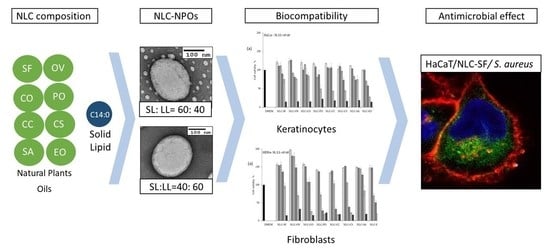
Biocompatibility and Antimicrobial Activity of Nanostructured Lipid Carriers for Topical Applications Are Affected by Type of Oils Used in Their Composition
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
| FFAs | Saturated, % | Monosaturated, % | Polysaturated, % |
|---|---|---|---|
| Sunflower oil 1 | 10 | 28 | 53 |
| Olive oil 1 | 15 | 68 | 8 |
| Corn oil 1 | 13 | 28 | 50 |
| Peanut oil 1 | 16 | 61 | 15 |
| Coconut oil 1 | 95 | 2 | 3 |
| Castor oil 2 | 2 | 91 | 7 |
| Sweet almond oil 2 | 11 | 70 | 19 |
| Eucalyptus oil 2 | 43 | 35 | 19 |
2.2. NLC-NPOs Preparation
2.3. Dynamic Light Scattering (DLS) for Physicochemical Characterization of NLC-NPOs
2.4. Morphologic and Structural Analysis
2.5. Lyophilization of NLC-NPOs
2.6. Evaluation of the Lipid Matrix Crystalline State by Differential Scanning Calorimetry
2.7. Cytotoxicity
2.8. Proliferation Test
2.9. Antimicrobial Activity Assays
2.10. Confocal Laser Scanning Microscopy (CLSM)
2.11. Fluorescence Microscopy of S. aureus with NLC-NPOs
2.12. Statistical Analysis
3. Results
3.1. Optimized NLC-NPOs with Stable Physicochemical Characteristics
3.1.1. Surfactant Concentration Influence Stability of the System
3.1.2. Stable NLC-NPOs Are Obtained with All Selected Oils
3.2. Crystallinity Studies
3.3. Biocompatibility Studies
3.3.1. Cytotoxicity
3.3.2. Proliferation
3.3.3. Internalization Analysis by Fluorescence Microscopy
3.4. The Type of Oil Used in NLC Formulations Influences the Antimicrobial Effect
3.4.1. Antimicrobial Effect NLC-NPO on S. aureus
3.4.2. Interaction of NLC-SF with S. aureus
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jaiswal, P.; Gidwani, B.; Vyas, A. Nanostructured lipid carriers and their current application in targeted drug delivery. Artif. Cells Nanomed. Biotechnol. 2016, 44, 27–40. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, I.; Yasir, M.; Verma, M.; Pratap Singh, A. Nanostructured Lipid Carriers: A Groundbreaking Approach for Transdermal Drug Delivery. Adv. Pharm. Bull. 2020, 10, 150–165. [Google Scholar] [CrossRef]
- Beloqui, A.; Ángeles Solinís, M.; Rodríguez-Gascón, A.; Almeida, A.J.; Préat, V. Nanostructured lipid carriers: Promising drug delivery systems for future clinics. Nanomedicine 2016, 12, 143–161. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, K.C.B.; Valle, A.B.C.d.S.; Paes, C.Q.; Tavares, G.D.; Pittella, F. Nanostructured Lipid Carriers for the Formulation of Topical Anti-Inflammatory Nanomedicines Based on Natural Substances. Pharmaceutics 2021, 13, 1454. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Hu, C.; Shao, L. The Antimicrobial Activity of Nanoparticles: Present Situation and Prospects for the Future. Int. J. Nanomed. 2017, 12, 1227–1249. [Google Scholar] [CrossRef] [Green Version]
