Preparation of Fluorescent Carbon Dots Composites and Their Potential Applications in Biomedicine and Drug Delivery—A Review
Abstract
:1. Introduction
2. Synthesis of Carbon Dots
2.1. “Top-Down” Method
2.1.1. Arc Discharge Method
2.1.2. Laser Ablation
2.1.3. Electrochemical Oxidation
2.1.4. Acid Oxidation
2.2. “Bottom-Up” Method
2.2.1. Water/Solvothermal Method
2.2.2. Microwave Method
2.2.3. Template Method
3. Synthesis of CDs Composites
4. Application of CDs and Composites
4.1. Biotherapy
4.1.1. Photodynamic Therapy and Photothermal Therapy
4.1.2. Cancer Theranostics
4.1.3. Gene Therapy
4.2. Drug Delivery
4.3. Biological Imaging
4.4. Biosensor
4.5. Capacitor
4.6. Electrocatalysis
4.7. Photocatalysis
4.8. Genotoxicity
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zheng, M.; Liu, K.M.; Su, Y. Carbon Dots for Biomedical Applications. Chin. J. Lumin. 2021, 42, 1233–1244. [Google Scholar] [CrossRef]
- Zhang, Z.; Qu, D.; An, L.; Wang, X.Y.; Sun, Z.C. Preparation, Luminescence Mechanism and Application of Fluorescent Carbon Dots. Chin. J. Lumin. 2021, 42, 1125–1140. [Google Scholar] [CrossRef]
- Cao, W.B.; Sun, Z.G.; Wu, Y.H.; Zhang, Y.H.; Zhan, Y. Progresses in preparation and application of organosilane functionalized carbon dots. Acta Mater. Compos. Sin. 2022, 39, 896–906. [Google Scholar]
- Wang, R.; Lu, K.-Q.; Zhang, F.; Tang, Z.-R.; Xu, Y.-J. 3D carbon quantum dots/graphene aerogel as a metal-free catalyst for enhanced photosensitization efficiency. Appl. Catal. B Environ. 2018, 233, 11–18. [Google Scholar] [CrossRef]
- Permatasari, F.A.; Fukazawa, H.; Ogi, T.; Iskandar, F.; Okuyama, K. Design of pyrrolic-N-rich carbon dots with absorption in the first near-infrared window for photothermal therapy. ACS Appl. Nano Mater. 2018, 1, 2368–2375. [Google Scholar] [CrossRef]
- Jaleel, J.A.; Pramod, K. Artful and multifaceted applications of carbon dot in biomedicine. J. Control. Release 2018, 269, 302–321. [Google Scholar] [CrossRef] [PubMed]
- Tao, H.; Yang, K.; Ma, Z.; Wan, J.; Zhang, Y.; Kang, Z.; Liu, Z. In vivo NIR fluorescence imaging, biodistribution, and toxicology of photoluminescent carbon dots produced from carbon nanotubes and graphite. Small 2012, 8, 281–290. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Chen, G.; Jia, Y.; Ji, C.; Wang, Y.; Zhou, Y.; Leblanc, R.M.; Peng, Z. Carbon dot composites for bioapplications: A review. J. Mater. Chem. B 2022, 10, 843–869. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Li, M.; Dong, H.; Liu, Y.; Hu, H.; Cai, Y.; Liang, Y.; Xiao, Y.; Zheng, M. Interconnected 3 D network of graphene-oxide nanosheets decorated with carbon dots for high-performance supercapacitors. ChemSusChem 2017, 10, 2626–2634. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.; Guo, J.; Zhao, T.; Guo, Z. Functionalized carbon dots on graphene as outstanding non-metal bifunctional oxygen electrocatalyst. Small 2019, 15, 1900296. [Google Scholar] [CrossRef]
- Du, F.; Zhang, L.; Zhang, L.; Zhang, M.; Gong, A.; Tan, Y.; Miao, J.; Gong, Y.; Sun, M.; Ju, H. Engineered gadolinium-doped carbon dots for magnetic resonance imaging-guided radiotherapy of tumors. Biomaterials 2017, 121, 109–120. [Google Scholar] [CrossRef]
- Liao, H.; Wang, Z.; Chen, S.; Wu, H.; Ma, X.; Tan, M. One-pot synthesis of gadolinium (III) doped carbon dots for fluorescence/magnetic resonance bimodal imaging. RSC Adv. 2015, 5, 66575–66581. [Google Scholar] [CrossRef]
- Ren, X.; Yuan, X.; Wang, Y.; Liu, C.; Qin, Y.; Guo, L.; Liu, L. Facile preparation of Gd3+ doped carbon quantum dots: Photoluminescence materials with magnetic resonance response as magnetic resonance/fluorescence bimodal probes. Opt. Mater. 2016, 57, 56–62. [Google Scholar] [CrossRef]
- Zhang, J.; Yu, S.-H. Carbon dots: Large-scale synthesis, sensing and bioimaging. Mater. Today 2016, 19, 382–393. [Google Scholar] [CrossRef]
- Hou, Y.; Lu, Q.; Deng, J.; Li, H.; Zhang, Y. One-pot electrochemical synthesis of functionalized fluorescent carbon dots and their selective sensing for mercury ion. Anal. Chim. Acta 2015, 866, 69–74. [Google Scholar] [CrossRef]
- Dong, Y.; Cai, J.; You, X.; Chi, Y. Sensing applications of luminescent carbon based dots. Analyst 2015, 140, 7468–7486. [Google Scholar] [CrossRef]
- Xu, X.; Ray, R.; Gu, Y.; Ploehn, H.J.; Gearheart, L.; Raker, K.; Scrivens, W.A. Electrophoretic analysis and purification of fluorescent single-walled carbon nanotube fragments. J. Am. Chem. Soc. 2004, 126, 12736–12737. [Google Scholar] [CrossRef]
- Hu, S.-L.; Niu, K.-Y.; Sun, J.; Yang, J.; Zhao, N.-Q.; Du, X.-W. One-step synthesis of fluorescent carbon nanoparticles by laser irradiation. J. Mater. Chem. 2009, 19, 484–488. [Google Scholar] [CrossRef]
