Anti-Inflammatory and Analgesic Evaluation of a Phytochemical Intercalated into Layered Double Hydroxide
Abstract
:1. Introduction
2. Material and Methods
2.1. Reagents
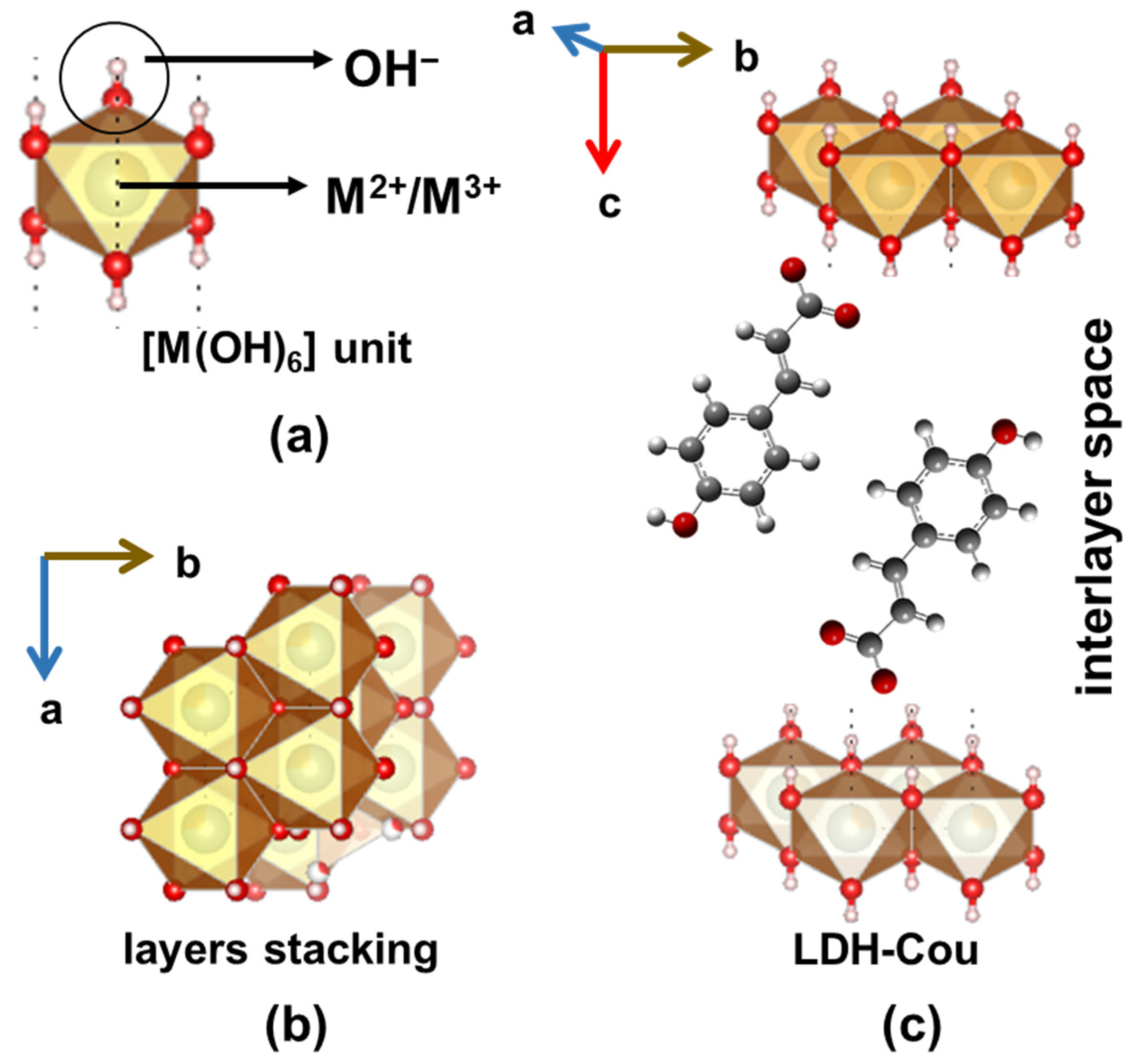
2.2. Preparation of LDH-Cou Material
2.3. In Vitro Assays
2.3.1. Release Kinetics Experiments
2.3.2. Neutral Red Cytotoxicity Assay
2.4. In Vivo Assays
2.4.1. Anti-Inflammatory and Analgesic Evaluation
Anti-Inflammatory Evaluation: Peritonitis Induced by Carrageenan
Anti-Inflammatory and Analgesic Evaluation (Antinociceptive Assay): Formalin Assay
Analgesic and Antinociception Evaluation: Acetic-Acid-Induced Writhing
Antinociception Evaluation: Tail-Flick Assay
2.5. Statistical Analysis
3. Results and Discussion
3.1. Drug Delivery System’s Performance: In Vitro Assays
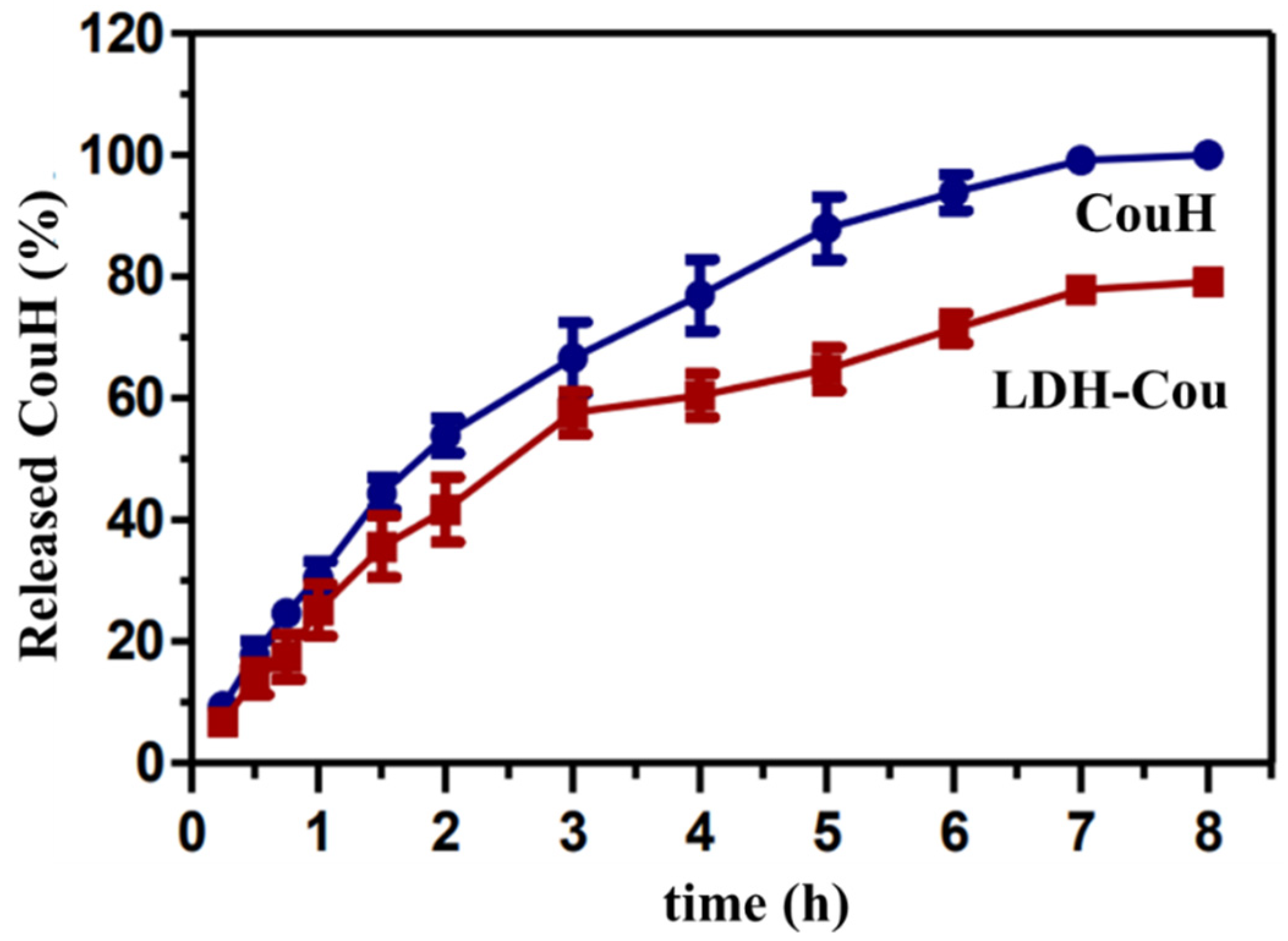
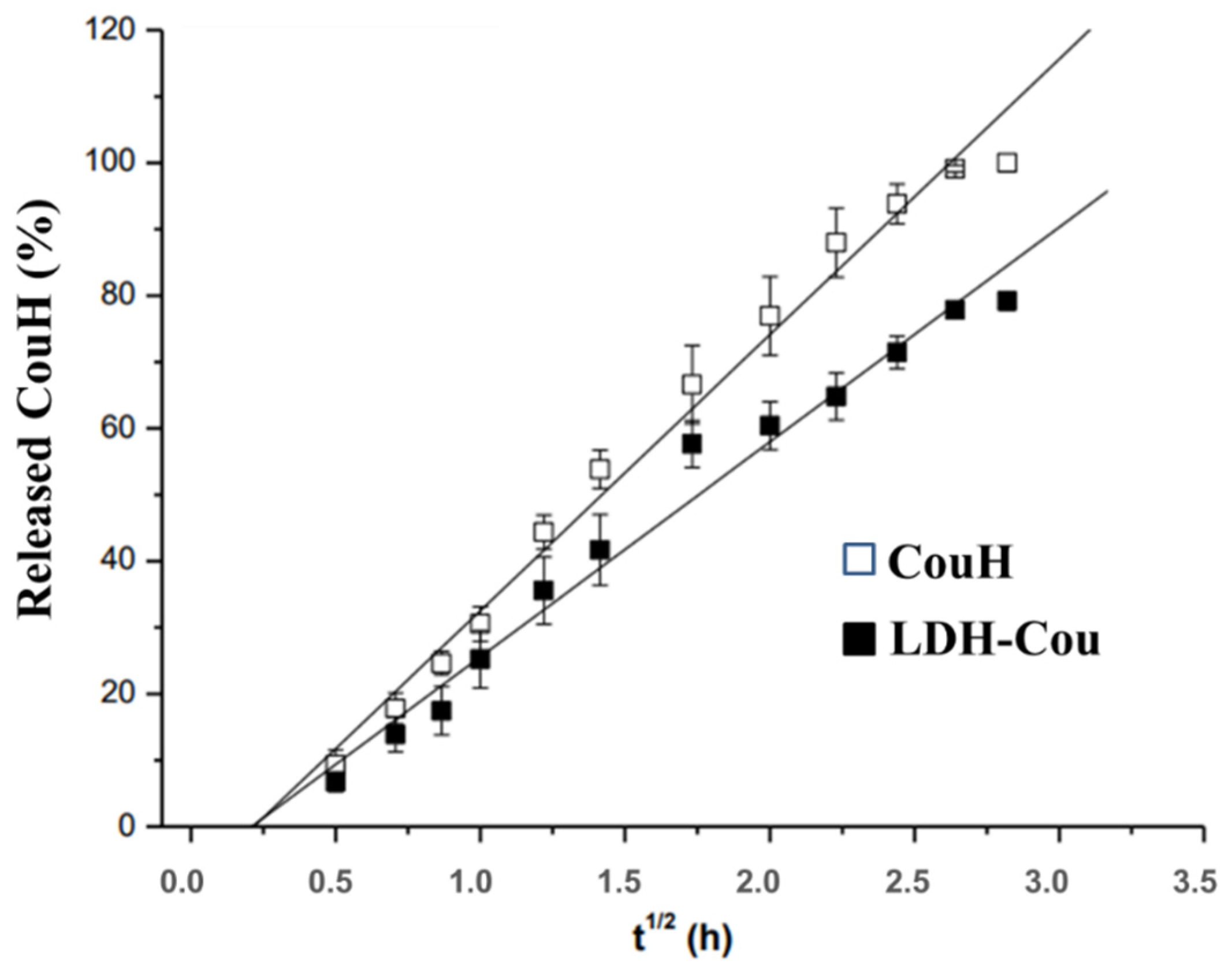
3.1.1. Kinetics Experiments
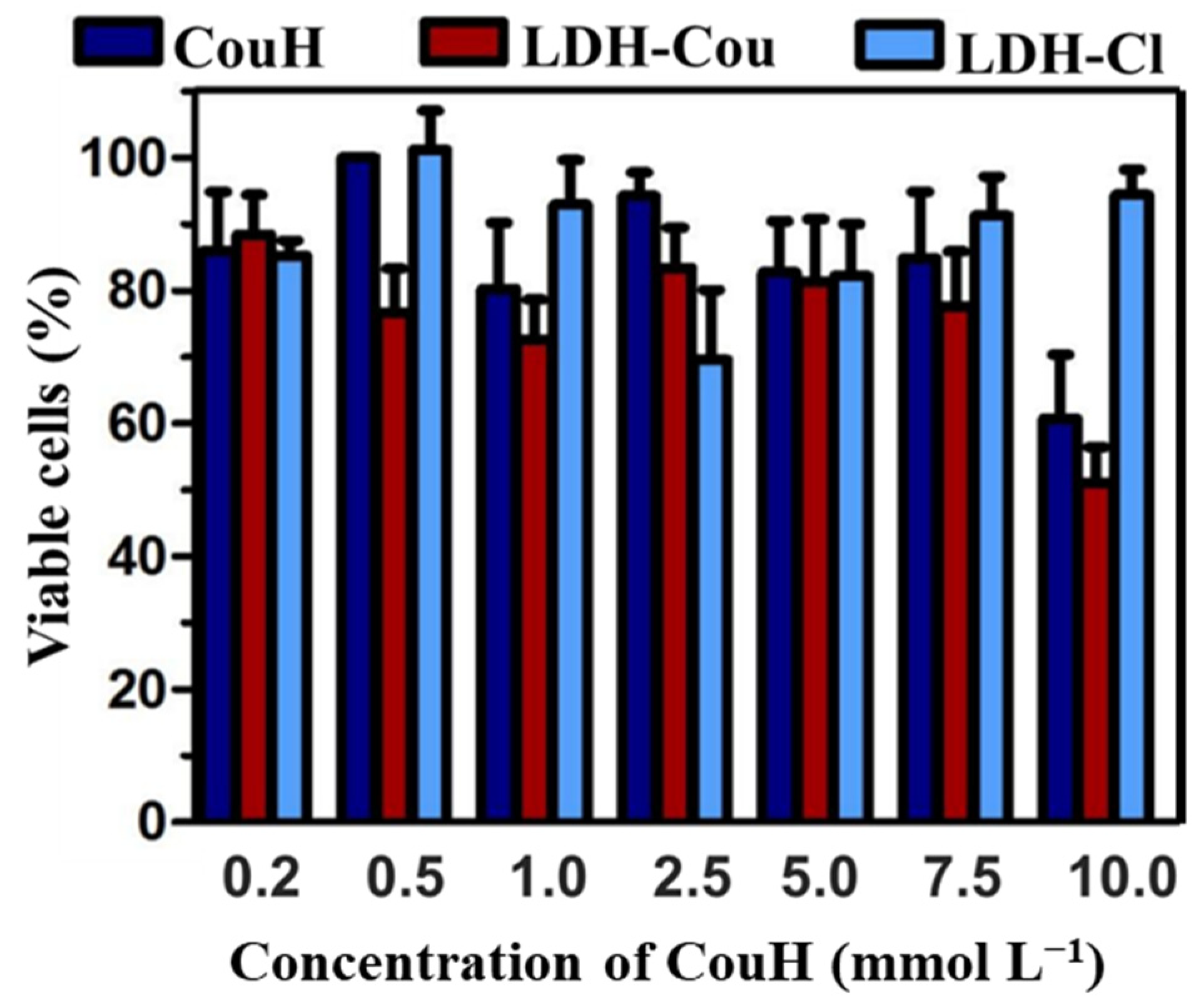
3.1.2. Cytotoxicity Evaluation by the Neutral Red Assay
3.2. In Vivo Pharmacological Assays
3.2.1. Anti-Inflammatory Evaluation: Peritonitis Induced by Carrageenan
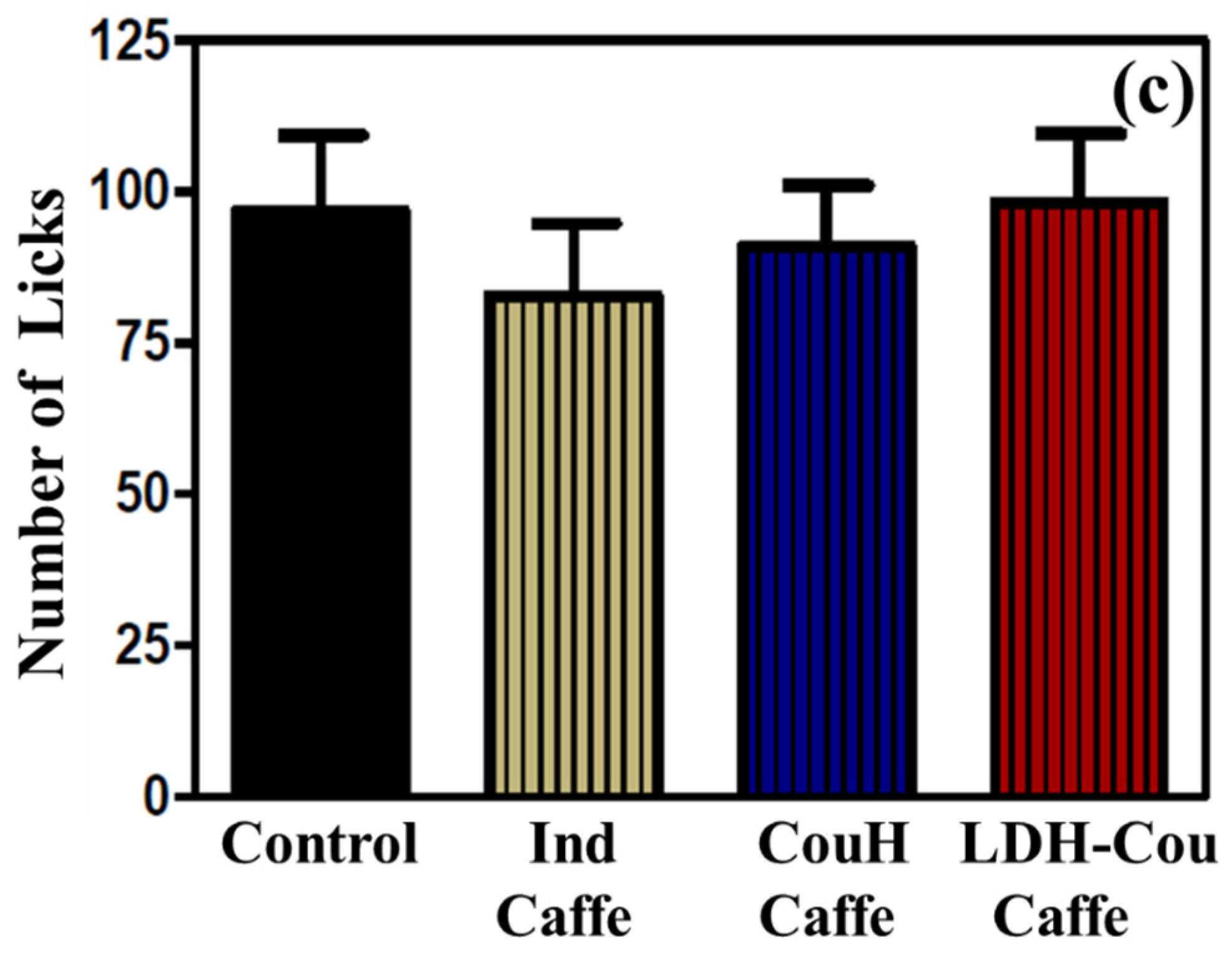
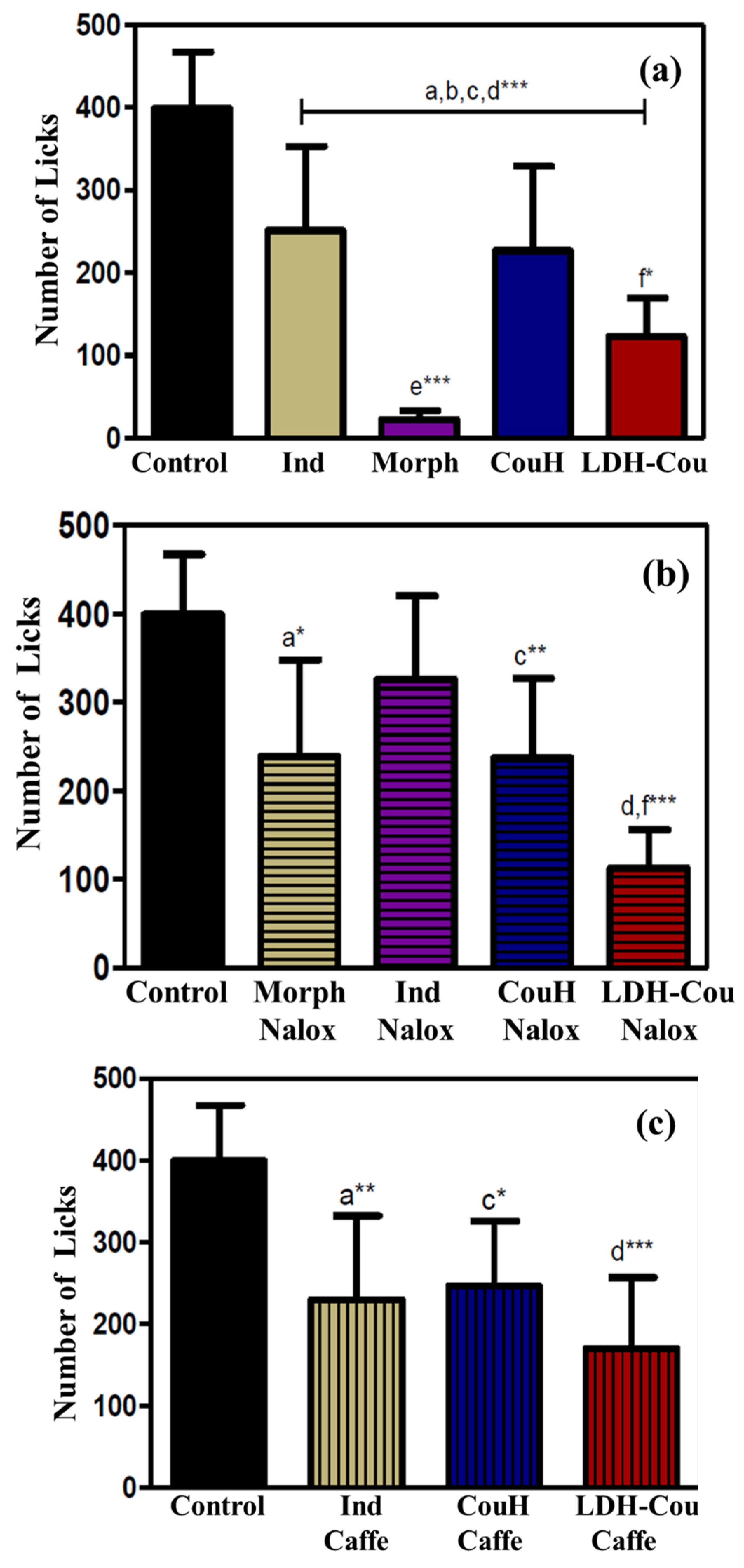
