Single -and Multi-Walled Carbon Nanotubes as Nanocarriers for the Delivery of 7-Hydroxyflavone
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Buffer Solutions
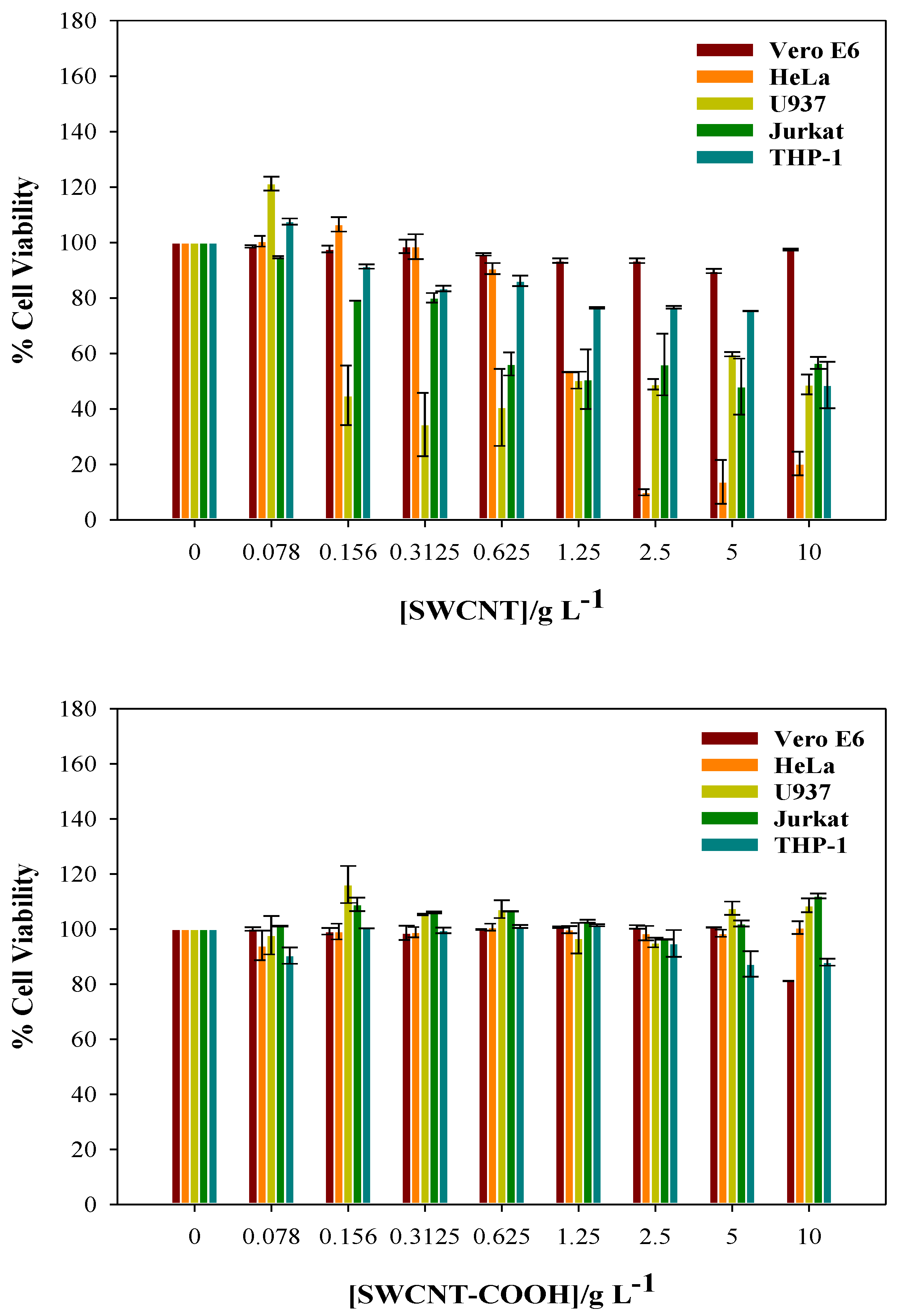
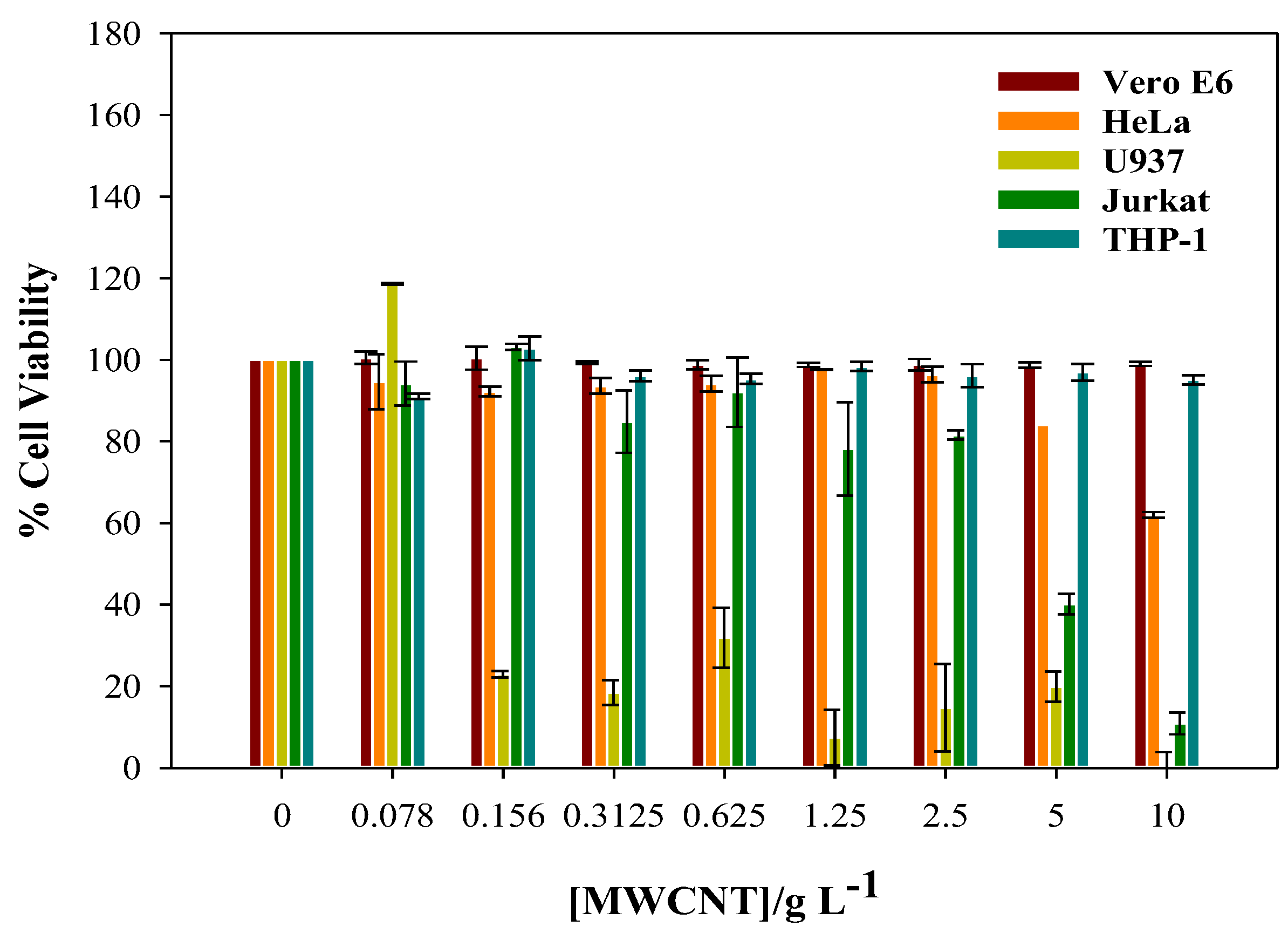
2.3. In vitro Cytotoxicity Assays
2.4. Fluorescence Measurements
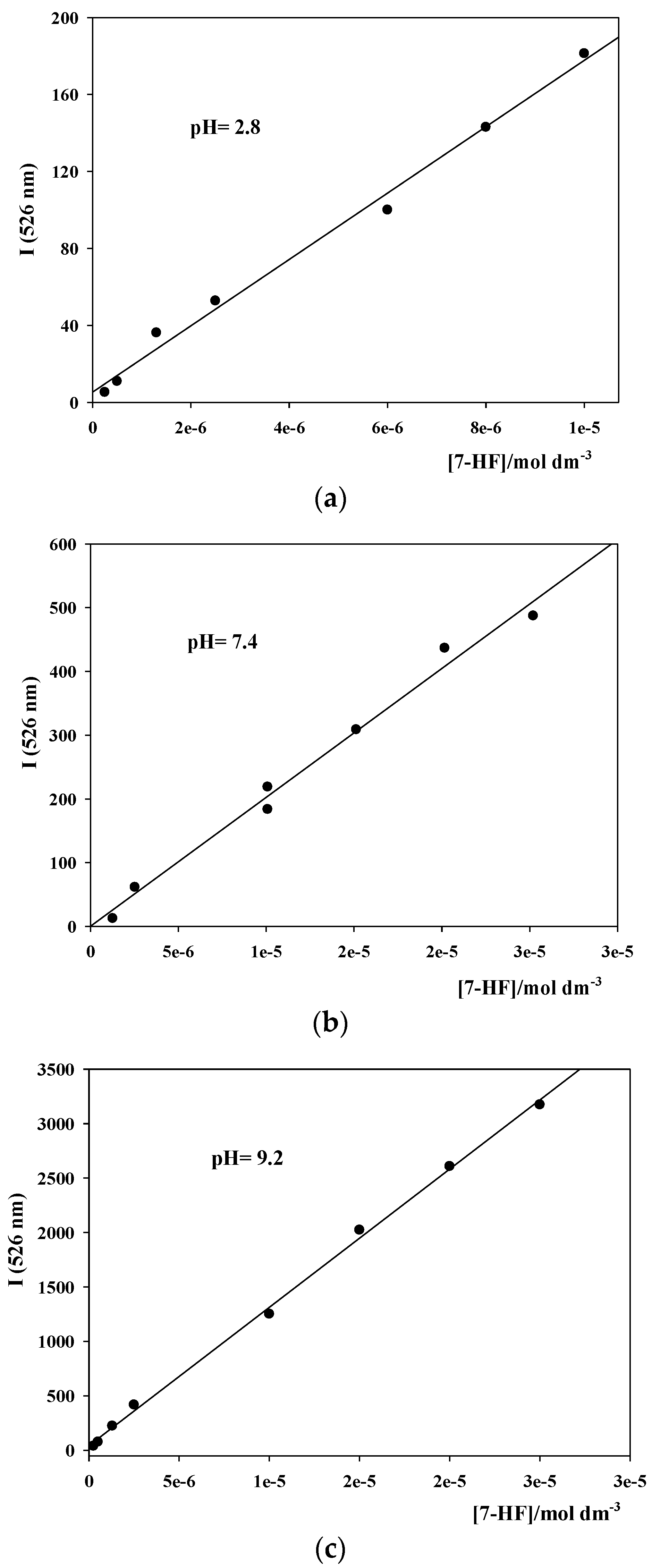
2.4.1. Fluorescence Emission Intensity Calibration Curves
2.5. Encapsulation of 7-HF in the CNTs
2.6. Encapsulation Efficiency
2.7. Zeta Potential Measurements
2.8. In Vitro 7-HF Release
2.9. Statistical Analysis
3. Results and Discussion
3.1. Fluorescence Emission Intensity Calibration Curves for 7-HF at Different pHs
3.2. Cytotoxicity of CNTs
3.3. Encapsulation of 7-HF in CNTs
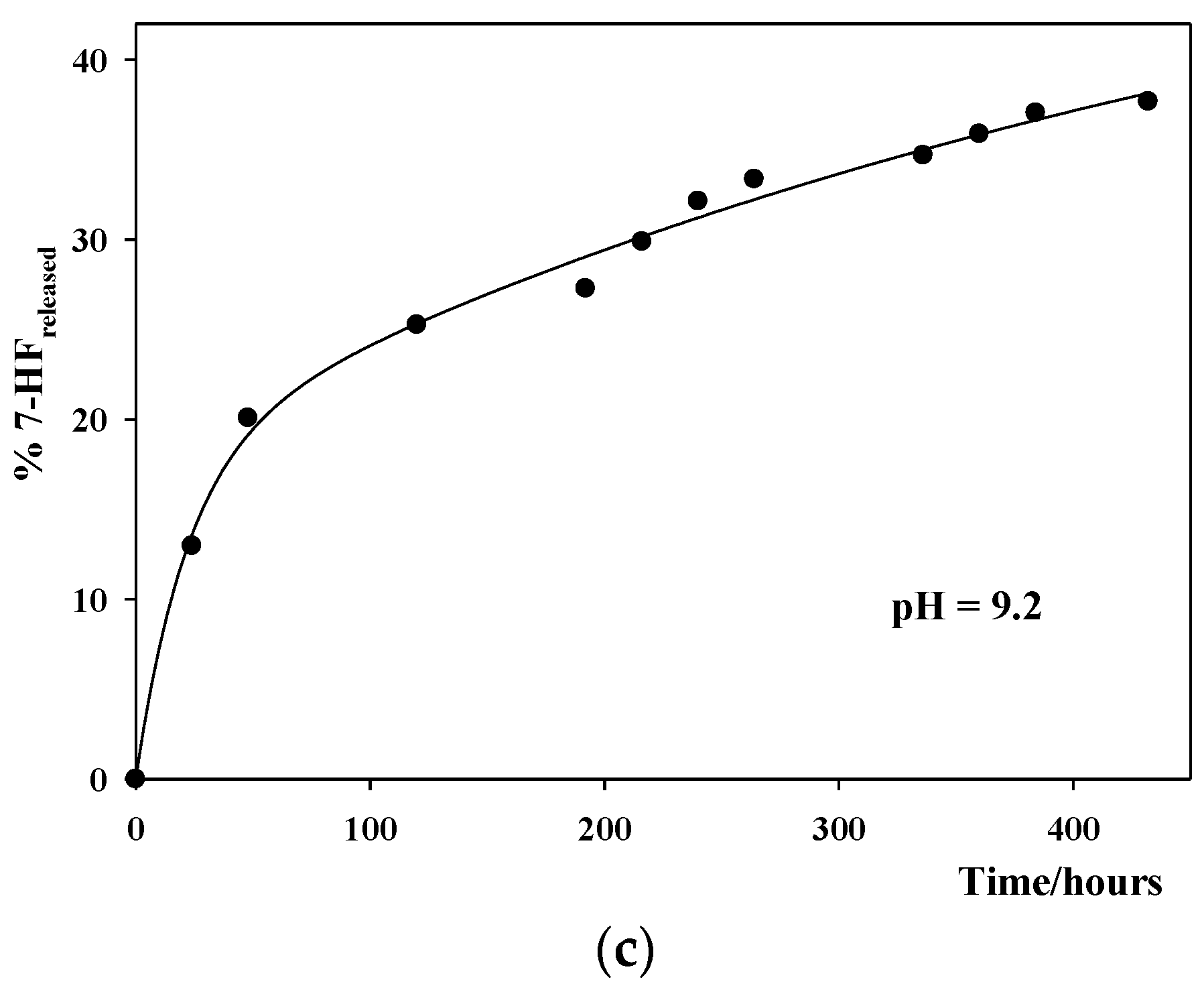
3.4. Study of the In Vitro 7-HF Release
