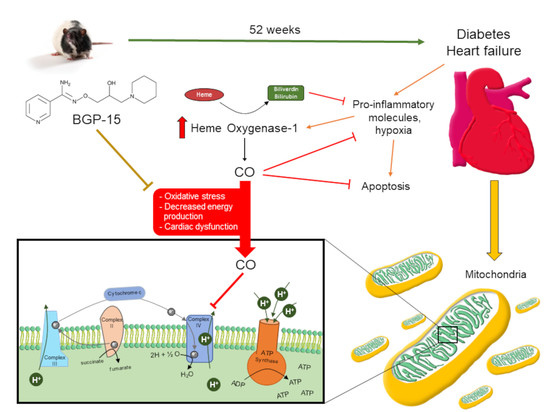
Cardioprotective Role of BGP-15 in Ageing Zucker Diabetic Fatty Rat (ZDF) Model: Extended Mitochondrial Longevity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animal Model
2.2. Chemicals
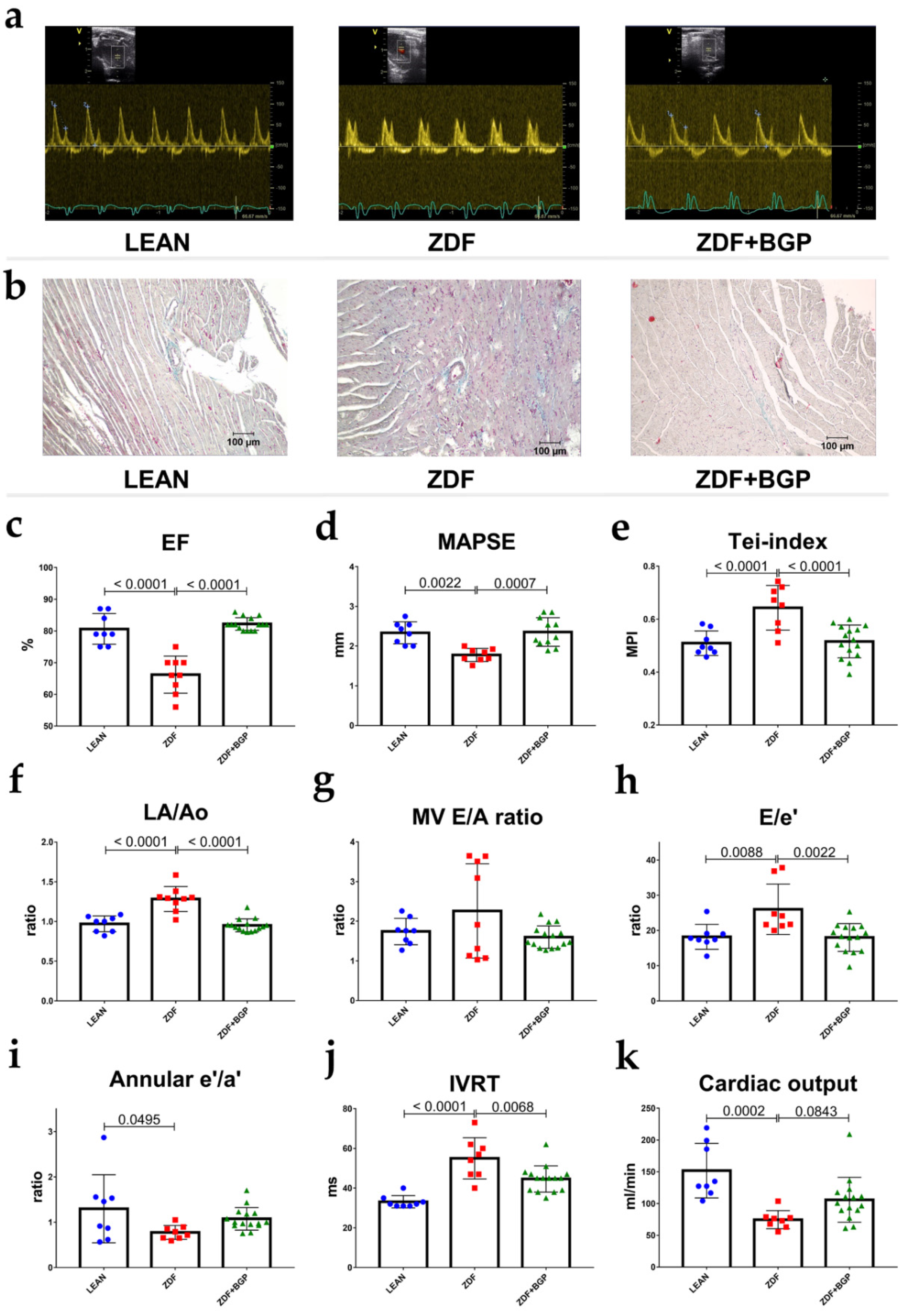
2.3. Echocardiographic Studies
2.4. Analysis of Serum Parameters
2.5. Morphometry
2.6. Histology
2.7. Western Blot
2.8. Cytochrome C Oxidase Enzyme Activity Microplate Assay
2.9. ATP Synthase Enzyme Activity Microplate Assay
2.10. Statistical Analyses
3. Results
3.1. BGP-15 Prevented Ageing and Type 2 Diabetes Mellitus-Associated Cardiac Dysfunction In Vivo
3.2. Severely Diabetic Rats Demonstrated Decreased Body Weight
3.3. Ageing and T2DM Elevates Serum Parameters
3.4. BGP-15 Has Mild Influence on Glucose Homeostasis
3.5. BPG-15 Decrease Myocardial Hypertrophy in Ageing Zucker Diabetic Fatty Rats
3.6. BGP-15 Improves Mitochondrial Function in Ageing Zucker Diabetic Fatty Rat Hearts
3.7. BGP-15 Treatment Induces MTCO and ATP Synthase Activity in Ageing Zucker Diabetic Fatty Rats
3.8. Ageing and T2DM Exerts an Antioxidant Response on ZDF Rat Hearts by HO-1 Dependent Activation
4. Discussion
4.1. General Characteristics of the ZDF Rats: Glucose and Lipid Homeostasis
4.2. Assessment of Cardiac Dysfunction and Remodelling
4.3. ROS Capacity
4.4. Upregulation of Cardiac ATP Synthase and Cytochrome C Oxidase
4.5. Translational Aspects and Future Directions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Maayah, Z.H.; McGinn, E.; Al Batran, R.; Gopal, K.; Ussher, J.R.; El-Kadi, A.O.S. Role of Cytochrome p450 and Soluble Epoxide Hydrolase Enzymes and Their Associated Metabolites in the Pathogenesis of Diabetic Cardiomyopathy. J. Cardiovasc. Pharmacol. 2019, 74, 235–245. [Google Scholar] [CrossRef] [PubMed]
- Abushouk, A.I.; El-Husseny, M.W.A.; Bahbah, E.; Elmaraezy, A.; Ali, A.A.; Ashraf, A.; Abdel-Daim, M.M. Peroxisome proliferator-activated receptors as therapeutic targets for heart failure. Biomed. Pharmacother. 2017, 95, 692–700. [Google Scholar] [CrossRef] [PubMed]
- Duncan, J.G. Mitochondrial dysfunction in diabetic cardiomyopathy. Biochim. Biophys. Acta 2011, 1813, 1351–1359. [Google Scholar] [CrossRef] [Green Version]
- Srivastava, S. Emerging therapeutic roles for NAD + metabolism in mitochondrial and age-related disorders. Clin. Transl. Med. 2016, 5, 25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pollak, N.; Dölle, C.; Ziegler, M. The power to reduce: Pyridine nucleotides-small molecules with a multitude of functions. Biochem. J. 2007, 402, 205–218. [Google Scholar] [CrossRef]