- Qiao, Z.-A.; Wang, Y.; Gao, Y.; Li, H.; Dai, T.; Liu, Y.; Huo, Q. Commercially activated carbon as the source for producing multicolor photoluminescent carbon dots by chemical oxidation. Chem. Commun. 2010, 46, 8812–8814. [Google Scholar] [CrossRef]
- Bottini, M.; Balasubramanian, C.; Dawson, M.I.; Bergamaschi, A.; Bellucci, S.; Mustelin, T. Isolation and characterization of fluorescent nanoparticles from pristine and oxidized electric arc-produced single-walled carbon nanotubes. J. Phys. Chem. B 2006, 110, 831–836. [Google Scholar] [CrossRef]
- Sun, Y.-P.; Zhou, B.; Lin, Y.; Wang, W.; Fernando, K.S.; Pathak, P.; Meziani, M.J.; Harruff, B.A.; Wang, X.; Wang, H. Quantum-sized carbon dots for bright and colorful photoluminescence. J. Am. Chem. Soc. 2006, 128, 7756–7757. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Lei, F.; Chen, H.; Yin, L.; Shi, Y.; Xie, J. One-step hydrothermal synthesis and optical properties of self-quenching-resistant carbon dots towards fluorescent ink and as nanosensors for Fe3+ detection. RSC Adv. 2019, 9, 8290–8299. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, J.; Booker, C.; Li, R.; Zhou, X.; Sham, T.-K.; Sun, X.; Ding, Z. An electrochemical avenue to blue luminescent nanocrystals from multiwalled carbon nanotubes (MWCNTs). J. Am. Chem. Soc. 2007, 129, 744–745. [Google Scholar] [CrossRef] [PubMed]
- Bao, L.; Zhang, Z.L.; Tian, Z.Q.; Zhang, L.; Liu, C.; Lin, Y.; Qi, B.; Pang, D.W. Electrochemical tuning of luminescent carbon nanodots: From preparation to luminescence mechanism. Adv. Mater. 2011, 23, 5801–5806. [Google Scholar] [CrossRef] [PubMed]
- Iannazzo, D.; Pistone, A.; Ferro, S.; De Luca, L.; Monforte, A.M.; Romeo, R.; Buemi, M.R.; Pannecouque, C. Graphene quantum dots based systems as HIV inhibitors. Bioconjug. Chem. 2018, 29, 3084–3093. [Google Scholar] [CrossRef]
- Atchudan, R.; Edison, T.N.J.I.; Chakradhar, D.; Perumal, S.; Shim, J.-J.; Lee, Y.R. Facile green synthesis of nitrogen-doped carbon dots using Chionanthus retusus fruit extract and investigation of their suitability for metal ion sensing and biological applications. Sens. Actuators B Chem. 2017, 246, 497–509. [Google Scholar] [CrossRef]
- Wang, B.; Yu, J.; Sui, L.; Zhu, S.; Tang, Z.; Yang, B.; Lu, S. Rational Design of Multi-Color-Emissive Carbon Dots in a Single Reaction System by Hydrothermal. Adv. Sci. 2021, 8, 2001453. [Google Scholar] [CrossRef]
- Peng, H.; Travas-Sejdic, J. Simple aqueous solution route to luminescent carbogenic dots from carbohydrates. Chem. Mater. 2009, 21, 5563–5565. [Google Scholar] [CrossRef]
- Zhu, S.; Meng, Q.; Wang, L.; Zhang, J.; Song, Y.; Jin, H.; Zhang, K.; Sun, H.; Wang, H.; Yang, B. Highly photoluminescent carbon dots for multicolor patterning, sensors, and bioimaging. Angew. Chem. 2013, 125, 4045–4049. [Google Scholar] [CrossRef]
- Jiang, K.; Sun, S.; Zhang, L.; Lu, Y.; Wu, A.; Cai, C.; Lin, H. Red, green, and blue luminescence by carbon dots: Full-color emission tuning and multicolor cellular imaging. Angew. Chem. Int. Ed. 2015, 54, 5360–5363. [Google Scholar] [CrossRef]
- Strauss, V.; Margraf, J.T.; Dolle, C.; Butz, B.; Nacken, T.J.; Walter, J.; Bauer, W.; Peukert, W.; Spiecker, E.; Clark, T. Carbon nanodots: Toward a comprehensive understanding of their photoluminescence. J. Am. Chem. Soc. 2014, 136, 17308–17316. [Google Scholar] [CrossRef]
- Zhu, H.; Wang, X.; Li, Y.; Wang, Z.; Yang, F.; Yang, X. Microwave synthesis of fluorescent carbon nanoparticles with electrochemiluminescence properties. Chem. Commun. 2009, 4, 5118–5120. [Google Scholar] [CrossRef]
- Tang, L.; Ji, R.; Cao, X.; Lin, J.; Jiang, H.; Li, X.; Teng, K.S.; Luk, C.M.; Zeng, S.; Hao, J. Deep ultraviolet photoluminescence of water-soluble self-passivated graphene quantum dots. ACS Nano 2012, 6, 5102–5110. [Google Scholar] [CrossRef]
- Kwon, W.; Do, S.; Rhee, S.W. Formation of highly luminescent nearly monodisperse carbon quantum dots via emulsion-templated carbonization of carbohydrates. RSC Adv. 2012, 2, 11223–11226. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Wu, D.; Han, S.; Hu, P.; Liu, R. Bottom-up fabrication of photoluminescent carbon dots with uniform morphology via a soft–hard template approach. Chem. Commun. 2013, 49, 4920–4922. [Google Scholar] [CrossRef]
- Hsiao, P.-H.; Timjan, S.; Kuo, K.-Y.; Juan, J.-C.; Chen, C.-Y. Optical management of CQD/AgNP@ SiNW arrays with highly efficient capability of dye degradation. Catalysts 2021, 11, 399. [Google Scholar] [CrossRef]
