al. [91] |
| Annona squamosa | Leaf | Escherichia coli, Bacillus subtilis, Staphylococcus aureus, Pseudomonas aeruginosa, Enterococcus faecium | 0.006–0.012 | Synergetic antibacterial potential against wound/burn infection causing bacteria | Ruddaraju et al. [69] |
| Artabotrys hexapetalu | Leaf | Streptococcus and Serratia | – | Showed better antibacterial performance against Gram positive and Gram negative bacteria | Shanavas et al. [68] |
| Bambusa vulgaris | |||||
| Tecoma castanifolia | Leaf | Bacillus subtilis, Staphylococcus aureus, Escherichia coli, Pseudomonas aeruginosa | 0.075–0.1 | Excellent antibacterial activity against Gram positive and Gram negative bacteria | Sharmila et al. [132] |
| Albizia lebbeck | Bark | Bacillus cereus, Staphylococcus aureus, Escherichia coli, Klebsiella pneumoniae,and Salmonella typhi | 35.5 | Strong antibacterial potential against Gram-negative and Gram-positive bacterial pathogens | Umar et al. [26] |
| Lycopersicon esculentum | Leaf | Enterococcus faecalis and Proteus vulgaris | 0.008–0.01 | A notable reduction in bacterial growth was observed | Vijayakumar et al. [137] |
| Costus igneus | Leaf | Streptococcus mutans, Lysinibacillus fusiformis, Proteus vulgaris,and Vibrio parahaemolyticus | 0.04–0.07 | Showed promising antibacterial activity against targeted pathogenic bacteria | Vinotha et al. [33] |
| Mucuna pruriens | Seed | Bacillus subtilis | 0.02 | Showed concentration dependent inhibition of the growth of B. subtilis | Agarwal et al. [27] |
| Sambucus ebulus | Leaf | Bacillus cereus, Staphylococcus aureus,and Escherichia coli | 0.1 | Exhibited antibacterial activity over all three bacteria | Alamdari et al. [124] |
| Cinnamomum verum | Bark | Escherichia coli and Staphylococcus aureus | 0.062–0.125 | Inhibited the growth of harmful pathogens | Ansari et al. [2] |
| Citrus sinensis | Fruit Peel | Escherichia coli and Staphylococcus aureus | 0.020–0.040 | Showed stronger antibacterial activity | Gao et al. [19] |
| Mussaenda frondosa | Leaf, Stem and Leaf-derived callus | Staphylococcus aureus, Bacillus subtilis, Escherichia coli and Pseudomonas aeruginosa | 0.019–0.185 | Showed inhibition against bacterial strains | Jayappa et al. [97] |
| Cratoxylum formosum | Leaf | Bacillus subtilis, Staphylococcus epidermidis, Escherichia coli | 5 | Inhibited Gram positive and Gram negative bacterial growth | Jevapatarakul et al. [105] |
| Aegle marmelos | Juice | Staphylococcus aureus, Bacillus cereus, Micrococcus luteus, Escherichia coli, Klebsiella pneumonia, Enterobacter aerogenes, Pseudomonas fluorescens, Pseudomonas aeruginosa and Salmonella enteritidis | 3.84–8.65 | Showed good bactericidal activity | Mallikarjunaswamy et al. [98] |
| Cassia fistula and Melia azadarach | Leaf | Escherichia coli and Staphylococcus aureus | 0.05 | Showed strong antimicrobial activity against clinical pathogens | Naseer et al. [126] |
| Beta vulgaris | Plant | Escherichia coli and Staphylococcus aureus | – | Shown antibacterial activity both Gram negative and Gram positive bacteria | Pillai et al. [25] |
| Cinnamomum tamala | |||||
| Cinnamomum verum | |||||
| Brassica oleracea | |||||
| Zea mays | Husk | Enterococcus faecalis | – | Excellent antibacterial activity against E. faecalis compared to zinc oxide synthesized without plant extract and commercial zinc oxide | Quek et al. [31] |
