Development and Evaluation of Novel Water-Based Drug-in-Adhesive Patches for the Transdermal Delivery of Ketoprofen
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of PVPAA
2.3. Characterization of PVPAA
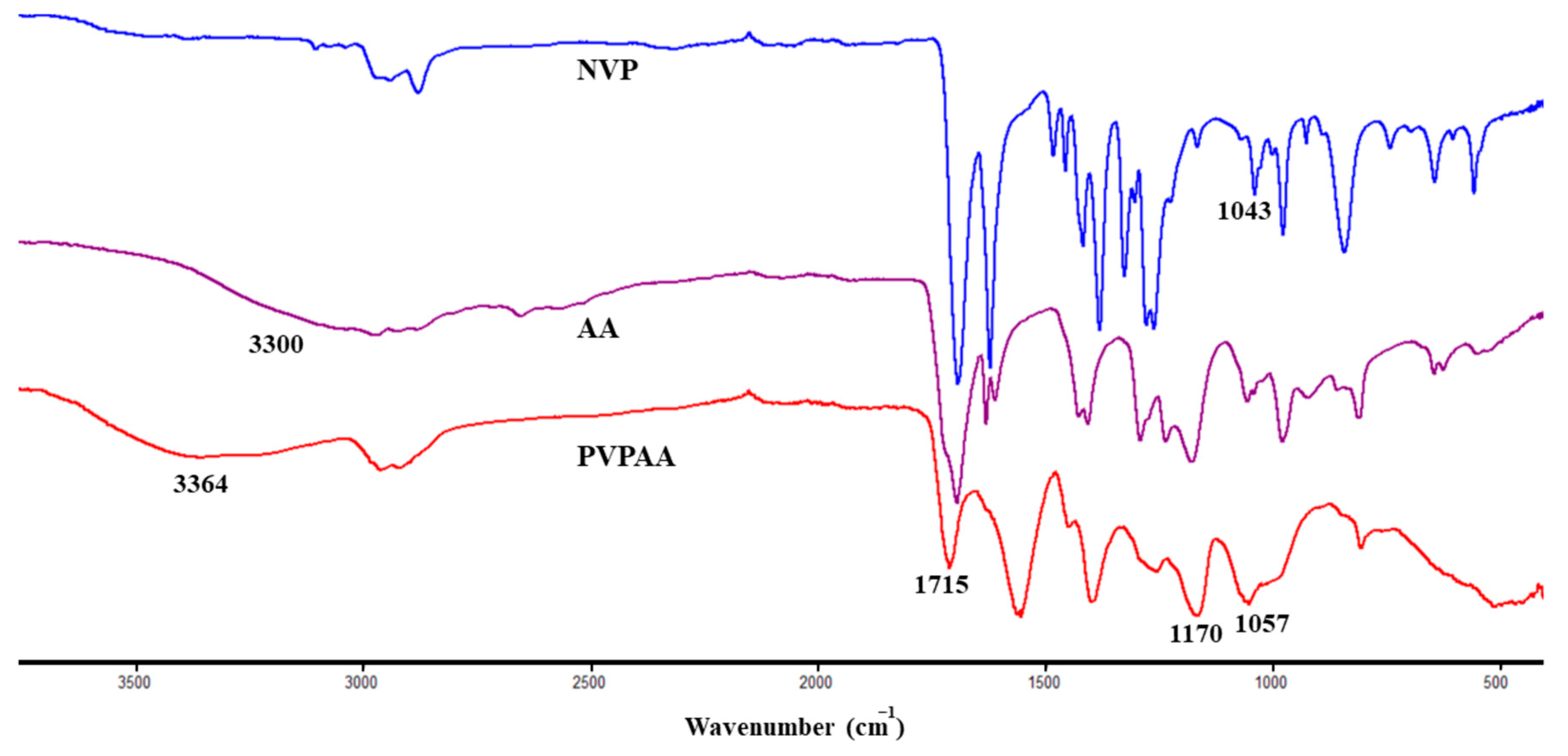
2.3.1. H-NMR
2.3.2. ATR-FTIR
2.3.3. Cytotoxicity Evaluation of PVPAA
2.4. Preparation of Water-based PVPAA/PMVEMA PSAs
2.5. Adhesive Evaluation
2.5.1. Tacking Strength
2.5.2. Peeling Strength
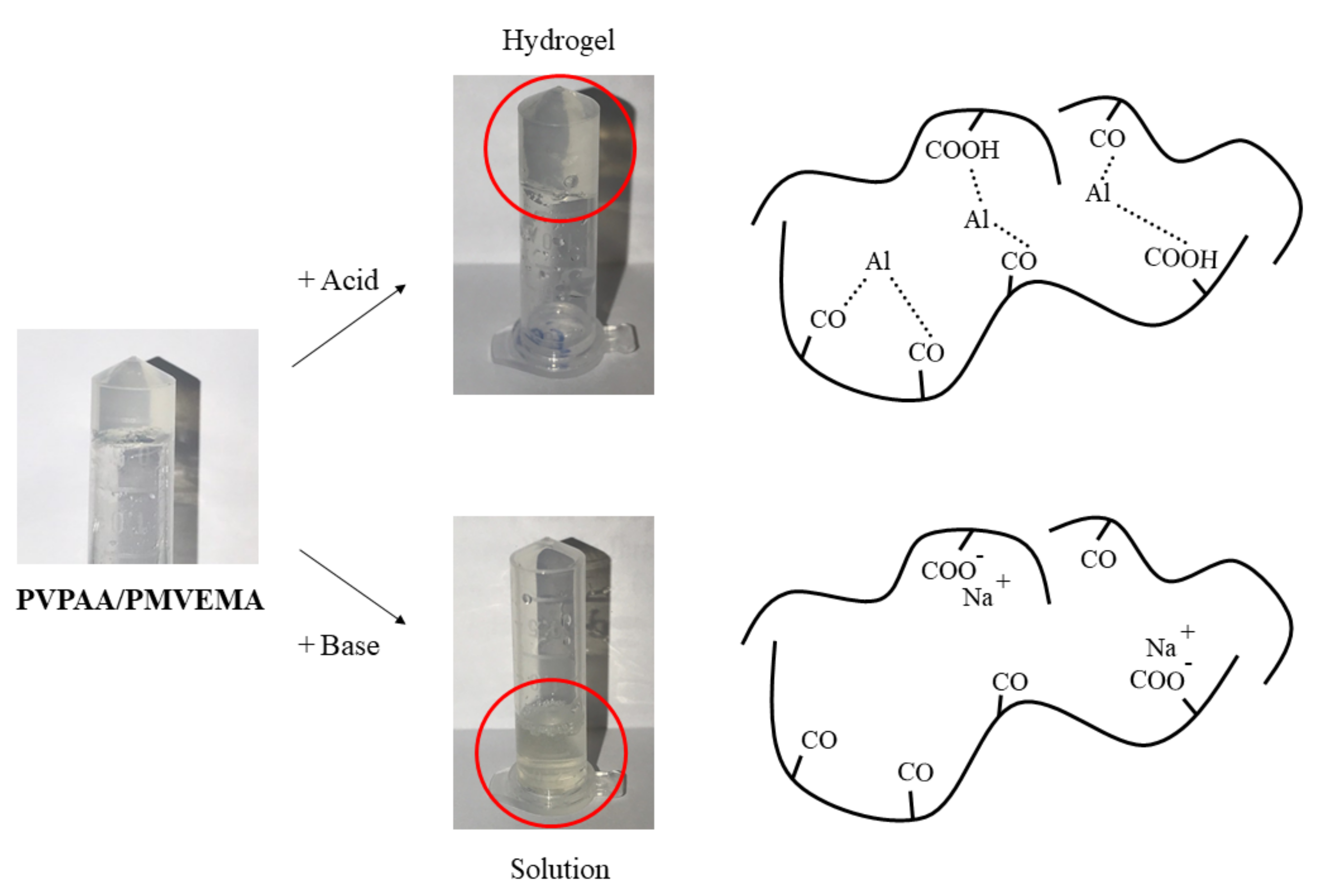
2.6. Metal-Coordination Complex Formation of the Hydrogel PSAs
2.7. Content Determination
2.8. In Vitro Skin Permeation
2.8.1. Skin Preparation
2.8.2. In Vitro Skin Permeation Studies
2.9. Stability Studies
2.10. In Vivo Skin Irritation Test
2.11. Safety Evaluation and In Vivo Skin Adhesion Performance
2.12. Statistical Analysis
3. Results and Discussion
3.1. Synthesis of PVPAA
3.2. Preparation and Metal-Coordination Complex Formation of the PVPAA/PMVEMA PSAs
3.3. Adhesive Evaluation
3.4. Drug Content Assay
3.5. In Vitro Skin Permeation
3.6. Stability Studies
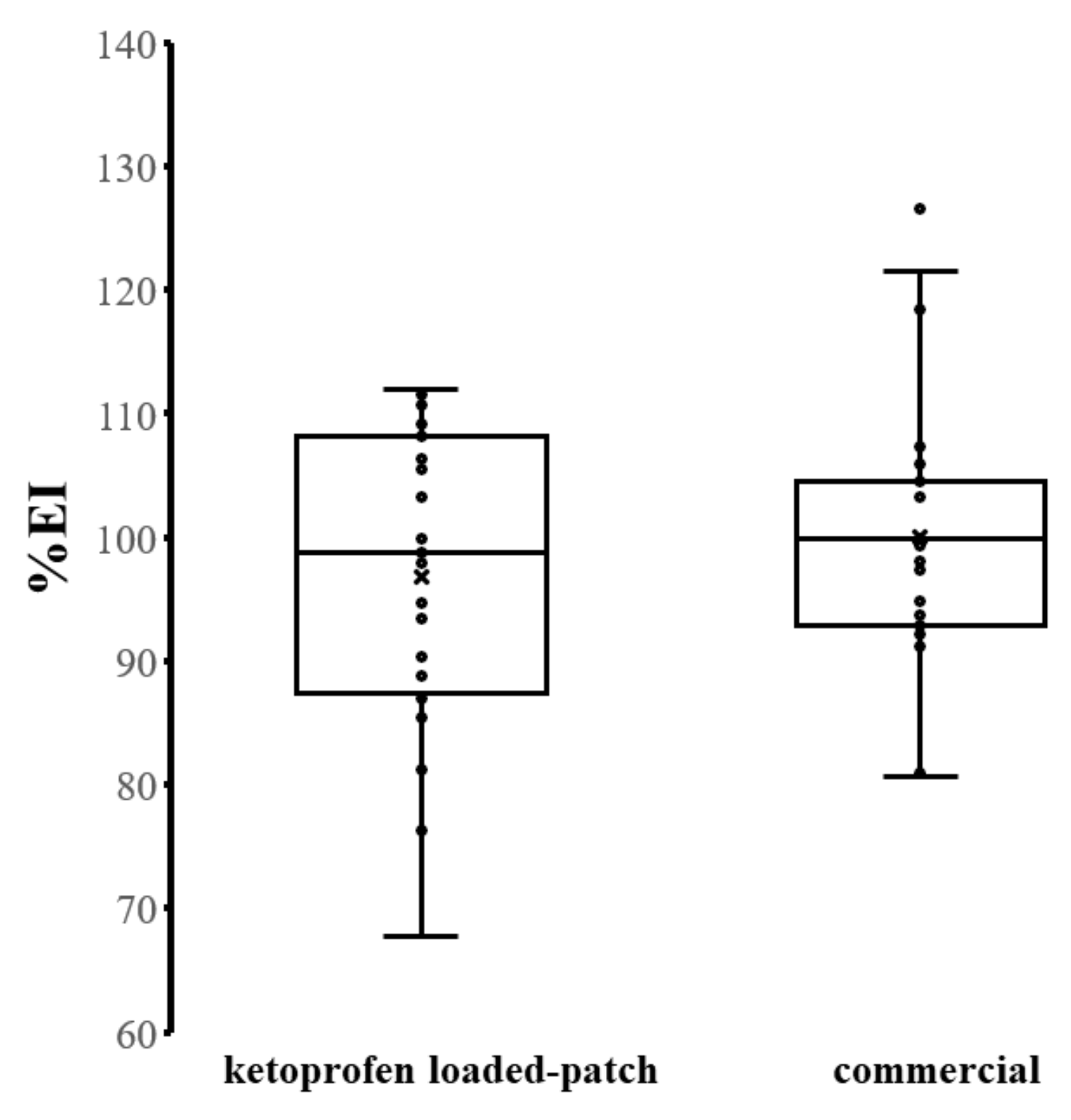
3.7. Safety Evaluation and In Vivo Skin Irritation Test
3.8. In Vivo Skin Adhesion Performance
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Prausnitz, M.R.; Langer, R. Transdermal drug delivery. Nat. Biotechnol. 2008, 26, 1261–1268. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Marepally, S.K.; Vemula, P.K.; Xu, C. Inorganic Nanoparticles for Transdermal Drug Delivery and Topical Application. In Nanoscience in Dermatology; Academic Press: Cambridge, MA, USA, 2016; pp. 57–72. [Google Scholar] [CrossRef]
