Cyclodextrin Stabilized Freeze-Dried Silica/Chitosan Nanoparticles for Improved Terconazole Ocular Bioavailability
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Preparation of Terconazole-Loaded Silica/Chitosan Nanoparticles (SCNs)
2.2.2. Statistical Analysis of the Experimental Design, Evaluation and Optimization of the Prepared SCNs
Determination of Particle Size (PS), Polydispersity Index (PDI) and Zeta Potential (ZP)
Determination of the Percentage Yield
Drug Loading (DL)
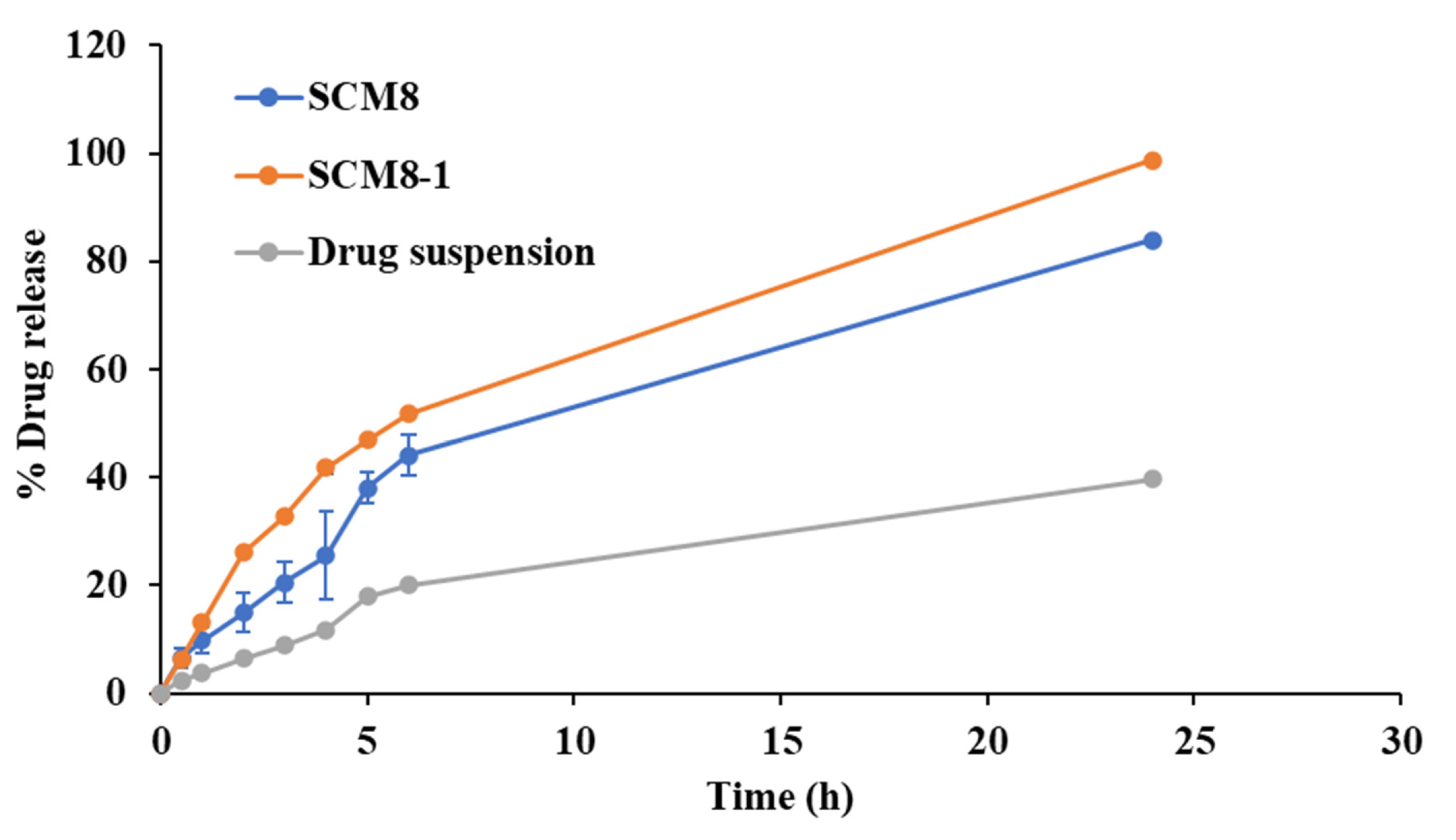
In Vitro Drug Release Studies
2.3. Preparation of the Modified Terconazole-Loaded Silica/Chitosan Nanoparticles (SCNs)
2.3.1. Characterization of the Modified Terconazole-Loaded Silica/Chitosan Nanoparticles (SCNs)
Fourier Transform Infrared Spectroscopy (FTIR)
Differential Scanning Calorimetry (DSC)
X-ray Diffraction Studies (XRD)
Scanning Electron Microscopy (SEM)
Transmission Electron Microscopy (TEM)
Mucoadhesion Study
2.3.2. In Vivo Histopathological Studies
2.3.3. In Vivo Evaluation of the Selected Terconazole-Loaded Silica/Chitosan Nanoparticles (SCNs)
Study Design
Tear Film Sampling
Chromatographic Conditions
3. Results and Discussion
3.1. Preparation of the Terconazole-Loaded Silica/Chitosan Nanoparticles (SCNs)
3.2. Statistical Analysis of the Experimental Design, Evaluation and Optimization of the Prepared SCNs
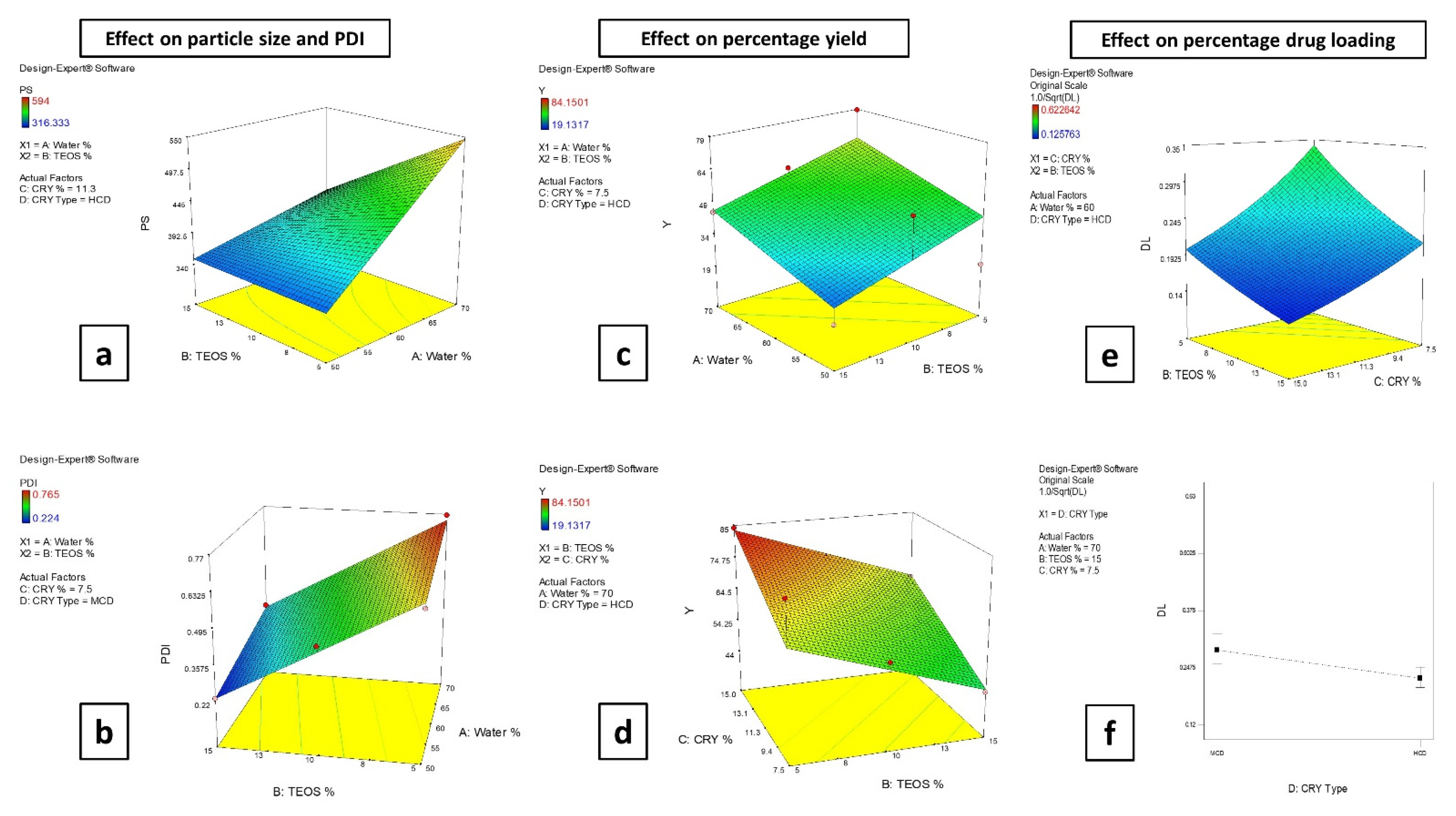
3.2.1. Effect of the Formulation Factors on the Particle Size (PS) and Polydispersity Index (PDI)