| Artocarpus heterophyllus | Peel | ||||
| Punica granatum | |||||
| Crotalaria verrucosa | Leaf | Escherichia coli, Staphylococcus aureus, Proteus vulgaris,and Klebsiella pneumonia | 0.1 | Exhibited significant antibacterial potentiality against Gram positive and Gram negative pathogenic bacteria | Sana et al. [71] |
| Aloe vera | Leaf | Bacillus subtilis, Staphylococcus aureus and Escherichia coli | 0.195–3.125 | Showed antibacterial activity against pathogenic bacteria | Sharma et al. [127] |
| Thlaspi arvense | Plant | Escherichia coli | 0.015 | Exhibited a significant antibacterial activity against Gram negative E. coli | Ullah et al. [108] |
| Myristica fragrans | Fruit | Klebsiella pneumoniae, Escherichia coli, Pseudomonas aeruginosa,and Staphylococcus aureus | 1 | Shown successful capacity against bacterial strains | Faisal et al. [11] |
| ANTIFUNGAL ACTIVITY | |||||
| Azadirachta indica | Leaf | Candida albicans and Candida tropicalis | 0.006–0.05 | Showed significant inhibition against fungal strains in a dose-dependent manner | Elumalai and Velmurugan [13] |
| Vitex trifolia | Leaf | Candida albicans and Candida tropicalis | 0.006–0.05 | Excellent antifungal activity against human pathogenic fungi | Elumalai et al. [72] |
| Boswellia ovalifoliolata | Bark | Meyerozyma caribbica, Aspergillus parvisclerotigenus, Meyerozyma guilliermondii, Rhizopus oryzae, Uncultured fungus clone, Aspergillus oryzae, Trichoderma asperellum | – | Showed good antifungal activity at 170 ppm compared to 50 and 100 ppm | Supraja et al. [61] |
| Pongamia pinnata | Seed | Candida albicans | 0.05 | Effectively inhibited the biofilm formation of C. albicans | Malaikozhundan et al. [111] |
| Ziziphus nummularia | Leaf | Candida albicans, Candida glabrata and Cryptococcus neoformans | 1.25–10 | Showed very good antifungal activity against clinical isolates | Padalia and Chanda [7] |
| Nyctanthes arbor-tristis | Flower | Alternaria alternata, Aspergillus niger, Botrytis cinerea, Fusarium oxysporum and Penicillium expansum | 0.016 | Showed good antifungal potential against fungal phytopathogens | Jamdagni et al. [32] |
| Costus pictus | Leaf | Aspergillus niger and Candida albicans | 0.1 | Exhibited strong antimicrobial behavior against fungal species | Suresh et al. [66] |
| Atalantia monophylla | Leaf | Candida albicans and Aspergillus niger | – | Showed antimicrobial potential against pathogenic fungi | Vijayakumar et al. [103] |
| Trianthema portulacastrum | Plant | Aspergillus niger, Aspergillus flavus and Aspergillus fumigatus | 0.1 | Showed significant antifungal property | Khan et al. [39] |
| Lycopersicon esculentum | Leaf | Candida albicans | 0.013 | A notable reduction in fungal growth was observed | Vijayakumar et al. [137] |
| Eucalyptus globules | Leaf | Alternaria mali, Botryosphaeria dothidea and Diplodia seriata | – | Showed considerable fungicidal property against phytopathogenic fungi | Ahmad et al. [94] |
| Citrus sinensis | Peel | Botrytis cinerea | 0.2 | Showed stronger antifungal activity against B. cinerea | Gao et al. [19] |
| Beta vulgaris | Plant | Candida albicans and Aspergillus niger | – | Shown activity against the fungal strains | Pillai et al. [25] |
| Cinnamomum tamala | |||||
| Cinnamomum verum | |||||
| Brassica oleracea | |||||
| Plant Name | Description | Concentration | Maximum Activity | Results | Reference |
|---|---|---|---|---|---|