- Akhtar, N.; Singh, V.; Yusuf, M.; Khan, R.A. Non-invasive drug delivery technology: Development and current status of transdermal drug delivery devices, techniques and biomedical applications. Biomed. Tech. 2020, 65, 243–272. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghosh, A.; Banerjee, S.; Kaity, S.; Wong, T.W. Current pharmaceutical design on adhesive based transdermal drug delivery systems. Curr. Pharm. Des. 2015, 21. [Google Scholar] [CrossRef]
- Lobo, S.; Sachdev, S.; Goswami, T. Role of pressure-sensitive adhesives in transdermal drug delivery systems. Ther. Deliv. 2016, 7, 33–48. [Google Scholar] [CrossRef]
- Tan, H.S.; Pfister, W.R. Pressure-sensitive adhesives for transdermal drug delivery systems. PSTT 1999, 2, 60–69. [Google Scholar] [CrossRef]
- Banerjee, S.; Chattopadhyay, P.; Ghosh, A.; Datta, P.; Veer, V. Aspect of adhesives in transdermal drug delivery systems. Int. J. Adhes. Adhes. 2014, 50, 70–84. [Google Scholar] [CrossRef]
- Zhang, J.; Deng, L.; Zhao, H.; Liu, M.; Jin, H.; Li, J.; Dong, A. Pressure-sensitive adhesive properties of poly(N-vinyl pyrrolidone)/D,L-lactic acid oligomer/glycerol/water blends for TDDS. J. Biomater. Sci. Polym. Ed. 2010, 21, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Chalykh, A.A.; Chalykh, A.E.; Novikov, M.B.; Feldstein, M.M. Pressure-sensitive adhesion in the blends of poly(N-vinyl pyrrolidone) and poly(ethylene glycol) of disparate chain lengths. J. Adhes. 2010, 78, 667–694. [Google Scholar] [CrossRef]
