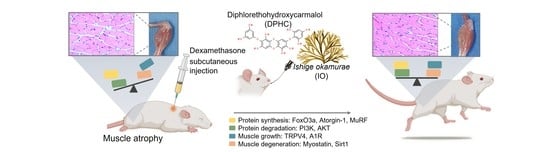
Diphlorethohydroxycarmalol Derived from Ishige okamurae Improves Behavioral and Physiological Responses of Muscle Atrophy Induced by Dexamethasone in an In-Vivo Model
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Dex-Induced Muscle Atrophy Model
2.3. Grip Strength
2.4. Ladder Climbing Exercise
2.5. Measurement of Calf, Gastrocnemius and Soleus Muscle Thickness
2.6. Evaluation of Muscle Histopathology
2.7. Extraction of RNA and Quantitative Real-Time-Polymerase Chain Reaction (qRT-PCR)
2.8. Statistical Analysis
3. Results
3.1. IO Extract and Its Active Component DPHC Improved the Muscle Behavioral Response in DEX-Induced Skeletal Muscle Atrophy-In Vivo Model
3.2. IO Extract and Its Active Component DPHC Improved Calf Skeletal Muscle and Muscle Types in DEX-Induced Skeletal Muscle Atrophy in an In Vivo Model
3.3. IO Extract and Its Active Component DPHC Improved the Metabolic Properties of the Gastrocnemius Muscle in the DEX-Induced Skeletal Muscle Atrophy-In Vivo Model
3.4. IO Extract and Its Active Component DPHC Improved the Metabolic Properties of Soleus Muscle in DEX-Induced Skeletal Muscle Atrophy-In Vivo Model
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yang, H.-W.; Fernando, K.H.N.; Oh, J.-Y.; Li, X.; Jeon, Y.-J.; Ryu, B. Anti-obesity and anti-diabetic effects of Ishige okamurae. Mar. Drugs 2019, 17, 202. [Google Scholar] [CrossRef] [Green Version]
- Yang, H.-W.; Son, M.; Choi, J.; Oh, S.; Jeon, Y.-J.; Byun, K.; Ryu, B. Effect of ishophloroglucin A, a component of Ishige okamurae, on glucose homeostasis in the pancreas and muscle of high fat diet-fed mice. Mar. Drugs 2019, 17, 608. [Google Scholar] [CrossRef] [Green Version]
- Yang, H.-W.; Jiang, Y.-F.; Lee, H.-G.; Jeon, Y.-J.; Ryu, B. Ca2+-Dependent Glucose Transport in Skeletal Muscle by Diphlorethohydroxycarmalol, an Alga Phlorotannin: In Vitro and In Vivo Study. Oxid. Med. Cell. Longev. 2021, 2021, 8893679. [Google Scholar] [CrossRef]
- Jayawardena, T.U.; Kim, S.-Y.; Jeon, Y.-J. Sarcopenia; functional concerns, molecular mechanisms involved, and seafood as a nutritional intervention–review article. Crit. Rev. Food Sci. Nutr. 2021, 1–21. [Google Scholar] [CrossRef]
- Jayawardena, T.U.; Nagahawatta, D.P.; Lu, Y.-A.; Yang, H.-W.; Je, J.-G.; Kim, S.-Y.; Jeon, Y.-J. Ishige okamurae and diphloroethohydoxycarmalol inhibit palmitic acid-impaired skeletal myogenesis and improve muscle regenerative potential. J. Funct. Foods 2021, 87, 104832. [Google Scholar] [CrossRef]
- Kim, S.-Y.; Ahn, G.; Kim, H.-S.; Je, J.-G.; Kim, K.-N.; Jeon, Y.-J. Diphlorethohydroxycarmalol (DPHC) isolated from the brown alga Ishige okamurae acts on inflammatory myopathy as an inhibitory agent of TNF-α. Mar. Drugs 2020, 18, 529. [Google Scholar] [CrossRef]
- Kandarian, S.C.; Jackman, R.W. Intracellular signaling during skeletal muscle atrophy. Muscle Nerve Off. J. Am. Assoc. Electrodiagn. Med. 2006, 33, 155–165. [Google Scholar] [CrossRef]