Anti-Inflammatory and Analgesic Evaluation: Formalin Assay
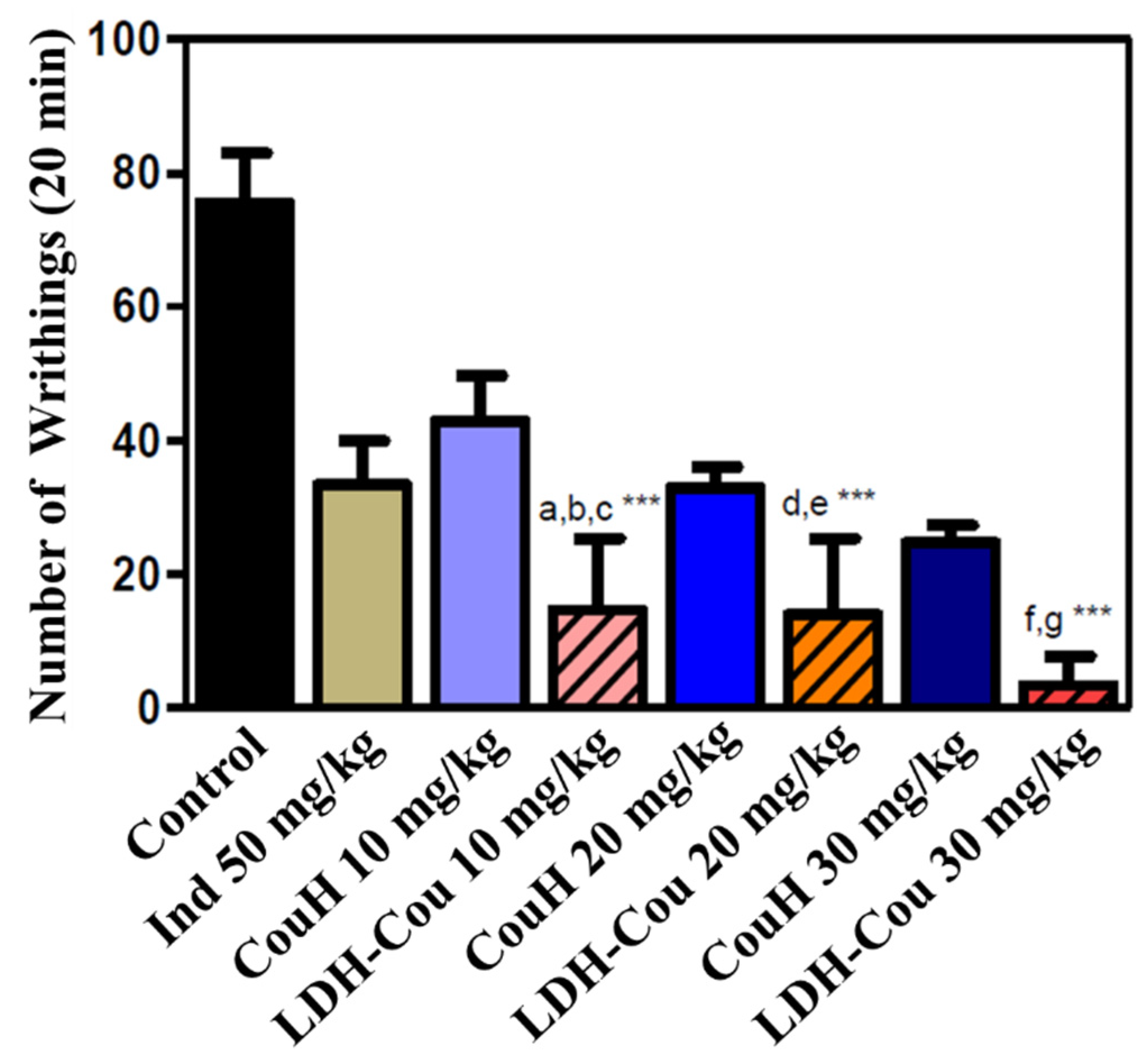
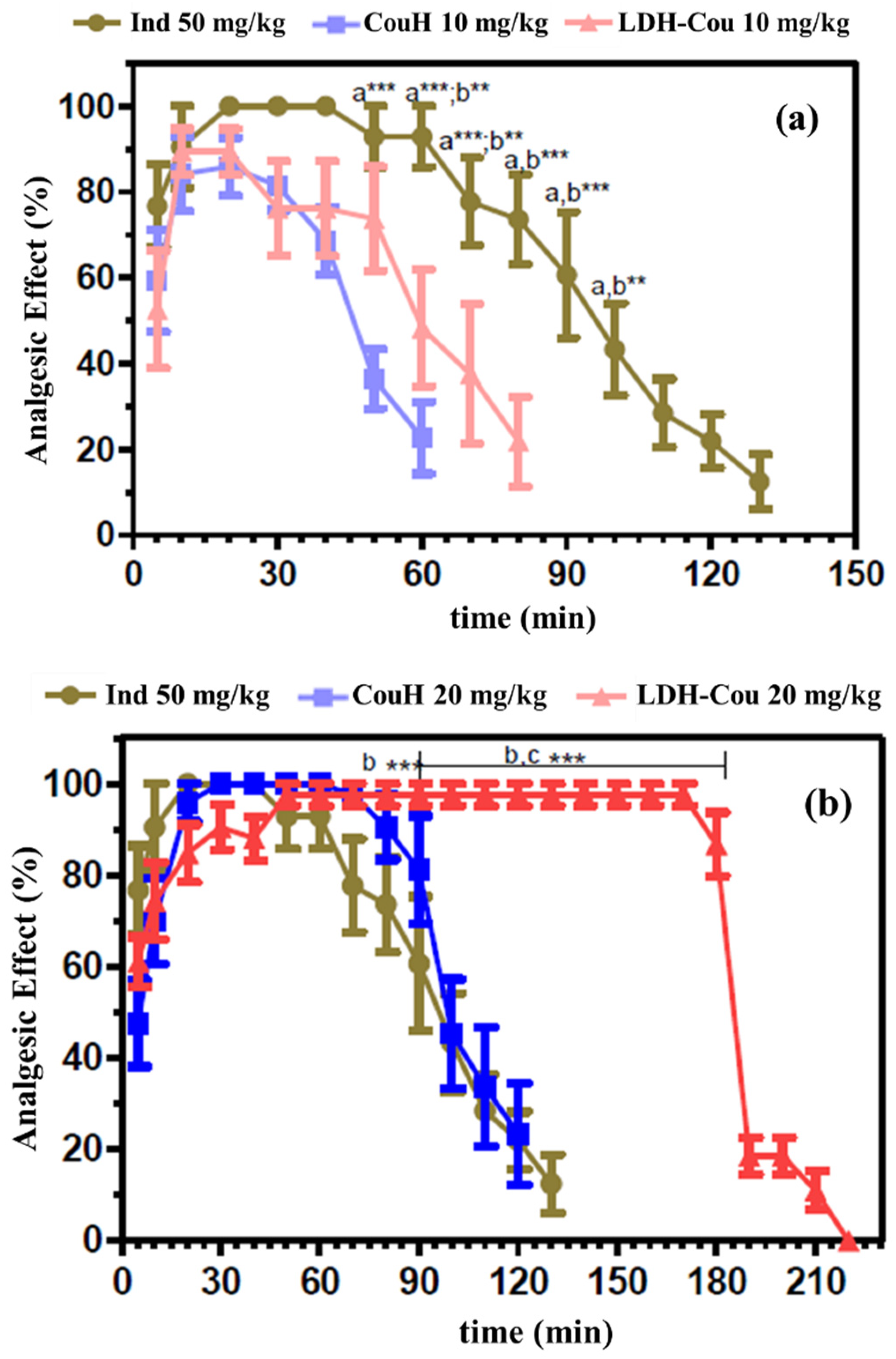
Anti-Inflammatory and Analgesic Evaluation: Acetic-Acid-Induced Writhing and Tail-Flick Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Goldsby, R.; Kindt, T.J.; Osborne, B.A.; Kuby, J. Immunology, 5th ed.; W.H. Freeman and Company: New York, NY, USA, 2003; p. 765. [Google Scholar]
- Furman, D.; Campisi, J.; Verdin, E.; Carrera-Bastos, P.; Targ, S.; Franceschi, C.; Ferrucci, L.; Gilroy, D.W.; Fasano, A.; Miller, G.W.; et al. Chronic inflammation in the etiology of disease across the life span. Nat. Med. 2019, 25, 1822–1832. [Google Scholar] [PubMed]
- Murakami, A.; Ohigashi, H. Targeting NOX, INOS and COX-2 in inflammatory cells: Chemoprevention using food phytochemicals. Int. J. Cancer 2007, 121, 2357–2363. [Google Scholar] [CrossRef]
- Willoughby, D.A.; Moore, A.R.; Colville-Nash, P.R. COX-1, COX-2, and COX3 and the future treatment of chronic inflammatory disease. Lancet 2000, 355, 646–648. [Google Scholar] [CrossRef]
- Rang, H.; Dale, M.; Ritter, J. Farmacologia, 5th ed.; Elsevier: Rio de Janeiro, Brazil, 2003; p. 901. [Google Scholar]