- Bai, J.; Lu, Y.; Li, P.-Y.; Liu, C.-M.; Wu, H.-C.; Wen, R.; Du, S.-Y. Development and in vitro evaluation of a transdermal hydrogel patch for ferulic acid. Pak. J. Pharm. Sci. 2014, 27, 369–375. [Google Scholar] [PubMed]
- Cilurzo, F.; Minghetti, P.; Gennari, C.G.M.; Casiraghi, A.; Montanari, L. A novel polymethylmethacrylate hydrophilic adhesive matrix intended for transdermal patch formulations. Drug Deliv. 2010, 17, 171–177. [Google Scholar] [CrossRef] [Green Version]
- Pornpitchanarong, C.; Rojanarata, T.; Opanasopit, P.; Patrojanasophon, P.; Ngawhirunpat, T. Synthesis of N-vinylpyrrolidone/Acrylic acid nanoparticles for drug delivery: Method optimization. MATEC Web Conf. 2018, 192. [Google Scholar] [CrossRef] [Green Version]
- Hao, J.S.; Chan, L.W.; Shen, Z.X.; Heng, P.W. Complexation between PVP and Gantrez polymer and its effect on release and bioadhesive properties of the composite PVP/Gantrez films. Pharm. Dev. Technol. 2004, 9, 379–386. [Google Scholar] [CrossRef]
- Shinkai, N.; Korenaga, K.; Okumura, Y.; Mizu, H.; Yamauchi, H. Microdialysis assessment of percutaneous penetration of ketoprofen after transdermal administration to hairless rats and domestic pigs. Eur. J. Pharm. Biopharm. 2011, 78, 415–421. [Google Scholar] [CrossRef]