- Pirinen, E.; Auranen, M.; Khan, N.A.; Brilhante, V.; Urho, N.; Pessia, A.; Hakkarainen, A.; Kuula, J.; Heinonen, U.; Schmidt, M.S.; et al. Niacin Cures Systemic NAD+ Deficiency and Improves Muscle Performance in Adult-Onset Mitochondrial Myopathy. Cell Metab. 2020, 31, 1078–1090.e5. [Google Scholar] [CrossRef]
- Pető, Á.; Kósa, D.; Fehér, P.; Ujhelyi, Z.; Sinka, D.; Vecsernyés, M.; Szilvássy, Z.; Juhász, B.; Csanádi, Z.; Vígh, L.; et al. Pharmacological Overview of the BGP-15 Chemical Agent as a New Drug Candidate for the Treatment of Symptoms of Metabolic Syndrome. Molecules 2020, 25, 429. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Literati-Nagy, B.; Kulcsar, E.; Literati-Nagy, Z.; Buday, B.; Peterfai, E.; Horvath, T.; Tory, K.; Kolonics, A.; Fleming, A.; Mandl, J.; et al. Improvement of Insulin Sensitivity by a Novel Drug, BGP-15, in Insulin-resistant Patients: A Proof of Concept Randomized Double-blind Clinical Trial. Horm. Metab. Res. 2009, 41, 374–380. [Google Scholar] [CrossRef]
- Szabados, E.; Literati-Nagy, P.; Farkas, B.; Sumegi, B. BGP-15, a nicotinic amidoxime derivate protecting heart from ischemia reperfusion injury through modulation of poly(ADP-ribose) polymerase. Biochem. Pharmacol. 2000, 59, 937–945. [Google Scholar] [CrossRef]
- Halmosi, R.; Berente, Z.; Osz, E.; Toth, K.; Literati-Nagy, P.; Sümegi, B. Effect of Poly(ADP-Ribose) Polymerase Inhibitors on the Ischemia-Reperfusion-Induced Oxidative Cell Damage and Mitochondrial Metabolism in Langendorff Heart Perfusion System. Mol. Pharmacol. 2001, 59, 1497–1505. [Google Scholar] [CrossRef]
- Kennedy, T.L.; Swiderski, K.; Murphy, K.T.; Gehrig, S.M.; Curl, C.L.; Chandramouli, C.; Febbraio, M.A.; Delbridge, L.M.; Koopman, R.; Lynch, G.S. BGP-15 Improves Aspects of the Dystrophic Pathology in mdx and dko Mice with Differing Efficacies in Heart and Skeletal Muscle. Am. J. Pathol. 2016, 186, 3246–3260. [Google Scholar] [CrossRef] [Green Version]
- Sapra, G.; Tham, Y.K.; Cemerlang, N.; Matsumoto, A.; Kiriazis, H.; Bernardo, B.C.; Henstridge, D.C.; Ooi, J.Y.Y.; Pretorius, L.; Boey, E.J.H.; et al. The small-molecule BGP-15 protects against heart failure and atrial fibrillation in mice. Nat. Commun. 2014, 5, 5705. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bombicz, M.; Priksz, D.; Gesztelyi, R.; Kiss, R.; Hollos, N.; Varga, B.; Nemeth, J.; Toth, A.; Papp, Z.; Szilvassy, Z.; et al. The Drug Candidate BGP-15 Delays the Onset of Diastolic Dysfunction in the Goto-Kakizaki Rat Model of Diabetic Cardiomyopathy. Molecules 2019, 24, 586. [Google Scholar] [CrossRef] [Green Version]