| Polygala tenuifolia | Root | 1 mg·mL−1 | 45.47% | Moderate antioxidant activity by scavenging DPPH free radical | Nagajyothi et al. [17] |
| Cassia fistula | Leaf | 2853 µg·mL−1 | 50% | Inhibiting DPPH free radical scavenging activity | Suresh et al. [76] |
| Artocarpus gomezianus | Fruit | 10.8 mg·mL−1 | 50% | Inhibiting DPPH free radical scavenging activity | Suresh et al. [77] |
| Rosa canina | Fruit | 0.2 mg·mL−1 | >90% | DPPH free radical scavenging attribute | Jafarirad et al. [57] |
| Azadirachta indica | Leaf | 8355 μg·mL−1 | 92% | Inhibiting DPPH free radical scavenging activity | Madan et al. [36] |
| Heritiera fomes and Sonneratia apetala | Bark and leaf | 53.64 μg·mL−1 | 50% | Strong DPPH free radical scavenging potential | Thatoi et al. [16] |
| Citrullus colocynthis | Fruit, seed and pulp | 0.22 mg·mL−1 (Fruit), 0.29 mg·mL−1 (Seed) and 0.26 mg·mL−1 (Pulp) | 50% | Inhibiting DPPH free radical scavenging activity | Azizi et al. [80] |
| Ceropegia candelabrum | Leaf | 95.09 μg·mL−1 | 55.43% | DPPH free radical scavenging activity | Murali et al. [5] |
| Azadirachta indica, Hibiscus rosa-sinensis, Murraya koenigii, Moringa oleifera and Tamarindus indica | Leaf | 11.03–31.51 µg·mL−1 (ABTS), 11.49–37.8 µg·mL−1 (DPPH), 23.31–45.9 µg·mL−1 (hydroxyl), 24.4–53.2 µg·mL−1 (superoxide) and 31.4–58.4 µg·mL−1 (hydrogen peroxide) | 50% | Inhibition of ABTS, DPPH, hydroxyl, superoxide and hydrogen peroxide radical scavenging activities | Rehana et al. [22] |
| Eucalyptus globulus | Leaf | 46.62 μg·mL−1 | 82% | DPPH free radical scavenging inhibition | Siripireddy and Mandal [83] |
| Andrographis paniculata | Leaf | 500 μg·mL−1 | 61.32% | DPPH free radical scavenging inhibition | Rajakumar et al. [34] |
| Mangifera indica | Leaf | 30 μg·mL−1 | 65% | DPPH free radical scavenging activity | Rajeshkumar et al. [65] |
| Coccinia abyssinica | Tuber | 127.74 μg·mL−1 | 50% | DPPH free radical scavenging activity | Safawo et al. [117] |
| Kalanchoe pinnata | Leaf | 700 μg·mL−1 | 50% | Reduce DPPH free radical scavenging capacity | Agarwal and Shanmugam [21] |
| Berberis aristata | Leaf | 3.55 μg·mL−1 | 50% | DPPH free radical scavenging activity | Chandra et al. [118] |
| Trianthema portulacastrum | Plant | 500 μg·mL−1 | 75% | Efficient DPPH free radical inhibition | Khan et al. [39] |
| Tecoma castanifolia | Leaf | 100 μg·mL−1 | 67% | DPPH free radical scavenging activity | Sharmila et al. [132] |
| Scutellaria baicalensis | Root | 1000 µg·mL−1 | 56.11% | Scavenging DPPH free radicals | Tettey and Shin [70] |
| Albizia lebbeck | Stem bark | 48.5 µg·mL−1 | 50% | Showed the concentration dependent effect in hydrogen peroxide (H2O2) free radical scavenging activity | Umar et al. [26] |
| Costus igneus | Leaf | 100 μg·mL−1 | 75% | DPPH free radical scavenging activity | Vinotha et al. [33] |
| Sambucus ebulus | Leaf | 43 µg·mL−1 | 50% | Exhibited hydrogen peroxide (H2O2) free radical scavenging activity | Alamdari et al. [124] |
| Mussaenda frondosa | Leaf, stem and leaf-derived callus | 824 µg·mL−1 (Leaf), 752 µg·mL−1 (Stem) and 857 µg·mL−1 (Callus) | 50% | Quenching the DPPH free radical scavenging | Jayappa et al. [97] |
| Aegle marmelos | Juice | 5.75–6.78 mg·mL−1 (DPPH), 4.45–5.05 mg·mL−1 (ABTS) and 7.86–9.05 mg·mL−1 (Superoxide) | 50% | ABTS cation radical, DPPH free radical, and superoxide anion radical scavenging activities | Mallikarjunaswamy et al. [98] |