- Rhee, Y.-S.; Choi, J.-G.; Park, E.-S.; Chi, S.-C. Transdermal delivery of ketoprofen using microemulsions. Int. J. Pharm. 2001, 228, 161–170. [Google Scholar] [CrossRef]
- Herwadkar, A.; Sachdeva, V.; Taylor, L.F.; Silver, H.; Banga, A.K. Low frequency sonophoresis mediated transdermal and intradermal delivery of ketoprofen. Int. J. Pharm. 2012, 423, 289–296. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Li, L.; Xie, J.; Sun, Y.; Ling, G.; He, Z. Transdermal Adhesive Patches Loaded with Ketoprofen Evaluated by Dynamic Detection of Percutaneous Absorption. AAPS PharmSciTech 2017, 18, 2141–2148. [Google Scholar] [CrossRef]
- Bempong, D.K.; Bhattacharyya, L. Development and validation of a stability-indicating high-performance liquid chromatographic assay for ketoprofen topical penetrating gel. J. Chromatogr. A 2005, 1073, 341–346. [Google Scholar] [CrossRef]
- Bajaj, S.; Whiteman, A.; Brandner, B. Transdermal drug delivery in pain management. Contin. Educ. Anaesth. Crit. Care Pain 2011, 11, 39–43. [Google Scholar] [CrossRef] [Green Version]
- Slabzhennikov, S.N.; Ryabchenko, O.B.; Kuarton, L.A. Interpretation of the IR spectra of aluminum, gallium, and indium tris(acetylacetonates). Russ. J. Coord. Chem. 2006, 32, 545–551. [Google Scholar] [CrossRef]
- Zheng, S.Y.; Ding, H.; Qian, J.; Yin, J.; Wu, Z.L.; Song, Y.; Zheng, Q. Metal-coordination complexes mediated physical hydrogels with high toughness, stick–slip tearing behavior, and good processability. Macromolecules 2016, 49, 9637–9646. [Google Scholar] [CrossRef]