- Pei, Y.; Song, H.; Liu, Y.; Cheng, Y.; Li, W.; Chen, Y.; Fan, Y.; Liu, B.; Lu, S. Boron–nitrogen-doped carbon dots on multi-walled carbon nanotubes for efficient electrocatalysis of oxygen reduction reactions. J. Colloid Interface Sci. 2021, 600, 865–871. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, M.; Li, H.; Ge, H.; Bian, Z. The enhanced photoreduction of Cr (VI) to Cr (III) using carbon dots coupled TiO2 mesocrystals. Appl. Catal. B Environ. 2018, 226, 213–219. [Google Scholar] [CrossRef]
- Sarkar, T.; Rawat, K.; Solanki, P.R.; Bohidar, H. Carbon dots-embedded fluorescent silica xerogel. Colloids Surf. A Physicochem. Eng. Asp. 2019, 583, 123844. [Google Scholar] [CrossRef]
- Li, S.-H.; Wang, R.; Tang, Z.-R.; Xu, Y.-J. Efficient visible-light-driven water remediation by 3D graphene aerogel-supported nitrogen-doped carbon quantum dots. Catal. Today 2019, 335, 160–165. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, L.; Zhang, K.; Lu, Y.; Chen, J.; Wang, S.; Hu, B.; Wang, X. Application of carbon dots and their composite materials for the detection and removal of radioactive ions: A review. Chemosphere 2022, 287 Pt 3, 132313. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, K.; Jia, K.; Liu, G.; Ren, S.; Li, K.; Long, X.; Li, M.; Qiu, J. Preparation of coal-based graphene quantum dots/α-Fe2O3 nanocomposites and their lithium-ion storage properties. Fuel 2019, 241, 646–652. [Google Scholar] [CrossRef]
- Huang, Q.; Lin, X.; Tong, L.; Tong, Q.-X. Graphene quantum dots/multiwalled carbon nanotubes composite-based electrochemical sensor for detecting dopamine release from living cells. ACS Sustain. Chem. Eng. 2020, 8, 1644–1650. [Google Scholar] [CrossRef]
- Song, B.; Wang, Q.; Wang, L.; Lin, J.; Wei, X.; Murugadoss, V.; Wu, S.; Guo, Z.; Ding, T.; Wei, S. Carbon nitride nanoplatelet photocatalysts heterostructured with B-doped carbon nanodots for enhanced photodegradation of organic pollutants. J. Colloid Interface Sci. 2020, 559, 124–133. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Guan, L.; Song, J.; Zheng, H. Rational design of a sandwiched structure Ni(OH)2 nanohybrid sustained by amino-functionalized graphene quantum dots for outstanding capacitance. Appl. Surf. Sci. 2019, 480, 727–737. [Google Scholar] [CrossRef]
- Jin, C.; Su, K.; Tan, L.; Liu, X.; Cui, Z.; Yang, X.; Li, Z.; Liang, Y.; Zhu, S.; Yeung, K.W.K.; et al. Near-infrared light photocatalysis and photothermy of carbon quantum dots and au nanoparticles loaded titania nanotube array. Mater. Des. 2019, 177, 107845. [Google Scholar] [CrossRef]
- Li, Y.; Feng, X.; Lu, Z.; Yin, H.; Liu, F.; Xiang, Q. Enhanced photocatalytic H2-production activity of C-dots modified g-C3N4/TiO2 nanosheets composites. J. Colloid Interface Sci. 2018, 513, 866–876. [Google Scholar] [CrossRef]
- Tang, C.; Liu, C.; Han, Y.; Guo, Q.; Ouyang, W.; Feng, H.; Wang, M.; Xu, F. Nontoxic carbon quantum dots/g-C3N4 for efficient photocatalytic inactivation of Staphylococcus aureus under visible light. Adv. Healthc. Mater. 2019, 8, 1801534. [Google Scholar] [CrossRef]
- Cao, X.; Xu, C.; Ma, J.; Dong, Y.; Dong, C.; Yue, M.; Ding, Y. Enhanced Photoelectrochemical Performance of WO3-Based Composite Photoanode Coupled with Carbon Quantum Dots and NiFe Layered Double Hydroxide. ChemSusChem 2019, 12, 4685–4692. [Google Scholar] [CrossRef]
- Ma, Y.; Zhang, X.; Bai, J.; Huang, K.; Ren, L. Facile, controllable tune of blue shift or red shift of the fluorescence emission of solid-state carbon dots. Chem. Eng. J. 2019, 374, 787–792. [Google Scholar] [CrossRef]
- Yun, X.; Li, J.; Chen, X.; Chen, H.; Xiao, L.; Xiang, K.; Chen, W.; Liao, H.; Zhu, Y. Porous Fe2O3 modified by nitrogen-doped carbon quantum dots/reduced graphene oxide composite aerogel as a high-capacity and high-rate anode material for alkaline aqueous batteries. ACS Appl. Mater. Interfaces 2019, 11, 36970–36984. [Google Scholar] [CrossRef]
- Kumawat, M.K.; Thakur, M.; Bahadur, R.; Kaku, T.; Prabhuraj, R.; Ninawe, A.; Srivastava, R. Preparation of graphene oxide-graphene quantum dots hybrid and its application in cancer theranostics. Mater. Sci. Eng. C 2019, 103, 109774. [Google Scholar] [CrossRef]
- Wang, S.; Li, L.; Zhu, Z.; Zhao, M.; Zhang, L.; Zhang, N.; Wu, Q.; Wang, X.; Li, G. Remarkable Improvement in Photocatalytic Performance for Tannery Wastewater Processing via SnS2 Modified with N-Doped Carbon Quantum Dots: Synthesis, Characterization, and 4-Nitrophenol-Aided Cr (VI) Photoreduction. Small 2019, 15, 1804515. [Google Scholar] [CrossRef]