- Gao, W.; Chen, Y.; Zhang, Y.; Zhang, Q.; Zhang, L. Nanoparticle-Based Local Antimicrobial Drug Delivery. Adv. Drug Deliv. Rev. 2018, 127, 46–57. [Google Scholar] [CrossRef]
- Pardeike, J.; Hommoss, A.; Müller, R.H. Lipid nanoparticles (SLN, NLC) in cosmetic and pharmaceutical dermal products. Int. J. Pharm. 2009, 366, 170–184. [Google Scholar] [CrossRef]
- Ghasemiyeh, P.; Mohammadi-Samani, S. Solid lipid nanoparticles and nanostructured lipid carriers as novel drug delivery systems: Applications, advantages and disadvantages. Res. Pharm. Sci. 2018, 4, 288–303. [Google Scholar]
- Subramaniam, B.; Siddik, Z.H.; Nagoor, N.H. Optimization of nanostructured lipid carriers: Understanding the types, designs, and parameters in the process of formulations. J. Nanoparticle Res. 2020, 22, 141. [Google Scholar] [CrossRef]
- Yang, Y.; Corona, A.; Schubert, B.; Reeder, R.; Henson, M.A. The effect of oil type on the aggregation stability of nanostructured lipid carriers. J. Colloid Interface Sci. 2014, 418, 261–272. [Google Scholar] [CrossRef]
- Pinto, F.; de Barros, D.P.C.; Fonseca, L.P. Design of multifunctional nanostructured lipid carriers enriched with α-tocopherol using vegetable oils. Ind. Crops Prod. 2018, 118, 149–159. [Google Scholar] [CrossRef]
- Pinto, F.; de Barros, D.P.C.; Reis, C.; Fonseca, L.P. Optimization of nanostructured lipid carriers loaded with retinoids by central composite design. J. Mol. Liq. 2019, 293, 111468. [Google Scholar] [CrossRef]
- D’Souza, A.; Shegokar, R. Potential of oils in development of nanostructured lipid carriers. In Essential Oils and Nanotechnology for Treatment of Microbial Diseases; Rai, M., Zacchino, S., Derita, M., Eds.; Chapter 12; CRC Press, Taylor & Francis Group LLC: Boca Raton, FL, USA, 2017; pp. 242–251. [Google Scholar]
- Lercker, G.; Rodriguez-Estrada, M.T. Chromatographic analysis of unsaponifiable compounds of olive oils and fat-containing foods. J. Chromatogr. A 2000, 881, 105–129. [Google Scholar] [CrossRef]
- Bonaccorso, A.; Cimino, C.; Manno, D.E.; Tomasello, B.; Serra, A.; Musumeci, T.; Puglisi, G.; Pignatello, R.; Carbone, C. Essential Oil-Loaded NLC for Potential Intranasal Administration. Pharmaceutics 2021, 13, 1166. [Google Scholar] [CrossRef]
- Lin, T.-K.; Zhong, L.; Santiago, J.L. Anti-Inflammatory and Skin Barrier Repair Effects of Topical Application of Some Plant Oils. Int. J. Mol. Sci. 2018, 19, 70. [Google Scholar] [CrossRef] [Green Version]
- Desbois, A.P.; Smith, V.J. Antibacterial free fatty acids: Activities, mechanisms of action and biotechnological potential. Appl. Microbiol. Biotechnol. 2010, 85, 1629–1642. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trommer, H.; Neubert, R.H.H. Overcoming the Stratum Corneum: The Modulation of Skin Penetration, Skin. Pharmacol. Physiol. 2006, 19, 106–121. [Google Scholar] [CrossRef] [PubMed]
- Dr Rimpler. Available online: http://cheerful.com.my/brands/dr-rimpler/ (accessed on 30 October 2021).