- Ngawhirunpat, T.; Thipwichai, S.; Opanasopit, P.; Rojanarata, T.; Panomsuk, S. Development and Evaluation of Ketoprofen Acrylic Transdermal Patches. Trop. J. Pharm. Res. 2012, 11. [Google Scholar] [CrossRef] [Green Version]
- Costa, C.; Santos, V.H.S.; Araujo, P.H.H.; Sayer, C.; Santos, A.F.; Dariva, C.; Fortuny, M. Rapid decomposition of a cationic azo-initiator under microwave irradiation. J. Appl. Polym. Sci. 2010. [Google Scholar] [CrossRef]
- Pornpitchanarong, C.; Rojanarata, T.; Opanasopit, P.; Ngawhirunpat, T.; Patrojanasophon, P. Synthesis of novel N-vinylpyrrolidone/acrylic acid nanoparticles as drug delivery carriers of cisplatin to cancer cells. Colloids Surf. B Biointerfaces 2020, 185, 110566. [Google Scholar] [CrossRef] [PubMed]
- Ito, T.; Otani, N.; Fujii, K.; Mori, K.; Eriguchi, M.; Koyama, Y. Bioadhesive and biodissolvable hydrogels consisting of water-swellable poly(acrylic acid)/poly(vinylpyrrolidone) complexes. J. Biomed. Mater. Res. B Appl. Biomater. 2020, 108, 503–512. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Liu, Z.; Du, H.; Zeng, Y.; Deng, L.; Xing, J.; Dong, A. A novel hydrophilic adhesive matrix with self-enhancement for drug percutaneous permeation through rat skin. Pharm. Res. 2009, 26, 1398–1406. [Google Scholar] [CrossRef] [PubMed]
