The Monoterpenoid Perillyl Alcohol: Anticancer Agent and Medium to Overcome Biological Barriers
Abstract
:1. Introduction
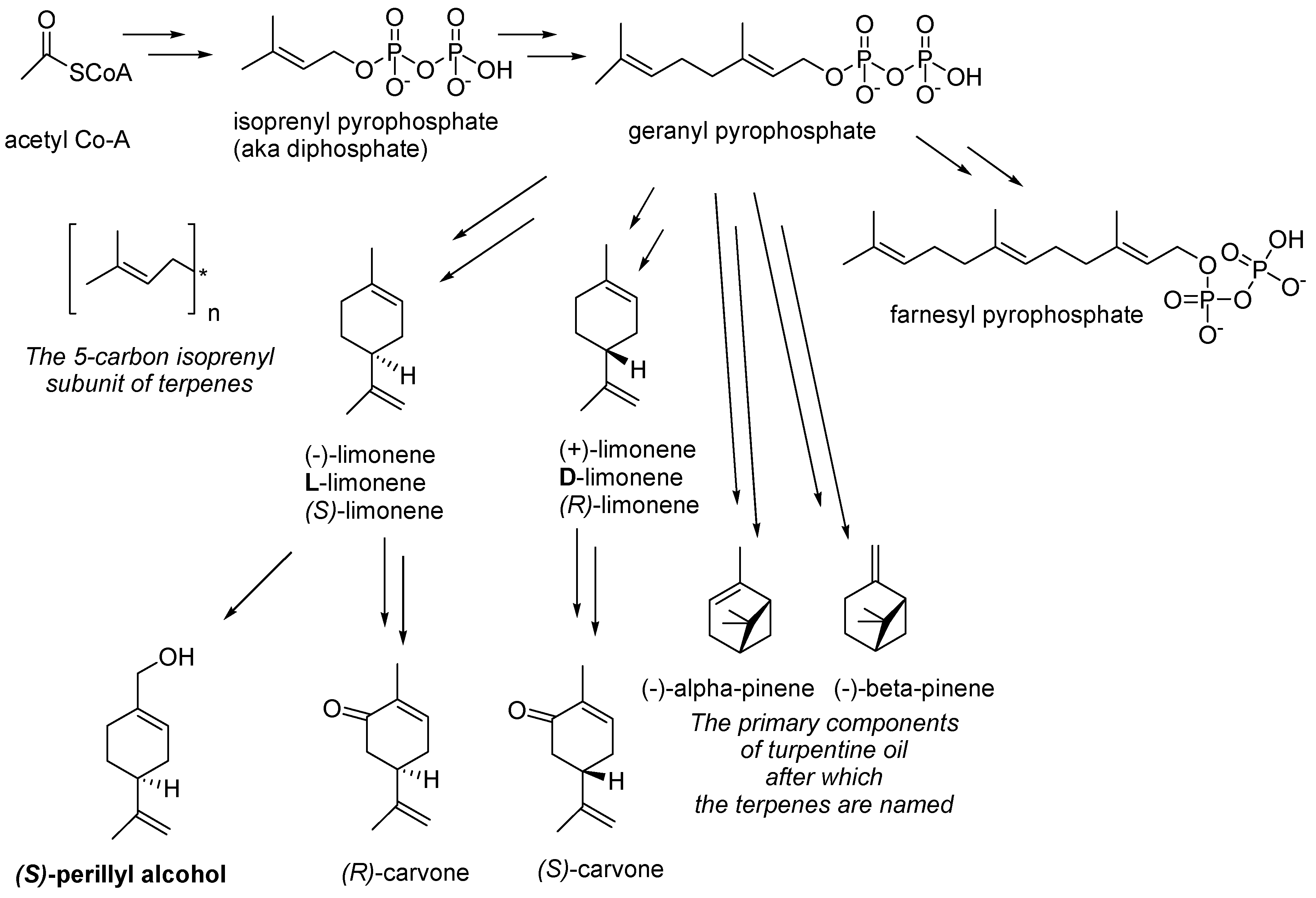
2. The Monoterpenoid Perillyl Alcohol (POH)
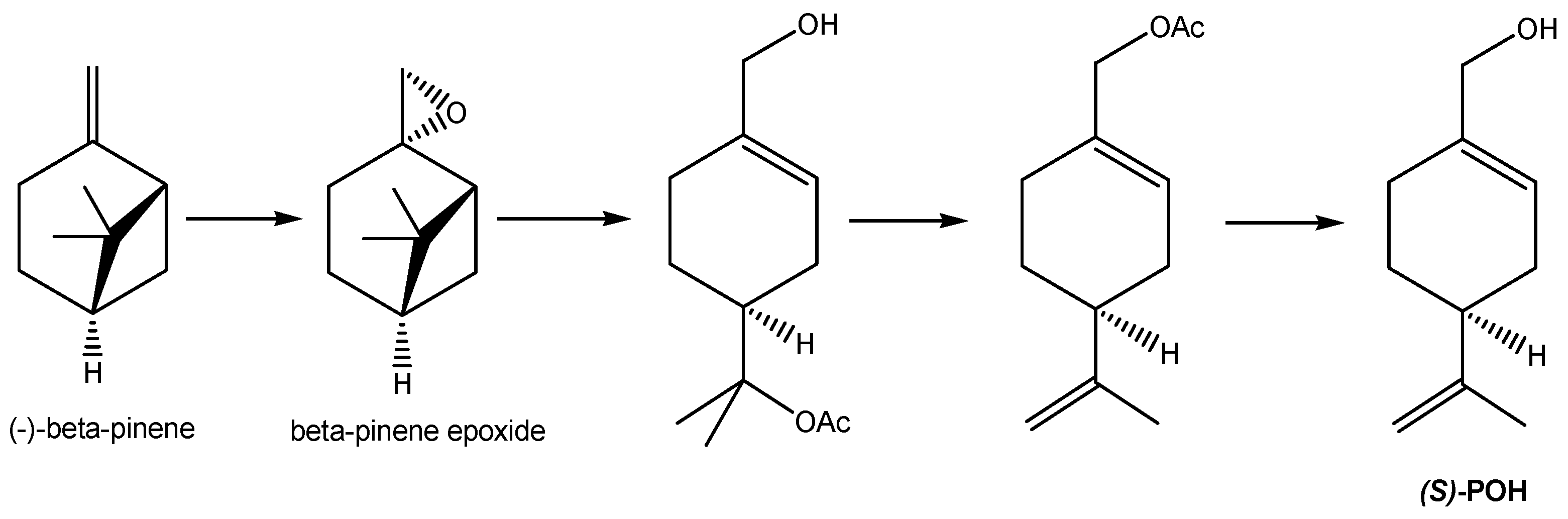
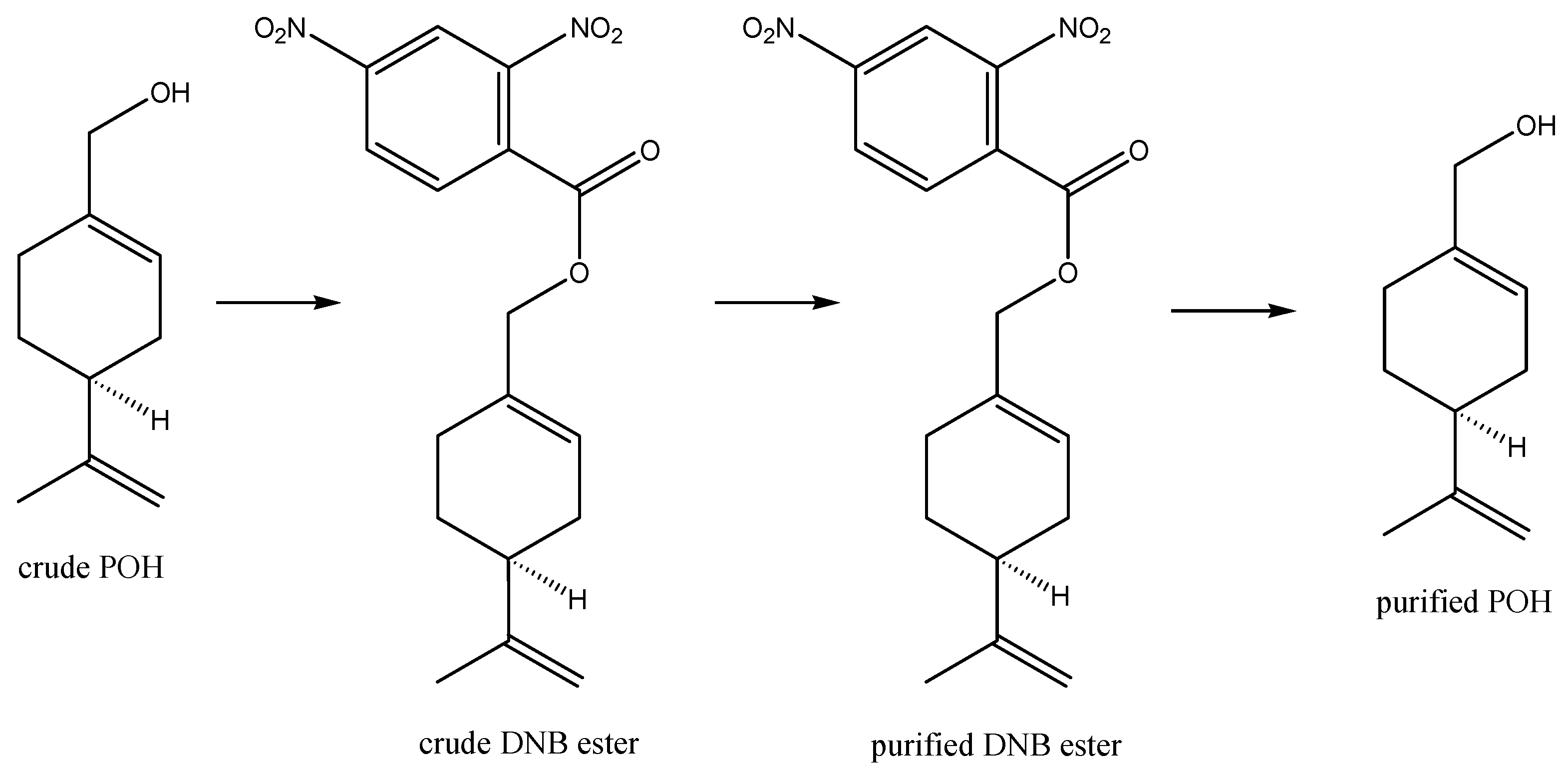
2.1. Biochemical Description of POH
2.2. Recognizing POH’s Potential for Anticancer Purposes
2.3. Mechanisms of POH Anticancer Function
3. Clinical Evaluation of Oral POH
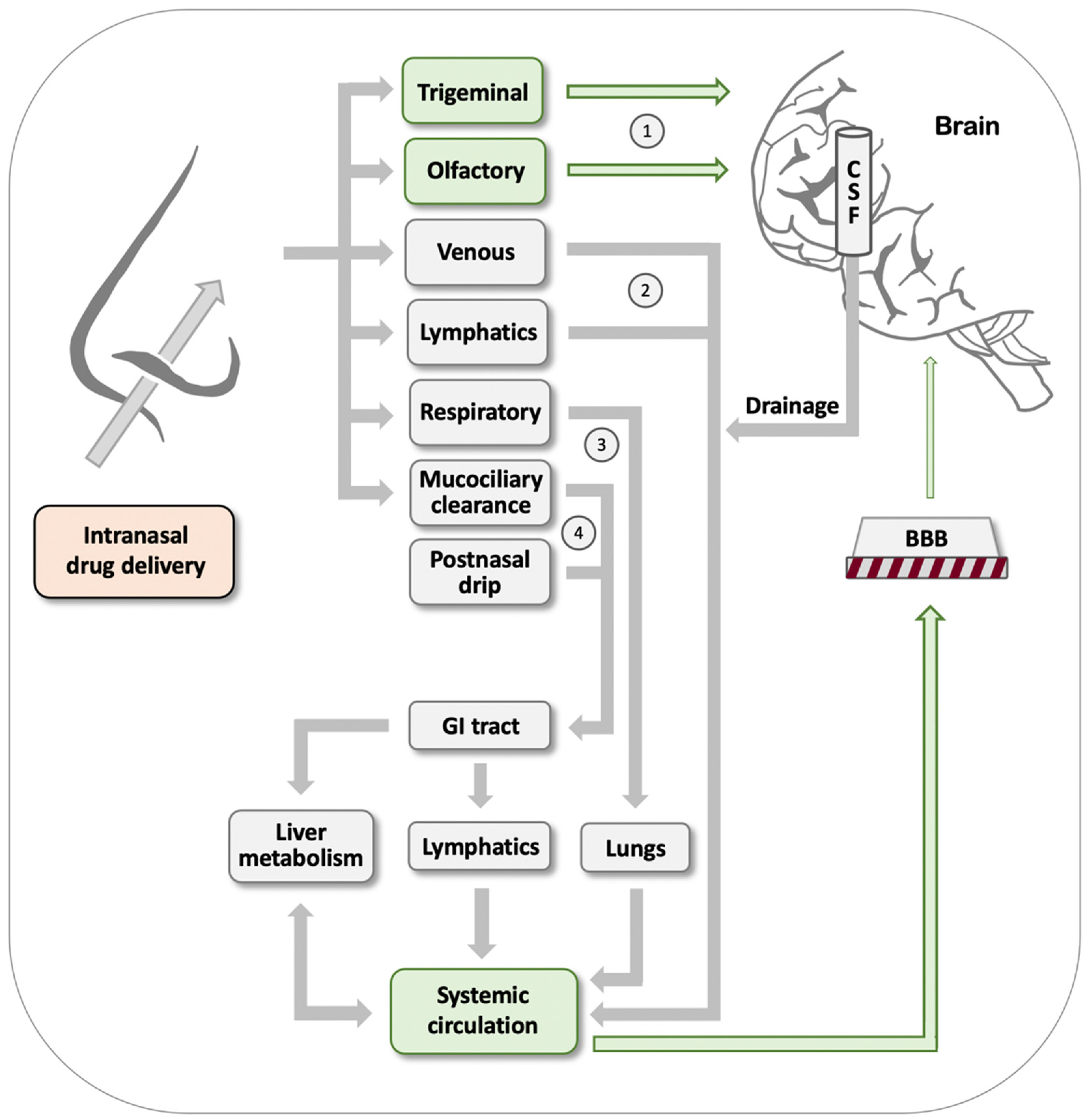
4. Intranasal Perillyl Alcohol
4.1. Intranasal POH as Monotherapy
4.2. Intranasal POH as a Co-Delivery System
5. Intra-Arterial Perillyl Alcohol
6. Perillyl Alcohol Derivatives, Analogs, and Conjugates
6.1. Conjugation with POH to Enhance Permeability
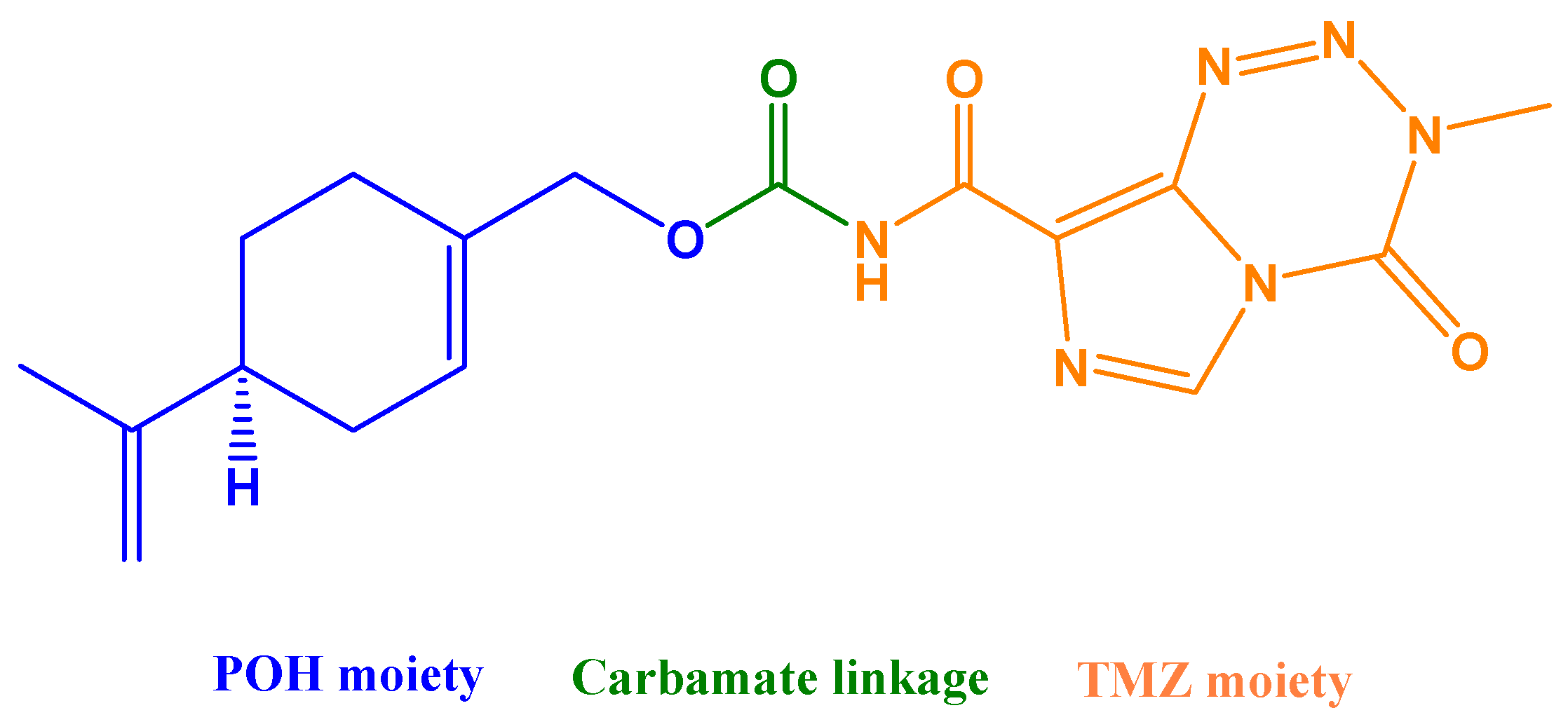
6.1.1. POH Conjugated to Temozolomide (NEO212)
6.1.2. POH Conjugated to Rolipram or 3-Bromopyruvate or Valproic Acid
6.2. POH and Penetration of the Skin
7. Conclusions and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| API | Active pharmacologic ingredient |
| BBB | Blood–brain barrier |
| CGMP | Current Good Manufacturing Practices |
| CNS | Central nervous system |
| GBM | Glioblastoma |
| IDH | Isocitrate dehydrogenase |
| MRI | Magnetic resonance imaging |
| POH | Perillyl alcohol |
| RT | Radiation therapy |
| TMZ | Temozolomide |
References
- Smith, Q.R. A Review of Blood–Brain Barrier Transport Techniques. Methods Mol. Med. 2003, 89, 193–208. [Google Scholar] [CrossRef] [PubMed]
- Obermeier, B.; Daneman, R.; Ransohoff, R.M. Development, maintenance and disruption of the blood-brain barrier. Nat. Med. 2013, 19, 1584–1596. [Google Scholar] [CrossRef]
- Langen, U.H.; Ayloo, S.; Gu, C. Development and Cell Biology of the Blood-Brain Barrier. Annu. Rev. Cell Dev. Biol. 2019, 35, 591–613. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharif, Y.; Jumah, F.; Coplan, L.; Krosser, A.; Sharif, K.; Tubbs, R.S. Blood brain barrier: A review of its anatomy and physiology in health and disease. Clin. Anat. 2018, 31, 812–823. [Google Scholar] [CrossRef] [PubMed]
- Frisk, G.; Svensson, T.; Bäcklund, L.M.; Lidbrink, E.; Blomqvist, P.; E Smedby, K. Incidence and time trends of brain metastases admissions among breast cancer patients in Sweden. Br. J. Cancer 2012, 106, 1850–1853. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, Y.-J.; Kim, J.-S.; Kim, I.A. Molecular subtype predicts incidence and prognosis of brain metastasis from breast cancer in SEER database. J. Cancer Res. Clin. Oncol. 2018, 144, 1803–1816. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, M.; Krishnamurthy, J.; Shonka, N. Targeting the Sanctuary Site: Options when Breast Cancer Metastasizes to the Brain. Oncology 2019, 33, 683730. [Google Scholar] [PubMed]
- Garcia-Alvarez, A.; Papakonstantinou, A.; Oliveira, M. Brain Metastases in HER2-Positive Breast Cancer: Current and Novel Treatment Strategies. Cancers 2021, 13, 2927. [Google Scholar] [CrossRef]
- Aulakh, S.; DeDeo, M.R.; Free, J.; Rosenfeld, S.S.; Quinones-Hinojosa, A.; Paulus, A.; Manna, A.; Manochakian, R.; Chanan-Khan, A.A.; Ailawadhi, S. Survival trends in glioblastoma and association with treating facility volume. J. Clin. Neurosci. 2019, 68, 271–274. [Google Scholar] [CrossRef]
- Efremov, L.; Abera, S.F.; Bedir, A.; Vordermark, D.; Medenwald, D. Patterns of glioblastoma treatment and survival over a 16-years period: Pooled data from the german cancer registries. J. Cancer Res. Clin. Oncol. 2021, 147, 3381–3390. [Google Scholar] [PubMed]
- Louis, D.N.; Perry, A.; Wesseling, P.; Brat, D.J.; A Cree, I.; Figarella-Branger, D.; Hawkins, C.; Ng, H.K.; Pfister, S.M.; Reifenberger, G.; et al. The 2021 WHO Classification of Tumors of the Central Nervous System: A summary. Neuro-Oncology 2021, 23, 1231–1251. [Google Scholar] [CrossRef] [PubMed]
- Frappaz, D.; Bonneville-Levard, A.; Ricard, D.; Carrie, S.; Schiffler, C.; Xuan, K.H.; Weller, M. Assessment of Karnofsky (KPS) and WHO (WHO-PS) performance scores in brain tumour patients: The role of clinician bias. Support. Care Cancer 2021, 29, 1883–1891. [Google Scholar] [CrossRef]
- Stupp, R.; Mason, W.P.; van den Bent, M.J.; Weller, M.; Fisher, B.; Taphoorn, M.J.; Belanger, K.; Brandes, A.A.; Marosi, C.; Bogdahn, U.; et al. Radiotherapy plus Concomitant and Adjuvant Temozolomide for Glioblastoma. N. Engl. J. Med. 2005, 352, 987–996. [Google Scholar] [CrossRef] [PubMed]
- Stupp, R.; Hegi, M.E.; Mason, W.P.; van den Bent, M.J.; Taphoorn, M.J.B.; Janzer, R.C.; Ludwin, S.K.; Allgeier, A.; Fisher, B.; Belanger, K.; et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009, 10, 459–466. [Google Scholar] [CrossRef]
- Kazda, T.; Dziacky, A.; Burkon, P.; Pospisil, P.; Slavik, M.; Řehák, Z.; Jancalek, R.; Slampa, P.; Slaby, O.; Lakomy, R. Radiotherapy of glioblastoma 15 years after the landmark Stupp’s trial: More controversies than standards? Radiol. Oncol. 2018, 52, 121–128. [Google Scholar] [CrossRef] [Green Version]
- Ostermann, S.; Csajka, C.; Buclin, T.; Leyvraz, S.; Lejeune, F.; Decosterd, L.; Stupp, R. Plasma and Cerebrospinal Fluid Population Pharmacokinetics of Temozolomide in Malignant Glioma Patients. Clin. Cancer Res. 2004, 10, 3728–3736. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Portnow, J.; Badie, B.; Chen, M.; Liu, A.; Blanchard, S.; Synold, T. The Neuropharmacokinetics of Temozolomide in Patients with Resectable Brain Tumors: Potential Implications for the Current Approach to Chemoradiation. Clin. Cancer Res. 2009, 15, 7092–7098. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guyon, J.; Chapouly, C.; Andrique, L.; Bikfalvi, A.; Daubon, T. The Normal and Brain Tumor Vasculature: Morphological and Functional Characteristics and Therapeutic Targeting. Front. Physiol. 2021, 12, 622615. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Wang, C.; Wang, L.; Chen, Y. A comprehensive review in improving delivery of small-molecule chemotherapeutic agents overcoming the blood-brain/brain tumor barriers for glioblastoma treatment. Drug Deliv. 2019, 26, 551–565. [Google Scholar] [CrossRef]
- Boogerd, W.; Groenveld, F.; Linn, S.; Baars, J.W.; Brandsma, D.; Van Tinteren, H. Chemotherapy as primary treatment for brain metastases from breast cancer: Analysis of 115 one-year survivors. J. Cancer Res. Clin. Oncol. 2012, 138, 1395–1403. [Google Scholar] [CrossRef] [PubMed]
- Franciosi, V.; Cocconi, G.; Michiara, M.; Di Costanzo, F.; Fosser, V.; Tonato, M.; Carlini, P.; Boni, C.; Di Sarra, S. Front-line chemotherapy with cisplatin and etoposide for patients with brain metastases from breast carcinoma, nonsmall cell lung carcinoma, or malignant melanoma: A prospective study. Cancer 1999, 85, 1599–1605. [Google Scholar] [CrossRef]