- Sumegi, K.; Fekete, K.; Antus, C.; Debreceni, B.; Gallyas, F., Jr.; Sumegi, B.; Szabo, A. BGP-15 Protects against Oxidative Stress- or Lipopolysaccharide-Induced Mitochondrial Destabilization and Reduces Mitochondrial Production of Reactive Oxygen Species. PLoS ONE 2017, 12, e0169372. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for Cardiac Chamber Quantification by Echocardiography in Adults: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2015, 28, 1–39.e14. [Google Scholar] [CrossRef] [Green Version]
- Nagueh, S.F.; Smiseth, O.A.; Appleton, C.P.; Byrd, B.F.; Dokainish, H.; Edvardsen, T.; Flachskampf, F.A.; Gillebert, T.C.; Klein, A.L.; Lancellotti, P.; et al. Recommendations for the Evaluation of Left Ventricular Diastolic Function by Echocardiography: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur. Hear. J. Cardiovasc. Imaging 2016, 17, 1321–1360. [Google Scholar] [CrossRef]
- Marsh, S.A.; Powell, P.C.; Agarwal, A.; Dell’Italia, L.J.; Chatham, J.C. Cardiovascular dysfunction in Zucker obese and Zucker diabetic fatty rats: Role of hydronephrosis. Am. J. Physiol. Circ. Physiol. 2007, 293, H292–H298. [Google Scholar] [CrossRef]
- Brom, C.E.V.D.; Huisman, M.C.; Vlasblom, R.; Boontje, N.M.; Duijst, S.; Lubberink, M.; Molthoff, C.F.; Lammertsma, A.A.; van der Velden, J.; Boer, C.; et al. Altered myocardial substrate metabolism is associated with myocardial dysfunction in early diabetic cardiomyopathy in rats: Studies using positron emission tomography. Cardiovasc. Diabetol. 2009, 8, 39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Daniels, A.; Linz, D.; van Bilsen, M.; Rütten, H.; Sadowski, T.; Ruf, S.; Juretschke, H.-P.; Neumann-Haefelin, C.; Munts, C.; van der Vusse, G.J.; et al. Long-term severe diabetes only leads to mild cardiac diastolic dysfunction in Zucker diabetic fatty rats. Eur. J. Heart Fail. 2012, 14, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Siwy, J.; Zoja, C.; Klein, J.; Benigni, A.; Mullen, W.; Mayer, B.; Mischak, H.; Jankowski, J.; Stevens, R.; Vlahou, A.; et al. Evaluation of the Zucker Diabetic Fatty (ZDF) Rat as a Model for Human Disease Based on Urinary Peptidomic Profiles. PLoS ONE 2012, 7, e51334. [Google Scholar] [CrossRef]
- Peterson, R.G.; Shaw, W.N.; Neel, M.-A.; Little, L.A.; Eichberg, J. Zucker Diabetic Fatty Rat as a Model for Non-insulin-dependent Diabetes Mellitus. ILAR J. 1990, 32, 16–19. [Google Scholar] [CrossRef] [Green Version]
- Horvath, O.; Ordog, K.; Bruszt, K.; Deres, L.; Gallyas, F.; Sumegi, B.; Toth, K.; Halmosi, R. BGP-15 Protects against Heart Failure by Enhanced Mitochondrial Biogenesis and Decreased Fibrotic Remodelling in Spontaneously Hypertensive Rats. Oxidative Med. Cell. Longev. 2021, 2021, 1250858. [Google Scholar] [CrossRef]