| Zea mays, Artocarpus heterophyllus and Punica granatum | Husk (Z. mays) and peel (A. heterophyllus and P. granatum) | 395.2 µg·mL−1 (P. granatum) | 50% | Inhibitory of DPPH radical scavenger | Quek et al. [31] |
| Myristica fragrans | Fruit | 400 μg·mL−1 | 82.12 TEAC (ABTS); 66.3% FRSA (DPPH); 71.1 μg AAE/mg (TAC); 63.41 μg AAE/mg (TRP) | Excellent free radical scavenging activities (ABTS, DPPH, TAC and TRP) | Faisal et al. [11] |
| Plant Name | Description | Concentration | Activity (IC50Value) * (mg mL−1) | Results | Reference |
|---|---|---|---|---|---|
| Hibiscus subdariffa | Leaf | 8 mg·kg−1 of body weight | – | Streptozotocin (STZ: 100 mg/kg of body weight) induced diabetes was cured by intraperitoneal injection of zinc oxide in mice | Bala et al. [35] |
| Heritiera fomes (HF)and Sonneratia apetala (SA) | Bark and leaf | 100 μL | 0.33 (HF) and 0.39 (SA) | Exhibited better anti-diabetic activity in terms of α-amylase inhibition activity | Thatoi et al. [16] |
| Azadirachta indica, Hibiscus rosa-sinensis, Murraya koenigii, Moringa oleifera and Tamarindus indica | Leaf | 100–1.52 µg·mL−1 | α-amylase: 0.025–0.05 α-glucosidase: 0.012–0.05 | Exhibited higher α-amylase and α-glucosidase inhibition activity | Rehana et al. [22] |
| Vaccinium arctostaphylos | Fruit | – | – | Exhibited great treating efficacy on alloxan-diabetic rats compared to chemically synthesized zinc oxide | Bayrami et al. [63] |
| Andrographis paniculata | Leaf | 100 μL | 0.12 | Exhibited better anti-diabetic activity in terms of exhibiting moderate α-amylase inhibitory activity | Rajakumar et al. [34] |
| Costus igneus | Leaf | 100 μg·mL−1 | – | Increased the percentage of α-amylase and α-glucosidase inhibition with increased concentration of nanoparticles | Vinotha et al. [33] |
| Mussaenda frondosa | Leaf, stem and leaf-derived callus | 20 μL | α-amylase: 0.014- 0.055 α-glucosidase: 0.014–0.035 | Exhibited on par α-amylase inhibitory activity and α-glucosidase inhibitory activity | Jayappa et al. [97] |
| Myristica fragrans | Fruit | 400 μg·mL−1 | – | Excellent α-amylase and α-glucosidase inhibition activity | Faisal et al. [11] |
| Plant Name | Description | Cell Lines Used | Activity (IC50Value) | Results | Reference |
|---|---|---|---|---|---|
| Anchusa italica | Flower | Vero cells | 142 μg·mL−1 | Showed concentration-dependent cytotoxicity on the growth of Vero cells | Azizi et al. [6] |
| Rosa canina | Fruit | Alveolar adenocarcinoma (A549) cells | >0.1 mg·mL−1 | Exhibited dose-dependent toxicity to A549 cells | Jafarirad et al. [57] |
| Citrullus colocynthis | Fruit, seed and pulp | 3T3 cells | 0.258 mg·mL−1 (Fruit), 0.160 mg·mL−1 (Seed) and 0.210 mg·mL−1 (Pulp) | Showed a dose dependent toxicity on the growth of 3T3 cells with non-toxic effect of concentration below 0.26 mg/mL | Azizi et al. [80] |
| Pongamia pinnata | Seed | Human MCF-7 breast cancer cell lines | 50 μg·mL−1 | More successful in control of human MCF-7 breast cancer cells compared to the seed extract and bulk zinc oxide (positive control) | Malaikozhundan et al. [111] |
| Ziziphus nummularia | Leaf | HeLa cancer cell lines | 50 and 200 μg·mL−1 | Showed potent dose-dependent cytotoxic effect against HeLa cancer cell lines | Padalia and Chanda [7] |
| Mangifera indica | Leaf | Lung cancer A549 cell lines | 25 μg·mL−1 | Significant cytotoxic effect against lung cancer A549 cell lines | Rajeshkumar et al. [65] |