- Lockman, P.; Mittapalli, R.K.; Taskar, K.S.; Rudraraju, V.; Gril, B.; Bohn, K.A.; Adkins, C.E.; Roberts, A.; Thorsheim, H.R.; Gaasch, J.A.; et al. Heterogeneous Blood–Tumor Barrier Permeability Determines Drug Efficacy in Experimental Brain Metastases of Breast Cancer. Clin. Cancer Res. 2010, 16, 5664–5678. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sarkaria, J.N.; Hu, L.S.; Parney, I.F.; Pafundi, D.H.; Brinkmann, D.H.; Laack, N.N.; Giannini, C.; Burns, T.C.; Kizilbash, S.; Laramy, J.K.; et al. Is the blood–brain barrier really disrupted in all glioblastomas? A critical assessment of existing clinical data. Neuro-Oncology 2018, 20, 184–191. [Google Scholar] [CrossRef] [PubMed]
- Pitz, M.W.; Desai, A.; Grossman, S.A.; Blakeley, J.O. Tissue concentration of systemically administered antineoplastic agents in human brain tumors. J. Neuro-Oncol. 2011, 104, 629–638. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arvanitis, C.D.; Ferraro, G.B.; Jain, R.K. The blood–brain barrier and blood–tumour barrier in brain tumours and metastases. Nat. Rev. Cancer 2020, 20, 26–41. [Google Scholar] [CrossRef] [PubMed]
- Marcucci, F.; Corti, A.; Ferreri, A. Breaching the Blood–Brain Tumor Barrier for Tumor Therapy. Cancers 2021, 13, 2391. [Google Scholar] [CrossRef] [PubMed]
- Crowell, P.L.; Elson, C.E. Isoprenoids, health and disease. In Neutraceuticals and Functional Foods; Wildman, R.E.C., Ed.; CRC Press: Boca Raton, FL, USA, 2001. [Google Scholar]
- Pan, J.; Xu, Z.; Ji, L.; Zhao, Z.; Tang, X. Constituents of Essential Oils from Leaves, Stems, and Fruits of Perilla frutescens (L.) britt. Zhongguo Zhong Yao Za Zhi 1992, 17, 164–165, 192. [Google Scholar] [PubMed]
- Shojaei, S.; Kiumarsi, A.; Moghadam, A.R.; Alizadeh, J.; Marzban, H.; Ghavami, S. Perillyl Alcohol (Monoterpene Alcohol), Limonene. Struct. Funct. Regul. Tor Complexes Yeasts Mamm. Part B 2014, 36, 7–32. [Google Scholar] [CrossRef]
- Kekulé, A. Lehrbuch der Organischen Chemie; Verlag von Ferdinand Enke: Erlangen, Germany, 1866; Volume 2. [Google Scholar]
- Newman, A.A. Chemistry of Terpenes and Terpenoids; Academic Press: Cambridge, MA, USA, 1972; p. 449. [Google Scholar]
- Dionísio, A.P.; Molina, G.; de Carvalho, D.S.; dos Santos, R.; Bicas, J.; Pastore, G. Natural flavourings from biotechnology for foods and beverages. In Natural Food Additives, Ingredients and Flavourings; Elsevier: Amsterdam, The Netherlands, 2012; pp. 231–259. [Google Scholar]
- Leitereg, T.J.; Guadagni, D.G.; Harris, J.; Mon, T.R.; Teranishi, R. Evidence for the Difference between the Odours of the Optical Isomers (+)- and (−)-Carvone. Nat. Cell Biol. 1971, 230, 455–456. [Google Scholar] [CrossRef]
- Sato, T.; Kobayakawa, R.; Kobayakawa, K.; Emura, M.; Itohara, S.; Kizumi, M.; Hamana, H.; Tsuboi, A.; Hirono, J. Supersensitive detection and discrimination of enantiomers by dorsal olfactory receptors: Evidence for hierarchical odour coding. Sci. Rep. 2015, 5, 14073. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moss, G.P. Basic terminology of stereochemistry (IUPAC Recommendations 1996). Pure Appl. Chem. 1996, 68, 2193–2222. [Google Scholar] [CrossRef]
- Chastain, D.E.; Mody, N.; Majetich, G. Method of preparing perillyl alcohol and perillyl acetate. United. U.S. Patent US5994598, 30 November 1999. [Google Scholar]
- Alonso-Gutierrez, J.; Chan, R.; Batth, T.S.; Adams, P.; Keasling, J.; Petzold, C.; Lee, T.S. Metabolic engineering of Escherichia coli for limonene and perillyl alcohol production. Metab. Eng. 2013, 19, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Liu, S.; Jin, G.; Yang, X.; Zhou, Y.J. Microbial production of limonene and its derivatives: Achievements and perspectives. Biotechnol. Adv. 2020, 44, 107628. [Google Scholar] [CrossRef] [PubMed]
- Soares-Castro, P.; Soares, F.; Santos, P.M. Current Advances in the Bacterial Toolbox for the Biotechnological Production of Monoterpene-Based Aroma Compounds. Molecules 2020, 26, 91. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Levin, D.; Puppali, S. Pharmaceutical compositions comprising monoterpenes. United. U.S. Patent US9700524B2, 11 July 2017. [Google Scholar]
- Crowell, P.L.; Lin, S.; Vedejs, E.; Gould, M.N. Identification of metabolites of the antitumor agent d-limonene capable of inhibiting protein isoprenylation and cell growth. Cancer Chemother. Pharmacol. 1992, 31, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Crowell, P.L.; Ren, Z.; Lin, S.; Vedejs, E.; Gould, M.N. Structure-activity relationships among monoterpene inhibitors of protein isoprenylation and cell proliferation. Biochem. Pharmacol. 1994, 47, 1405–1415. [Google Scholar] [CrossRef]
- Haag, J.D.; Gould, M.N. Mammary carcinoma regression induced by perillyl alcohol, a hydroxylated analog of limonene. Cancer Chemother. Pharmacol. 1994, 34, 477–483. [Google Scholar] [CrossRef] [PubMed]
- Elegbede, J.; Elson, C.; Qureshi, A.; Tanner, M.; Gould, M. Inhibition of DMBA-induced mammary cancer by the monoterpene d-limonene. Carcinogenesis 1984, 5, 661–664. [Google Scholar] [CrossRef] [PubMed]
- Elegbede, J.A.; Elson, C.E.; Qureshi, A.; Tanner, M.A.; Gould, M.N. Regression of Rat Primary Mammary Tumors Following Dietary d-Limonene2. J. Natl. Cancer Inst. 1986, 76, 323–325. [Google Scholar] [CrossRef] [PubMed]
- Homburger, F.; Treger, A.; Boger, E. Inhibition of Murine Subcutaneous and Intravenous Benzo(rst)pentaphene Carcinogenesis by Sweet Orange Oils and d-Limonene. Oncology 1971, 25, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Wattenberg, L.W.; Coccia, J.B. Inhibition of 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone carcinogenesis in mice by D-limonene and citrus fruit oils. Carcinogenesis 1991, 12, 115–117. [Google Scholar] [CrossRef] [PubMed]
- Gelb, M.H.; Tamanoi, F.; Yokoyama, K.; Ghomashchi, F.; Esson, K.; Gould, M.N. The inhibition of protein prenyltransferases by oxygenated metabolites of limonene and perillyl alcohol. Cancer Lett. 1995, 91, 169–175. [Google Scholar] [CrossRef]
- Crowell, P.; Chang, R.; Ren, Z.; Elson, C.; Gould, M. Selective inhibition of isoprenylation of 21-26-kDa proteins by the anticarcinogen d-limonene and its metabolites. J. Biol. Chem. 1991, 266, 17679–17685. [Google Scholar] [CrossRef]
- Da Fonseca, C.O.; Linden, R.; Futuro, D.; Gattass, C.R.; Quirico-Santos, T. Ras pathway activation in gliomas: A strategic target for intranasal administration of perillyl alcohol. Arch. Immunol. Ther. Exp. 2008, 56, 267–276. [Google Scholar] [CrossRef] [Green Version]
- Gould, M.N. Prevention and therapy of mammary cancer by monoterpenes. J. Cell. Biochem. 1995, 59, 139–144. [Google Scholar] [CrossRef]
- Holstein, S.A.; Hohl, R.J. Monoterpene regulation of Ras and Ras-related protein expression. J. Lipid Res. 2003, 44, 1209–1215. [Google Scholar] [CrossRef] [Green Version]