- Lubasova, D.; Niu, H.; Zhao, X.; Lin, T. Hydrogel properties of electrospun polyvinylpyrrolidone and polyvinylpyrrolidone/poly(acrylic acid) blend nanofibers. RSC Adv. 2015, 5, 54481–54487. [Google Scholar] [CrossRef]
- Shah, K.R. Polyacrylate-N-vinyl lactam polymer or copolymer blends as pressure-sensitive adhesives. J. Adhes. Sci. Technol. 1987, 1, 159–164. [Google Scholar] [CrossRef]
- Liu, Y.; Hsu, S.H. Synthesis and biomedical applications of self-healing hydrogels. Front. Chem. 2018, 6, 449. [Google Scholar] [CrossRef] [Green Version]
- Larraneta, E.; Henry, M.; Irwin, N.J.; Trotter, J.; Perminova, A.A.; Donnelly, R.F. Synthesis and characterization of hyaluronic acid hydrogels crosslinked using a solvent-free process for potential biomedical applications. Carbohydr. Polym. 2018, 181, 1194–1205. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Zhou, C.; Xu, L.; Yao, F.; Cen, L.; Fu, G.D. Stimuli-responsive hydrogels prepared by simultaneous “click chemistry” and metal–ligand coordination. RSC Adv. 2015, 5, 18242–18251. [Google Scholar] [CrossRef]
- Crowley, J.D.; Goshe, A.J.; Bosnich, B. Molecular recognition. Electrostatic effects in supramolecular self-assembly. Chem. Commun. 2003. [Google Scholar] [CrossRef]
- Karwoski, A.C.; Plaut, R.H. Experiments on peeling adhesive tapes from human forearms. Skin Res. Technol. 2004, 10, 271–277. [Google Scholar] [CrossRef] [PubMed]
- PubChem Compound Summary for CID 3825, Ketoprofen. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Ketoprofen (accessed on 16 May 2021).