| Costus pictus | Leaf | Daltons lymphoma ascites (DLA) cells | 50 µg·mL−1 | Exhibited strong anticancer behavior against DLA bearing mice cell lines | Suresh et al. [66] |
| Anacardium occidentale | Leaf | Human normal fibroblast cell line (Hu02) and human pancreatic cancer cell lines (Panc-1 and AsPC-1) | 40 μM (Panc-1) and 30 μM (AsPC-1) | Exhibited the concentration-dependent cytotoxicity against human pancreatic cancer cell lines | Zhao et al. [88] |
| Kalanchoe pinnata | Leaf | Murine macrophage RAW 264.7 cells | – | Exhibited no significant cytotoxicity up to 1 mg/mL in RAW 264.7 cells | Agarwal and Shanmugam [21] |
| Gracilaria edulis | Aqueous extract | Cervical carcinoma cells (SiHa cells) | 35 μg·mL−1 | Exhibited cytotoxic effect against SiHa cells in a dose dependent manner | Asik et al. [89] |
| Juglans regia | Leaf | Human skin fibroblasts | 200 μg·mL−1 | Have less cytotoxicity than chemical zinc oxide nanoparticles | Darvishi et al. [119] |
| Cucurbita pepo | Leaf | Mammalian osteoblast-like MG63 cells | 20 ppm | Induced cytotoxicity that affected the proliferation of MG63 cells in the concentration dependent manner | Hu et al. [120] |
| Pandanus odorifer | Leaf | Breast cancer (MCF-7), liver cancer (HepG2), and lung cancer (A-549) cells | 100 μg·mL−1 | Apoptotic and necrosis effect on MCF-7, HepG2, and A549 cancer cell lines | Hussain et al. [121] |
| Trianthema portulacastrum | Plant | Mouse pre-osteoblast cell line (MC3T3-E1) | – | Showed no toxic effect and the cells were found viable | Khan et al. [39] |
| Cucumis melo inodorus | Rough shell | Human (Michigan Cancer Foundation-7 [MCF7]) and murine (TUBO) breast cancer cell lines | 40 µg·mL−1 (MCF7); 20 µg·mL−1 (TUBO) | Found as a powerful apoptosis inducer in breast cancer cells in human cell line (MCF7) and murine (TUBO cell line and cancer model) | Mahdizadeh et al. [101] |
| Artocarpus heterophyllus | Leaf | Human colon cancer HCT-116 cell lines | 20 μg·mL−1 | Showed excellent cytotoxic effect against human colon cancer HCT-116 cell lines | Majeed et al. [93] |
| Hyssops officinalis | Plant | MDA-MB231 breast cancer cell line | 125 μg·mL−1 | Inhibitory effects on the growth of breast cancer cells and induction of cytotoxicity depending on nanoparticle concentration and time of exposure | Rahimi Kalateh Shah Mohammad et al. [102] |
| Annona squamosa | Leaf | Cervical cancer cells (HeLa cell lines) | 50 μg·mL−1 | Anticancer activity against HeLa cell lines in a dose dependent pattern with a defensive prospect towards mammalian (HEK-293) cells | Ruddaraju et al. [69] |
| Tecoma castanifolia | Leaf | Human lung carcinoma cells (A549) | 65 μg·mL−1 | Conferred better cytotoxic effects on proliferation of A549 cell line | Sharmila et al. [132] |
| Scutellaria baicalensis | Root | HeLa cells (Human cervical cancer cell line) and RAW 264.7 murine macrophage cells | 1000 µg·mL−1 | Showed dose-dependent antiproliferative activity against the growth of HeLa cells and no toxicity on RAW 264.7 macrophages (normal immune system cells) | Tettey and Shin [70] |
| Albizia lebbeck | Stem bark | Human breast cancer cell lines (MDA-MB 231 and MCF-7) | Cytotoxicity: 100 µg·mL−1 (MDA-MB 231) and 5 µg·mL−1 (MCF-7); Proliferation: 100 µg·mL−1 (MDA-MB 231 and MCF-7) | Inhibited the cell viability and cell number (proliferation) of MDA-MB 231 and MCF-7 cells in concentration dependent manner | Umar et al. [26] |