- Gautam, R.; Jachak, S.M. Recent developments in anti-inflammatory natural products. Med. Res. Rev. 2009, 29, 767–820. [Google Scholar] [PubMed]
- Cavalcanti, I.L.; Gozzani, J.L.; Sampaio Filho, A.A.; Alves Neto, O.; Garcia, J.B.S. Dor Pós-Operatória-Sociedade Brasileira de Anestesiologia, 1st ed.; Sociedade Brasileira de Anestesiologia SBA: Rio de Janeiro, Brazil, 2004; p. 425. [Google Scholar]
- Dunder, R.J. Avaliação da atividade analgésica e anti-inflamatória da fração hexânica de Agave sisalana. Master’s Dissertation, Universidade Estadual de Campinas-Instituto de Biologia, Campinas, São Paulo, Brazil, 2009. [Google Scholar]
- Lima, F.O. Envolvimento dos receptores periféricos de adenosina na hipernocicepção inflamatória. Master’s Dissertation, Universidade Federal de São Paulo-Instituto de Ciências Biológicas, Ribeirão Preto, São Paulo, Brazil, 2008. [Google Scholar]
- De Miranda, F.G.; Vilar, J.C.; Alves, I.A.; Cavalcanti, S.C.; Antoniolli, A.R. Antinociceptive and antiedematogenic properties and acute toxicity of Tabebuia avellanedae Lor. ex Griseb. inner bark aqueous extract. BMC Pharmacol. 2001, 1, 6. [Google Scholar] [CrossRef] [Green Version]