- Sandri, M. Signaling in muscle atrophy and hypertrophy. Physiology 2008, 23, 160–170. [Google Scholar] [CrossRef] [Green Version]
- Short, K.R.; Bigelow, M.L.; Kahl, J.; Singh, R.; Coenen-Schimke, J.; Raghavakaimal, S.; Nair, K.S. Decline in skeletal muscle mitochondrial function with aging in humans. Proc. Natl. Acad. Sci. USA 2005, 102, 5618–5623. [Google Scholar] [CrossRef] [Green Version]
- Thomas, D.R. Loss of skeletal muscle mass in aging: Examining the relationship of starvation, sarcopenia and cachexia. Clin. Nutr. 2007, 26, 389–399. [Google Scholar] [CrossRef]
- Mitchell, W.K.; Atherton, P.J.; Williams, J.; Larvin, M.; Lund, J.N.; Narici, M. Sarcopenia, dynapenia, and the impact of advancing age on human skeletal muscle size and strength; a quantitative review. Front. Physiol. 2012, 3, 260. [Google Scholar] [CrossRef] [Green Version]
- Falcon, L.J.; Harris-Love, M.O. Sarcopenia and the new ICD-10-CM code: Screening, staging, and diagnosis considerations. Fed. Pract. 2017, 34, 24. [Google Scholar]
- Schakman, O.; Kalista, S.; Barbé, C.; Loumaye, A.; Thissen, J.-P. Glucocorticoid-induced skeletal muscle atrophy. Int. J. Biochem. Cell Biol. 2013, 45, 2163–2172. [Google Scholar] [CrossRef]
- De Kloet, E.R.; Joëls, M.; Holsboer, F. Stress and the brain: From adaptation to disease. Nat. Rev. Neurosci. 2005, 6, 463–475. [Google Scholar] [CrossRef]
- Kuo, T.; Harris, C.A.; Wang, J.-C. Metabolic functions of glucocorticoid receptor in skeletal muscle. Mol. Cell. Endocrinol. 2013, 380, 79–88. [Google Scholar] [CrossRef] [Green Version]
- Bhasin, S.; Storer, T.W.; Berman, N.; Callegari, C.; Clevenger, B.; Phillips, J.; Bunnell, T.J.; Tricker, R.; Shirazi, A.; Casaburi, R.; et al. Activation of the ubiquitin pathway in rat skeletal muscle by catabolic doses of glucocorticoids. N. Engl. J. Med. 1997, 272, C1007–C1016. [Google Scholar]
- Komamura, K.; Shirotani-Ikejima, H.; Tatsumi, R.; Tsujita-Kuroda, Y.; Kitakaze, M.; Miyatake, K.; Sunagawa, K.; Miyata, T. Differential gene expression in the rat skeletal and heart muscle in glucocorticoid-induced myopathy: Analysis by microarray. Cardiovasc. Drugs Ther. 2003, 17, 303–310. [Google Scholar] [CrossRef]
- Cea, L.A.; Balboa, E.; Puebla, C.; Vargas, A.A.; Cisterna, B.A.; Escamilla, R.; Regueira, T.; Sáez, J.C. Dexamethasone-induced muscular atrophy is mediated by functional expression of connexin-based hemichannels. Biochim. Biophys. Acta Mol. Basis Dis. 2016, 1862, 1891–1899. [Google Scholar] [CrossRef]
- Gilson, H.; Schakman, O.; Combaret, L.; Lause, P.; Grobet, L.; Attaix, D.; Ketelslegers, J.-M.; Thissen, J.-P. Myostatin gene deletion prevents glucocorticoid-induced muscle atrophy. Endocrinology 2007, 148, 452–460. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.W.; Ku, S.-K.; Han, M.H.; Kim, K.Y.; Kim, S.G.; Kim, G.-Y.; Hwang, H.J.; Kim, B.W.; Kim, C.M.; Choi, Y.H. The administration of Fructus Schisandrae attenuates dexamethasone-induced muscle atrophy in mice. Int. J. Mol. Med. 2015, 36, 29–42. [Google Scholar] [CrossRef] [Green Version]