3.2.2. Effect of the Formulation Factors on the Percentage Yield
3.2.3. Effect of the Formulation Factors on the Drug Loading (DL)
3.2.4. Effect of the Formulation Factors on Q2 and Q6
3.3. Characterization of the Modified Terconazole-Loaded Silica/Chitosan Nanoparticles (SCNs)
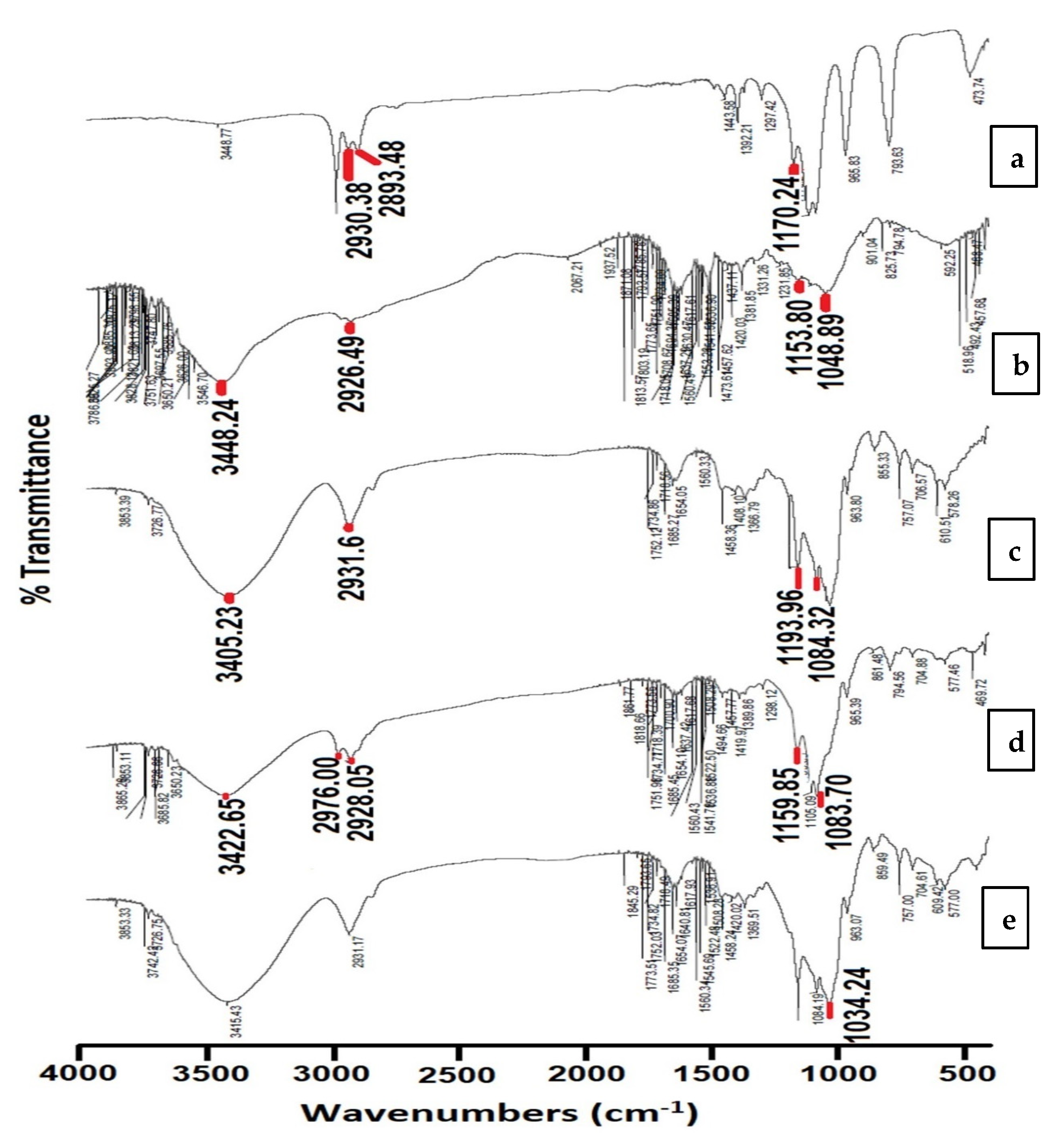
3.3.1. Fourier Transform Infrared Spectroscopy (FTIR)
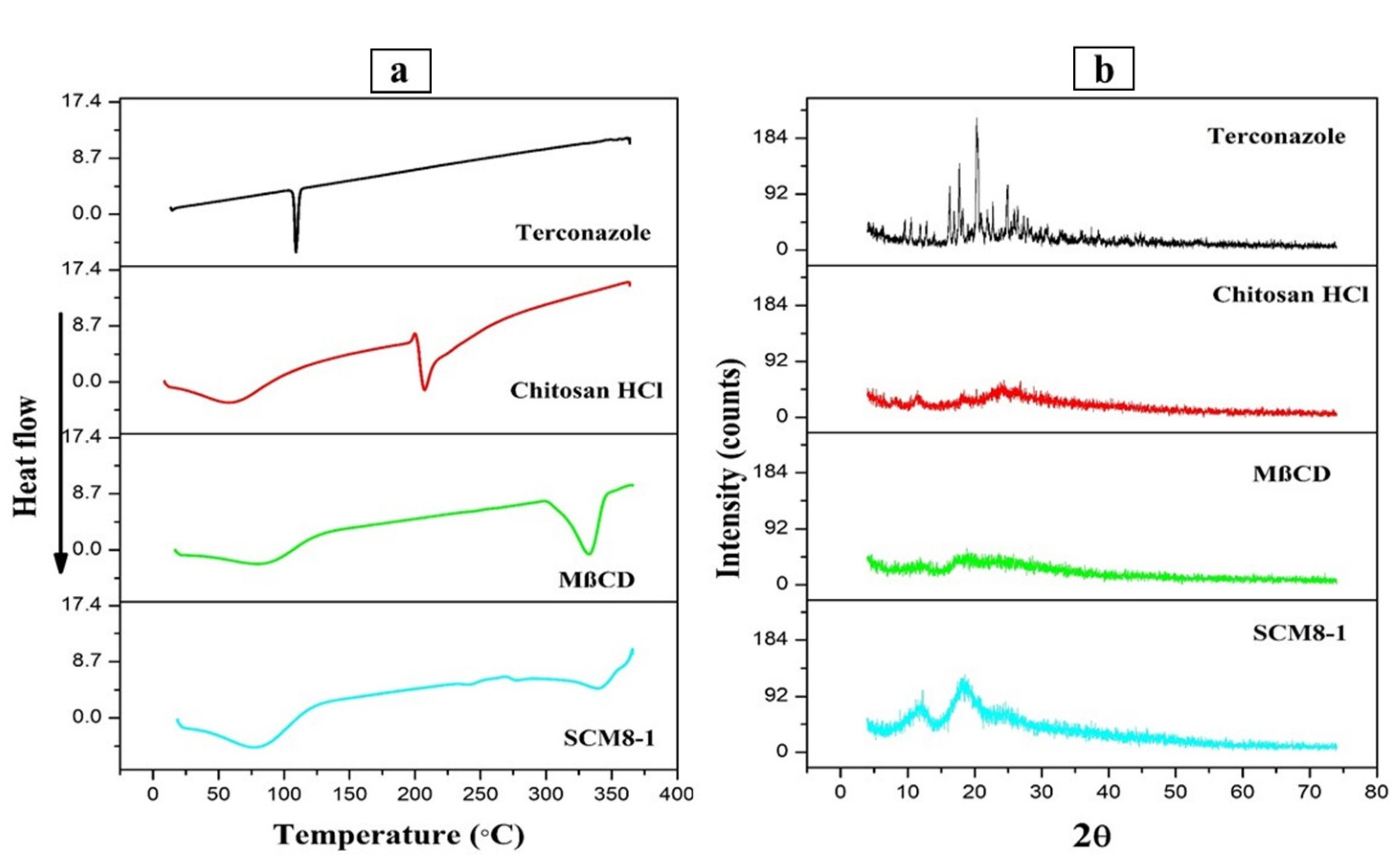
3.3.2. Differential Scanning Calorimetry Study
3.3.3. X-ray Diffraction Study (XRD)
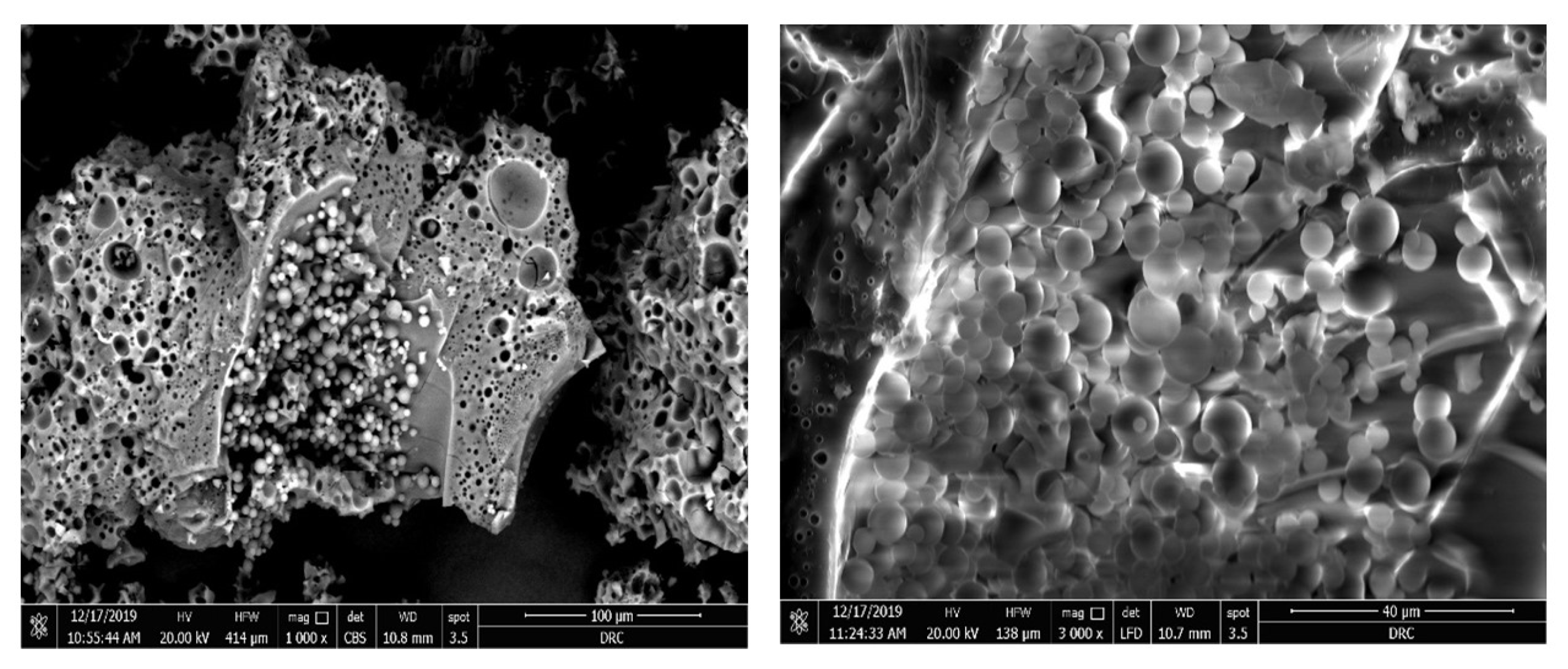
3.3.4. Scanning Electron Microscopy (SEM)
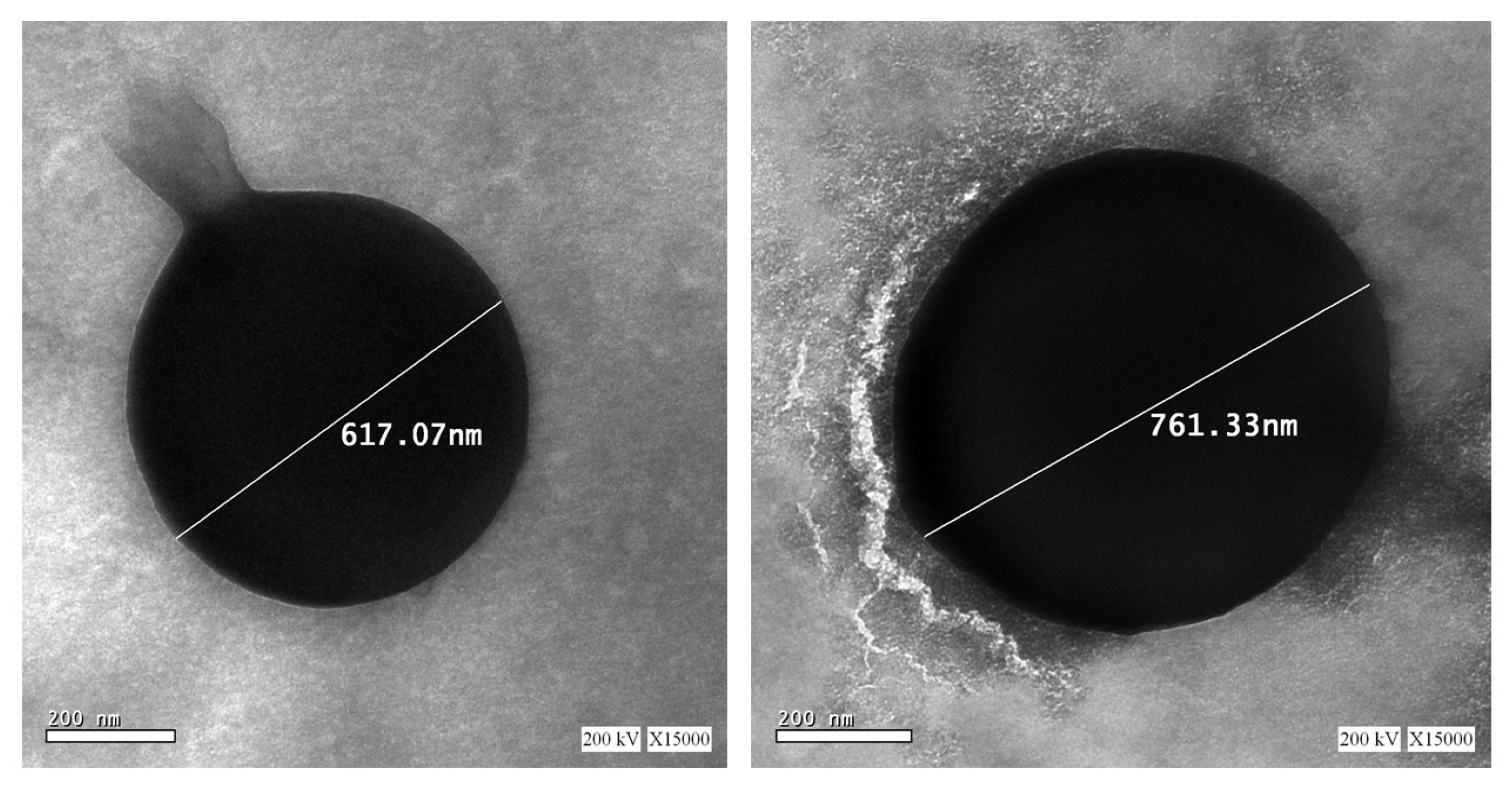
3.3.5. Transmission Electron Microscopy (TEM)
3.3.6. Mucoadhesion Study
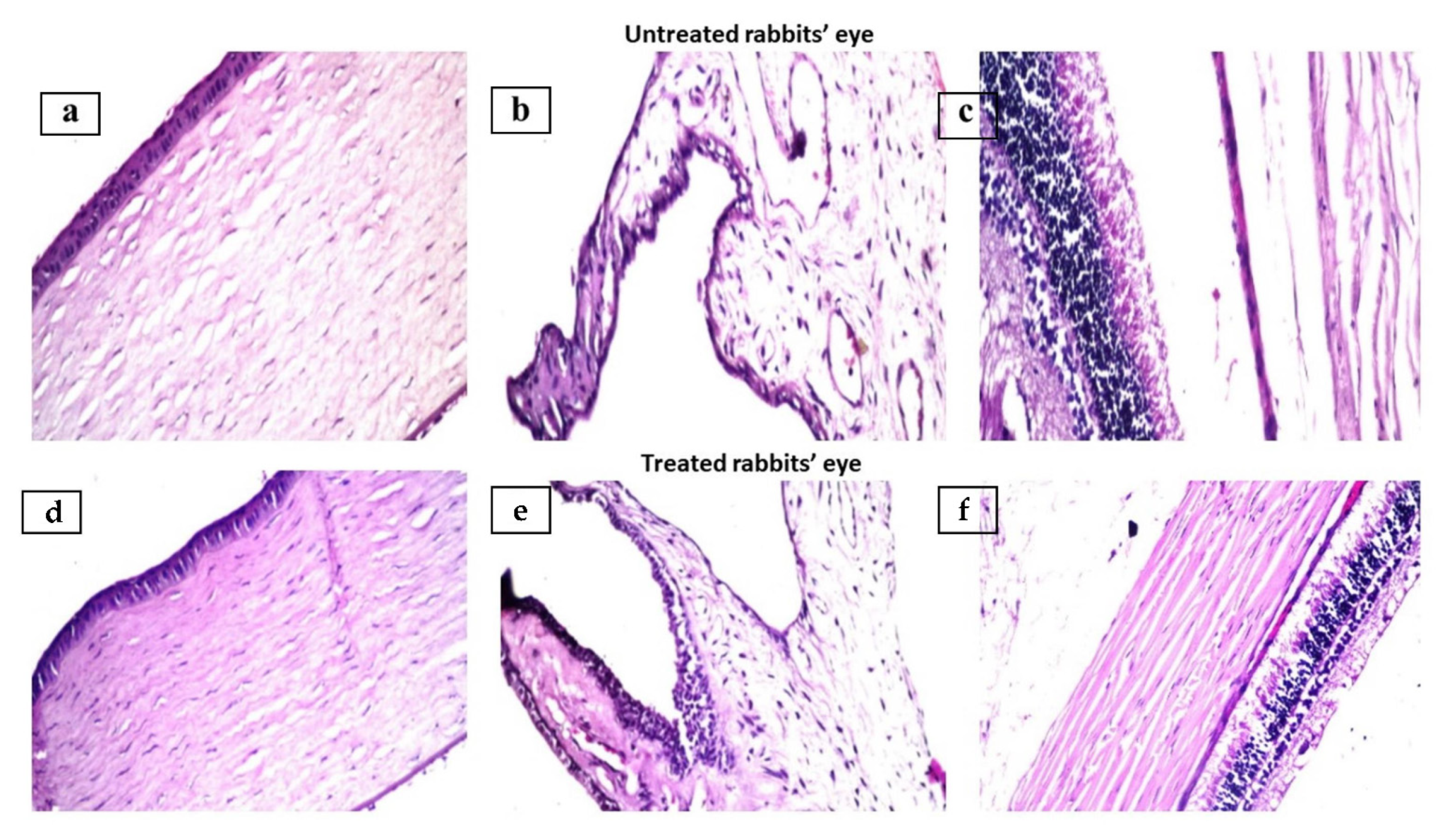
3.4. Ocular Irritation Study
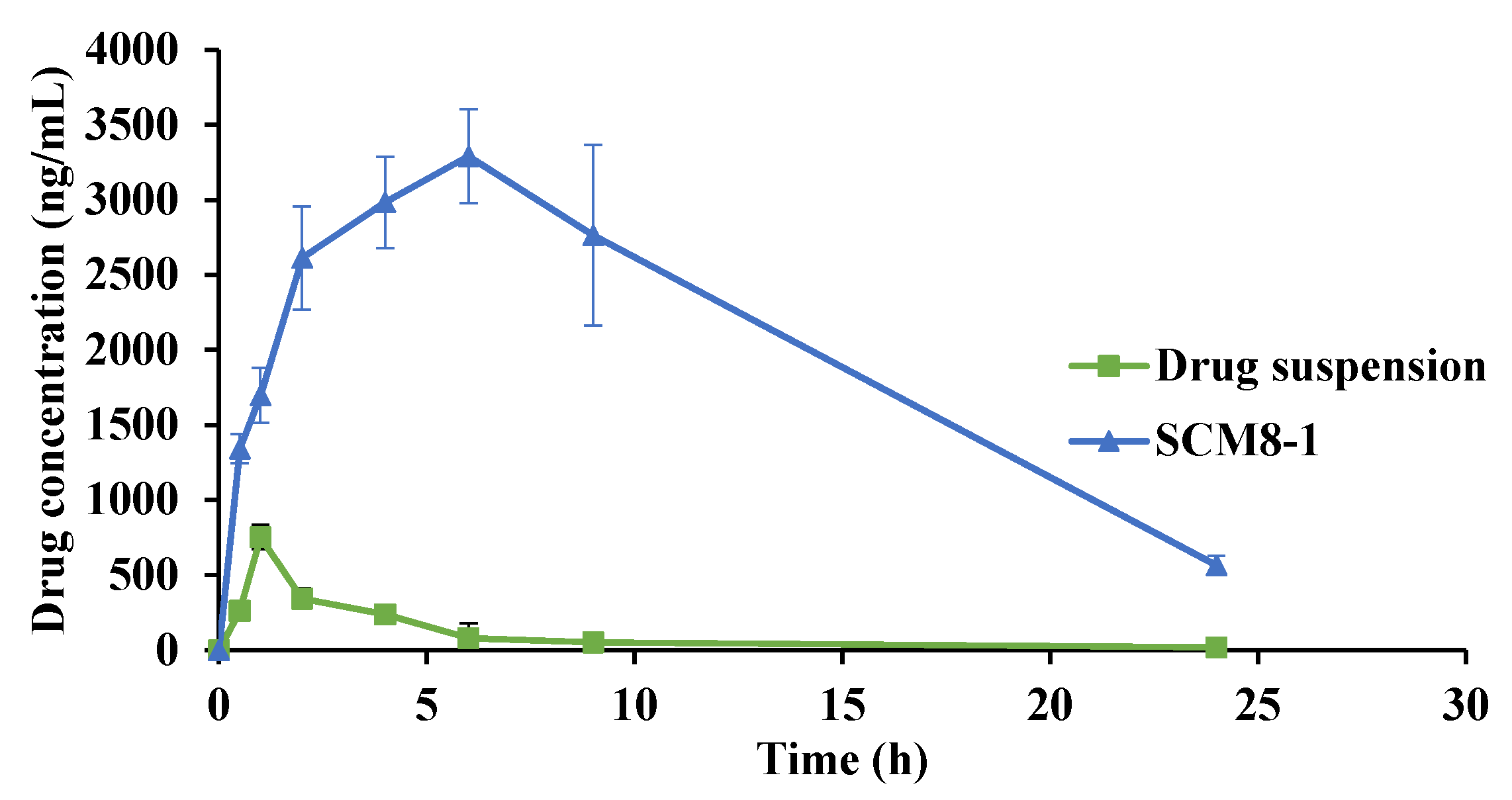
3.5. In Vivo Evaluation of the Selected Terconazole-Loaded Silica/Chitosan Nanoparticles (SCNs)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Whitcher, J.P.; Srinivasan, M.; Upadhyay, M.P. Corneal blindness: A global perspective. Bull. World Health Organ. 2001, 79, 214–221. [Google Scholar] [PubMed]
- Garg, P.; Krishna, P.; Stratis, A.; Gopinathan, U. The value of corneal transplantation in reducing blindness. Eye 2005, 19, 1106–1114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gupta, N.; Tandon, R.; Gupta, S.K.; Sreenivas, V.; Vashist, P. Burden of corneal blindness in India. Indian J. Commun. Med. 2013, 38, 198. [Google Scholar]
- Brown, L.; Leck, A.K.; Gichangi, M.; Burton, M.J.; Denning, D.W. The global incidence and diagnosis of fungal keratitis. Lancet Infect. Dis. 2021, 21, e49–e57. [Google Scholar] [CrossRef]