- Sharma, A.; Madhunapantula, S.V.; Robertson, G.P. Toxicological considerations when creating nanoparticle-based drugs and drug delivery systems. Expert Opin. Drug Metab. Toxicol. 2012, 8, 47–69. [Google Scholar] [CrossRef]
- Pinilla, C.M.B.; Lopes, N.A.; Brandelli, A. Lipid-Based Nanostructures for the Delivery of Natural Antimicrobials. Molecules 2021, 26, 3587. [Google Scholar] [CrossRef]
- Parlet, C.P.; Brown, M.M.; Horswill, A.R. Commensal Staphylococci Influence Staphylococcus aureus Skin Colonization and Disease. Trends Microbiol. 2019, 27, 497–507. [Google Scholar] [CrossRef]
- Tong, S.Y.; Davis, J.S.; Eichenberger, E.; Holland, T.L.; Fowler, V.G., Jr. Staphylococcus aureus infections: Epidemiology, pathophysiology, clinical manifestations, and management. Clin. Microbiol. Rev. 2015, 28, 603–661. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ki, V.; Rotstein, C. Bacterial skin and soft tissue infections in adults: A review of their epidemiology, pathogenesis, diagnosis, treatment and site of care. Can. J. Infect. Dis. Med. Microbiol. 2008, 19, 173–184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nichols, R.L.; Florman, S. Clinical presentations of soft-tissue infections and surgical site infections. Clin. Infect. Dis. 2001, 33 (Suppl. S2), S84–S93. [Google Scholar] [CrossRef] [Green Version]
- Van Bambeke, F.; Barcia-Macay, M.; Lemaire, S.; Tulkens, P.M. Cellular pharmacodynamics and pharmacokinetics of antibiotics: Current views and perspectives. Curr. Opin. Drug Discov. Dev. 2006, 9, 218–230. [Google Scholar]
- Umerska, A.; Cassisa, V.; Matougui, N.; Joly-Guillou, M.-L.; Eveillard, M.; Saulnier, P. Antibacterial action of lipid nanocapsules containing fatty acids or monoglycerides as co-surfactants. Eur. J. Pharm. Biopharm. 2016, 108, 100–110. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization—WHO. Global Action Plan on Antimicrobial Resistance. Available online: https://www.who.int/publications/i/item/9789241509763 (accessed on 30 October 2021).
- Da Silva, L.C.N.; da Silva, M.V.; Correia, M.T.S. Editorial: New frontiers in the search of antimicrobials agents from natural products. Front. Microbiol. 2017, 8, 210. [Google Scholar] [CrossRef]
- Khezri, K.; Farahpour, M.R.; Mounesi Rad, S. Accelerated infected wound healing by topical application of encapsulated Rosemary essential oil into nanostructured lipid carriers. Artif. Cells Nanomed. Biotechnol. 2019, 47, 980–988. [Google Scholar] [CrossRef] [Green Version]
- Saporito, F.; Sandri, G.; Bonferoni, M.C.; Rossi, S.; Boselli, C.; Icaro Cornaglia, A.; Mannucci, B.; Grisoli, P.; Vigani, B.; Ferrari, F. Essential oil-loaded lipid nanoparticles for wound healing. Int. J. Nanomed. 2017, 13, 175–186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, L.; Pornpattananangkul, D.; Hu, C.M.J.; Huang, C.M. Development of nanoparticles for antimicrobial drug delivery. Curr. Med. Chem. 2010, 17, 585–594. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brandelli, A.; Pinilla, C.M.B.; Lopes, N.A. Nanoliposomes as a plataform for delivery of antimicrobials. In Nanotechnology Applied to Pharmaceutical Technology; Rai, M., Santos, C.A., Eds.; Springer: Cham, Switzerland, 2017; pp. 55–90. [Google Scholar]