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Es-Safi, N.E.; Ghidouche, S.; Ducrot, P.H. Flavonoids: Hemysinthesis, reactivity, characterization and free radical scavenging activity. Molecules 2007, 12, 2228–2258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shen, N.; Wang, T.; Gan, Q.; Liu, S.; Wang, L.; Jin, B. Plant flavonoids: Classification, distribution, biosynthesis, and antioxidant activity. Food Chem. 2022, 383, 132531. [Google Scholar] [CrossRef] [PubMed]
- Sabino, R.K.; Ranjit, A.; Sharma, K.; Prada’s, P.; Shang, X. Bio active compounds of citrus fruits: A review of composition and health benefits of carotenoids, flavonoids, limonoida, and terpenes. Antioxidants 2022, 11, 239. [Google Scholar] [CrossRef]
- Singh, N.; Kaufman, M.; Silakari, O. Flavones: An important scaffold for medicinal chemistry. Eur. J. Med. Chem. 2014, 12, 206–239. [Google Scholar] [CrossRef] [PubMed]
- Kali, A.E.; Habila, J.D. Flavonoids: Isolation, characterization, and health benefits. J. Basic Appl. Sci. 2020, 9, 45. [Google Scholar] [CrossRef]
- Cushnie, T.P.T.; Lamb, A.J. Antimicrobial activity of flavonoids. Int. J. Antimicrob. Agents 2005, 26, 343–356. [Google Scholar] [CrossRef]
- Havsteen, B. Flavonoids, a class of natural products of high pharmacological potency. Biochem. Pharmacol. 1983, 32, 1141–1148. [Google Scholar] [CrossRef]
- Middleton, E.; Chithan, K. The impact of plant flavonoids on mammalian biology: Implications for immunity, inflammation and cancer. In The Flavonoids: Advances in Research Since 1986; Harborne, J.B., Ed.; Chapman and Hall: London, UK, 1993; pp. 145–166. [Google Scholar]
- Liang, Y.; Xie, M.; Li, J.; Liu, L.; Cao, Y. Influence of 3-Hydroxyflavone on Colloidal Stability and Internationalization of Ag Nanomaterials Into THP-1 Macrophages. Dose Response 2019, 17, 1–9. [Google Scholar] [CrossRef]