- Rowinsky, E.K.; Windle, J.J.; Von Hoff, D.D. Ras Protein Farnesyltransferase: A Strategic Target for Anticancer Therapeutic Development. J. Clin. Oncol. 1999, 17, 3631–3652. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ren, Z.; Elson, C.E.; Gould, M.N. Inhibition of type I and type II geranylgeranyl-protein transferases by the monoterpene perillyl alcohol in NIH3T3 cells. Biochem. Pharmacol. 1997, 54, 113–120. [Google Scholar] [CrossRef]
- A Ariazi, E.; Satomi, Y.; Ellis, M.J.; Haag, J.D.; Shi, W.; A Sattler, C.; Gould, M.N. Activation of the transforming growth factor beta signaling pathway and induction of cytostasis and apoptosis in mammary carcinomas treated with the anticancer agent perillyl alcohol. Cancer Res. 1999, 59, 1917–1928. [Google Scholar]
- Koyama, M.; Sowa, Y.; Hitomi, T.; Iizumi, Y.; Watanabe, M.; Taniguchi, T.; Ichikawa, M.; Sakai, T. Perillyl alcohol causes g1 arrest through p15(ink4b) and p21(waf1/cip1) induction. Oncol. Rep. 2013, 29, 779–784. [Google Scholar] [CrossRef] [Green Version]
- Wiseman, D.; Werner, S.R.; Crowell, P.L. Cell Cycle Arrest by the Isoprenoids Perillyl Alcohol, Geraniol, and Farnesol Is Mediated by p21Cip1and p27Kip1in Human Pancreatic Adenocarcinoma Cells. J. Pharmacol. Exp. Ther. 2006, 320, 1163–1170. [Google Scholar] [CrossRef] [PubMed]
- Yuri, T.; Danbara, N.; Tsujita-Kyutoku, M.; Kiyozuka, Y.; Senzaki, H.; Shikata, N.; Kanzaki, H.; Tsubura, A. Perillyl Alcohol Inhibits Human Breast Cancer Cell Growth in vitro and in vivo. Breast Cancer Res. Treat. 2004, 84, 251–260. [Google Scholar] [CrossRef] [PubMed]
- Satomi, Y.; Miyamoto, S.; Gould, M.N. Induction of AP-1 activity by perillyl alcohol in breast cancer cells. Carcinogenesis 1999, 20, 1957–1961. [Google Scholar] [CrossRef] [Green Version]
- Sundin, T.; Peffley, D.M.; Gauthier, D.; Hentosh, P. The isoprenoid perillyl alcohol inhibits telomerase activity in prostate cancer cells. Biochimie 2012, 94, 2639–2648. [Google Scholar] [CrossRef]
- Sundin, T.; Peffley, D.M.; Hentosh, P. Disruption of an htert-mtor-raptor protein complex by a phytochemical perillyl alcohol and rapamycin. Mol. Cell. Biochem. 2013, 375, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Peffley, D.M.; Sharma, C.; Hentosh, P.; Buechler, R.D. Perillyl alcohol and genistein differentially regulate PKB/Akt and 4E-BP1 phosphorylation as well as eIF4E/eIF4G interactions in human tumor cells. Arch. Biochem. Biophys. 2007, 465, 266–273. [Google Scholar] [CrossRef] [PubMed]
- Sundin, T.; Peffley, D.; Hentosh, P. eIF4E-Overexpression imparts perillyl alcohol and rapamycin-mediated regulation of telomerase reverse transcriptase. Exp. Cell Res. 2013, 319, 2103–2112. [Google Scholar] [CrossRef] [PubMed]
- Garcia, D.G.; Amorim, L.M.F.; Faria, M.V.D.C.; Freire, A.S.; Santelli, R.E.; Da Fonseca, C.O.; Quirico-Santos, T.; Burth, P. The anticancer drug perillyl alcohol is a Na/K-ATPase inhibitor. Mol. Cell. Biochem. 2010, 345, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Garcia, D.G.; de Castro-Faria-Neto, H.C.; da Silva, C.I.; Souza, K.F.C.D.S.E.; Gonçalves-De-Albuquerque, C.F.; Silva, A.R.; Amorim, L.M.D.F.D.; Freire, A.S.; Santelli, R.E.; Diniz, L.P.; et al. Na/K-ATPase as a target for anticancer drugs: Studies with perillyl alcohol. Mol. Cancer 2015, 14, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, Y.; Bian, J.; Zhang, F. Inhibition of perillyl alcohol on cell invasion and migration depends on the Notch signaling pathway in hepatoma cells. Mol. Cell. Biochem. 2015, 411, 307–315. [Google Scholar] [CrossRef] [PubMed]
- Berchtold, C.M.; Chen, K.S.; Miyamoto, S.; Gould, M.N. Perillyl alcohol inhibits a calcium-dependent constitutive nuclear factor-kappab pathway. Cancer Res. 2005, 65, 8558–8566. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, A.Q.; Nafees, S.; Sultana, S. Perillyl alcohol protects against ethanol induced acute liver injury in Wistar rats by inhibiting oxidative stress, NFκ-B activation and proinflammatory cytokine production. Toxicology 2011, 279, 108–114. [Google Scholar] [CrossRef]
- Tabassum, R.; Vaibhav, K.; Shrivastava, P.; Khan, A.; Ahmed, M.E.; Ashafaq, M.; Khan, M.B.; Islam, F.; Safhi, M.M.; Islam, F. Perillyl alcohol improves functional and histological outcomes against ischemia-reperfusion injury by attenuation of oxidative stress and repression of cox-2, nos-2 and nf-kappab in middle cerebral artery occlusion rats. Eur. J. Pharmacol. 2015, 747, 190–199. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Li, J.; Wang, K.S.; Mi, C.; Piao, L.X.; Xu, G.H.; Li, X.; Lee, J.J.; Jin, X. Perillyl alcohol efficiently scavenges activity of cellular ROS and inhibits the translational expression of hypoxia-inducible factor-1α via mTOR/4E-BP1 signaling pathways. Int. Immunopharmacol. 2016, 39, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.-Y.; Wang, W.; Jhaveri, N.; Torres, S.; Tseng, J.; Leong, M.N.; Lee, D.J.; Goldkorn, A.; Xu, T.; Petasis, N.; et al. Perillyl Alcohol for the Treatment of Temozolomide-Resistant Gliomas. Mol. Cancer Ther. 2012, 11, 2462–2472. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nishitoh, H. CHOP is a multifunctional transcription factor in the ER stress response. J. Biochem. 2012, 151, 217–219. [Google Scholar] [CrossRef] [Green Version]
- Schönthal, A.H. Endoplasmic Reticulum Stress: Its Role in Disease and Novel Prospects for Therapy. Scientifica 2012, 2012, 1–26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schönthal, A.H. Pharmacological targeting of endoplasmic reticulum stress signaling in cancer. Biochem. Pharmacol. 2013, 85, 653–666. [Google Scholar] [CrossRef] [PubMed]
- Ripple, G.H.; Gould, M.N.; A Stewart, J.; Tutsch, K.D.; Arzoomanian, R.Z.; Alberti, D.; Feierabend, C.; Pomplun, M.; Wilding, G.; Bailey, H.H. Phase I clinical trial of perillyl alcohol administered daily. Clin. Cancer Res. 1998, 4, 1159–1164. [Google Scholar]
- Ripple, G.H.; Gould, M.N.; Arzoomanian, R.Z.; Alberti, D.; Feierabend, C.; Simon, K.; Binger, K.; Tutsch, K.D.; Pomplun, M.; Wahamaki, A.; et al. Phase I clinical and pharmacokinetic study of perillyl alcohol administered four times a day. Clin. Cancer Res. 2000, 6, 390–396. [Google Scholar] [PubMed]
- Bailey, H.H.; Levy, D.; Harris, L.S.; Schink, J.C.; Foss, F.; Beatty, P.; Wadler, S. A Phase II Trial of Daily Perillyl Alcohol in Patients with Advanced Ovarian Cancer: Eastern Cooperative Oncology Group Study E2E96. Gynecol. Oncol. 2002, 85, 464–468. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Oettel, K.; Bailey, H.; Van Ummersen, L.; Tutsch, K.; Staab, M.J.; Horvath, D.; Alberti, N.; Arzoomanian, R.; Rezazadeh, H.; et al. Phase II trial of perillyl alcohol (NSC 641066) administered daily in patients with metastatic androgen independent prostate cancer. Investig. New Drugs 2003, 21, 367–372. [Google Scholar] [CrossRef] [PubMed]