- Huang, E.-J.; Kuo, W.-W.; Chen, Y.-J.; Chen, T.-H.; Chang, M.-H.; Lu, M.-C.; Tzang, B.-S.; Hsu, H.-H.; Huang, C.-Y.; Lee, S.-D. Homocysteine and other biochemical parameters in Type 2 diabetes mellitus with different diabetic duration or diabetic retinopathy. Clin. Chim. Acta 2006, 366, 293–298. [Google Scholar] [CrossRef]
- Bansal, S.; Biswas, G.; Avadhani, N.G. Mitochondria-targeted heme oxygenase-1 induces oxidative stress and mitochondrial dysfunction in macrophages, kidney fibroblasts and in chronic alcohol hepatotoxicity. Redox Biol. 2013, 2, 273–283. [Google Scholar] [CrossRef] [Green Version]
- Abraham, N.; Lin, J.-C.; Schwartzman, M.; Levere, R.; Shibahara, S. The physiological significance of heme oxygenase. Int. J. Biochem. 1988, 20, 543–558. [Google Scholar] [CrossRef]
- Llesuy, S.F.; Tomaro, M.L. Heme oxygenase and oxidative stress. Evidence of involvement of bilirubin as physiological protector against oxidative damage. Biochim. Biophys. Acta (BBA) Bioenerg. 1994, 1223, 9–14. [Google Scholar] [CrossRef]
- Di Noia, M.A.; Van Driesche, S.; Palmieri, F.; Yang, L.-M.; Quan, S.; Goodman, A.I.; Abraham, N.G. Heme Oxygenase-1 Enhances Renal Mitochondrial Transport Carriers and Cytochrome c Oxidase Activity in Experimental Diabetes. J. Biol. Chem. 2006, 281, 15687–15693. [Google Scholar] [CrossRef] [Green Version]
- Alonso, J.-R.; Cardellach, F.; López, S.; Casademont, J.; Miró, Ò. Carbon Monoxide Specifically Inhibits Cytochrome C Oxidase of Human Mitochondrial Respiratory Chain. Pharmacol. Toxicol. 2003, 93, 142–146. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, S.; Avadhani, N.G. Cytochrome c oxidase dysfunction in oxidative stress. Free Radic. Biol. Med. 2012, 53, 1252–1263. [Google Scholar] [CrossRef] [Green Version]
- Goodarzi, M.T.; Varmaziar, L.; Navidi, A.A.; Parivar, K. Study of oxidative stress in type 2 diabetic patients and its relationship with glycated hemoglobin. Saudi Med. J. 2008, 29, 503–506. [Google Scholar] [PubMed]
- Sivitz, W.I.; Yorek, M.A. Mitochondrial Dysfunction in Diabetes: From Molecular Mechanisms to Functional Significance and Therapeutic Opportunities. Antioxid. Redox Signal. 2010, 12, 537–577. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vazquez, E.J.; Berthiaume, J.M.; Kamath, V.; Achike, O.; Buchanan, E.; Montano, M.M.; Chandler, M.P.; Miyagi, M.; Rosca, M.G. Mitochondrial complex I defect and increased fatty acid oxidation enhance protein lysine acetylation in the diabetic heart. Cardiovasc. Res. 2015, 107, 453–465. [Google Scholar] [CrossRef] [PubMed] [Green Version]