- Foster, C.S. Fungal keratitis. Infect. Dis. Clin. N. Am. 1992, 6, 851–857. [Google Scholar] [CrossRef]
- Spierer, O.; Dugar, J.; Miller, D.; O’Brien, T.P. Comparative antifungal susceptibility analysis of Candida albicans versus non-albicans Candida corneal isolates. Cornea 2015, 34, 576–579. [Google Scholar] [CrossRef] [PubMed]
- Gaudana, R.; Ananthula, H.K.; Parenky, A.; Mitra, A.K. Ocular drug delivery. AAPS J. 2010, 12, 348–360. [Google Scholar] [CrossRef]
- Patel, A.; Cholkar, K.; Agrahari, V.; Mitra, A.K. Ocular drug delivery systems: An overview. World J. Pharmacol. 2013, 2, 47–64. [Google Scholar] [CrossRef] [PubMed]
- Li, P.-H.; Chen, C.-C.; Liou, S.-W. Candida parapsilosis keratitis treated successfully with topical and oral fluconazole. Taiwan J. Ophthalmol. 2016, 6, 155–157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsumoto, Y.; Murat, D.; Kojima, T.; Shimazaki, J.; Tsubota, K. The comparison of solitary topical micafungin or fluconazole application in the treatment of Candida fungal keratitis. Br. J. Ophthalmol. 2011, 95, 1406–1409. [Google Scholar] [CrossRef]
- Al-Badriyeh, D.; Neoh, C.F.; Stewart, K.; Kong, D.C. Clinical utility of voriconazole eye drops in ophthalmic fungal keratitis. Clin. Ophthalmol. 2010, 4, 391–405. [Google Scholar]
- Mohsen, A.M. Cationic Polymeric Nanoparticles for Improved Ocular Delivery and Antimycotic Activity of Terconazole. J. Pharm. Sci. 2021, 111, 458–468. [Google Scholar] [CrossRef] [PubMed]