- Bailey, H.H.; Attia, S.; Love, R.R.; Fass, T.; Chappell, R.; Tutsch, K.; Harris, L.; Jumonville, A.; Hansen, R.; Shapiro, G.R.; et al. Phase II trial of daily oral perillyl alcohol (NSC 641066) in treatment-refractory metastatic breast cancer. Cancer Chemother. Pharmacol. 2007, 62, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Matos, J.M.; Schmidt, C.M.; Thomas, H.J.; Cummings, O.W.; Wiebke, E.A.; Madura, J.A.; Patrick, L.J.; Crowell, P. A Pilot Study of Perillyl Alcohol in Pancreatic Cancer. J. Surg. Res. 2008, 147, 194–199. [Google Scholar] [CrossRef] [PubMed]
- Meadows, S.M.; Mulkerin, D.; Berlin, J.; Bailey, H.; Kolesar, J.; Warren, D.; Thomas, J.P. Phase II Trial of Perillyl Alcohol in Patients with Metastatic Colorectal Cancer. Int. J. Pancreatol. 2002, 32, 125–128. [Google Scholar] [CrossRef]
- Durço, A.O.; Conceição, L.S.R.; de Souza, D.S.; Lima, C.A.; Quintans, J.D.S.S.; dos Santos, M.R.V. Perillyl alcohol as a treatment for cancer: A systematic review. Phytomedicine Plus 2021, 1, 100090. [Google Scholar] [CrossRef]
- Erdő, F.; Bors, L.A.; Farkas, D.; Bajza, Á.; Gizurarson, S. Evaluation of intranasal delivery route of drug administration for brain targeting. Brain Res. Bull. 2018, 143, 155–170. [Google Scholar] [CrossRef]
- Keller, L.-A.; Merkel, O.; Popp, A. Intranasal drug delivery: Opportunities and toxicologic challenges during drug development. Drug Deliv. Transl. Res. 2021, 1–23. [Google Scholar] [CrossRef]
- Pardeshi, C.V.; Belgamwar, V.S. Direct nose to brain drug delivery via integrated nerve pathways bypassing the blood-brain barrier: An excellent platform for brain targeting. Epert Opin. Drug Deliv. 2013, 10, 957–972. [Google Scholar] [CrossRef] [PubMed]
- Dhas, N.; Yadav, D.; Singh, A.; Garkal, A.; Kudarha, R.; Bangar, P.; Savjani, J.; Pardeshi, C.V.; Garg, N.; Mehta, T. Direct transport theory: From the nose to the brain. In Direct Nose-to-Brain Drug Delivery; Pardeshi, C.V., Souto, E.B., Eds.; Academic Press: San Diego, CA, USA; Cambridge, MA, USA; Oxford, UK; London, UK, 2021; pp. 15–38. [Google Scholar]
- Khunt, D.; Misra, M. Basic considerations of anatomical and physiological aspects of the nose and the brain. In Direct Nose-to-Brain Drug Delivery; Pardeshi, C.V., Souto, E.B., Eds.; Academic Press: San Diego, CA, USA; Cambridge, MA, USA; Oxford, UK; London, UK, 2021; pp. 3–14. [Google Scholar]
- Laddha, U.D.; Tagalpallewar, A.A. Physicochemical, biopharmaceutical, and practical considerations for efficient nose-to-brain drug delivery. In Direct Nose-to-Brain Drug Delivery; Pardeshi, C.V., Souto, E.B., Eds.; Academic Press: San Diego, CA, USA; Cambridge, MA, USA; Oxford, UK; London, UK, 2021; pp. 39–56. [Google Scholar]
- Gänger, S.; Schindowski, K. Tailoring Formulations for Intranasal Nose-to-Brain Delivery: A Review on Architecture, Physico-Chemical Characteristics and Mucociliary Clearance of the Nasal Olfactory Mucosa. Pharmaceutics 2018, 10, 116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crowe, T.; Greenlee, M.H.W.; Kanthasamy, A.; Hsu, W.H. Mechanism of intranasal drug delivery directly to the brain. Life Sci. 2018, 195, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Djupesland, P.G.; Messina, J.C.; A Mahmoud, R. The nasal approach to delivering treatment for brain diseases: An anatomic, physiologic, and delivery technology overview. Ther. Deliv. 2014, 5, 709–733. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, T.C.; Da Fonseca, C.O.; Schönthal, A.H. Preclinical development and clinical use of perillyl alcohol for chemoprevention and cancer therapy. Am. J. Cancer Res. 2015, 5, 1580–1593. [Google Scholar]
- Da Fonseca, C.O.; Masini, M.; Futuro, D.; Caetano, R.; Gattass, C.R.; Quirico-Santos, T. Anaplastic oligodendroglioma responding favorably to intranasal delivery of perillyl alcohol: A case report and literature review. Surg. Neurol. 2006, 66, 611–615. [Google Scholar] [CrossRef] [PubMed]
- da Fonseca, C.O.; Schwartsmann, G.; Fischer, J.; Nagel, J.; Futuro, D.; Quirico-Santos, T.; Gattass, C.R. Preliminary results from a phase I/II study of perillyl alcohol intranasal administration in adults with recurrent malignant gliomas. Surg. Neurol. 2008, 70, 259–266. [Google Scholar] [CrossRef]
- Da Fonseca, C.O.; Simão, M.; Lins, I.R.; Caetano, R.O.; Futuro, D.; Quirico-Santos, T. Efficacy of monoterpene perillyl alcohol upon survival rate of patients with recurrent glioblastoma. J. Cancer Res. Clin. Oncol. 2010, 137, 287–293. [Google Scholar] [CrossRef] [PubMed]
- Da Fonseca, C.O.; Teixeira, R.M.; Silva, J.C.T.; Fischer, J.D.S.D.G.; Meirelles, O.C.; Landeiro, J.A.; Quirico-Santos, T. Long-term outcome in patients with recurrent malignant glioma treated with Perillyl alcohol inhalation. Anticancer Res. 2013, 33, 5625–5631. [Google Scholar]
- Schönthal, A.H.; Peereboom, D.M.; Wagle, N.; Lai, R.; Mathew, A.J.; Hurth, K.M.; Simmon, V.F.; Howard, S.P.; Taylor, L.P.; Chow, F.; et al. Phase I trial of intranasal NEO100, highly purified perillyl alcohol, in adult patients with recurrent glioblastoma. Neuro-Oncol. Adv. 2021, 3, vdab005. [Google Scholar] [CrossRef]
- National Cancer Institute. Common Terminology Criteria for Adverse Events (CTCAE) Version 5.0. Available online: https://ctep.cancer.gov/protocoldevelopment/electronic_applications/ctc.htm#ctc_50 (accessed on 15 July 2021).
- Barker, F.G., II; Chang, S.M.; Gutin, P.H.; Malec, M.K.; McDermott, M.W.; Prados, M.D.; Wilson, C.B. Survival and functional status after resection of recurrent glioblastoma multiforme. Neurosurgery 1998, 42, 709–720, discussion 720–703. [Google Scholar] [CrossRef] [Green Version]
- Batchelor, T.T.; Mulholland, P.; Neyns, B.; Nabors, L.B.; Campone, M.; Wick, A.; Mason, W.; Mikkelsen, T.; Phuphanich, S.; Ashby, L.S.; et al. Phase III Randomized Trial Comparing the Efficacy of Cediranib As Monotherapy, and in Combination With Lomustine, Versus Lomustine Alone in Patients With Recurrent Glioblastoma. J. Clin. Oncol. 2013, 31, 3212–3218. [Google Scholar] [CrossRef] [Green Version]
- Brandes, A.A.; Finocchiaro, G.; Zagonel, V.; Reni, M.; Caserta, C.; Fabi, A.; Clavarezza, M.; Maiello, E.; Eoli, M.; Lombardi, G.; et al. AVAREG: A phase II, randomized, noncomparative study of fotemustine or bevacizumab for patients with recurrent glioblastoma. Neuro-Oncology 2016, 18, 1304–1312. [Google Scholar] [CrossRef]
- Desjardins, A.; Herndon, J.E., II; McSherry, F.; Ravelo, A.; Lipp, E.S.; Healy, P.; Peters, K.B.; Sampson, J.H.; Randazzo, D.; Sommer, N.; et al. Single-institution retrospective review of patients with recurrent glioblastoma treated with bevacizumab in clinical practice. Health Sci. Rep. 2019, 2, e114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghiaseddin, A.; Peters, K.B. Use of bevacizumab in recurrent glioblastoma. CNS Oncol. 2015, 4, 157–169. [Google Scholar] [CrossRef]