| Lycopersicon esculentum | Leaf | Murine macrophage cells (RAW 264.7) and Human cervical cancer (HeLa) cells | 100 µg·mL−1 | Zinc oxide nanoparticles were non-toxic to macrophage cells, as no alterations in viability. Treatment of HeLa cells with zinc oxide nanoparticles induced cell growth retardation, cell clumping, cell bursting, and loss of membrane stability and they prevented the proliferation of HeLa cells | Vijayakumar et al. [137] |
| Rehmanniae radix | Plant | Bone cancer cell line MG-63 | 30 μg·mL−1 | Exhibited strong anticancer activity and inducing apoptosis on MG-63 cells via stimulating increased generation of ROS | Cheng et al. [104] |
| Citrus sinensis | Peel | Human umbilical vein endothelial cells (HUVECs) | Below 25 mg·L−1 | Cytotoxicity towards HUVECs exhibited when the concentration exceeded 12.5 mg L−1 | Gao et al. [19] |
| Mussaenda frondosa | Leaf, stem and leaf-derived callus | Human lung adenocarcinoma cells (A549) | 67.75 µg·mL−1 (Callus) and 85.66 µg·mL−1 (Stem) | Exhibited on par cytotoxic activity on A549 cells in a dose-dependent action | Jayappa et al. [97] |
| Hyssopus officinalis | Leaf | Human prostate cancer (PC3) cells | 8.07 µg·mL−1 (24 h) and 5 µg·mL−1 (48 h) | Demonstrated the dose-dependent cytotoxicity effect and induced apoptosis on PC3 cells | Rahimi Kalateh Shah Mohammad et al. [107] |
| Crotalaria verrucosa | Leaf | HeLa and DU145 cell lines | 7.07 µg·mL−1 (HeLa); 6.30 µg·mL−1 (DU145) | Exhibited the dose-dependent inhibition curve with IC50 value of 7.07 µg/mL and 6.30 µg/mL in HeLa and DU145 cells, respectively | Sana et al. [71] |
| Deverra tortuosa | Plant | Human colorectal epithelial adenocarcinoma (Caco-2), human lung epithelial carcinoma (A549) and normal human lung fibroblast cell line (WI38) | 83.47 μg·mL−1 (A549), 50.81 μg·mL−1 (Caco-2) and 434.60 μg·mL−1 (WI38) | Exhibited the profound selective concentration dependent cytotoxic effect on Caco-2 and A549 cancer cell lines with appreciable lower cytotoxic activity on normal WI38 cells | Selim et al. [99] |
| Euphorbia fischeriana | Root | Lung cancer (A549) cells | 14.5 µg·mL−1 | Induced cytotoxicity and also activated apoptosis during increased ROS formation, decreased mitochondrial membrane potential, inhibited cell migration, altered AO/EtBr staining and induced pro-apoptotic and inhibited anti-apoptotic protein | Zhang et al. [129] |
| Myristica fragrans | Fruit | Streptomyces 85E strain for protein kinase inhibition capability | 5 mg·mL−1 | Clear zones were observed against Streptomyces 85E strain which used to elucidate the protein kinase inhibition capability | Faisal et al. [11] |
| Raphanus sativus | Leaf | Lung cancer cell line (A549) | 40 μg·mL−1 | Showed a better anticancer activity by reducing cell viability | Umamaheswari et al. [29] |
| Plant Name | Description | Assay/Model | Activity (IC50Value) | Results | Reference |
|---|---|---|---|---|---|
| Polygala tenuifolia | Root | LPS-stimulated RAW 264.7 murine macrophage cells | 1 mg·mL−1 | Showed anti-inflammatory activity by suppressing the LPS-induced mRNA and protein expressions of iNOS, COX-2, and anti-inflammatory cytokines in LPS-stimulated RAW 264.7 murine macrophage cells | Nagajyothi et al. [17] |