- Tolman, E.; Isaacson, D.; Rosenthale, M.; McGuire, J.; Van Cutsem, J.; Borgers, M.; Van den Bossche, H. Anticandidal activities of terconazole, a broad-spectrum antimycotic. Antimicrob. Agents Chemother. 1986, 29, 986–991. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weisberg, M. Terconazole—A new antifungal agent for vulvovaginal candidiasis. Clin. Ther. 1989, 11, 659–668. [Google Scholar] [PubMed]
- Srilakshmi, M.; Devi, G.R.; Sharmila, S.; Rahaman, S.A. spectrophotometric determination of poorly water soluble drug terconazole using hydrotropic solubilization technique. Indo Am. J. Pharm. Res 2013, 3, 3527–3534. [Google Scholar]
- Selvarajan, V.; Obuobi, S.; Ee, P.L.R. Silica nanoparticles—A versatile tool for the treatment of bacterial infections. Front. Chem. 2020, 602. [Google Scholar] [CrossRef]
- Kirtane, A.R.; Verma, M.; Karandikar, P.; Furin, J.; Langer, R.; Traverso, G. Nanotechnology approaches for global infectious diseases. Nat. Nanotechnol. 2021, 16, 369–384. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, Q.; Han, N.; Bai, L.; Li, J.; Liu, J.; Che, E.; Hu, L.; Zhang, Q.; Jiang, T. Mesoporous silica nanoparticles in drug delivery and biomedical applications. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 313–327. [Google Scholar] [CrossRef]
- Slowing, I.I.; Trewyn, B.G.; Giri, S.; Lin, V.Y. Mesoporous silica nanoparticles for drug delivery and biosensing applications. Adv. Funct. Mater. 2007, 17, 1225–1236. [Google Scholar] [CrossRef]