- Weller, M.; Le Rhun, E. How did lomustine become standard of care in recurrent glioblastoma? Cancer Treat. Rev. 2020, 87, 102029. [Google Scholar] [CrossRef] [PubMed]
- Da Fonseca, C.O.; Silva, J.T.; Lins, I.R.; Simão, M.; Arnobio, A.; Futuro, D.; Quirico-Santos, T. Correlation of tumor topography and peritumoral edema of recurrent malignant gliomas with therapeutic response to intranasal administration of perillyl alcohol. Investig. New Drugs 2009, 27, 557–564. [Google Scholar] [CrossRef] [PubMed]
- Faria, G.M.; Soares, I.D.P.; D’Alincourt Salazar, M.; Amorim, M.R.; Pessoa, B.L.; da Fonseca, C.O.; Quirico-Santos, T. Intranasal perillyl alcohol therapy improves survival of patients with recurrent glioblastoma harboring mutant variant for mthfr rs1801133 polymorphism. BMC Cancer 2020, 20, 294. [Google Scholar] [CrossRef] [PubMed]
- Waitkus, M.S.; Diplas, B.H.; Yan, H. Biological Role and Therapeutic Potential of IDH Mutations in Cancer. Cancer Cell 2018, 34, 186–195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, H.; Ye, D.; Guan, K.-L.; Xiong, Y. IDH1 and IDH2 Mutations in Tumorigenesis: Mechanistic Insights and Clinical Perspectives. Clin. Cancer Res. 2012, 18, 5562–5571. [Google Scholar] [CrossRef] [Green Version]
- Huang, L.E. Friend or foe—IDH1 mutations in glioma 10 years on. Carcinogenesis 2019, 40, 1299–1307. [Google Scholar] [CrossRef] [PubMed]
- Tabei, Y.; Kobayashi, K.; Saito, K.; Shimizu, S.; Suzuki, K.; Sasaki, N.; Shiokawa, Y.; Nagane, M. Survival in patients with glioblastoma at a first progression does not correlate with isocitrate dehydrogenase (IDH)1 gene mutation status. Jpn. J. Clin. Oncol. 2021, 51, 45–53. [Google Scholar] [CrossRef]
- Da Fonseca, C.; Soares, I.P.; Clemençon, D.S.; Rochlin, S.; Cardeman, L.; Quirico-Santos, T. Perillyl alcohol inhalation concomitant with oral temozolomide halts progression of recurrent inoperable glioblastoma: A case report. J. Histol. Histopathol. 2015, 2, 12. [Google Scholar] [CrossRef]
- Santos, J.G.; Da Cruz, W.M.S.; Schönthal, A.H.; Salazar, M.D.; Fontes, C.A.P.; Quirico-Santos, T.; Da Fonseca, C.O. Efficacy of a ketogenic diet with concomitant intranasal perillyl alcohol as a novel strategy for the therapy of recurrent glioblastoma. Oncol. Lett. 2017, 15, 1263–1270. [Google Scholar] [CrossRef] [PubMed]
- Santos, J.G.; Faria, G.; Cruz, W.D.C.S.D.; Fontes, C.A.; Schönthal, A.H.; Quirico-Santos, T.; Da Fonseca, C.O. Adjuvant effect of low-carbohydrate diet on outcomes of patients with recurrent glioblastoma under intranasal perillyl alcohol therapy. Surg. Neurol. Int. 2020, 11, 389. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Chen, H.; Chan, K.K.; Budd, T.; Ganapathi, R. Gas chromatographic–mass spectrometric analysis of perillyl alcohol and metabolites in plasma. J. Chromatogr. B Biomed. Sci. Appl. 1999, 728, 85–95. [Google Scholar] [CrossRef]
- Santos, J.D.S.; Diedrich, C.; Machado, C.S.; da Fonseca, C.O.; Khalil, N.M.; Mainardes, R.M. Intranasal administration of perillyl alcohol–loaded nanoemulsion and pharmacokinetic study of its metabolite perillic acid in plasma and brain of rats using ultra-performance liquid chromatography/tandem mass spectrometry. Biomed. Chromatogr. 2021, 35, e5037. [Google Scholar] [CrossRef] [PubMed]
- De Lima, D.C.; Rodrigues, S.V.; Boaventura, G.T.; Cho, H.; Chen, T.C.; Schönthal, A.H.; Da Fonseca, C.O. Simultaneous measurement of perillyl alcohol and its metabolite perillic acid in plasma and lung after inhalational administration in Wistar rats. Drug Test. Anal. 2019, 12, 268–279. [Google Scholar] [CrossRef] [PubMed]
- Marin, A.A.; Murillo, O.; Sussmann, R.A.; Ortolan, L.S.; Battagello, D.S.; Quirino, T.D.C.; Bittencourt, J.C.; Epiphanio, S.; Katzin, A.M.; Carvalho, L.J.M. Perillyl Alcohol Reduces Parasite Sequestration and Cerebrovascular Dysfunction during Experimental Cerebral Malaria. Antimicrob. Agents Chemother. 2021, 65. [Google Scholar] [CrossRef] [PubMed]
- Nehra, G.; Andrews, S.; Rettig, J.; Gould, M.N.; Haag, J.D.; Howard, S.P.; Thorne, R.G. Intranasal administration of the chemotherapeutic perillyl alcohol results in selective delivery to the cerebrospinal fluid in rats. Sci. Rep. 2021, 11, 1–11. [Google Scholar] [CrossRef]
- Eftekhari, R.B.; Maghsoudnia, N.; Samimi, S.; Zamzami, A.; Dorkoosh, F.A. Co-Delivery Nanosystems for Cancer Treatment: A Review. Pharm. Nanotechnol. 2019, 7, 90–112. [Google Scholar] [CrossRef]
- Guo, P.; He, Y.; Xu, T.; Pi, C.; Jiang, Q.; Wei, Y.; Zhao, L. Co-delivery system of chemotherapy drugs and active ingredients from natural plants: A brief overview of preclinical research for cancer treatment. Expert Opin. Drug Deliv. 2020, 17, 665–675. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, N.N.; Junior, E.D.O.; Granja, S.; Boni, F.I.; Ferreira, L.M.; Cury, B.S.; Santos, L.C.; Reis, R.M.; Lima, E.M.; Baltazar, F.; et al. Nose-to-brain co-delivery of drugs for glioblastoma treatment using nanostructured system. Int. J. Pharm. 2021, 603, 120714. [Google Scholar] [CrossRef] [PubMed]
- Mignani, S.; Shi, X.; Karpus, A.; Majoral, J.-P. Non-invasive intranasal administration route directly to the brain using dendrimer nanoplatforms: An opportunity to develop new CNS drugs. Eur. J. Med. Chem. 2021, 209, 112905. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Swenson, S.; Cho, H.-Y.; Hofman, F.M.; Schönthal, A.H.; Chen, T.C. Efficient brain targeting and therapeutic intracranial activity of bortezomib through intranasal co-delivery with NEO100 in rodent glioblastoma models. J. Neurosurg. 2020, 132, 959–967. [Google Scholar] [CrossRef] [PubMed]
- Scott, K.; Hayden, P.J.; Will, A.; Wheatley, K.; Coyne, I. Bortezomib for the treatment of multiple myeloma. Cochrane Database Syst. Rev. 2016, 4, CD010816. [Google Scholar] [CrossRef] [Green Version]
- Hemeryck, A.; Geerts, R.; Monbaliu, J.; Hassler, S.; Verhaeghe, T.; Diels, L.; Verluyten, W.; Van Beijsterveldt, L.; Mamidi, R.N.V.S.; Janssen, C.; et al. Tissue distribution and depletion kinetics of bortezomib and bortezomib-related radioactivity in male rats after single and repeated intravenous injection of 14C-bortezomib. Cancer Chemother. Pharmacol. 2007, 60, 777–787. [Google Scholar] [CrossRef] [PubMed]
- Pinel, S.; Labussiere, M.; Delfortrie, S.; Plenat, F.; Chastagner, P. Proteasome inhibition by bortezomib does not translate into efficacy on two malignant glioma xenografts. Oncol. Rep. 1994, 20, 1283–1287. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.; Cho, H.-Y.; Rosenstein-Sisson, R.; Ramos, N.I.M.; Price, R.; Hurth, K.; Schönthal, A.H.; Hofman, F.M.; Chen, T.C. Intratumoral delivery of bortezomib: Impact on survival in an intracranial glioma tumor model. J. Neurosurg. 2018, 128, 695–700. [Google Scholar] [CrossRef] [Green Version]