- Benetti, D.; Jokar, E.; Yu, C.-H.; Fathi, A.; Zhao, H.; Vomiero, A.; Diau, E.-W.-G.; Rosei, F. Hole-extraction and photostability enhancement in highly efficient inverted perovskite solar cells through carbon dot-based hybrid material. Nano Energy 2019, 62, 781–790. [Google Scholar] [CrossRef]
- Borenstein, A.; Strauss, V.; Kowal, M.D.; Anderson, M.; Kaner, R.B. Laser-Assisted Lattice Recovery of Graphene by Carbon Nanodot Incorporation. Small 2019, 15, 1904918. [Google Scholar] [CrossRef]
- Ali, G.A.; Thalji, M.R.; Soh, W.C.; Algarni, H.; Chong, K.F. One-step electrochemical synthesis of MoS2/graphene composite for supercapacitor application. J. Solid State Electrochem. 2020, 24, 25–34. [Google Scholar] [CrossRef]
- Wang, X.-F.; Wang, G.-G.; Li, J.-B.; Liu, Z.; Chen, Y.-X.; Liu, L.-F.; Han, J.-C. Direct white emissive Cl-doped graphene quantum dots-based flexible film as a single luminophore for remote tunable UV-WLEDs. Chem. Eng. J. 2019, 361, 773–782. [Google Scholar] [CrossRef]
- Pan, L.; Sun, S.; Zhang, A.; Jiang, K.; Zhang, L.; Dong, C.; Huang, Q.; Wu, A.; Lin, H. Truly fluorescent excitation-dependent carbon dots and their applications in multicolor cellular imaging and multidimensional sensing. Adv. Mater. 2015, 27, 7782–7787. [Google Scholar] [CrossRef]
- Li, Q.; Bai, Z.; Xi, X.; Guo, Z.; Liu, C.; Liu, X.; Zhao, X.; Li, Z.; Cheng, Y.; Wei, Y. Rapid microwave-assisted green synthesis of guanine-derived carbon dots for highly selective detection of Ag+ in aqueous solution. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2021, 248, 119208. [Google Scholar] [CrossRef]
- Zheng, K.; Li, X.; Chen, M.; Gong, Y.; Tang, A.; Wang, Z.; Wei, Z.; Guan, L.; Teng, F. Controllable synthesis highly efficient red, yellow and blue carbon nanodots for photo-luminescent light-emitting devices. Chem. Eng. J. 2020, 380, 122503. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, X.; Liu, J.; Han, B.; Hu, X.; Yang, F.; Xu, Z.; Li, Y.; Jia, S.; Li, Z. Carbon quantum dot implanted graphite carbon nitride nanotubes: Excellent charge separation and enhanced photocatalytic hydrogen evolution. Angew. Chem. 2018, 130, 5867–5873. [Google Scholar] [CrossRef]
- Yang, P.; Zhang, Z.-W.; Zou, G.-D.; Huang, Y.; Li, N.; Fan, Y. Template thermolysis to create a carbon dots-embedded mesoporous titanium-oxo sulfate framework for visible-light photocatalytic applications. Inorg. Chem. 2020, 59, 2062–2069. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Xu, C.; Lu, Y.; Wu, F.; Ye, G.; Wei, G.; Sun, T.; Chen, J. Visualization of adsorption: Luminescent mesoporous silica-carbon dots composite for rapid and selective removal of U (VI) and in situ monitoring the adsorption behavior. ACS Appl. Mater. Interfaces 2017, 9, 7392–7398. [Google Scholar] [CrossRef] [PubMed]
- Tam, T.V.; Kang, S.G.; Kim, M.H.; Lee, S.G.; Hur, S.H.; Chung, J.S.; Choi, W.M. Novel graphene hydrogel/B-doped graphene quantum dots composites as trifunctional electrocatalysts for Zn− air batteries and overall water splitting. Adv. Energy Mater. 2019, 9, 1900945. [Google Scholar] [CrossRef]
- Chabu, J.M.; Zeng, K.; Jin, G.; Zhang, M.; Li, Y.; Liu, Y.N. Simple approach for the preparation of nitrogen and sulfur codoped carbon dots/reduced graphene oxide as host for high-rate lithiumsulfur batteries. Mater. Chem. Phys. 2019, 229, 226–231. [Google Scholar] [CrossRef]
- Sankaranarayanan, S.; Vishnukumar, P.; Hariram, M.; Vivekanandhan, S.; Camus, C.; Buschmann, A.H.; Navia, R. Hydrothermal synthesis, characterization and seed germination effects of green-emitting graphene oxide-carbon dot composite using brown macroalgal bio-oil as precursor. J. Chem. Technol. Biotechnol. 2019, 94, 3269–3275. [Google Scholar] [CrossRef]
- Zheng, X.; Ding, G.; Wang, H.; Cui, G.; Zhang, P. One-step hydrothermal synthesis of carbon dots-polymer composites with solid-state photoluminescence. Mater. Lett. 2019, 238, 22–25. [Google Scholar] [CrossRef]
- Dong, Y.; Wang, R.; Li, G.; Chen, C.; Chi, Y.; Chen, G. Polyamine-functionalized carbon quantum dots as fluorescent probes for selective and sensitive detection of copper ions. Anal. Chem. 2012, 84, 6220–6224. [Google Scholar] [CrossRef]
- Yang, S.T.; Cao, L.; Luo, P.G.; Lu, F.; Sun, Y.P. Carbon Dots for Optical Imaging in Vivo. J. Am. Chem. Soc. 2009, 131, 11308–11309. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; He, X.; Kang, Z.; Huang, H.; Liu, Y.; Liu, J.; Lian, S.; Tsang, C.H.A.; Yang, X.; Lee, S.T. Water-soluble fluorescent carbon quantum Carbon quantum dots and their applications dots and photocatalyst design. Angew. Chem. Int. Ed. 2010, 49, 4430–4434. [Google Scholar] [CrossRef]
- Wang, Q.; Huang, X.; Long, Y.; Wang, X.; Zhang, H.; Zhu, R.; Liang, L.; Teng, P.; Zheng, H. Hollow luminescent carbon dots for drug delivery. Carbon 2013, 59, 192–199. [Google Scholar] [CrossRef]