- Jan, D.B.; Meinardi, M.M.H.M. The 500 Dalton rule for the skin penetration of chemical compounds and drugs. Exp. Dermatol. 2000, 9, 165–169. [Google Scholar]
- Lee, T.-W.; Kim, J.-C.; Hwang, S.-J. Hydrogel patches containing Triclosan for acne treatment. Eur. J. Pharm. Biopharm. 2003, 56, 407–412. [Google Scholar] [CrossRef]
- Tansathien, K.; Suriyaaumporn, P.; Charoenputtakhun, P.; Ngawhirunpat, T.; Opanasopit, P.; Rangsimawong, W. Development of sponge microspicule cream as a transdermal delivery system for protein and growth factors from deer antler velvet extract. Biol. Pharm. Bull. 2019, 42, 1207–1215. [Google Scholar] [CrossRef] [Green Version]
- Zagorska-Dziok, M.; Sobczak, M. Hydrogel-Based Active Substance Release Systems for Cosmetology and Dermatology Application: A Review. Pharmaceutics 2020, 12, 396. [Google Scholar] [CrossRef] [PubMed]






| Polymer | V50 (%wt) | % Yields |
|---|---|---|
| PVPAA-01 | 0.05 | 21.42 ± 0.25 |
| PVPAA-02 | 0.1 | 4.57 ± 0.14 |
| PVPAA-03 | 0.2 | 0.55 ± 0.89 |
| Formulation | Ratio of PVPAA: PMVEMA | Tacking Strength (N) | Peeling Strength (N) |
|---|---|---|---|
| F1 | 0:1 | 0.12 * ± 0.00 | 0.07 ± 0.01 |
| F2 | 1:1 | 0.33 ± 0.02 | 0.07 ± 0.01 |
| F3 | 1:2 | 0.22 * ± 0.01 | 0.07 ± 0.01 |
| Ketoprofen-loaded PVPAA/PMVEMA patches | 1:1 | 0.24 ** ± 0.00 | 0.09 ± 0.01 |
| Commercial (Hisamitsu®) | - | 0.08 ± 0.01 | 0.06 ± 0.01 |
| Formulation | Drug Content (%) | Steady-State Flux (µg/cm2/h) | Permeability Coefficient (cm/h) |
|---|---|---|---|
| Ketoprofen-loaded patches | 98.98 ± 0.58 | 4.77 ± 1.00 | 8.15 ± 1.00 |
| Commercial (Hisamitsu®) | 98.78 ± 0.97 | 4.33 ± 0.80 | 8.99 ± 0.80 |
| Time (Months) | Study | Parameters | |||
|---|---|---|---|---|---|
| Physical Appearance | Tacking Strength (N) | Peeling Strength (N) | Drug Content (%) | ||
| 0 | colorless hydrogels | 0.445 ± 0.03 | 0.136 ± 0.01 | 95.39 ± 0.73 | |
| 3 | accelerated | colorless hydrogels | 0.409 ± 0.06 | 0.137 ± 0.01 | 94.85 ± 0.83 |
| long-term | colorless hydrogels | 0.454 ± 0.01 | 0.134 ± 0.00 | 94.55 ± 0.51 | |
| 6 | accelerated | colorless hydrogels | 0.494 ± 0.08 | 0.125 ± 0.00 | 94.25 ± 0.58 |
| long-term | colorless hydrogels | 0.503 ± 0.04 | 0.128 ± 0.01 | 94.36 ± 0.74 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arunprasert, K.; Pornpitchanarong, C.; Rojanarata, T.; Ngawhirunpat, T.; Opanasopit, P.; Aumklad, P.; Patrojanasophon, P. Development and Evaluation of Novel Water-Based Drug-in-Adhesive Patches for the Transdermal Delivery of Ketoprofen. Pharmaceutics 2021, 13, 789. https://doi.org/10.3390/pharmaceutics13060789
Arunprasert K, Pornpitchanarong C, Rojanarata T, Ngawhirunpat T, Opanasopit P, Aumklad P, Patrojanasophon P. Development and Evaluation of Novel Water-Based Drug-in-Adhesive Patches for the Transdermal Delivery of Ketoprofen. Pharmaceutics. 2021; 13(6):789. https://doi.org/10.3390/pharmaceutics13060789
Chicago/Turabian StyleArunprasert, Kwanputtha, Chaiyakarn Pornpitchanarong, Theerasak Rojanarata, Tanasait Ngawhirunpat, Praneet Opanasopit, Porawan Aumklad, and Prasopchai Patrojanasophon. 2021. "Development and Evaluation of Novel Water-Based Drug-in-Adhesive Patches for the Transdermal Delivery of Ketoprofen" Pharmaceutics 13, no. 6: 789. https://doi.org/10.3390/pharmaceutics13060789