- Friday, B.B.; Anderson, S.K.; Buckner, J.; Yu, C.; Giannini, C.; Geoffroy, F.; Schwerkoske, J.; Mazurczak, M.; Gross, H.; Pajon, E.; et al. Phase II trial of vorinostat in combination with bortezomib in recurrent glioblastoma: A north central cancer treatment group study. Neuro-Oncology 2011, 14, 215–221. [Google Scholar] [CrossRef] [Green Version]
- Odia, Y.; Kreisl, T.N.; Aregawi, D.; Innis, E.K.; Fine, H.A. A phase II trial of tamoxifen and bortezomib in patients with recurrent malignant gliomas. J. Neuro-Oncol. 2015, 125, 191–195. [Google Scholar] [CrossRef] [PubMed]
- Raizer, J.J.; Chandler, J.P.; Ferrarese, R.; Grimm, S.A.; Levy, R.M.; Muro, K.; Rosenow, J.; Helenowski, I.; Rademaker, A.; Paton, M.; et al. A phase II trial evaluating the effects and intra-tumoral penetration of bortezomib in patients with recurrent malignant gliomas. J. Neuro-Oncol. 2016, 129, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Bota, D.A.; Alexandru, D.; Keir, S.T.; Bigner, D.; Vredenburgh, J.; Friedman, H.S. Proteasome inhibition with bortezomib induces cell death in GBM stem-like cells and temozolomide-resistant glioma cell lines, but stimulates GBM stem-like cells’ VEGF production and angiogenesis. J. Neurosurg. 2013, 119, 1415–1423. [Google Scholar] [CrossRef] [Green Version]
- Kardosh, A.; Golden, E.B.; Pyrko, P.; Uddin, J.; Hofman, F.M.; Chen, T.C.; Louie, S.G.; Petasis, N.; Schönthal, A.H. Aggravated Endoplasmic Reticulum Stress as a Basis for Enhanced Glioblastoma Cell Killing by Bortezomib in Combination with Celecoxib or Its Non-Coxib Analogue, 2,5-Dimethyl-Celecoxib. Cancer Res. 2008, 68, 843–851. [Google Scholar] [CrossRef] [Green Version]
- Styczynski, J.; Olszewska-Slonina, D.; Kolodziej, B.; Napieraj, M.; Wysocki, M. Activity of bortezomib in glioblastoma. Anticancer. Res. 2007, 26, 4499–4503. [Google Scholar]
- Joshi, S.; Ergin, A.; Wang, M.; Reif, R.; Zhang, J.; Bruce, J.N.; Bigio, I.J. Inconsistent blood brain barrier disruption by intraarterial mannitol in rabbits: Implications for chemotherapy. J. Neuro-Oncol. 2010, 104, 11–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Joshi, S.; Meyers, P.M.; Ornstein, E. Intracarotid delivery of drugs: The potential and the pitfalls. Anesthesiology 2008, 109, 543–564. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lundqvist, H.; Rosander, K.; Lomanov, M.; Lukjashin, V.; Shimchuk, G.; Zolotov, V.; Minakova, E. Permeability of the Blood-Brain Barrier in the Rat after Local Proton Irradiation. Acta Radiol. Oncol. 1982, 21, 267–271. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- A Peña, L.; Fuks, Z.; Kolesnick, R.N. Radiation-induced apoptosis of endothelial cells in the murine central nervous system: Protection by fibroblast growth factor and sphingomyelinase deficiency. Cancer Res. 2000, 60, 321–327. [Google Scholar] [PubMed]
- Sprowls, S.A.; Arsiwala, T.; Bumgarner, J.; Shah, N.; Lateef, S.S.; Kielkowski, B.N.; Lockman, P.R. Improving CNS Delivery to Brain Metastases by Blood–Tumor Barrier Disruption. Trends Cancer 2019, 5, 495–505. [Google Scholar] [CrossRef] [PubMed]
- Dasgupta, A.; Liu, M.; Ojha, T.; Storm, G.; Kiessling, F.; Lammers, T. Ultrasound-mediated drug delivery to the brain: Principles, progress and prospects. Drug Discov. Today Technol. 2016, 20, 41–48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abrahao, A.; Meng, Y.; Llinas, M.; Huang, Y.; Hamani, C.; Mainprize, T.; Aubert, I.; Heyn, C.; Black, S.E.; Hynynen, K.; et al. First-in-human trial of blood–brain barrier opening in amyotrophic lateral sclerosis using MR-guided focused ultrasound. Nat. Commun. 2019, 10, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Carpentier, A.; Canney, M.; Vignot, A.; Reina, V.; Beccaria, K.; Horodyckid, C.; Karachi, C.; Leclercq, D.; Lafon, C.; Chapelon, J.-Y.; et al. Clinical trial of blood-brain barrier disruption by pulsed ultrasound. Sci. Transl. Med. 2016, 8, 343re2. [Google Scholar] [CrossRef] [PubMed]
- Arvanitis, C.D.; Askoxylakis, V.; Guo, Y.; Datta, M.; Kloepper, J.; Ferraro, G.B.; Bernabeu, M.O.; Fukumura, D.; McDannold, N.; Jain, R.K. Mechanisms of enhanced drug delivery in brain metastases with focused ultrasound-induced blood–tumor barrier disruption. Proc. Natl. Acad. Sci. USA 2018, 115, E8717–E8726. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, W.; He, H.; Marín-Ramos, N.I.; Zeng, S.; Swenson, S.; Cho, H.-Y.; Fu, J.; Beringer, P.M.; Neman, J.; Chen, L.; et al. Enhanced brain delivery and therapeutic activity of trastuzumab after blood-brain barrier opening by neo100 in mouse models of brain-metastatic breast cancer. Neuro Oncol. 2021. e-pub ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; I Marín-Ramos, N.; He, H.; Zeng, S.; Cho, H.-Y.; Swenson, S.D.; Zheng, L.; Epstein, A.L.; Schönthal, A.H.; Hofman, F.M.; et al. NEO100 enables brain delivery of blood–brain barrier impermeable therapeutics. Neuro-Oncology 2021, 23, 63–75. [Google Scholar] [CrossRef]
- Aouad-Maroun, M.; Raphael, C.K.; Sayyid, S.K.; Farah, F.; Akl, E.A. Ultrasound-guided arterial cannulation for paediatrics. Cochrane Database Syst. Rev. 2016, 9, CD011364. [Google Scholar] [CrossRef] [PubMed]
- Hou, X.-T.; Sun, Y.-Q.; Zhang, H.-J.; Zheng, S.-H.; Liu, Y.-Y.; Wang, J.-G. Femoral Artery Cannulation in Stanford Type a Aortic Dissection Operations. Asian Cardiovasc. Thorac. Ann. 2006, 14, 35–37. [Google Scholar] [CrossRef]
- Saadat, S.; Schultheis, M.; Azzolini, A.; Romero, J.; Dombrovskiy, V.; Odroniec, K.; Scholz, P.; Lemaire, A.; Batsides, G.; Lee, L. Femoral cannulation: A safe vascular access option for cardiopulmonary bypass in minimally invasive cardiac surgery. Perfusion 2016, 31, 131–134. [Google Scholar] [CrossRef]
- Bangalore, S.; Bhatt, D.L. Femoral Arterial Access and Closure. Circulation 2011, 124, e147–e156. [Google Scholar] [CrossRef] [Green Version]
- Scheer, B.V.; Perel, A.; Pfeiffer, U.J. Clinical review: Complications and risk factors of peripheral arterial catheters used for haemodynamic monitoring in anaesthesia and intensive care medicine. Crit. Care 2002, 6, 199–204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fusco, D.S.; Shaw, R.K.; Tranquilli, M.; Kopf, G.S.; Elefteriades, J.A. Femoral Cannulation is Safe for Type A Dissection Repair. Ann. Thorac. Surg. 2004, 78, 1285–1289. [Google Scholar] [CrossRef] [PubMed]
- Sattenberg, R.J.; Meckler, J.; Saver, J.L.; Gobin, Y.P.; Liebeskind, D.S. Cerebral angiography. In Stroke: Pathophysiology, Diagnosis, and Management, 6th ed.; Grotta, J.C., Ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2016; pp. 790–805. [Google Scholar]
- Gantt, R.W.; Peltier-Pain, P.; Thorson, J.S. Enzymatic methods for glyco(diversification/randomization) of drugs and small molecules. Nat. Prod. Rep. 2011, 28, 1811–1853. [Google Scholar] [CrossRef] [PubMed]