- Huguet-Casquero, A.; Moreno-Sastre, M.; López-Méndez, T.B.; Gainza, E.; Pedraz, J.L. Encapsulation of Oleuropein in Nanostructured Lipid Carriers: Biocompatibility and Antioxidant Efficacy in Lung Epithelial Cells. Pharmaceutics 2020, 12, 429. [Google Scholar] [CrossRef]
- Gonzalez-Mira, E.; Egea, M.A.; Garcia, M.L.; Souto, E.B. Design and ocular tolerance of flurbiprofen loaded ultrasound-engineered NLC. Colloids Surf. B Biointerfaces 2010, 81, 412–421. [Google Scholar] [CrossRef]
- Jawahar, N.; Hingarh, P.K.; Arun, R.; Selvaraj, J.; Anbarasan, A.; Sathianarayanan, S.; Nagaraju, G. Enhanced oral bioavailability of an antipsychotic drug through nanostructured lipid carriers. Int. J. Biol. Macromol. 2018, 11, 269–275. [Google Scholar] [CrossRef]
- Yoon, B.K.; Jackman, J.A.; Valle-González, E.R.; Cho, N.J. Antibacterial Free Fatty Acids and Monoglycerides: Biological Activities, Experimental Testing, and Therapeutic Applications. Int. J. Mol. Sci. 2018, 19, 1114. [Google Scholar] [CrossRef] [Green Version]
- Zuzarte, M.; Salgueiro, L. Essential Oils Chemistry. In Bioactive Essential Oils and Cancer; de Sousa, D., Ed.; Springer: Cham, Switzerland, 2015. [Google Scholar]
- Eromosele, I.C.; Eromosele, C.O.; Akintoye, A.O.; Komolafe, T.O. Characterization of oils and chemical analyses of the seeds of wild plants. Plant Foods Hum. Nutr. 1994, 46, 361–365. [Google Scholar] [CrossRef] [PubMed]
- Rekkab, S.; Zarrok, H.; Salghi, R.; Zarrouk, A.; Bazzi, L.; Hammouti, B.; Kabouche, Z.; Touzani, R.; Zougagh, M. Green Corrosion Inhibitor from Essential Oil of Eucalyptus globulus (Myrtaceae) for C 38 Steel in Sulfuric Acid Solution. J. Mater. Environ. Sci. 2012, 3, 613–627. [Google Scholar]
- Douvartzides, S.L.; Charisiou, N.D.; Papageridis, K.N.; Goula, M.A. Green Diesel: Biomass Feedstocks, Production Technologies, Catalytic Research, Fuel Properties and Performance in Compression Ignition Internal Combustion Engines. Energies 2019, 12, 809. [Google Scholar] [CrossRef] [Green Version]
- Cecilia, J.A.; Ballesteros Plata, D.; Alves Saboya, R.M.; Tavares de Luna, F.M.; Cavalcante, C.L., Jr.; Rodríguez-Castellón, E. An Overview of the Biolubricant Production Process: Challenges and Future Perspectives. Processes 2020, 8, 257. [Google Scholar] [CrossRef] [Green Version]
- Mawatari, T.; Fukuda, R.; Mori, H.; Mia, S.; Ohno, N. High Pressure Rheology of Environmentally Friendly Vegetable Oils. Tribol. Lett. 2013, 51, 273–280. [Google Scholar] [CrossRef]
- Akhtar, S.; Khalid, N.; Ahmed, I.; Shahzad, A.; Suleria, H.A. Physicochemical characteristics, functional properties, and nutritional benefits of peanut oil: A review. Crit. Rev. Food Sci. Nutr. 2014, 54, 1562–1575. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, G.D.; Gómez-Coca, R.B.; Pérez-Camino, M.C.; Moreda, W.; Barrera-Arellano, D. Chemical Characterization of Major and Minor Compounds of Nut Oils: Almond, Hazelnut, and Pecan Nut. J. Chem. 2017, 2017, 11. [Google Scholar] [CrossRef] [Green Version]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Andrews, J.M. Determination of minimum inhibitory concentrations. J. Antimicrob. Chemother. 2001, 48 (Suppl. S1), 5–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Araujo, V.H.S.; Bento da Silva, P.; Oliveira Szlachetka, I.; William da Silva, S.; Fonseca-Santos, B.; Chorilli, M.; Ganassin, R.; de Oliveira, G.R.T.; da Rocha, M.C.O.; Fernandes, R.P.; et al. The influence of NLC composition on curcumin loading under a physicochemical perspective and in vitro evaluation. Colloids Surf. A Physicochem. Eng. Asp. 2020, 602, 125070. [Google Scholar] [CrossRef]