- Manzano, M.; Vallet-Regí, M. Mesoporous silica nanoparticles for drug delivery. Adv. Funct. Mater. 2020, 30, 1902634. [Google Scholar] [CrossRef]
- Wu, S.-H.; Mou, C.-Y.; Lin, H.-P. Synthesis of mesoporous silica nanoparticles. Chem. Soc. Rev. 2013, 42, 3862–3875. [Google Scholar] [CrossRef]
- Kim, M.; Park, J.H.; Jeong, H.; Hong, J.; Choi, W.S.; Lee, B.H.; Park, C.Y. An evaluation of the in vivo safety of nonporous silica nanoparticles: Ocular topical administration versus oral administration. Sci. Rep. 2017, 7, 8238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Z.; Barnes, J.C.; Bosoy, A.; Stoddart, J.F.; Zink, J.I. Mesoporous silica nanoparticles in biomedical applications. Chem. Soc. Rev. 2012, 41, 2590–2605. [Google Scholar] [CrossRef] [PubMed]
- Jokinen, M.; Leino, L.; Griffin, C.; Pitkäkatu, I. New solutions for ophthalmic drug delivery using biodegradable silica matrix. OnDrugDelivery 2016, 63, 28–30. [Google Scholar]
- Dargelas, F.; Forsback, A.; Mattila, A.; Leino, L.; Noppari, P.; Reay, M.; Terjung, C.; Unger, F.; Baerfacker, L. Development of injectable long-acting controlled release intravitreal depot of BAY224 using biodegradable silica microparticle-silica hydrogel composite. Acta Ophthalmol. 2021, 99, 1357. [Google Scholar] [CrossRef]
- Ways, T.M.; Ng, K.W.; Lau, W.M.; Khutoryanskiy, V.V. Silica nanoparticles in transmucosal drug delivery. Pharmaceutics 2020, 12, 751. [Google Scholar] [CrossRef]
- Delsitech, August 2021. Available online: https://www.delsitech.com/pipeline (accessed on 11 February 2022).