- Rahman, M.; Rahman, S.; Islam, R.; Rahman, F.; Mithi, F.M.; Alqahtani, T.; Almiklafi, M.A.; Alghamdi, S.Q.; Alruwaili, A.S.; Hossain, S.; et al. Role of phenolic compounds in human disease: Current knowledge and future prospects. Molecules 2022, 27, 233. [Google Scholar] [CrossRef] [PubMed]
- Contardi, M.; Lenzuni, M.; Fiorentini, F.; Summa, M.; Bertorelli, R.; Suarato, G.; Athanassiou, A. Hydroxycinnamic Acids and Derivatives Formulations for Skin Damages and Disorders: A Review. Pharmaceutics 2021, 13, 999. [Google Scholar] [CrossRef]
- Roychodhury, S.; Sinha, B.; Choudhury, B.P.; Jha, N.K.; Palit, P.; Kundu, S.; Mandal, S.C.; Kolesarova, A.; Yousef, M.I.; Ruokolainen, J.; et al. Scavenging Properties of Plant-Derived Natural Biomolecule Para-Coumaric Acid in the Prevention of Oxidative Stress-Induced Diseases. Antioxidants 2021, 10, 1205. [Google Scholar] [CrossRef] [PubMed]
- Kiliç, I.; Yesiloglu, T. Spectroscopic studies on the antioxidant activity of p-coumaric acid. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2013, 115, 719–724. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Yang, Z.; Liu, W.; Pan, Z.; Zhang, X.; Li, M.; Liu, X.; Zheng, Q.; Li, D. The Anti-tumor Effects of p-Coumaric Acid on Melanoma A375 and B16 Cells. Front. Oncol. 2020, 10, 558414. [Google Scholar] [CrossRef]
- Boz, H. p-Coumaric acid in cereals: Presence, antioxidant and antimicrobial effects. Int. J. Food Sci. 2015, 50, 2323–2328. [Google Scholar] [CrossRef]
- Lou, Z.; Wang, H.; Rao, S.; Sun, J.; Ma, C.; Li, J. p-Coumaric acid kills bacteria through dual damage mechanisms. Food Control. 2012, 25, 550–554. [Google Scholar] [CrossRef]
- Amalan, V.; Vijayakumar, N.; Indumathi, D.; Ramakrishman, A. Antidiabetic and antihyperlipidemic activity of p-Coumaric acid in diabetic rats, role of pancreatic GLUT 2: In vivo approach. Biomed. Pharmacother. 2016, 84, 230–236. [Google Scholar] [CrossRef] [PubMed]
- Vauzour, D.; Corona, G.; Spencer., J.P. Caffeic acid, tyrosol and p-Coumaric acid are potent inhibitors of 5-S-cysteinyldopamine induced neurotoxicity. Arch. Biochem. Biophys. 2010, 501, 106–111. [Google Scholar] [CrossRef] [PubMed]
- Pragasam, S.J.; Venkatesan, V.; Rasool, M. Immunomodulatory and anti-inflammatory effect of p-Coumaric acid, a common dietary polyphenol on experimental inflammation in rats. Inflammation 2013, 36, 169–176. [Google Scholar] [CrossRef]