| Heritiera fomes (HF) and Sonneratia apetala (SA) | Bark and leaf | Inhibition of protein denaturationin vitroassay | 72.35 μg·mL−1 (HF) and 63.29 μg·mL−1 (SA) | Anti-inflammation activity inhibiting protein (heat induced albumin) denaturation | Thatoi et al. [16] |
| Andrographis paniculata | Leaf | Inhibition of protein denaturationin vitroassay | 66.78 μg·mL−1 | Anti-inflammatory activity by inhibiting protein denaturation | Rajakumar et al. [34] |
| Kalanchoe pinnata | Leaf | LPS-induced Murine Raw 264.7 cell lines; Detection of the mRNA expressions of TNF-α, IL-1β, IL-6, and COX-2 | – | Reduced the expression of pro-inflammatory cytokines, attenuated the release of IL-1β, IL-6, and TNF-α by inhibiting mRNA expression, inhibited the gene expression of COX-2 enzyme and suppressed NO production | Agarwal and Shanmugam [21] |
| Hyssops officinalis | Plant | Reduction of mouse paw edema | 5 mg·kg−1 | Reduction of inflammation by significantly reducing the thickness of mouse paw edema | Mohammad et al. [102] |
| Mussaenda frondosa | Leaf, stem & leaf-derived callus | Human red blood cells membrane stabilization method | 500 µg·mL−1 | Exhibited varying degrees of human RBCs membrane and lysosomal membrane stabilizing activity in a dose-dependent manner | Jayappa et al. [97] |
| Vernonia amygdalina | Leaf | Swiss Albino male mice | 2.5, 5, and 7.5 mg·kg−1 | Exhibited the potent anti-inflammatory activity against carrageenan induced-inflammation in mice | Liu et al. [125] |
| Plant Name | Dye Degraded | Solar Irradiation Time | pH Range | Degradation Efficiency (%) | Reference |
|---|---|---|---|---|---|
| Phyllanthus niruri | Methylene blue (MB) | 30 min | – | 99% | Anbuvannan et al. [53] |
| Anisochilus carnosus | Methylene blue (MB) | 90 min | – | 100% | Anbuvannan et al. [54] |
| Vitex trifolia | Methylene blue (MB) | 90 min | – | 92.13% | Elumalai et al. [72] |
| Plectranthus amboinicus | Methyl red (MR) | 180 min | – | 92.45% | Fu and Fu [73] |
| Allium sativum, Allium cepa and Petroselinum crispum | Methylene blue | 180 min | – | >90% | Stan et al. [74] |
| Cassia fistula | Methylene blue | 120 min | 7 | 90% (for 5 ppm) | Suresh et al. [76] |
| Artocarpus gomezianus | Methylene blue (MB) | 120 min | 10 | 100% (for 5 ppm, sun light); 65% (for 5 ppm, UV light) | Suresh et al. [77] |
| Corymbia citriodora | Methylene blue | 90 min | – | 83.45% | Zheng et al. [78] |
| Mimosa pudica | Methylene blue | 120 min | – | 90% | Fatimah et al. [110] |
| Coffea arabica | Methylene blue | 120 min | – | 98% | |
| Carissa edulis | Congo red | 130 min | – | 97% | Fowsiya et al. [56] |
| Nephelium lappaceum | Methyl orange (MO) | 120 min | 7.01 | 83.99% | Karnan and Selvakumar [58] |
| Azadirachta indica | Methylene blue (MB) | 120 min | – | >80% | Madan et al. [36] |
| Terminalia chebula | Rhodamine B (RhB) | 5 h | – | 70% (for 5 ppm) | Rana et al. [79] |
| Carica papaya | Alizarin Red-S | 120 min | – | 99% | Sharma [60] |
| Sedum alfredii | 2-chlorophenol (2-CP) | 120 min | 6.3 | 96.93% | Wang et al. [133] |
| Camellia sinensis | Methylene blue (MB) | 240 min | – | 100% | Nava et al. [62] |
| Eucalyptus globulus | Methylene blue (MB) and Methyl orange (MO) | 50 min (MB) and 1 h (MO) | – | 98.2% (MB) and 96.6% (MO) | Siripireddy and Mandal [83] |
| Acacia senegal | Direct blue 129 (DB129) | 105 min | – | 95% | Taghavi Fardood et al. [84] |