- Mehmood, A.; Ghafar, H.; Yaqoob, S.; Gohar, U.F.; Ahmad, B. Mesoporous silica nanoparticles: A review. J. Dev. Drugs 2017, 6, 1–14. [Google Scholar] [CrossRef]
- Zaky, R.; Hessien, M.; El-Midany, A.; Khedr, M.; Abdel-Aal, E.; El-Barawy, K. Preparation of silica nanoparticles from semi-burned rice straw ash. Powder Technol. 2008, 185, 31–35. [Google Scholar] [CrossRef]
- Lenza, R.F.; Vasconcelos, W.L. Preparation of silica by sol-gel method using formamide. Mater. Res. 2001, 4, 189–194. [Google Scholar] [CrossRef] [Green Version]
- Yang, X.; Liu, X.; Zhang, A.; Lu, D.; Li, G.; Zhang, Q.; Liu, Q.; Jiang, G. Distinguishing the sources of silica nanoparticles by dual isotopic fingerprinting and machine learning. Nat. Commun. 2019, 10, 1620. [Google Scholar] [CrossRef]
- Kierys, A.; Zaleski, R.; Grochowicz, M.; Gorgol, M.; Sienkiewicz, A. Polymer–mesoporous silica composites for drug release systems. Microporous Mesoporous Mater. 2020, 294, 109881. [Google Scholar] [CrossRef]
- Finnie, K.S.; Bartlett, J.R.; Barbé, C.J.; Kong, L. Formation of silica nanoparticles in microemulsions. Langmuir 2007, 23, 3017–3024. [Google Scholar] [CrossRef] [PubMed]
- Balthis, J.H. Preparation of Silica Sols from Finely Divided Silicon. U.S. Patent No. 2614994, 21 October 1952. [Google Scholar]
- Guo, J.; Liu, X.; Cheng, Y.; Li, Y.; Xu, G.; Cui, P. Size-controllable synthesis of monodispersed colloidal silica nanoparticles via hydrolysis of elemental silicon. J. Colloid Interface Sci 2008, 326, 138–142. [Google Scholar] [CrossRef] [PubMed]
- Dubashynskaya, N.; Poshina, D.; Raik, S.; Urtti, A.; Skorik, Y.A. Polysaccharides in ocular drug delivery. Pharmaceutics 2020, 12, 22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dubashynskaya, N.V.; Bokatyi, A.N.; Golovkin, A.S.; Kudryavtsev, I.V.; Serebryakova, M.K.; Trulioff, A.S.; Dubrovskii, Y.A.; Skorik, Y.A. Synthesis and Characterization of Novel Succinyl Chitosan-Dexamethasone Conjugates for Potential Intravitreal Dexamethasone Delivery. Int. J. Mol. Sci. 2021, 22, 10960. [Google Scholar] [CrossRef] [PubMed]
- De Campos, A.M.; Diebold, Y.; Carvalho, E.L.; Sánchez, A.; José Alonso, M. Chitosan nanoparticles as new ocular drug delivery systems: In vitro stability, in vivo fate, and cellular toxicity. Pharm. Res. 2004, 21, 803–810. [Google Scholar] [CrossRef] [PubMed]
- Alonso, M.J.; Sánchez, A. The potential of chitosan in ocular drug delivery. J. Pharm. Pharmacol. 2003, 55, 1451–1463. [Google Scholar] [CrossRef] [PubMed]
- He, P.; Davis, S.S.; Illum, L. In vitro evaluation of the mucoadhesive properties of chitosan microspheres. Int. J. Pharm. 1998, 166, 75–88. [Google Scholar] [CrossRef]
- Zamboulis, A.; Nanaki, S.; Michailidou, G.; Koumentakou, I.; Lazaridou, M.; Ainali, N.M.; Xanthopoulou, E.; Bikiaris, D.N. Chitosan and its derivatives for ocular delivery formulations: Recent advances and developments. Polym. J. 2020, 12, 1519. [Google Scholar] [CrossRef]
- De Campos, A.M.; Sánchez, A.; Alonso, M.J. Chitosan nanoparticles: A new vehicle for the improvement of the delivery of drugs to the ocular surface. Application to cyclosporin A. Int. J. Pharm. 2001, 224, 159–168. [Google Scholar] [CrossRef]
- Salama, A.H.; Abdelkhalek, A.A.; Elkasabgy, N.A. Etoricoxib-loaded bio-adhesive hybridized polylactic acid-based nanoparticles as an intra-articular injection for the treatment of osteoarthritis. Int. J. Pharm. 2020, 578, 119081. [Google Scholar] [CrossRef] [PubMed]
- Loftsson, T.; Brewster, M.E. Cyclodextrins as functional excipients: Methods to enhance complexation efficiency. J. Pharm. Sci. 2012, 101, 3019–3032. [Google Scholar] [CrossRef] [PubMed]
- Pandey, A. Cyclodextrin-based nanoparticles for pharmaceutical applications: A review. Environ. Chem. Lett. 2021, 19, 4297–4310. [Google Scholar] [CrossRef]
- Arora, D.; Saneja, A.; Jaglan, S. Cyclodextrin-based delivery systems for dietary pharmaceuticals. Environ. Chem. Lett. 2019, 17, 1263–1270. [Google Scholar] [CrossRef]
- Vega, E.; Egea, M.A.; Calpena, A.C.; Espina, M.; García, M.L. Role of hydroxypropyl-β-cyclodextrin on freeze-dried and gamma-irradiated PLGA and PLGA–PEG diblock copolymer nanospheres for ophthalmic flurbiprofen delivery. Int. J. Nanomed. 2012, 7, 1357–1371. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gharib, R.; Haydar, S.; Charcosset, C.; Fourmentin, S.; Greige-Gerges, H. First study on the release of a natural antimicrobial agent, estragole, from freeze-dried delivery systems based on cyclodextrins and liposomes. J. Drug Deliv. Sci. Technol. 2019, 52, 794–802. [Google Scholar] [CrossRef]
- Van den Hoven, J.M.; Metselaar, J.M.; Storm, G.; Beijnen, J.H.; Nuijen, B. Cyclodextrin as membrane protectant in spray-drying and freeze-drying of PEGylated liposomes. Int. J. Pharm. 2012, 438, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.Y.J.; Al-Salami, H.; Dass, C.R. β-Cyclodextrin-containing chitosan-oligonucleotide nanoparticles improve insulin bioactivity, gut cellular permeation and glucose consumption. J. Pharm. Pharmacol. 2021, 73, 726–739. [Google Scholar] [CrossRef]
- Mahmoud, A.A.; Salama, A.H.; Shamma, R.N.; Farouk, F. Bioavailability enhancement of aripiprazole via silicosan particles: Preparation, characterization and in vivo evaluation. AAPS PharmSciTech 2018, 19, 3751–3762. [Google Scholar] [CrossRef] [PubMed]
- Ryan, C.; Alcock, E.; Buttimer, F.; Schmidt, M.; Clarke, D.; Pemble, M.; Bardosova, M. Synthesis and characterisation of cross-linked chitosan composites functionalised with silver and gold nanoparticles for antimicrobial applications. Sci. Technol. Adv. Mater. 2017, 18, 528–540. [Google Scholar] [CrossRef]
- Ryan, C.; Delezuk, J.; Pavinatto, A.; Oliveira, O.; Fudouzi, H.; Pemble, M.; Bardosova, M. Silica-based photonic crystals embedded in a chitosan-TEOS matrix: Preparation, properties and proposed applications. J. Mater. Sci. 2016, 51, 5388–5396. [Google Scholar] [CrossRef]