- Meyer, M.; Deniselle, M.C.G.; Hunt, H.; de Kloet, E.R.; De Nicola, A.F. The selective glucocorticoid receptor modulator CORT108297 restores faulty hippocampal parameters in Wobbler and corticosterone-treated mice. J. Steroid Biochem. Mol. Biol. 2014, 143, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.E.; Fournier, M.; Da, X.; Lewis, M.I. Time course expression of Foxo transcription factors in skeletal muscle following corticosteroid administration. J. Appl. Physiol. 2010, 108, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Senf, S.M.; Dodd, S.L.; Judge, A.R. FOXO signaling is required for disuse muscle atrophy and is directly regulated by Hsp70. Am. J. Physiol. Physiol. 2010, 298, C38–C45. [Google Scholar] [CrossRef] [Green Version]
- Lim, J.; Lee, Y.J.; Cho, H.; Park, D.; Jung, G.; Ku, S.K.; CHoI, J. Extracellular polysaccharides purified from Aureobasidium pullulans SM-2001 (Polycan) inhibit dexamethasone-induced muscle atrophy in mice. Int. J. Mol. Med. 2018, 41, 1245–1264. [Google Scholar] [CrossRef]
- Pritschow, B.W.; Lange, T.; Kasch, J.; Kunert-Keil, C.; Liedtke, W.; Brinkmeier, H. Functional TRPV4 channels are expressed in mouse skeletal muscle and can modulate resting Ca2+ influx and muscle fatigue. Pflügers Arch. J. Physiol. 2011, 461, 115–122. [Google Scholar] [CrossRef]
- Schnyder, S.; Handschin, C. Skeletal muscle as an endocrine organ: PGC-1α, myokines and exercise. Bone 2015, 80, 115–125. [Google Scholar] [CrossRef] [Green Version]
- Jang, Y.J.; Son, H.J.; Kim, J.-S.; Jung, C.H.; Ahn, J.; Hur, J.; Ha, T.Y.; Lee, S.-J.; Bae, J.H.; Lee, H.H.; et al. Ginsenoside Rg3 upregulates myotube formation and mitochondrial function, thereby protecting myotube atrophy induced by tumor necrosis factor-alpha. J. Ethnopharmacol. 2018, 9, 112054. [Google Scholar]
- Go, G.-Y.; Jo, A.; Seo, D.-W.; Kim, W.-Y.; Kim, Y.K.; So, E.-Y.; Chen, Q.; Kang, J.-S.; Bae, G.-U.; Lee, S.-J. Ginsenoside Rb1 and Rb2 upregulate Akt/mTOR signaling–mediated muscular hypertrophy and myoblast differentiation. J. Ginseng Res. 2020, 44, 435–441. [Google Scholar] [CrossRef]
- Cho, S.; Hong, R.; Yim, P.; Yeom, M.; Lee, B.; Yang, W.M.; Hong, J.; Lee, H.S.; Hahm, D. An herbal formula consisting of Schisandra chinensis (Turcz.) Baill, Lycium chinense Mill and Eucommia ulmoides Oliv alleviates disuse muscle atrophy in rats. J. Ethnopharmacol. 2018, 213, 328–339. [Google Scholar] [CrossRef]
- Tong, T.; Kim, M.; Park, T. α-Cedrene, a Newly Identified Ligand of MOR23, Increases Skeletal Muscle Mass and Strength. Mol. Nutr. Food Res. 2018, 62, 1800173. [Google Scholar] [CrossRef]