- Fujita, T.; Ohira, K.; Miyatake, K.; Nakano, Y.; Nakayama, M. Inhibitory Effect of Perillosides A and C, and Related Monoterpene Glucosides on Aldose Reductase and Their Structure-Activity Relationships. Chem. Pharm. Bull. 1995, 43, 920–926. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arafa, H.M. Possible contribution of beta-glycosidases and caspases in the cytotoxicity of novel glycoconjugates in colon cancer cells. Invest New Drugs 2010, 28, 306–317. [Google Scholar] [CrossRef]
- Nandurkar, N.S.; Zhang, J.; Ye, Q.; Ponomareva, L.V.; She, Q.-B.; Thorson, J.S. The Identification of Perillyl Alcohol Glycosides with Improved Antiproliferative Activity. J. Med. Chem. 2014, 57, 7478–7484. [Google Scholar] [CrossRef] [PubMed]
- Xanthakis, E.; Magkouta, S.; Loutrari, H.; Stamatis, H.; Roussos, C.; Kolisis, F.N. Enzymatic synthesis of perillyl alcohol derivatives and investigation of their antiproliferative activity. Biocatal. Biotransformation 2009, 27, 170–178. [Google Scholar] [CrossRef]
- Said, B.; Montenegro, I.; Valenzuela, M.; Olguín, Y.; Caro, N.; Werner, E.; Godoy, P.; Villena, J.; Madrid, A. Synthesis and Antiproliferative Activity of New Cyclodiprenyl Phenols against Select Cancer Cell Lines. Molecules 2018, 23, 2323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rezende, A.A.; Santos, R.S.; Andrade, L.N.; Amaral, R.G.; Pereira, M.M.; Bani, C.; Chen, M.; Priefer, R.; da Silva, C.F.; de Albuquerque Junior, R.L.C.; et al. Anti-tumor efficiency of perillylalcohol/beta-cyclodextrin inclusion complexes in a sarcoma s180-induced mice model. Pharmaceutics 2021, 13, 245. [Google Scholar] [CrossRef]
- Da Silva, C.E.H.; Gosmann, G.; de Andrade, S.F. Limonene and perillyl alcohol derivatives: Synthesis and anticancer activity. Mini Rev. Med. Chem. 2021, 21, 1813–1829. [Google Scholar] [CrossRef] [PubMed]
- Zielińska-Błajet, M.; Pietrusiak, P.; Feder-Kubis, J. Selected Monocyclic Monoterpenes and Their Derivatives as Effective Anticancer Therapeutic Agents. Int. J. Mol. Sci. 2021, 22, 4763. [Google Scholar] [CrossRef] [PubMed]
- Hopkins, A.L. Network pharmacology: The next paradigm in drug discovery. Nat. Chem. Biol. 2008, 4, 682–690. [Google Scholar] [CrossRef] [PubMed]
- Szumilak, M.; Wiktorowska-Owczarek, A.; Stanczak, A. Hybrid Drugs—A Strategy for Overcoming Anticancer Drug Resistance? Molecules 2021, 26, 2601. [Google Scholar] [CrossRef] [PubMed]
- Vendrusculo, V.; de Souza, V.P.; LA, M.F.; MG, M.D.O.; Banzato, T.P.; Monteiro, P.A.; Pilli, R.A.; de Carvalho, J.E.; Russowsky, D. Synthesis of novel perillyl-dihydropyrimidinone hybrids designed for antiproliferative activity. Medchemcomm 2018, 9, 1553–1564. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, B.; Behbahani, F.K. Recent developments in the synthesis and applications of dihydropyrimidin-2(1H)-ones and thiones. Mol. Divers. 2018, 22, 405–446. [Google Scholar] [CrossRef]
- Chen, T.C.; Cho, H.-Y.; Wang, W.; Barath, M.; Sharma, N.; Hofman, F.M.; Schönthal, A.H. A Novel Temozolomide–Perillyl Alcohol Conjugate Exhibits Superior Activity against Breast Cancer Cells In Vitro and Intracranial Triple-Negative Tumor Growth In Vivo. Mol. Cancer Ther. 2014, 13, 1181–1193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, T.C.; Cho, H.-Y.; Wang, W.; Wetzel, S.J.; Singh, A.; Nguyen, J.; Hofman, F.M.; Schönthal, A.H. Chemotherapeutic effect of a novel temozolomide analog on nasopharyngeal carcinoma in vitro and in vivo. J. Biomed. Sci. 2015, 22, 71–80. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schönthal, A.; Swenson, S.; Minea, R.; Kim, H.; Cho, H.; Mohseni, N.; Kim, Y.-M.; Chen, T. Potentially Curative Therapeutic Activity of NEO212, a Perillyl Alcohol-Temozolomide Conjugate, in Preclinical Cytarabine-Resistant Models of Acute Myeloid Leukemia. Cancers 2021, 13, 3385. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.C.; Cho, H.-Y.; Wang, W.; Nguyen, J.; Jhaveri, N.; Rosenstein-Sisson, R.; Hofman, F.M.; Schönthal, A.H. A novel temozolomide analog, NEO212, with enhanced activity against MGMT-positive melanoma in vitro and in vivo. Cancer Lett. 2015, 358, 144–151. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.-Y.; Swenson, S.; Thein, T.Z.; Wang, W.; Wijeratne, N.R.; I Marín-Ramos, N.; E Katz, J.; Hofman, F.M.; Schönthal, A.H.; Chen, T.C. Pharmacokinetic properties of the temozolomide perillyl alcohol conjugate (NEO212) in mice. Neuro-Oncol. Adv. 2020, 2, vdaa160. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.-Y.; Wang, W.; Jhaveri, N.; Lee, D.J.; Sharma, N.; Dubeau, L.; Schönthal, A.H.; Hofman, F.M.; Chen, T.C. NEO212, Temozolomide Conjugated to Perillyl Alcohol, Is a Novel Drug for Effective Treatment of a Broad Range of Temozolomide-Resistant Gliomas. Mol. Cancer Ther. 2014, 13, 2004–2017. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jhaveri, N.; Agasse, F.; Armstrong, D.; Peng, L.; Commins, D.; Wang, W.; Rosenstein-Sisson, R.; Vaikari, V.P.; Santiago, S.V.; Santos, T.; et al. A novel drug conjugate, NEO212, targeting proneural and mesenchymal subtypes of patient-derived glioma cancer stem cells. Cancer Lett. 2016, 371, 240–250. [Google Scholar] [CrossRef] [PubMed]
- Di Francia, R.; Crisci, S.; De Monaco, A.; Cafiero, C.; Re, A.; Iaccarino, G.; De Filippi, R.; Frigeri, F.; Corazzelli, G.; Micera, A.; et al. Response and Toxicity to Cytarabine Therapy in Leukemia and Lymphoma: From Dose Puzzle to Pharmacogenomic Biomarkers. Cancers 2021, 13, 966. [Google Scholar] [CrossRef]
- Zhang, J.; Stevens, M.F.; Bradshaw, T.D. Temozolomide: Mechanisms of action, repair and resistance. Curr. Mol. Pharmacol. 2012, 5, 102–114. [Google Scholar] [CrossRef]
- Zhu, J.; Mix, E.; Winblad, B. The Antidepressant and Antiinflammatory Effects of Rolipram in the Central Nervous System. CNS Drug Rev. 2001, 7, 387–398. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.C.; Wadsten, P.; Su, S.; Rawlinson, N.; Hofman, F.M.; Hill, C.K.; Schonthal, A.H. The type iv phosphodiesterase inhibitor rolipram induces expression of the cell cycle inhibitors p21(cip1) and p27(kip1), resulting in growth inhibition, increased differentiation, and subsequent apoptosis of malignant a-172 glioma cells. Cancer Biol. Ther. 2002, 1, 268–276. [Google Scholar] [CrossRef] [PubMed]