- Neog, M.K.; Pragasam, S.J.; Krishnan, M.; Rasool, M. p-Coumaric acid, a dietary polyphenol ameliorates inflammation and curtails cartilage and bone erosion in the rheumatoid arthritis rat model. BioFactors 2017, 43, 698–717. [Google Scholar] [CrossRef]
- Monzote, L.; Córdova, W.H.P.; García, M.; Piñón, A.; Setzer, W.N. In-vitro and in-vivo Activities of Phenolic Compounds against Cutaneous Leishmaniasis. Rec. Nat. Prod. 2016, 10, 269–276. [Google Scholar]
- Luceri, C.; Giannini, L.; Lodovici, M.; Antonucci, E.; Abbate, R.; Masini, E.; Dolara, P. p-Coumaric acid, a common dietary phenol, inhibits platelet activity in vitro and in vivo. Br. J. Nutr. 2007, 97, 458–463. [Google Scholar] [CrossRef] [Green Version]
- Brewster, M.E.; Noppe, M.; Peeters, J.; Loftsson, T. Effect of the unstirred water layer on permeability enhancement by hydrophilic cyclodextrins. Int. J. Pharm. 2007, 1, 250–253. [Google Scholar] [CrossRef] [PubMed]
- Loftsson, T.; Duchêne, D. Cyclodextrins and their pharmaceutical applications. Int. J. Pharm. 2007, 329, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Constantino, V.R.L.; Cunha, V.R.R.; Rocha, M.A.; Figueiredo, M.P.; Magri, V.R.; Eulálio, D.; Perotti, G.F.; Bizeto, M.A.; Zambuzzi, W.F.; Koh, I.H.J. Layered Double Hydroxides: Characterization, Biocompatibility, and Therapeutical Purposes. In Series on Chemistry, Energy and the Environment: Progress in Layered Double Hydroxides, From Synthesis to New Applications; Nocchetti, M., Costantino, U., Eds.; World Scientific: Singapore, 2022; Volume 8, pp. 413–482. [Google Scholar] [CrossRef]
- Cavani, F.; Trifirò, F.; Vaccari, A. Hydrotalcite-type anionic clays: Preparation, properties and applications. Catal. Today 1991, 11, 173–301. [Google Scholar] [CrossRef]
- Hydrotalcite. Available online: https://www.drugs.com/international/hydrotalcite.html (accessed on 28 February 2022).
- Choi, G.; Choy, J.H. Recent progress in layered double hydroxide as a cancer theranostic nanoplatform. Wires Nanomed. Nanobiotechnol. 2020, 13, e1679. [Google Scholar] [CrossRef]
- Rives, V.; del Arco, M.; Martín, C. Layered Double Hydroxides as drug carriers and for controlled release of non-steroidal anti-inflammatory drugs (NASIDs): A review. J. Control. Release 2013, 169, 28–39. [Google Scholar] [CrossRef] [PubMed]