| LEAN | ZDF | ZDF+BGP-15 | ||
|---|---|---|---|---|
| N | 8 | 7 | 9 | |
| Parameter | Unit | Mean ± SD | Mean ± SD | Mean ± SD |
| BW | g | 453.6 ± 14.76 | # 279.3 ± 53.76 | # 279.9 ± 40.84 |
| HW | g | 1.221 ± 0.15 | 1.09 ± 0.12 | # 1.02 ± 0.18 |
| HW/TL | g/cm | 0.25 ± 0.05 | 0.24 ± 0.03 | 0.23 ± 0.04 |
| BW/TL | g/cm | 91.14 ± 6.79 | # 60.13 ± 11.2 | # 58.86 ± 9.59 |
| HW/BW | g/kg | 2.69 ± 0.36 | # 4 ± 0.7 | # 3.76 ± 0.95 |
| lung w/d | 0.19 ± 0.02 | 0.19 ± 0.01 | 0.18 ± 0.01 | |
| kidney w/d | 0.26 ± 0.03 | 0.23 ± 0.07 | 0.25 ± 0.03 |
| LEAN | ZDF | ZDF+BGP-15 | ||
|---|---|---|---|---|
| N | 8 | 6 | 8 | |
| Parameter | Unit | Mean ± SD | Mean ± SD | Mean ± SD |
| Total Cholesterol | mmol/L | 2.19 ± 0.36 | # 8.09 ± 4.21 | # 5.62 ± 2.59 |
| LDLc | mmol/L | 0.23 ± 0.08 | # 1.08 ± 0.68 | * 0.51 ± 0.33 |
| HDLc | mmol/L | 0.49 ± 0.15 | 1.16 ± 0.68 | 0.69 ± 0.58 |
| Creatinine | µmol/L | 26.7 ± 12.85 | # 141 ± 221.1 | # 66.88 ± 53.83 |
| CK | U/L | 380.8 ± 304.6 | 548.8 ± 312.4 | * 182.1 ± 124.9 |
| LDH | U/L | 1143 ± 1559 | 1871 ± 1256 | 1114 ± 856.8 |
| Troponin T | ng/L | 3263 ± 2356 | 4162 ± 3293 | 3349 ± 2276 |
| Control | ZDF | ZDF+BGP-15 | ||
|---|---|---|---|---|
| N | 8 | 6 | 8 | |
| Parameter | Unit | Mean ± SD | Mean ± SD | Mean ± SD |
| Glucose | mmol/litre | 7.05 ± 0.59 | # 18.42 ± 7.93 | * 10.74 ± 3.34 |
| Insulin | mU/litre | 5.81 ± 1.48 | # 2.05 ± 1.02 | * 5.81 ± 1.48 |
| HOMA-IR | 1.79 ± 0.40 | 1.87 ± 1.50 | # 3.19 ± 0.88 | |
| HOMA-B | 13.3 ± 5.15 | # −0.89 ± 1.54 | * 11.92 ± 10.57 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kozma, M.; Bombicz, M.; Varga, B.; Priksz, D.; Gesztelyi, R.; Tarjanyi, V.; Kiss, R.; Szekeres, R.; Takacs, B.; Menes, A.; et al. Cardioprotective Role of BGP-15 in Ageing Zucker Diabetic Fatty Rat (ZDF) Model: Extended Mitochondrial Longevity. Pharmaceutics 2022, 14, 226. https://doi.org/10.3390/pharmaceutics14020226
Kozma M, Bombicz M, Varga B, Priksz D, Gesztelyi R, Tarjanyi V, Kiss R, Szekeres R, Takacs B, Menes A, et al. Cardioprotective Role of BGP-15 in Ageing Zucker Diabetic Fatty Rat (ZDF) Model: Extended Mitochondrial Longevity. Pharmaceutics. 2022; 14(2):226. https://doi.org/10.3390/pharmaceutics14020226
Chicago/Turabian StyleKozma, Mate, Mariann Bombicz, Balazs Varga, Daniel Priksz, Rudolf Gesztelyi, Vera Tarjanyi, Rita Kiss, Reka Szekeres, Barbara Takacs, Akos Menes, and et al. 2022. "Cardioprotective Role of BGP-15 in Ageing Zucker Diabetic Fatty Rat (ZDF) Model: Extended Mitochondrial Longevity" Pharmaceutics 14, no. 2: 226. https://doi.org/10.3390/pharmaceutics14020226