- Wang, F.; Chen, Y.; Liu, C.; Ma, D. White light-emitting devices based on carbon dots’ electroluminescence. Chem. Commun. 2011, 47, 3502–3504. [Google Scholar] [CrossRef]
- Lightcap, I.V.; Kamat, P.V. Graphitic design: Prospects of graphene-based nanocomposites for solar energy conversion, storage, and sensing. Acc. Chem. Res. 2013, 46, 2235–2243. [Google Scholar] [CrossRef]
- Lim, S.Y.; Shen, W.; Gao, Z. Carbon quantum dots and their applications. Chem. Soc. Rev. 2015, 44, 362–381. [Google Scholar] [CrossRef]
- Zuo, P.; Lu, X.; Sun, Z.; Guo, Y.; He, H. A review on syntheses, properties, characterization and bioanalytical applications of fluorescent carbon dots. Microchim. Acta 2016, 183, 519–542. [Google Scholar] [CrossRef]
- Song, Y.; Zhu, S.; Yang, B. Bioimaging based on fluorescent carbon dots. RSC Adv. 2014, 4, 27184–27200. [Google Scholar] [CrossRef]
- Luo, P.G.; Yang, F.; Yang, S.T.; Sonkar, S.K.; Yang, L.; Broglie, J.J.; Liu, Y.; Sun, Y.P. Carbon-based quantum dots for fluorescence imaging of cells and tissues. RSC Adv. 2014, 4, 10791–10807. [Google Scholar] [CrossRef]
- Shen, G.; Hu, M.; Qi, J. Progress of Luminescent Carbon Dots in Biomedicine Engineering. Nano Biomed. Eng. 2016, 8, 190–202. [Google Scholar] [CrossRef] [Green Version]
- Zheng, D.-W.; Li, B.; Li, C.-X.; Fan, J.-X.; Lei, Q.; Li, C.; Xu, Z.; Zhang, X.-Z. Carbon-dot-decorated carbon nitride nanoparticles for enhanced photodynamic therapy against hypoxic tumor via water splitting. ACS Nano 2016, 10, 8715–8722. [Google Scholar] [CrossRef]
- Jia, Q.; Ge, J.; Liu, W.; Zheng, X.; Chen, S.; Wen, Y.; Zhang, H.; Wang, P. A magnetofluorescent carbon dot assembly as an acidic H2O2-driven oxygenerator to regulate tumor hypoxia for simultaneous bimodal imaging and enhanced photodynamic therapy. Adv. Mater. 2018, 30, 1706090. [Google Scholar] [CrossRef]
- Yang, W.; Wei, B.; Yang, Z.; Sheng, L. Facile synthesis of novel carbon-dots/hemin nanoplatforms for synergistic photo-thermal and photo-dynamic therapies. J. Inorg. Biochem. 2019, 193, 166–172. [Google Scholar] [CrossRef] [PubMed]
- Suriamoorthy, P.; Zhang, X.; Hao, G.; Joly, A.G.; Singh, S.; Hossu, M.; Sun, X.; Chen, W. Folic acid-CdTe quantum dot conjugates and their applications for cancer cell targeting. Cancer Nanotechnol. 2010, 1, 19–28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhunia, S.K.; Maity, A.R.; Nandi, S.; Stepensky, D.; Jelinek, R. Imaging Cancer Cells Expressing the Folate Receptor with Carbon Dots Produced from Folic Acid. Chembiochem 2016, 17, 614–619. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Zhang, P.; Zhai, X.; Tian, F.; Li, W.; Yang, J.; Liu, Y.; Wang, H.; Wang, W.; Liu, W. Nano-carrier for gene delivery and bioimaging based on carbon dots with PEI-passivation enhanced fluorescence. Biomaterials 2012, 33, 3604–3613. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Zhang, Z.; Zhang, X.; Zhu, Q. Energy-preserving finite volume element method for the improved Boussinesq equation. J. Comput. Phys. 2014, 270, 58–69. [Google Scholar] [CrossRef]
- Ghosh, S.; Ghosal, K.; Mohammad, S.A.; Sarkar, K. Dendrimer functionalized carbon quantum dot for selective detection of breast cancer and gene therapy. Chem. Eng. J. 2019, 373, 468–484. [Google Scholar] [CrossRef]
- Calabrese, G.; De Luca, G.; Nocito, G.; Rizzo, M.G.; Lombardo, S.P.; Chisari, G.; Forte, S.; Sciuto, E.L.; Conoci, S. Carbon Dots: An Innovative Tool for Drug Delivery in Brain Tumors. Int. J. Mol. Sci. 2021, 22, 11783. [Google Scholar] [CrossRef]
- Naik, K.; Chaudhary, S.; Ye, L.; Parmar, A.S. A Strategic Review on Carbon Quantum Dots for Cancer-Diagnostics and Treatment. Front. Bioeng. Biotechnol. 2022, 10, 882100. [Google Scholar] [CrossRef]
- Li, J.; Yang, S.; Deng, Y.; Chai, P.; Yang, Y.; He, X.; Xie, X.; Kang, Z.; Ding, G.; Zhou, H. Emancipating target-functionalized carbon dots from autophagy vesicles for a novel visualized tumor therapy. Adv. Funct. Mater. 2018, 28, 1800881. [Google Scholar] [CrossRef]
- Yan, F.; Jiang, Y.; Sun, X.; Wei, J.; Chen, L.; Zhang, Y. Multicolor carbon dots with concentration-tunable fluorescence and solvent-affected aggregation states for white light-emitting diodes. Nano Res. 2020, 13, 52–60. [Google Scholar] [CrossRef]
- Sung, S.-Y.; Su, Y.-L.; Cheng, W.; Hu, P.-F.; Chiang, C.-S.; Chen, W.-T.; Hu, S.-H. Graphene quantum dots-mediated theranostic penetrative delivery of drug and photolytics in deep tumors by targeted biomimetic nanosponges. Nano Lett. 2018, 19, 69–81. [Google Scholar] [CrossRef]