- Se Gupta, N.; Sahihi, M.; Dehkhodaei, M.; Kelly, D.; Arany, I. Differential role of 3-hydroxyflavone and 7-hydroxyflavone against nicotine-induced oxidative stress in rat renal proximal tubule cells. PLoS ONE 2017, 12, e0179777. [Google Scholar] [CrossRef] [Green Version]
- Jin, Z.; Yang, Y.Z.; Chen, J.X.; Tang, Y.Z. Inhibition of pro-inflammatory mediators in RAW264.7 cells by 7-hydroxyflavone and 7,8-dihydroxyflavone. J. Pharm. Pharmacol. 2017, 69, 865–894. [Google Scholar] [CrossRef]
- Ferraz, C.R.; Carvalho, T.T.; Manchope, M.F.; Atero, N.A.; Rásquele-Oliveira, F.S.; Fattori, V.; Casageande, R.; Verri, W.A. Therapeutic potential of flavonoids in pain and inflammation: Mechanisms of action, pre-clinicall and clonical data, and pharmaceutical development. Molecules 2020, 25, 762. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kawaii, S.; Ishikawa, Y.; Yoshizawa, Y. Relationship between the structure of methoxylated and hydroxylated flavones and their antiproliferative activitty in HL60 cells. Anticancer Res. 2018, 38, 5679–5684. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Su, H.; Zhang, T.; Du, J.; Cui, S.; Yang, F.; Jin, Q. Inhibition of enterovirus 71 replication by 7-hydroxyflavone and diidopropyl-flavones-7-yl phosphate. PLoS ONE 2014, 9, e92565. [Google Scholar] [CrossRef]
- Ayaz, M.; Sadiq, A.; Junaid, M.; Ullah, F.; Ovais, M.; Ullah, I.; Ahmed, J.; Shahid, M. Flavonoids as prospective neuroprotectant and their therapeutic propensity in aging associated neurological disorders. Front. Aging Neurosci. 2019, 11, 155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ganguly, S.; Das, P.; Margel, S. Containers for drug delivery. In Composite Sciences and Technology; Book Series; Available online: https://link.springer.com/chapter/10.1007/978-981-16-8146-2_6 (accessed on 11 February 2022).