- Danaei, M.; Dehghankhold, M.; Ataei, S.; Hasanzadeh Davarani, F.; Javanmard, R.; Dokhani, A.; Khorasani, S.; Mozafari, M.R. Impact of Particle Size and Polydispersity Index on the Clinical Applications of Lipidic Nanocarrier Systems. Pharmaceutics 2018, 10, 57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Worldwide, M.I. Dynamic Light Scattering, Common Terms Defined; Malvern Instruments Limited: Malvern, UK, 2011; pp. 1–6, Inform White Paper. [Google Scholar]
- Bunjes, H. Current Opinion in Colloid & Interface Science Structural properties of solid lipid based colloidal drug delivery systems. Curr. Opin. Colloid Interface Sci. 2011, 16, 405–411. [Google Scholar]
- Zheng, M.; Falkeborg, M.; Zheng, Y.; Yang, T.; Xu, X. Formulation and characterization of nanostructured lipid carriers containing a mixed lipids core. Colloids Surf. A Physicochem. Eng. Asp. 2013, 430, 76–84. [Google Scholar] [CrossRef]
- Puri, A.; Loomis, K.; Smith, B.; Lee, J.-H.; Yavlovich, A.; Heldman, E.; Blumenthal, R. Lipid-based nanoparticles as pharmaceutical drug carriers: From concepts to clinic. Crit. Rev. Ther. Drug Carr. Syst. 2009, 26, 523–580. [Google Scholar] [CrossRef] [Green Version]
- Doktorovova, S.; Souto, E.B.; Silva, A.M. Nanotoxicology applied to solid lipid nanoparticles and nanostructured lipid carriers—A systematic review of in vitro data. Eur. J. Pharm. Biopharm. 2014, 87, 1–18. [Google Scholar] [CrossRef]
- Doktorovová, S.; Kovačević, A.B.; Garcia, M.L.; Souto, E.B. Preclinical safety of solid lipid nanoparticles and nanostructured lipid carriers: Current evidence from in vitro and in vivo evaluation. Eur. J. Pharm. Biopharm. 2016, 108, 235–252. [Google Scholar] [CrossRef]
- Ferreira, L.E.; Muniz, B.V.; Burga-Sánchez, J.; Volpato, M.C.; de Paula, E.; Rosa, E.A.; Groppo, F.C. The effect of two drug delivery systems in ropivacaine cytotoxicity and cytokine release by human keratinocytes and fibroblasts. J. Pharm. Pharmacol. 2017, 69, 161–171. [Google Scholar] [CrossRef]
- ISO. ISO 10993-5:2009: Biological Evaluation of Medical Devices—Part 5: Tests for In Vitro Cytotoxicity. Available online: https://www.iso.org/standard/36406.html (accessed on 30 October 2021).
- Ghodrati, M.; Farahpour, M.R.; Hamishehkar, H. Encapsulation of Peppermint essential oil in nanostructured lipid carriers: In-vitro antibacterial activity and accelerative effect on infected wound healing. Colloids Surf. A Physicochem. Eng. Asp. 2019, 564, 161–169. [Google Scholar] [CrossRef]
- Quan, C.; Cho, M.K.; Shao, Y.; Mianecki, L.E.; Liao, E.; Perry, D.; Quan, T. Dermal fibroblast expression of stromal cell-derived factor-1 (SDF-1) promotes epidermal keratinocyte proliferation in normal and diseased skin. Protein Cell 2015, 6, 890–903. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iwig, M.; Glaesser, D.; Fass, U.; Struck, H.G. Fatty acid cytotoxicity to human lens epithelial cells. Exp. Eye Res. 2004, 79, 689–704. [Google Scholar] [CrossRef]