- Zhou, D.; Zhai, Y.; Qu, S.; Li, D.; Jing, P.; Ji, W.; Shen, D.; Rogach, A.L. Electrostatic assembly guided synthesis of highly luminescent carbon-nanodots@ BaSO4 hybrid phosphors with improved stability. Small 2017, 13, 1602055. [Google Scholar] [CrossRef]
- Du, F.; Li, J.; Hua, Y.; Zhang, M.; Zhou, Z.; Yuan, J.; Wang, J.; Peng, W.; Zhang, L.; Xia, S. Multicolor nitrogen-doped carbon dots for live cell imaging. J. Biomed. Nanotechnol. 2015, 11, 780–788. [Google Scholar] [CrossRef]
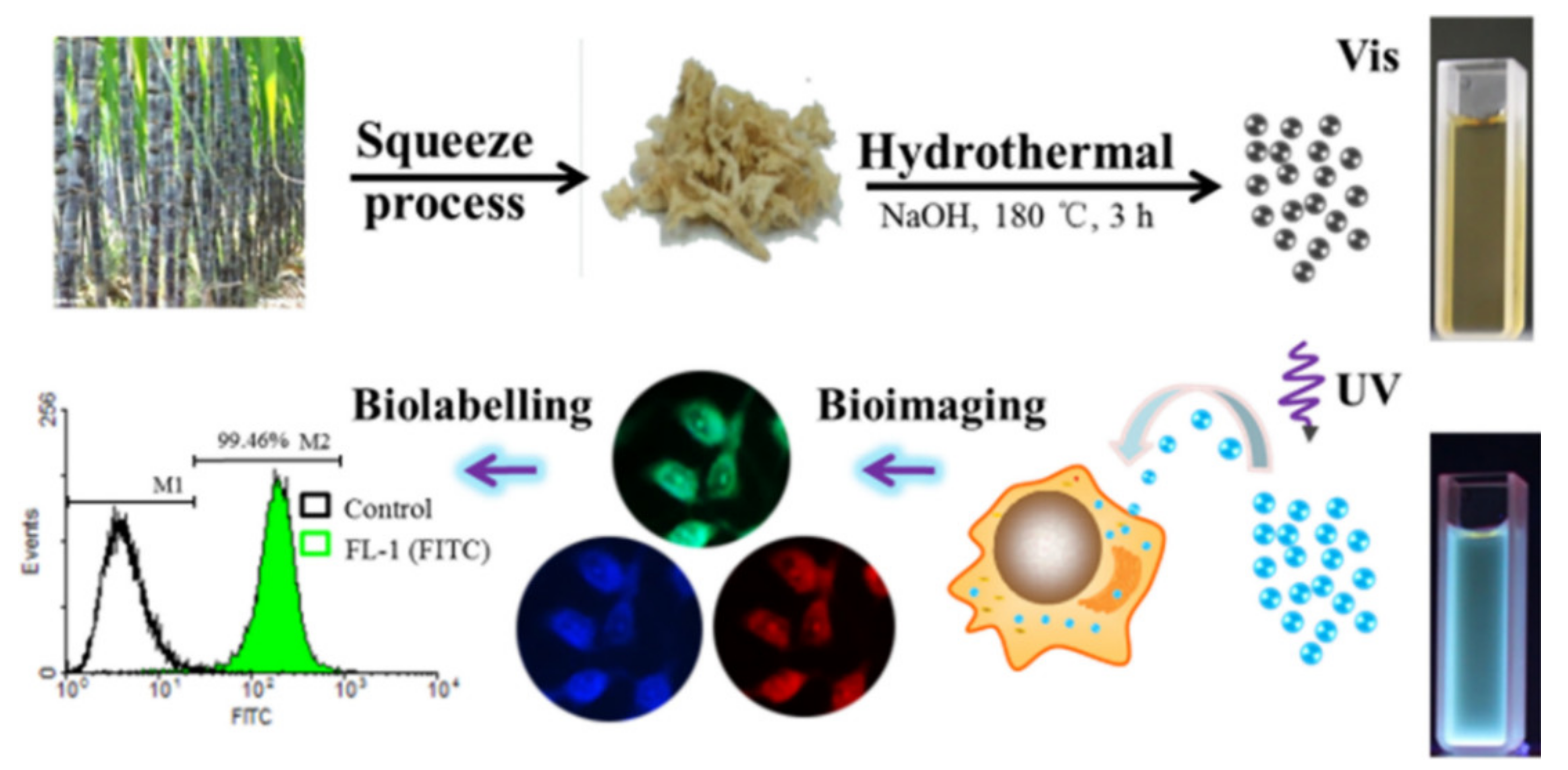
- Du, F.; Zhang, M.; Li, X.; Li, J.; Jiang, X.; Li, Z.; Hua, Y.; Shao, G.; Jin, J.; Shao, Q. Economical and green synthesis of bagasse-derived fluorescent carbon dots for biomedical applications. Nanotechnology 2014, 25, 315702. [Google Scholar] [CrossRef]
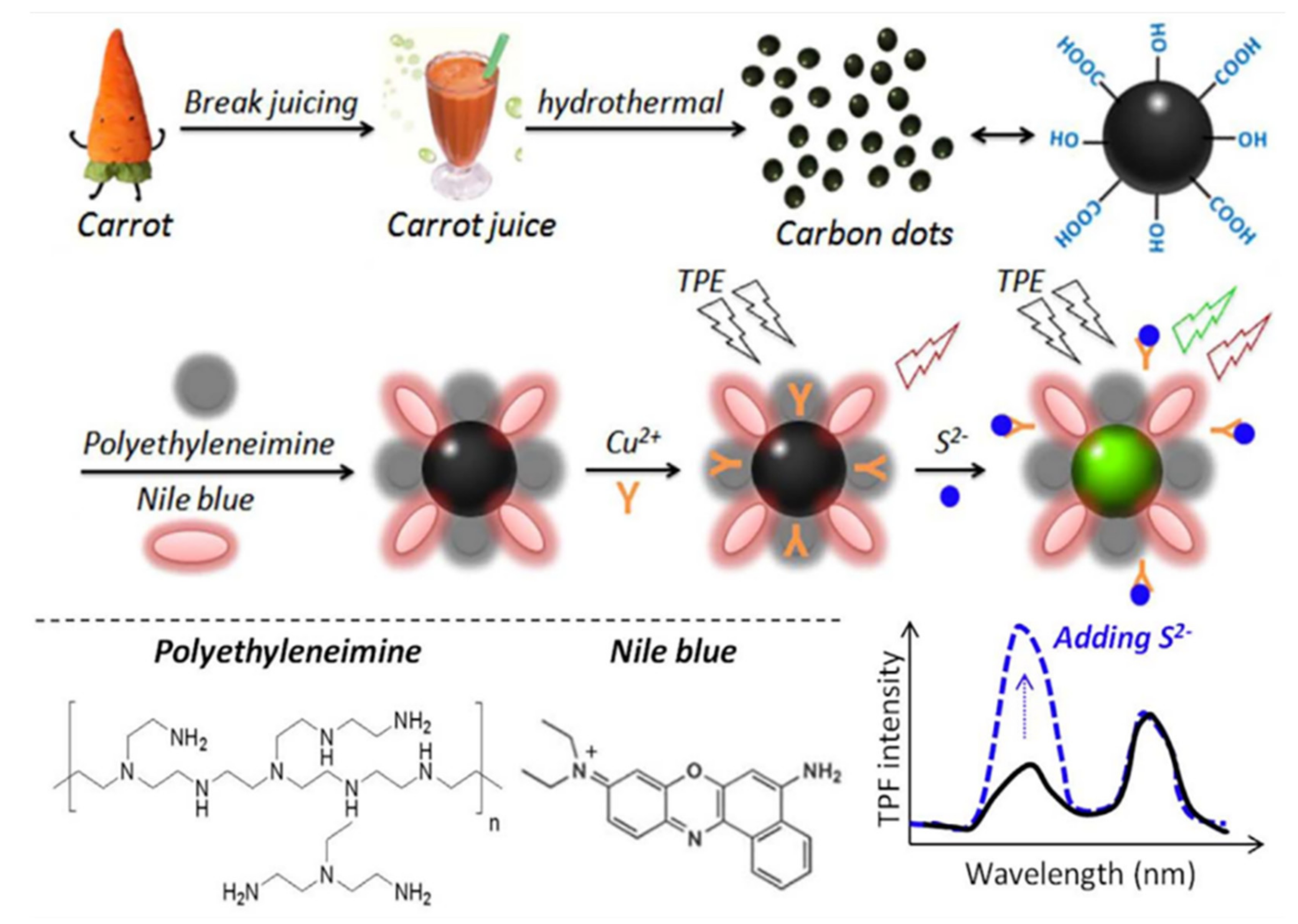
- Jin, H.; Gui, R.; Wang, Y.; Sun, J. Carrot-derived carbon dots modified with polyethyleneimine and nile blue for ratiometric two-photon fluorescence turn-on sensing of sulfide anion in biological fluids. Talanta 2017, 169, 141–148. [Google Scholar] [CrossRef]
- Wang, B.; Wang, Y.; Wu, H.; Song, X.; Guo, X.; Zhang, D.; Ma, X.; Tan, M. A mitochondria-targeted fluorescent probe based on TPP-conjugated carbon dots for both one-and two-photon fluorescence cell imaging. RSC Adv. 2014, 4, 49960–49963. [Google Scholar] [CrossRef]
- Tong, G.; Wang, J.; Wang, R.; Guo, X.; He, L.; Qiu, F.; Wang, G.; Zhu, B.; Zhu, X.; Liu, T. Amorphous carbon dots with high two-photon fluorescence for cellular imaging passivated by hyperbranched poly (amino amine). J. Mater. Chem. B 2015, 3, 700–706. [Google Scholar] [CrossRef]
- Wang, H.; Wang, K.; Mu, Q.; Stephen, Z.R.; Yu, Y.; Zhou, S.; Zhang, M. Mesoporous carbon nanoshells for high hydrophobic drug loading, multimodal optical imaging, controlled drug release, and synergistic therapy. Nanoscale 2017, 9, 1434–1442. [Google Scholar] [CrossRef] [Green Version]
- Tan, M.; Li, X.; Wu, H.; Wang, B.; Wu, J. N-doped carbon dots derived from bovine serum albumin and formic acid with one-and two-photon fluorescence for live cell nuclear imaging. Colloids Surf. B Biointerfaces 2015, 136, 141–149. [Google Scholar] [CrossRef]
- Li, B.; Wang, X.; Guo, Y.; Iqbal, A.; Dong, Y.; Li, W.; Liu, W.; Qin, W.; Chen, S.; Zhou, X. One-pot synthesis of polyamines improved magnetism and fluorescence Fe3O4–carbon dots hybrid NPs for dual modal imaging. Dalton Trans. 2016, 45, 5484–5491. [Google Scholar] [CrossRef] [Green Version]
- Rodrigues, C.V.; Correa, J.R.; Aiube, C.M.; Andrade, L.P.; Galvão, P.M.; Costa, P.A.; Campos, A.L.; Pereira, A.J.; Ghesti, G.F.; Felix, J.F. Down-and up-conversion photoluminescence of carbon-dots from brewing industry waste: Application in live cell-imaging experiments. J. Braz. Chem. Soc. 2015, 26, 2623–2628. [Google Scholar] [CrossRef]
- Liu, J.-H.; Cao, L.; LeCroy, G.E.; Wang, P.; Meziani, M.J.; Dong, Y.; Liu, Y.; Luo, P.G.; Sun, Y.-P. Carbon “quantum” dots for fluorescence labeling of cells. ACS Appl. Mater. Interfaces 2015, 7, 19439–19445. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Zhong, D.; Miao, H.; Yang, X. Carbon dots derived from rose flowers for tetracycline sensing. Talanta 2015, 140, 128–133. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; An, X.; Li, L. Easy synthesis of highly fluorescent carbon dots from albumin and their photoluminescent mechanism and biological imaging applications. Mater. Sci. Eng. C 2016, 58, 730–736. [Google Scholar] [CrossRef] [PubMed]
- Levine, R.L. Rapid benchtop method of alkaline hydrolysis of proteins. J. Chromatogr. A 1982, 236, 496–498. [Google Scholar] [CrossRef]
- Sies, H. Oxidative stress: A concept in redox biology and medicine. Redox Biol. 2015, 4, 180–183. [Google Scholar] [CrossRef] [Green Version]
- Xue, Y.; Elsanousi, A.; Fan, Y.; Lin, J.; Li, J.; Xu, X.; Lu, Y.; Zhang, L.; Zhang, T.; Tang, C. N,N-Dimethyl formamide facilitated formation of hexagonal boron nitride from boric acid. Solid State Sci. 2013, 24, 1–5. [Google Scholar] [CrossRef]
- He, M.; Shang, N.; Zheng, B.; Yue, G. An ultrasensitive colorimetric and fluorescence dual-readout assay for glutathione with a carbon dot–MnO2 nanosheet platform based on the inner filter effect. RSC Adv. 2021, 11, 21137–21144. [Google Scholar] [CrossRef]
- Wu, D.; Li, G.; Chen, X.; Qiu, N.; Shi, X.; Chen, G.; Sun, Z.; You, J.; Wu, Y. Fluorometric determination and imaging of glutathione based on a thiol-triggered inner filter effect on the fluorescence of carbon dots. Microchim. Acta 2017, 184, 1923–1931. [Google Scholar] [CrossRef]