| Conyza canadensis | Methylene blue (MB) and Methyl orange (MO) | 45 min (MO) and 20 min (MB) | – | 94.5% (MO) and 85.3% (MB) | Ali et al. [85] |
| Garcinia mangostana | Malachite green | 180 min | – | 99% | Aminuzzaman et al. [86] |
| Citrus sinensis | Methylene blue (MB) | 120 min | – | 83% | Luque et al. [64] |
| Ferulago angulata | Rhodamine B (RhB) | 150 min | – | 93% | Mehr et al. [115] |
| Laurus nobilis | Remazol Brilliant Red F3B (Reactive Red 180, RR180) | 45 min | Around 6.8 | 99% | Chemingui et al. [92] |
| Dolichos lablab | Methylene blue (MB), Rhodamine B (RhB) and Orange II (OII) | 210 min | 11 (MB), 9 (RhB) and 5 (OII) | 80% (MB), 95% (RhB), and 66% (OII) | Kahsay et al. [122] |
| Trianthema portulacastrum | Synozol navy blue K-BF | 150min | – | 91% | Khan et al. [39] |
| Abelmoschus esculentus | Methylene blue (MB) and Rhodamine B (RhB) | 60 min (MB) and 50 min (RhB) | ∼7 | 100% | Prasad et al. [123] |
| Artabotrys hexapetalu (AH) and Bambusa vulgaris (BV) | Rhodamine B (RhB) | 180 min | Neutral | 92% (AH) and 88% (BV) | Shanavas et al. [68] |
| Musa acuminata | Methylene blue (MB) | 7 h | 12 | 98.13% | Abdullah et al. [40] |
| Sambucus ebulus | Methylene blue | 200 min | – | 80% | Alamdari et al. [124] |
| Ziziphus jujuba | Methylene blue (MB) and Eriochrome black-T (ECBT) | 5 h | – | 92% (MB) and 86% (ECBT) | Golmohammadi et al. [96] |
| Mussaenda frondosa | Methylene blue | 100 min (Leaf), 100 min (Stem), and 120 min (Callus) | 7 | 30% (Leaf), 30% (Stem) and 90% (Callus) | Jayappa et al. [97] |
| Aegle marmelos | Methylene blue (MB) | 35 min | – | 96% | Mallikarjunaswamy et al. [98] |
| Quince | Methylene blue | 2 h | Normal | 80% | Moghaddas et al. [141] |
| Syzygium cumini | Rhodamine B (RhB) | 100 min | 9 | 98% | Rafique et al. [106] |
| Aloe vera | Methyl orange (MO) | 140–160 min | – | 95% | Sharma et al. [127] |
| Thlaspi arvense | Methylene blue (MB) | 2 h | – | 100% | Ullah et al. [108] |
| Calliandra haematocephala | Methylene blue (MB) | 270 min | – | 88% | Vinayagam et al. [128] |
| Myristica fragrans | Methylene blue | 140 min | – | 88% | Faisal et al. [11] |
| Bridelia retusa | Rhodamine B | 165 min | – | Upto 94.74% | Vinayagam et al. [130] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Murali, M.; Kalegowda, N.; Gowtham, H.G.; Ansari, M.A.; Alomary, M.N.; Alghamdi, S.; Shilpa, N.; Singh, S.B.; Thriveni, M.C.; Aiyaz, M.; et al. Plant-Mediated Zinc Oxide Nanoparticles: Advances in the New Millennium towards Understanding Their Therapeutic Role in Biomedical Applications. Pharmaceutics 2021, 13, 1662. https://doi.org/10.3390/pharmaceutics13101662
Murali M, Kalegowda N, Gowtham HG, Ansari MA, Alomary MN, Alghamdi S, Shilpa N, Singh SB, Thriveni MC, Aiyaz M, et al. Plant-Mediated Zinc Oxide Nanoparticles: Advances in the New Millennium towards Understanding Their Therapeutic Role in Biomedical Applications. Pharmaceutics. 2021; 13(10):1662. https://doi.org/10.3390/pharmaceutics13101662
Chicago/Turabian StyleMurali, Mahadevamurthy, Nataraj Kalegowda, Hittanahallikoppal G. Gowtham, Mohammad Azam Ansari, Mohammad N. Alomary, Saad Alghamdi, Natarajamurthy Shilpa, Sudarshana B. Singh, M. C. Thriveni, Mohammed Aiyaz, and et al. 2021. "Plant-Mediated Zinc Oxide Nanoparticles: Advances in the New Millennium towards Understanding Their Therapeutic Role in Biomedical Applications" Pharmaceutics 13, no. 10: 1662. https://doi.org/10.3390/pharmaceutics13101662