- Behzadi, S.; Serpooshan, V.; Tao, W.; Hamaly, M.A.; Alkawareek, M.Y.; Dreaden, E.C.; Brown, D.; Alkilany, A.M.; Farokhzad, O.C.; Mahmoudi, M. Cellular uptake of nanoparticles: Journey inside the cell. Chem. Soc. Rev. 2017, 46, 4218–4244. [Google Scholar] [CrossRef] [PubMed]
- Brandelli, A. The interaction of nanostructured antimicrobials with biological systems: Cellular uptake, trafficking and potential toxicity. Food Sci. Hum. Wellness 2020, 9, 8–20. [Google Scholar] [CrossRef]
- Silva, E.; Barreiros, L.; Segundo, M.A.; Costa Lima, S.A.; Reis, S. Cellular interactions of a lipid-based nanocarrier model with human keratinocytes: Unravelling transport mechanisms. Acta Biomater. 2017, 53, 439–449. [Google Scholar] [CrossRef]
- Teskac, K.; Kristl, J. The evidence for solid lipid nanoparticles mediated cell uptake of resveratrol. Int. J. Pharm. 2010, 390, 61–69. [Google Scholar] [CrossRef]
- Rajendran, L.; Knölker, H.J.; Simons, K. Subcellular targeting strategies for drug design and delivery. Nat. Rev. Drug Discov. 2010, 9, 29–42. [Google Scholar] [CrossRef]
- Neves, A.R.; Queiroz, J.F.; Costa Lima, S.A.; Figueiredo, F.; Fernandes, R.; Reis, S. Cellular uptake and transcytosis of lipid-based nanoparticles across the intestinal barrier: Relevance for oral drug delivery. J. Colloid Interface Sci. 2016, 463, 258–265. [Google Scholar] [CrossRef]
- Da Silva, J.S.; Zilly, A.; da Silva, R.M.M.; Librelotto, C.S.; Ferreira, H. Evaluation of Antibacterial Activity of Sunflower Oil: Support for Nursing. Res. Soc. Dev. 2021, 10, e8710917941. [Google Scholar]
- Lacey, R.W.; Lord, V.L. Sensitivity of staphylococci to fatty acids: Novel inactivation of linolenic acid by serum. J. Med. Microbiol. 1981, 14, 41–49. [Google Scholar] [CrossRef] [Green Version]
- Lukowski, G.; Lindequist, U.; Mundt, S.; Kramer, A.; Jülich, W.-D. Inhibition of Dermal MRSA Colonization by Microalgal Micro-and Nanoparticles. Skin Pharmacol. Physiol. 2008, 21, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Tabassum, N.; Vidyasagar, G.M. In vitro antimicrobial activity of edible oils against human pathogens causing skin infections. Int. J. Pharm. Sci. Res. IJPSR 2014, 5, 4493–4498. [Google Scholar]
- Kisich, K.O.; Howell, M.D.; Boguniewicz, M.; Heizer, H.R.; Watson, N.U.; Leung, D.Y. The constitutive capacity of human keratinocytes to kill Staphylococcus aureus is dependent on beta-defensin 3. J. Investig. Dermatol. 2007, 127, 2368–2380. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Esfanjani, A.F.; Assadpour, E.; Jafari, S.M. Improving the bioavailability of phenolic compounds by loading them within lipid-based nanocarriers. Trends Food Sci. Technol. 2018, 76, 56–66. [Google Scholar] [CrossRef]












| Oils | SF | OV | CO | PO | CC | CS | SA | EO 2 | |
|---|---|---|---|---|---|---|---|---|---|
| FFAs,% | |||||||||
| C12:0 | 0.1 | - | - | - | 50–52 | - | - | - | |
| C14:0 | 0.1 | - | - | 0.6 | 19–21 | - | 0.07 | - | |
| C16:0 | 6.1–6.6 | 11–14 | 10–11 | 0.31–19 | 7.5–9.3 | 0.8–1.1 | 4.7–16 | 36 | |