- Cai, Q.-Y.; Li, J.; Ge, J.; Zhang, L.; Hu, Y.-L.; Li, Z.-H.; Qu, L.-B. A rapid fluorescence “switch-on” assay for glutathione detection by using carbon dots–MnO2 nanocomposites. Biosens. Bioelectron. 2015, 72, 31–36. [Google Scholar] [CrossRef]
- Yang, C.; Deng, W.; Liu, H.; Ge, S.; Yan, M. Turn-on fluorescence sensor for glutathione in aqueous solutions using carbon dots–MnO2 nanocomposites. Sens. Actuators B Chem. 2015, 216, 286–292. [Google Scholar] [CrossRef]
- Abedini, F.; Hosseinkhani, H.; Ismail, M.; Domb, A.J.; Omar, A.R.; Chong, P.P.; Hong, P.D.; Yu, D.S.; Farber, I.Y. Cationized dextran nanoparticle-encapsulated CXCR4-siRNA enhanced correlation between CXCR4 expression and serum alkaline phosphatase in a mouse model of colorectal cancer. Int. J. Nanomed. 2012, 7, 4159. [Google Scholar]
- Zhang, Y.; Nie, Y.; Zhu, R.; Han, D.; Zhao, H.; Li, Z. Nitrogen doped carbon dots for turn-off fluorescent detection of alkaline phosphatase activity based on inner filter effect. Talanta 2019, 204, 74–81. [Google Scholar] [CrossRef]
- Loo, A.H.; Sofer, Z.; Bouša, D.; Ulbrich, P.; Bonanni, A.; Pumera, M. Carboxylic carbon quantum dots as a fluorescent sensing platform for DNA detection. ACS Appl. Mater. Interfaces 2016, 8, 1951–1957. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, S.; Ge, S.; Wang, S.; Yan, M.; Zang, D.; Yu, J. Facile and sensitive paper-based chemiluminescence DNA biosensor using carbon dots dotted nanoporous gold signal amplification label. Anal. Methods 2013, 5, 1328–1336. [Google Scholar] [CrossRef]
- Qaddare, S.H.; Salimi, A. Amplified fluorescent sensing of DNA using luminescent carbon dots and AuNPs/GO as a sensing platform: A novel coupling of FRET and DNA hybridization for homogeneous HIV-1 gene detection at femtomolar level. Biosens. Bioelectron. 2017, 89, 773–780. [Google Scholar] [CrossRef]
- Kudr, J.; Richtera, L.; Xhaxhiu, K.; Hynek, D.; Heger, Z.; Zitka, O.; Adam, V. Carbon dots based FRET for the detection of DNA damage. Biosens. Bioelectron. 2017, 92, 133–139. [Google Scholar] [CrossRef]
- Liang, S.-S.; Qi, L.; Zhang, R.-L.; Jin, M.; Zhang, Z.-Q. Ratiometric fluorescence biosensor based on CdTe quantum and carbon dots for double strand DNA detection. Sens. Actuators B Chem. 2017, 244, 585–590. [Google Scholar] [CrossRef]
- Li, N.; Liu, S.G.; Zhu, Y.D.; Liu, T.; Lin, S.M.; Shi, Y.; Luo, H.Q.; Li, N.B. Tuning gold nanoparticles growth via DNA and carbon dots for nucleic acid and protein detection. Sens. Actuators B Chem. 2017, 251, 455–461. [Google Scholar] [CrossRef]
- Huang, Q.; Li, Q.; Chen, Y.; Tong, L.; Lin, X.; Zhu, J.; Tong, Q. High quantum yield nitrogen-doped carbon dots: Green synthesis and application as “off-on” fluorescent sensors for the determination of Fe3+ and adenosine triphosphate in biological samples. Sens. Actuators B Chem. 2018, 276, 82–88. [Google Scholar] [CrossRef]
- Wu, Z.; Chen, R.; Pan, S.; Liu, H.; Hu, X. A ratiometric fluorescence strategy based on dual-signal response of carbon dots and o-phenylenediamine for ATP detection. Microchem. J. 2021, 164, 105976. [Google Scholar] [CrossRef]
- Cheng, X.; Cen, Y.; Xu, G.; Wei, F.; Shi, M.; Xu, X.; Sohail, M.; Hu, Q. Aptamer based fluorometric determination of ATP by exploiting the FRET between carbon dots and graphene oxide. Microchim. Acta 2018, 185, 144. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Zhang, F.; Xu, L.; Liu, X.; Ma, P.; Sun, Y.; Wang, X.; Song, D. A fluorescence resonance energy transfer biosensor based on carbon dots and gold nanoparticles for the detection of trypsin. Sens. Actuators B Chem. 2018, 273, 1015–1021. [Google Scholar] [CrossRef]
- Zhu, H.; Wang, E.; Li, J.; Wang, J. L-tyrosine methyl ester-stabilized carbon dots as fluorescent probes for the assays of biothiols. Anal. Chim. Acta 2018, 1006, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Amjadi, M.; Hallaj, T.; Kouhi, Z. An enzyme-free fluorescent probe based on carbon dots–MnO2 nanosheets for determination of uric acid. J. Photochem. Photobiol. A Chem. 2018, 356, 603–609. [Google Scholar] [CrossRef]
- Huang, Q.; Lin, X.; Lin, C.; Zhang, Y.; Hu, S.; Wei, C. A high performance electrochemical biosensor based on Cu2O–carbon dots for selective and sensitive determination of dopamine in human serum. RSC Adv. 2015, 5, 54102–54108. [Google Scholar] [CrossRef]
- Huang, Q.; Hu, S.; Zhang, H.; Chen, J.; He, Y.; Li, F.; Weng, W.; Ni, J.; Bao, X.; Lin, Y. Carbon dots and chitosan composite film based biosensor for the sensitive and selective determination of dopamine. Analyst 2013, 138, 5417–5423. [Google Scholar] [CrossRef]