- Huang, Y.; Chen, K.; Ren, Q.; Yi, L.; Zhu, J.; Zhang, Q.; Mi, M. Dihydromyricetin attenuates dexamethasone-induced muscle atrophy by improving mitochondrial function via the PGC-1α pathway. Cell. Physiol. Biochem. 2018, 49, 758–779. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.-Y.; Ku, S.-K.; Lee, K.-W.; Song, C.-H.; An, W.G. Muscle-protective effects of Schisandrae Fructus extracts in old mice after chronic forced exercise. J. Ethnopharmacol. 2018, 212, 175–187. [Google Scholar] [CrossRef] [PubMed]
- Shen, S.; Liao, Q.; Liu, J.; Pan, R.; Lee, S.M.; Lin, L. Myricanol rescues dexamethasone-induced muscle dysfunction via a sirtuin 1-dependent mechanism. J. Cachexia. Sarcopenia Muscle 2019, 10, 429–444. [Google Scholar] [CrossRef] [PubMed]
- Schiaffino, S.; Reggiani, C. Molecular diversity of myofibrillar proteins: Gene regulation and functional significance. Physiol. Rev. 1996, 76, 371–423. [Google Scholar] [CrossRef]
- Gao, Y.; Arfat, Y.; Wang, H.; Goswami, N. Muscle atrophy induced by mechanical unloading: Mechanisms and potential countermeasures. Front. Physiol. 2018, 9, 235. [Google Scholar] [CrossRef]
- Schiaffino, S.; Dyar, K.A.; Ciciliot, S.; Blaauw, B.; Sandri, M. Mechanisms regulating skeletal muscle growth and atrophy. FEBS J. 2013, 280, 4294–4314. [Google Scholar] [CrossRef]
- Cohen, S.; Nathan, J.A.; Goldberg, A.L. Muscle wasting in disease: Molecular mechanisms and promising therapies. Nat. Rev. Drug Discov. 2015, 14, 58–74. [Google Scholar] [CrossRef]
- Glass, D.J. Molecular mechanisms modulating muscle mass. Trends Mol. Med. 2003, 9, 344–350. [Google Scholar] [CrossRef]
- Ma, K.; Mallidis, C.; Bhasin, S.; Mahabadi, V.; Artaza, J.; Gonzalez-Cadavid, N.; Arias, J.; Salehian, B. Glucocorticoid-induced skeletal muscle atrophy is associated with upregulation of myostatin gene expression. Am. J. Physiol. Metab. 2003, 285, E363–E371. [Google Scholar] [CrossRef]
- Nikawa, T.; Ulla, A.; Sakakibara, I. Polyphenols and their effects on muscle atrophy and muscle health. Molecules 2021, 26, 4887. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ryu, B.; Oh, S.; Yang, H.-W.; Sosorburam, B.; Chung, D.-M.; Seo, M.; Park, S.-J.; Byun, K.; Jeon, Y.-J. Diphlorethohydroxycarmalol Derived from Ishige okamurae Improves Behavioral and Physiological Responses of Muscle Atrophy Induced by Dexamethasone in an In-Vivo Model. Pharmaceutics 2022, 14, 719. https://doi.org/10.3390/pharmaceutics14040719
Ryu B, Oh S, Yang H-W, Sosorburam B, Chung D-M, Seo M, Park S-J, Byun K, Jeon Y-J. Diphlorethohydroxycarmalol Derived from Ishige okamurae Improves Behavioral and Physiological Responses of Muscle Atrophy Induced by Dexamethasone in an In-Vivo Model. Pharmaceutics. 2022; 14(4):719. https://doi.org/10.3390/pharmaceutics14040719
Chicago/Turabian StyleRyu, Bomi, Seyeon Oh, Hye-Won Yang, Batsukh Sosorburam, Dong-Min Chung, Minyoung Seo, Shin-Jae Park, Kyunghee Byun, and You-Jin Jeon. 2022. "Diphlorethohydroxycarmalol Derived from Ishige okamurae Improves Behavioral and Physiological Responses of Muscle Atrophy Induced by Dexamethasone in an In-Vivo Model" Pharmaceutics 14, no. 4: 719. https://doi.org/10.3390/pharmaceutics14040719