| C18:0 | 3.3–5.3 | 1.6–2.6 | 1.8–2 | 0.1–4.9 | 2–7.8 | 0.7–1.0 | 0.3–3.0 | 3.36 | |
| C20:0 | 0.3 | 0.4 | 0.4 | 0.15–2.3 | - | - | 0.04–0.2 | - | |
| C22:0 | 0.6 | 0.1 | 0.1 | 0.13–4.5 | - | - | - | - | |
| C24:0 | 0.2 | 0.1 | 0.2 | 0.09–2.5 | - | - | - | - | |
| C16:1 | 0.1 | 0.1–1.5 | 0.1 | 0.6 | - | - | - | - | |
| C18:1 | 21–30 | 64–78 | 26–28 | 16–69 | 7–8.6 | 2.2–3.3/ 88–90 1 | 50–86 | 27 | |
| C20:1 | 0.2 | 0.3 | 0.3 | 0.3–2.4 | - | - | - | - | |
| C18:2 | 58–66 | 6.2–16 | 56–60 | 14–60 | 1.8–9.2 | 4.1–4.7 | - | 19 | |
| C18:3 | 0.1–1.5 | 0.6–3.1 | 0.8–0.9 | 0.07 | 0.1 | 0.5–0.7 | 0.1–1.0 | - | |
| Ref. | [41,42,43] | [42,43] | [42] | [42,44] | [42,43] | [42,43] | [45] | [40] | |
| Scheme | SL, wt% | LL, wt% | Surfactant, wt% |
|---|---|---|---|
| 60:40 | 1.5 | 1 | 2 |
| 40:60 | 1 | 1.5 | 2 |
| SL:LL | Tm, °C | ΔH, J/g | CI, % | |||
|---|---|---|---|---|---|---|
| 60:40 | 40:60 | 60:40 | 40:60 | 60:40 | 40:60 | |
| NLC-SF | 49.4 | 51.0 | 76.5 | 73.4 | 40 | 38 |
| NLC-OV | 49.1 | 49.7 | 83.6 | 82.2 | 44 | 43 |
| NLC-CO | 49.8 | 50.1 | 80.7 | 79.9 | 42 | 42 |
| NLC-PO | 51.7 | 50.4 | 110.3 | 101.0 | 58 | 53 |
| NLC-CC | 47.7 | 48.4 | 47.1 | 83.1 | 25 | 43 |
| NLC-CS | 48.4 | 46.0 | 69.7 | 51.6 | 36 | 27 |
| NLC-SA | 50.7 | 49.2 | 104.7 | 102.4 | 55 | 54 |
| NLC-EO | 44.3 | 45.5 | 67.9 | 70.5 | 36 | 37 |
| Myristic acid | 55.7 | 190.9 | 100 | |||
| Total Lipids, mg/mL−1 | HaCaT | HFDn | ||
|---|---|---|---|---|
| NLC-SF | 1.25 | 1.25 | ||
| NLC-OV | 1.25 | 1.25 | ||
| NLC-CO | 1.25 | 0.833 | 1.25 | |
| NLC-PO | 0.833 | 0.625 | 0.833 | |
| NLC-CC | 0.833 | 0.625 | 0.833 | |
| NLC-CS | 0.833 | 0.625 | 1.25 | |
| NLC-SA | 1.25 | 1.25 | ||
| NLC-EO | 0.625 | 0.625 | 1.25 | |
| SL:LL, wt% | 60:40/40:60 | 60:40 | 60:40/40:60 | 40:60 |
| Lipid Ratio | SL:LL = 60:40 | SL:LL = 40:60 | ||
|---|---|---|---|---|
| NLC-NPOs | Last well in which growth was inhibited | Total lipids in well, mg/mL−1 | Last well in which growth was inhibited | Total lipids in well, mg/mL−1 |
| NLC-SF | 6 | 0.39 | 5 | 0.78 |
| NLC-OV | 6 | 0.39 | 5 | 0.78 |
| NLC-CO | 3 | 3.13 | 2 | 6.25 |
| NLC-PO | 4 | 1.56 | 5 | 0.78 |
| NLC-CC | 5 | 0.78 | 4 | 1.56 |
| NLC-CS | 4 | 1.56 | 4 | 1.56 |
| NLC-SA | 4 | 1.56 | 4 | 1.56 |
| NLC-EO | 4 | 1.56 | 5 | 0.78 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Barros, D.P.C.; Reed, P.; Alves, M.; Santos, R.; Oliva, A. Biocompatibility and Antimicrobial Activity of Nanostructured Lipid Carriers for Topical Applications Are Affected by Type of Oils Used in Their Composition. Pharmaceutics 2021, 13, 1950. https://doi.org/10.3390/pharmaceutics13111950
de Barros DPC, Reed P, Alves M, Santos R, Oliva A. Biocompatibility and Antimicrobial Activity of Nanostructured Lipid Carriers for Topical Applications Are Affected by Type of Oils Used in Their Composition. Pharmaceutics. 2021; 13(11):1950. https://doi.org/10.3390/pharmaceutics13111950
Chicago/Turabian Stylede Barros, Dragana P. C., Patricia Reed, Marta Alves, Rafaela Santos, and Abel Oliva. 2021. "Biocompatibility and Antimicrobial Activity of Nanostructured Lipid Carriers for Topical Applications Are Affected by Type of Oils Used in Their Composition" Pharmaceutics 13, no. 11: 1950. https://doi.org/10.3390/pharmaceutics13111950