- Li, L.; Yu, B.; You, T. Nitrogen and sulfur co-doped carbon dots for highly selective and sensitive detection of Hg (II) ions. Biosens. Bioelectron. 2015, 74, 263–269. [Google Scholar] [CrossRef]
- Li, C.; Wang, Y.; Zhang, X.; Guo, X.; Kang, X.; Du, L.; Liu, Y. Red fluorescent carbon dots with phenylboronic acid tags for quick detection of Fe (III) in PC12 cells. J. Colloid Interface Sci. 2018, 526, 487–496. [Google Scholar] [CrossRef]
- Borenstein, A.; Hanna, O.; Attias, R.; Luski, S.; Brousse, T.; Aurbach, D. Carbon-based composite materials for supercapacitor electrodes: A review. J. Mater. Chem. A 2017, 5, 12653–12672. [Google Scholar] [CrossRef]
- Da Silva, L.M.; Cesar, R.; Moreira, C.M.; Santos, J.H.; De Souza, L.G.; Pires, B.M.; Vicentini, R.; Nunes, W.; Zanin, H. Reviewing the fundamentals of supercapacitors and the difficulties involving the analysis of the electrochemical findings obtained for porous electrode materials. Energy Storage Mater. 2020, 27, 555–590. [Google Scholar] [CrossRef]
- Dong, D. Ternary composite MnO2@ MoS2/polypyrrole from in-situ synthesis for binder-free and flexible supercapacitor. J. Bioresour. Bioprod. 2019, 4, 242–250. [Google Scholar]
- Lv, L.; Fan, Y.; Chen, Q.; Zhao, Y.; Hu, Y.; Zhang, Z.; Chen, N.; Qu, L. Three-dimensional multichannel aerogel of carbon quantum dots for high-performance supercapacitors. Nanotechnology 2014, 25, 235401. [Google Scholar] [CrossRef]
- Li, J.; Yun, X.; Hu, Z.; Xi, L.; Li, N.; Tang, H.; Lu, P.; Zhu, Y. Three-dimensional nitrogen and phosphorus co-doped carbon quantum dots/reduced graphene oxide composite aerogels with a hierarchical porous structure as superior electrode materials for supercapacitors. J. Mater. Chem. A 2019, 7, 26311–26325. [Google Scholar] [CrossRef]
- Zhang, H.; Chen, J.; Li, Y.; Liu, P.; Wang, Y.; An, T.; Zhao, H. Nitrogen-doped carbon nanodots@ nanospheres as an efficient electrocatalyst for oxygen reduction reaction. Electrochim. Acta 2015, 165, 7–13. [Google Scholar] [CrossRef] [Green Version]
- Liang, K.; Wang, L.; Xu, Y.; Fang, Y.; Fang, Y.; Xia, W.; Liu, Y.-N. Carbon dots self-decorated heteroatom-doped porous carbon with superior electrocatalytic activity for oxygen reduction. Electrochim. Acta 2020, 335, 135666. [Google Scholar] [CrossRef]
- Chen, J.; Xiao, G.; Duan, G.; Wu, Y.; Zhao, X.; Gong, X. Structural design of carbon dots/porous materials composites and their applications. Chem. Eng. J. 2021, 421, 127743. [Google Scholar] [CrossRef]
- Wu, T.; Liu, X.; Liu, Y.; Cheng, M.; Liu, Z.; Zeng, G.; Shao, B.; Liang, Q.; Zhang, W.; He, Q. Application of QD-MOF composites for photocatalysis: Energy production and environmental remediation. Coord. Chem. Rev. 2020, 403, 213097. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, F.; Feng, Y.; Xie, Z.; Zhang, Q.; Jin, X.; Liu, H.; Liu, Y.; Lv, W.; Liu, G. Facile synthesis of carbon quantum dots loaded with mesoporous gC3N4 for synergistic absorption and visible light photodegradation of fluoroquinolone antibiotics. Dalton Trans. 2018, 47, 1284–1293. [Google Scholar] [CrossRef]
- Shen, Q.; You, Z.; Yu, Y.; Qin, T.; Su, Y.; Wang, H.; Wu, C.; Zhang, F.; Yang, H. A carbon quantum dots/porous InVO4 microsphere composite with enhanced photocatalytic activity. Eur. J. Inorg. Chem. 2018, 2018, 1080–1086. [Google Scholar] [CrossRef]
- Şenel, B.; Demir, N.; Büyükköroğlu, G.; Yıldız, M. Graphene quantum dots: Synthesis, characterization, cell viability, genotoxicity for biomedical applications. Saudi Pharm. J. 2019, 27, 846–858. [Google Scholar] [CrossRef] [PubMed]
- Saikia, M.; Singh, A.; Dihingia, A.; Khare, P.; Kalita, J.; Saikia, B.K. Scalable production, cell toxicity assessment, and plant growth promotion activities of carbon quantum dots derived from low-quality coal feedstock. Chem. Eng. J. 2022, 433, 133633. [Google Scholar] [CrossRef]














Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chai, Y.; Feng, Y.; Zhang, K.; Li, J. Preparation of Fluorescent Carbon Dots Composites and Their Potential Applications in Biomedicine and Drug Delivery—A Review. Pharmaceutics 2022, 14, 2482. https://doi.org/10.3390/pharmaceutics14112482
Chai Y, Feng Y, Zhang K, Li J. Preparation of Fluorescent Carbon Dots Composites and Their Potential Applications in Biomedicine and Drug Delivery—A Review. Pharmaceutics. 2022; 14(11):2482. https://doi.org/10.3390/pharmaceutics14112482
Chicago/Turabian StyleChai, Yaru, Yashan Feng, Kun Zhang, and Jingan Li. 2022. "Preparation of Fluorescent Carbon Dots Composites and Their Potential Applications in Biomedicine and Drug Delivery—A Review" Pharmaceutics 14, no. 11: 2482. https://doi.org/10.3390/pharmaceutics14112482