- Elnaggar, Y.S.; Talaat, S.M.; Bahey-El-Din, M.; Abdallah, O.Y. Novel lecithin-integrated liquid crystalline nanogels for enhanced cutaneous targeting of terconazole: Development, in vitro and in vivo studies. Int. J. Nanomed. 2016, 11, 5531–5547. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maged, A.; Mahmoud, A.A.; Ghorab, M.M. Nano spray drying technique as a novel approach to formulate stable econazole nitrate nanosuspension formulations for ocular use. Mol. Pharm. 2016, 13, 2951–2965. [Google Scholar] [CrossRef]
- Mahmoud, A.A.; El-Feky, G.S.; Kamel, R.; Awad, G.E. Chitosan/sulfobutylether-β-cyclodextrin nanoparticles as a potential approach for ocular drug delivery. Int. J. Pharm. 2011, 413, 229–236. [Google Scholar] [CrossRef]
- Banchroft, J.; Stevens, A.; Turner, D.J.N.Y. Theory and Practice of Histological Techniques, 4th ed.; Churchil Livingstone: London, UK; San Francisco, CA, USA; Tokyo, Japan, 1996. [Google Scholar]
- Mosallam, S.; Ragaie, M.H.; Moftah, N.H.; Elshafeey, A.H.; Abdelbary, A.A. Use of novasomes as a vesicular carrier for improving the topical delivery of terconazole: In vitro characterization, in vivo assessment and exploratory clinical experimentation. Int. J. Nanomed. 2021, 16, 119–132. [Google Scholar] [CrossRef] [PubMed]
- Patravale, V.; Date, A.A.; Kulkarni, R. Nanosuspensions: A promising drug delivery strategy. J. Pharm. Pharmacol. 2004, 56, 827–840. [Google Scholar] [CrossRef] [Green Version]
- El-Mahrouk, G.; Aboul-Einien, M.H.; Elkasabgy, N.A. Formulation and evaluation of meloxicam orally dispersible capsules. Asian J. Pharm. Sci 2009, 4, 8–22. [Google Scholar]
- Adel, I.M.; ElMeligy, M.F.; Abdelrahim, M.E.; Maged, A.; Abdelkhalek, A.A.; Abdelmoteleb, A.M.; Elkasabgy, N.A. Design and characterization of spray-dried proliposomes for the pulmonary delivery of curcumin. Int. J. Nanomed. 2021, 16, 2667–2687. [Google Scholar] [CrossRef]
- Donatti, D.A.; Ruiz, A.I.; Vollet, D.R. A dissolution and reaction modeling for hydrolysis of TEOS in heterogeneous TEOS–water–HCl mixtures under ultrasound stimulation. Ultrason. Sonochem. 2002, 9, 133–138. [Google Scholar] [CrossRef]
- Qi, D.; Lin, C.; Zhao, H.; Liu, H.; Lü, T. Size regulation and prediction of the SiO2 nanoparticles prepared via Stöber process. J. Dispers. Sci. Technol. 2017, 38, 70–74. [Google Scholar] [CrossRef]
- dos Santos, J.-F.R.; Couceiro, R.; Concheiro, A.; Torres-Labandeira, J.-J.; Alvarez-Lorenzo, C. Poly (hydroxyethyl methacrylate-co-methacrylated-β-cyclodextrin) hydrogels: Synthesis, cytocompatibility, mechanical properties and drug loading/release properties. Acta Biomater. 2008, 4, 745–755. [Google Scholar] [CrossRef]
- Mahmoud, A.A.; Elkasabgy, N.A.; Abdelkhalek, A.A. Design and characterization of emulsified spray dried alginate microparticles as a carrier for the dually acting drug roflumilast. Eur. J. Pharm. Sci. 2018, 122, 64–76. [Google Scholar] [CrossRef]
- Di Cagno, M.; Stein, P.C.; Skalko-Basnet, N.; Brandl, M.; Bauer-Brandl, A. Solubilization of ibuprofen with β-cyclodextrin derivatives: Energetic and structural studies. J. Pharm. Biomed. Anal. 2011, 55, 446–451. [Google Scholar] [CrossRef] [PubMed]
- Rizvi, S.A.; Saleh, A.M. Applications of nanoparticle systems in drug delivery technology. Saudi Pharm. J. 2018, 26, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Panyam, J.; Dali, M.M.; Sahoo, S.K.; Ma, W.; Chakravarthi, S.S.; Amidon, G.L.; Levy, R.J.; Labhasetwar, V. Polymer degradation and in vitro release of a model protein from poly (D, L-lactide-co-glycolide) nano-and microparticles. J. Control. Release 2003, 92, 173–187. [Google Scholar] [CrossRef]
- Williams, M. The Mathematics of Diffusion: By J. Crank; Clarendon Press: Oxford, UK, 1975; p. 414. [Google Scholar]
- Cantin, O.; Siepmann, F.; Willart, J.-F.; Danede, F.; Siepmann, J.; Karrout, Y. PEO hot melt extrudates for controlled drug delivery: Importance of the type of drug and loading. J. Drug Deliv. Sci. Technol. 2021, 61, 102238. [Google Scholar] [CrossRef]
- Kamel, R.; El-Wakil, N.A.; Abdelkhalek, A.A.; Elkasabgy, N.A. Nanofibrillated cellulose/cyclodextrin based 3D scaffolds loaded with raloxifene hydrochloride for bone regeneration. Int. J. Biol. Macromol. 2020, 156, 704–716. [Google Scholar] [CrossRef] [PubMed]
- Judefeind, A.; de Villiers, M.M. Drug loading into and in vitro release from nanosized drug delivery systems. In Nanotechnology in Drug Delivery; Springer: Berlin/Heidelberg, Germany, 2009; pp. 129–162. [Google Scholar]
- Kamel, R.; El-Wakil, N.A.; Abdelkhalek, A.A.; Elkasabgy, N.A. Topical cellulose nanocrystals-stabilized nanoemulgel loaded with ciprofloxacin HCl with enhanced antibacterial activity and tissue regenerative properties. J. Drug Deliv. Sci. Technol. 2021, 64, 102553. [Google Scholar] [CrossRef]
- Higazy, I.M.; Mahmoud, A.A.; Ghorab, M.M.; Ammar, H.O. Development and evaluation of polyvinyl alcohol stabilized polylactide-co-caprolactone-based nanoparticles for brain delivery. J. Drug Deliv. Sci. Technol. 2021, 61, 102274. [Google Scholar] [CrossRef]
- Silva, S.S.; Oliveira, J.M.; Mano, J.F.; Reis, R.L. Physicochemical characterization of novel chitosan-soy protein/TEOS porous hybrids for tissue engineering applications. Proc. Mater. Sci. Forum 2006, 514, 1000–1004. [Google Scholar] [CrossRef]
- Mantelli, F.; Argüeso, P. Functions of ocular surface mucins in health and disease. Curr. Opin. Allergy Clin. Immunol. 2008, 8, 477–483. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silva, C.A.; Nobre, T.M.; Pavinatto, F.J.; Oliveira, O.N., Jr. Interaction of chitosan and mucin in a biomembrane model environment. J. Colloid Interface Sci. 2012, 376, 289–295. [Google Scholar] [CrossRef]
- Sun, Y.; Huffman, K.; Freeman, W.R.; Sailor, M.J.; Cheng, L. Intravitreal safety profiles of sol-gel mesoporous silica microparticles and the degradation product (Si(OH)4). Drug Deliv. 2020, 27, 703–711. [Google Scholar] [CrossRef]
- Kim, J.-Y.; Park, J.-H.; Kim, M.; Jeong, H.; Hong, J.; Chuck, R.S.; Park, C.Y. Safety of nonporous silica nanoparticles in human corneal endothelial cells. Sci. Rep. 2017, 7, 14566. [Google Scholar] [CrossRef] [Green Version]
- Ahuja, M.; Verma, P.; Bhatia, M. Preparation and evaluation of chitosan–itraconazole co-precipitated nanosuspension for ocular delivery. J. Exp. Nanosci. 2015, 10, 209–221. [Google Scholar] [CrossRef]