- Shah, A.; Aftab, S.; Anisad, J.; Ashiq, M.N.; Iftikhar, F.J. Nanocarriers for targeted drug delivery. J. Drug Deliv. Sci. Technol. 2021, 63, 102426. [Google Scholar] [CrossRef]
- Ganguly, S.; Margel, S. Design of magnetic hydrogels for hyperthermia and drug delivery. Polymers 2021, 13, 4259. [Google Scholar] [CrossRef]
- Iacob, A.T.; Lupascu, F.G.; Apotrosoaei, M.; Vasincu, I.M.; Tauser, R.G.; Lupascu, D.; Giusca, S.E.; Caruntu, I.-D.; Profire, L. Recent biomedical approaches.for quitosano based materials as drug delivery nanocarriersBiomedical. Pharmaceutics 2021, 13, 587. [Google Scholar] [CrossRef]
- Ijima, S. Helical microtubules of graphitic carbon. Nature 1991, 354, 56–58. [Google Scholar] [CrossRef]
- Beg, S.; Rahman, M.; Jain, A.; Sabino, S.; Hasnain, M.S.; Swain, S.; Imam, S.; Kazmi, I.; Akhter, S. Emergence in the 4 functionalized carbon nanotubes as smart nanocarriers for drug delivery applications. In Fullernenes, Graphemes and Nanotubes: A Pharmaceutical Approach; Frumezescu, A.M., Ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2018; Chapter 4; pp. 105–133. [Google Scholar] [CrossRef]
- Gholozadeh, H.; Ghorbani-HasanSaraei, A.; Tahermansouri, H.; Shaidi, S.A. The simultaneous adsorption and desorption of flavonoids from bitter orange peel by the carboxylared multi-walled carbon nanotubes. Carbon Lett. 2019, 29, 273–279. [Google Scholar] [CrossRef]
- Daneshmehr, S. Carbon nanotubes for delivery of quercetin as anticancer drug: Theoretical study. Procedia Mater. Sci. 2015, 11, 131–136. [Google Scholar] [CrossRef] [Green Version]
- Murjani, B.O.; Kadu, P.S.; Bansod, M.; Vaisya, S.S.; Yadav, M.D. Carbon nanotubes in biomedical applications: Current status, promises, and challenges. Carbon Lett. 2022, 32, 1207–1226. [Google Scholar] [CrossRef]
- Hassani, M.; Tahghighi, A.; Rohani, M.; Ekmati, M.; Ahmadian, M.; Ahmavvand, H. Robust antibacterial activity of functionalized carbon nanotube-levofloxacine conjugate based on in vitro and in vivo studies. Sci. Rep. 2022, 12, 10064. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Xiao, Q.; Mei, Y.; He, S.; Zhang, Z.; Wang, R.; Wang, W. Insights on functionalized carbon nanotubes for cancer theranostics. J. Nanobiotechnol. 2021, 19, 423. [Google Scholar] [CrossRef] [PubMed]
- Pranantyo, D.; Kang, E.; Chan-Park, M.B. Smart nanomicelles with bacterial infection-responsive disassembly for selective antimicrobial applications. Biomater. Sci. 2021, 9, 1627–1638. [Google Scholar] [CrossRef] [PubMed]
- Strober, W. Trypan Blue Exclusion Test of Cell Viability. Curr. Protoc. Immunol. 2015, 111, A3.B.1–A3.B.3. [Google Scholar] [CrossRef]
- Molina-Velázquez, S. Final Master’s Project. 2021. [Google Scholar]