- Goldhoff, P.; Warrington, N.M.; Limbrick, D.D., Jr.; Hope, A.; Woerner, B.M.; Jackson, E.; Perry, A.; Piwnica-Worms, D.; Rubin, J.B. Targeted Inhibition of Cyclic AMP Phosphodiesterase-4 Promotes Brain Tumor Regression. Clin. Cancer Res. 2008, 14, 7717–7725. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cho, H.-Y.; Thein, T.Z.; Wang, W.; Swenson, S.D.; Fayngor, R.A.; Ou, M.; Marín-Ramos, N.I.; Schönthal, A.H.; Hofman, F.M.; Chen, T.C. The Rolipram–Perillyl Alcohol Conjugate (NEO214) Is A Mediator of Cell Death through the Death Receptor Pathway. Mol. Cancer Ther. 2019, 18, 517–530. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, T.C.; Chan, N.; Labib, S.; Yu, J.; Cho, H.-Y.; Hofman, F.M.; Schönthal, A.H. Induction of Pro-Apoptotic Endoplasmic Reticulum Stress in Multiple Myeloma Cells by NEO214, Perillyl Alcohol Conjugated to Rolipram. Int. J. Mol. Sci. 2018, 19, 277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fan, T.; Sun, G.; Sun, X.; Zhao, L.; Zhong, R.; Peng, Y. Tumor Energy Metabolism and Potential of 3-Bromopyruvate as an Inhibitor of Aerobic Glycolysis: Implications in Tumor Treatment. Cancers 2019, 11, 317. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Birsoy, K.; Wang, T.; Possemato, R.; Yilmaz, O.H.; E Koch, C.; Chen, W.; Hutchins, A.W.; Gultekin, Y.; Peterson, T.R.; Carette, J.; et al. MCT1-mediated transport of a toxic molecule is an effective strategy for targeting glycolytic tumors. Nat. Genet. 2013, 45, 104–108. [Google Scholar] [CrossRef] [Green Version]
- Chen, T.C.; Yu, J.; Nigjeh, E.N.; Wang, W.; Myint, P.T.; Zandi, E.; Hofman, F.M.; Schönthal, A.H. A perillyl alcohol-conjugated analog of 3-bromopyruvate without cellular uptake dependency on monocarboxylate transporter 1 and with activity in 3-BP-resistant tumor cells. Cancer Lett. 2017, 400, 161–174. [Google Scholar] [CrossRef] [PubMed]
- Göttlicher, M. Valproic acid: An old drug newly discovered as inhibitor of histone deacetylases. Ann. Hematol. 2004, 83 (Suppl. 1), S91–S92. [Google Scholar] [PubMed]
- Martirosian, V.; Deshpande, K.; Zhou, H.; Shen, K.; Smith, K.; Northcott, P.; Lin, M.; Stepanosyan, V.; Das, D.; Remsik, J.; et al. Medulloblastoma uses GABA transaminase to survive in the cerebrospinal fluid microenvironment and promote leptomeningeal dissemination. Cell Rep. 2021, 36, 109475. [Google Scholar] [CrossRef] [PubMed]
- Haque, T.; Talukder, M.U. Chemical Enhancer: A Simplistic Way to Modulate Barrier Function of the Stratum Corneum. Adv. Pharm. Bull. 2018, 8, 169–179. [Google Scholar] [CrossRef] [Green Version]
- Li, B.S.; Cary, J.H.; Maibach, H.I. Stratum corneum substantivity: Drug development implications. Arch. Dermatol. Res. 2018, 310, 537–549. [Google Scholar] [CrossRef]
- Kováčik, A.; Kopečná, M.; Vávrová, K. Permeation enhancers in transdermal drug delivery: Benefits and limitations. Expert Opin. Drug Deliv. 2020, 17, 145–155. [Google Scholar] [CrossRef]
- Lopes, L.B.; Garcia, M.T.J.; Bentley, M.V.L. Chemical penetration enhancers. Ther. Deliv. 2015, 6, 1053–1061. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Jiang, Q.-D.; Chai, Y.-P.; Zhang, H.; Peng, P.; Yang, X.-X. Natural Terpenes as Penetration Enhancers for Transdermal Drug Delivery. Molecules 2016, 21, 1709. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kopečná, M.; Macháček, M.; Nováčková, A.; Paraskevopoulos, G.; Roh, J.; Vávrová, K. Esters of terpene alcohols as highly potent, reversible, and low toxic skin penetration enhancers. Sci. Rep. 2019, 9, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Lluria-Prevatt, M.; Morreale, J.; Gregus, J.; Alberts, D.S.; Kaper, F.; Giaccia, A.; Powell, M.B. Effects of perillyl alcohol on melanoma in the TPras mouse model. Cancer Epidemiol. Biomark. Prev. 2002, 11, 573–579. [Google Scholar]
- Chaudhary, S.C.; Alam, M.S.; Siddiqui, M.; Athar, M. Perillyl alcohol attenuates Ras-ERK signaling to inhibit murine skin inflammation and tumorigenesis. Chem.-Biol. Interact. 2009, 179, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Barthelman, M.; Chen, W.; Gensler, H.L.; Huang, C.; Dong, Z.; Bowden, G.T. Inhibitory effects of perillyl alcohol on UVB-induced murine skin cancer and AP-1 transactivation. Cancer Res. 1998, 58, 711–716. [Google Scholar] [PubMed]
- Gupta, A.; Myrdal, P.B. Development of a perillyl alcohol topical cream formulation. Int. J. Pharm. 2004, 269, 373–383. [Google Scholar] [CrossRef] [PubMed]
- Stratton, S.P.; Saboda, K.L.; Myrdal, P.B.; Gupta, A.; McKenzie, N.E.; Brooks, C.; Salasche, S.J.; Warneke, J.A.; Ranger-Moore, J.; Bozzo, P.D.; et al. Phase 1 Study of Topical Perillyl Alcohol Cream for Chemoprevention of Skin Cancer. Nutr. Cancer 2008, 60, 325–330. [Google Scholar] [CrossRef] [PubMed]
- Stratton, S.P.; Alberts, D.S.; Einspahr, J.G.; Sagerman, P.M.; Warneke, J.A.; Curiel-Lewandrowski, C.; Myrdal, P.B.; Karlage, K.L.; Nickoloff, B.J.; Brooks, C.; et al. A Phase 2a Study of Topical Perillyl Alcohol Cream for Chemoprevention of Skin Cancer. Cancer Prev. Res. 2010, 3, 160–169. [Google Scholar] [CrossRef] [Green Version]
- D’Alessio, P.; Mirshahi, M.; Bisson, J.-F.; Bene, M. Skin Repair Properties of d-Limonene and Perillyl Alcohol in Murine Models. Anti-Inflamm. Anti-Allergy Agents Med. Chem. 2014, 13, 29–35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Araujo-Filho, H.G.; Pereira, E.W.M.; Heimfarth, L.; Souza Monteiro, B.; Santos Passos, F.R.; Siqueira-Lima, P.; Gandhi, S.R.; Viana Dos Santos, M.R.; Guedes da Silva Almeida, J.R.; Picot, L.; et al. Limonene, a food additive, and its active metabolite perillyl alcohol improve regeneration and attenuate neuropathic pain after peripheral nerve injury: Evidence for il-1beta, tnf-alpha, gap, ngf and erk involvement. Int. Immunopharmacol. 2020, 86, 106766. [Google Scholar] [CrossRef] [PubMed]
- Erickson, C.; Miller, S.J. Treatment options in melanoma in situ: Topical and radiation therapy, excision and Mohs surgery. Int. J. Dermatol. 2010, 49, 482–491. [Google Scholar] [CrossRef] [PubMed]
- Van Zyl, L.; du Preez, J.; Gerber, M.; du Plessis, J.; Viljoen, J. Essential Fatty Acids as Transdermal Penetration Enhancers. J. Pharm. Sci. 2016, 105, 188–193. [Google Scholar] [CrossRef] [PubMed]
- Viljoen, J.; Cowley, A.; du Preez, J.; Gerber, M.; Du Plessis, J. Penetration enhancing effects of selected natural oils utilized in topical dosage forms. Drug Dev. Ind. Pharm. 2015, 41, 2045–2054. [Google Scholar] [CrossRef] [PubMed]
- Swenson, S.; Silva-Hirschberg, C.; Wang, W.; Singh, A.; Hofman, F.M.; Chen, K.L.; Schönthal, A.H.; Chen, T.C. NEO412: A temozolomide analog with transdermal activity in melanoma in vitro and in vivo. Oncotarget 2018, 9, 37026–37041. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, T.C.; da Fonseca, C.O.; Levin, D.; Schönthal, A.H. The Monoterpenoid Perillyl Alcohol: Anticancer Agent and Medium to Overcome Biological Barriers. Pharmaceutics 2021, 13, 2167. https://doi.org/10.3390/pharmaceutics13122167
Chen TC, da Fonseca CO, Levin D, Schönthal AH. The Monoterpenoid Perillyl Alcohol: Anticancer Agent and Medium to Overcome Biological Barriers. Pharmaceutics. 2021; 13(12):2167. https://doi.org/10.3390/pharmaceutics13122167
Chicago/Turabian StyleChen, Thomas C., Clovis O. da Fonseca, Daniel Levin, and Axel H. Schönthal. 2021. "The Monoterpenoid Perillyl Alcohol: Anticancer Agent and Medium to Overcome Biological Barriers" Pharmaceutics 13, no. 12: 2167. https://doi.org/10.3390/pharmaceutics13122167