- Başaran, E.; Yazan, Y. Ocular application of chitosan. Expert Opin. Drug Deliv. 2012, 9, 701–712. [Google Scholar] [CrossRef]
- Chaudhari, P.; Ghate, V.M.; Lewis, S.A. Supramolecular cyclodextrin complex: Diversity, safety, and applications in ocular therapeutics. Exp. Eye Res. 2019, 189, 107829. [Google Scholar] [CrossRef]
- Mahmoudi, A.; Malaekeh-Nikouei, B.; Hanafi-Bojd, M.Y.; Toloei, M.; Hosseini, M.; Nikandish, M. Preliminary in vivo safety evaluation of a tacrolimus eye drop formulation using hydroxypropyl beta cyclodextrin after ocular administration in NZW rabbits. Clin. Ophthalmol. 2020, 14, 947–953. [Google Scholar] [CrossRef] [Green Version]
- Loftssona, T.; Järvinen, T. Cyclodextrins in ophthalmic drug delivery. Adv. Drug Deliv. Rev. 1999, 36, 59–79. [Google Scholar] [CrossRef]
- Irimia, T.; Ghica, M.V.; Popa, L.; Anuţa, V.; Arsene, A.-L.; Dinu-Pîrvu, C.-E. Strategies for improving ocular drug bioavailability and corneal wound healing with chitosan-based delivery systems. Polym. J. 2018, 10, 1221. [Google Scholar] [CrossRef] [Green Version]
- Boddupalli, B.M.; Mohammed, Z.N.; Nath, R.A.; Banji, D. Mucoadhesive drug delivery system: An overview. J. Adv. Pharm. Technol. Res. 2010, 1, 381–387. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yeh, T.-H.; Hsu, L.-W.; Tseng, M.T.; Lee, P.-L.; Sonjae, K.; Ho, Y.-C.; Sung, H.-W. Mechanism and consequence of chitosan-mediated reversible epithelial tight junction opening. Biomaterials 2011, 32, 6164–6173. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.-Y.; Hao, J.-L.; Wang, S.; Zheng, Y.; Zhang, W.-S. Nanoparticles in the ocular drug delivery. Int. J. Ophthalmol. 2013, 6, 390–396. [Google Scholar] [PubMed]
- Weng, Y.; Liu, J.; Jin, S.; Guo, W.; Liang, X.; Hu, Z. Nanotechnology-based strategies for treatment of ocular disease. Acta Pharm. Sin. B 2017, 7, 281–291. [Google Scholar] [CrossRef] [Green Version]


| Code * | Independent Variables | Responses | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Water (% v/v) in the Hydroalcoholic Solution | TEOS (%w/v) | Cryoprotectant Type; Percentage (%w/v) | Particle Size (PS; nm) | PDI | Yield (%) | Drug Loading (DL; %) | Q2 (%) | Q6 (%) | |
| SCM1 | 50 | 5 | MβCD; 7.5 | 399.50 ± 2.12 | 0.621 ± 0.012 | 30.44 ± 0.57 | 0.48 ± 0.01 | 21.92 ± 0.94 | 52.51 ± 0.42 |
| SCM2 | 50 | 5 | MβCD; 15 | 322.80 ± 37.68 | 0.634 ± 0.014 | 70.38 ± 0.36 | 0.24 ± 0.02 | 15.97 ± 0.40 | 43.62 ± 0.54 |
| SCM3 | 50 | 10 | MβCD; 7.5 | 329.50 ± 38.32 | 0.458 ± 0.009 | 57.36 ± 0.34 | 0.27 ± 0.03 | 19.66 ± 0.83 | 46.78 ± 0.70 |
| SCM4 | 50 | 10 | MβCD; 15 | 353.67 ± 41.67 | 0.465 ± 0.002 | 68.36 ± 0.23 | 0.21 ± 0.01 | 13.88 ± 0.35 | 43.69 ± 0.80 |
| SCM5 | 50 | 15 | MβCD; 7.5 | 316.33 ± 28.02 | 0.229 ± 0.009 | 23.48 ± 0.11 | 0.28 ± 0.03 | 23.32 ± 0.43 | 46.68 ± 0.89 |
| SCM6 | 50 | 15 | MβCD; 15 | 376.50 ± 7.78 | 0.238 ± 0.012 | 41.71 ± 0.21 | 0.19 ± 0.01 | 27.10 ± 0.30 | 51.63 ± 0.74 |
| SCM7 | 70 | 5 | MβCD; 7.5 | 560.14 ± 38.13 | 0.767 ± 0.097 | 62.55 ± 0.55 | 0.63 ± 0.01 | 21.34 ± 0.30 | 50.77 ± 0.71 |
| SCM8 | 70 | 5 | MβCD; 15 | 538.60 ± 33.15 | 0.747 ± 0.006 | 82.34 ± 0.23 | 0.30 ± 0.01 | 14.44 ± 0.64 | 44.60 ± 0.69 |
| SCM9 | 70 | 10 | MβCD; 7.5 | 451.33 ± 57.28 | 0.518 ± 0.008 | 58.38 ± 0.37 | 0.34 ± 0.02 | 22.42 ± 0.65 | 48.68 ± 0.50 |
| SCM10 | 70 | 10 | MβCD; 15 | 455.67 ± 60.87 | 0.551 ± 0.025 | 70.35 ± 0.32 | 0.20 ± 0.01 | 13.97 ± 0.33 | 38.31 ± 0.35 |
| SCM11 | 70 | 15 | MβCD; 7.5 | 363.00 ± 44.44 | 0.341 ± 0.007 | 42.44 ± 0.39 | 0.23 ± 0.01 | 24.99 ± 0.94 | 45.32 ± 0.09 |
| SCM12 | 70 | 15 | MβCD; 15 | 344.50 ± 41.33 | 0.319 ± 0.006 | 61.47 ± 0.19 | 0.18 ± 0.01 | 15.17 ± 0.25 | 40.14 ± 0.23 |
| SCH1 | 50 | 5 | HPβCD; 7.5 | 383.50 ± 21.92 | 0.633 ± 0.014 | 23.43 ± 0.40 | 0.36 ± 0.01 | 12.94 ± 0.83 | 44.50 ± 0.43 |
| SCH2 | 50 | 5 | HPβCD; 15 | 348.00 ± 39.10 | 0.623 ± 0.012 | 55.62 ± 0.42 | 0.17 ± 0.02 | 8.50 ± 0.08 | 35.06 ± 0.15 |
| SCH3 | 50 | 10 | HPβCD; 7.5 | 338.67 ± 35.23 | 0.449 ± 0.002 | 55.66 ± 0.46 | 0.34 ± 0.02 | 19.52 ± 0.35 | 39.11 ± 0.22 |
| SCH4 | 50 | 10 | HPβCD; 15 | 322.67 ± 37.81 | 0.427 ± 0.004 | 69.57 ± 0.58 | 0.13 ± 0.01 | 20.24 ± 0.96 | 41.72 ± 0.23 |
| SCH5 | 50 | 15 | HPβCD; 7.5 | 375.50 ± 14.85 | 0.228 ± 0.007 | 19.36 ± 0.32 | 0.24 ± 0.01 | 20.75 ± 0.35 | 42.84 ± 0.41 |
| SCH6 | 50 | 15 | HPβCD; 15 | 325.00 ± 46.78 | 0.224 ± 0.017 | 35.73 ± 0.19 | 0.13 ± 0.00 | 16.55 ± 0.33 | 30.47 ± 0.68 |
| SCH7 | 70 | 5 | HPβCD; 7.5 | 594.00 ± 28.28 | 0.746 ± 0.006 | 78.71 ± 0.21 | 0.35 ± 0.01 | 15.86 ± 0.16 | 39.90 ± 0.59 |
| SCH8 | 70 | 5 | HPβCD; 15 | 510.50 ± 10.61 | 0.756 ± 0.007 | 84.42 ± 0.37 | 0.25 ± 0.04 | 8.92 ± 0.09 | 28.85 ± 0.40 |
| SCH9 | 70 | 10 | HPβCD; 7.5 | 407.00 ± 21.92 | 0.518 ± 0.006 | 57.43 ± 0.29 | 0.25 ± 0.01 | 15.25 ± 0.09 | 36.32 ± 0.06 |
| SCH10 | 70 | 10 | HPβCD; 15 | 484.75 ± 33.87 | 0.550 ± 0.007 | 72.54 ± 0.45 | 0.15 ± 0.00 | 9.44 ± 0.57 | 32.13 ± 0.01 |
| SCH11 | 70 | 15 | HPβCD; 7.5 | 390.67 ± 34.27 | 0.314 ± 0.009 | 44.37 ± 0.24 | 0.22 ± 0.02 | 16.13 ± 0.47 | 43.00 ± 0.71 |
| SCH12 | 70 | 15 | HPβCD; 15 | 457.00 ± 49.81 | 0.338 ± 0.003 | 63.41 ± 0.37 | 0.16 ± 0.01 | 11.48 ± 0.58 | 14.75 ± 0.40 |
| Pharmacokinetic Parameter | Drug Suspension | SCM8-1 |
|---|---|---|
| Cmax (ng/mL) | 752.2 ± 81.2 | 3449.3 ± 148.6 |
| tmax (h) * | 1 | 7 |
| AUC (ng.h/mL) | 2494.1 ± 274.0 | 49197.4 ± 5235.7 |
| MRT (h) | 4.9 ± 0.3 | 8.3 ± 0.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zaghloul, N.; El Hoffy, N.M.; Mahmoud, A.A.; Elkasabgy, N.A. Cyclodextrin Stabilized Freeze-Dried Silica/Chitosan Nanoparticles for Improved Terconazole Ocular Bioavailability. Pharmaceutics 2022, 14, 470. https://doi.org/10.3390/pharmaceutics14030470
Zaghloul N, El Hoffy NM, Mahmoud AA, Elkasabgy NA. Cyclodextrin Stabilized Freeze-Dried Silica/Chitosan Nanoparticles for Improved Terconazole Ocular Bioavailability. Pharmaceutics. 2022; 14(3):470. https://doi.org/10.3390/pharmaceutics14030470
Chicago/Turabian StyleZaghloul, Nada, Nada M. El Hoffy, Azza A. Mahmoud, and Nermeen A. Elkasabgy. 2022. "Cyclodextrin Stabilized Freeze-Dried Silica/Chitosan Nanoparticles for Improved Terconazole Ocular Bioavailability" Pharmaceutics 14, no. 3: 470. https://doi.org/10.3390/pharmaceutics14030470