- Moyá, M.L.; Ostos, F.J.; Moreno, I.; García, D.; Moreno-Gordillo, P.; Rosado, I.V.; López-Cornejo, P. Metallo-Liposomes Derived from the [Ru(bpy)3]2+ complex as nanocarriers of therapeutic agents. Chemosensors 2021, 9, 90. [Google Scholar] [CrossRef]
- López-López, M.; Bernal, E.; Moyá, M.L.; Sánchez, F.; López-Cornejo, P. Study of ionic surfactants interactions with carboxylated single-walled carbon nanotubes by using ion-selective electrodes? Electrochem. Commun. 2016, 67, 31–34. [Google Scholar] [CrossRef]
- Serdiuk, I.E.; Varenikov, A.S.; Roshal, A.D. 7-Hydroxyflavone revisited: Spectral, acid−base properties, and interplay of the protolytic forms in the ground and excited states. J. Phys. Chem. A 2014, 118, 3068–3080. [Google Scholar] [CrossRef]
- Fredenberg, S.; Wahlgren, M.; Reslow, M.; Axelsson, A. The mechanisms of drug release in poly(lactic-co-glycolic acid)-based drug delivery systems—A review. Int. J. Pharm. 2011, 415, 34–52. [Google Scholar] [CrossRef]



| 100% 7-HFencapsulated | |||
|---|---|---|---|
| pH = 7.4 | [SWNCTs]/g·L−1 | [SWCNT-COOH]/g·L−1 | [MWNCTs]/g·L−1 |
| [7-HF] = 1.51·10−5 M | 0.20 | 0.025 | 0.090 |
| CNTs | Kmax/g−1 L |
|---|---|
| SWCNT | (1.5 ± 0.3)·103 |
| MWCNT | (1.2 ± 0.2)·103 |
| SWCNT-COOH | (1.5 ± 0.3)·103 |
| 7-HF/SWCNT | ||
| pH | 103 kn/s−1 | |
| 2.0 | 1.7 ± 0.4 | |
| pH | 103 kn/s−1 | 103 ka/s−1 |
| 7.4 | 1.8 ± 0.5 | 68 ± 10 |
| 9.2 | 1.9 ± 0.7 | 50 ± 14 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Espíndola, C.; Correa, A.J.; López-López, M.; López-Cornejo, P.; Bernal, E.; Lebrón, J.A.; Ostos, F.J.; Benhnia, M.R.-E.-I.; Moyá, M.L. Single -and Multi-Walled Carbon Nanotubes as Nanocarriers for the Delivery of 7-Hydroxyflavone. Pharmaceutics 2022, 14, 2806. https://doi.org/10.3390/pharmaceutics14122806
Espíndola C, Correa AJ, López-López M, López-Cornejo P, Bernal E, Lebrón JA, Ostos FJ, Benhnia MR-E-I, Moyá ML. Single -and Multi-Walled Carbon Nanotubes as Nanocarriers for the Delivery of 7-Hydroxyflavone. Pharmaceutics. 2022; 14(12):2806. https://doi.org/10.3390/pharmaceutics14122806
Chicago/Turabian StyleEspíndola, Cecilia, Alejandro Javier Correa, Manuel López-López, Pilar López-Cornejo, Eva Bernal, José Antonio Lebrón, Francisco José Ostos, Mohammed Rafii-El-Idrissi Benhnia, and María Luisa Moyá. 2022. "Single -and Multi-Walled Carbon Nanotubes as Nanocarriers for the Delivery of 7-Hydroxyflavone" Pharmaceutics 14, no. 12: 2806. https://doi.org/10.3390/pharmaceutics14122806
APA StyleEspíndola, C., Correa, A. J., López-López, M., López-Cornejo, P., Bernal, E., Lebrón, J. A., Ostos, F. J., Benhnia, M. R.-E.-I., & Moyá, M. L. (2022). Single -and Multi-Walled Carbon Nanotubes as Nanocarriers for the Delivery of 7-Hydroxyflavone. Pharmaceutics, 14(12), 2806. https://doi.org/10.3390/pharmaceutics14122806