- Cunha, V.R.R.; Guilherme, V.A.; de Paula, E.; de Araújo, D.R.; Silva, R.O.; Medeiros, J.V.R.; Leite, J.R.S.A.; Petersen, P.A.D.; Foldvari, M.; Petrilli, H.M.; et al. Delivery system for mefenamic acid based on the nanocarrier layered double hydroxide: Physicochemical characterization and evaluation of anti-inflammatory and antinociceptive potential. Mater. Sci. Eng. C 2016, 58, 629–638. [Google Scholar] [CrossRef] [Green Version]
- Gil, O.M.; Rocha, M.A.; Constantino, V.R.L.; Koh, I.H.J.; Faria, D.L.A. Modified drug release system based on sulindac and layered double hydroxide: An in vivo Raman investigation. Vib. Spectrosc. 2016, 87, 60–66. [Google Scholar] [CrossRef]
- Figueiredo, M.P.; Borrego-Sánchez, A.; García-Villén, F.; Miele, D.; Rossi, S.; Sandri, G.; Viseras, C.; Constantino, V.R.L. Polymer/iron-based layered double hydroxides as multifunctional wound dressings. Pharmaceutics 2020, 12, 1130. [Google Scholar] [CrossRef]
- Cunha, V.R.R.; Petersen, P.A.D.; Souza, R.B.; Martins, A.M.C.R.P.F.; Leroux, F.; Taviot-Gueho, C.; Petrilli, H.M.; Koh, I.H.J.; Constantino, V.R.L. Phytochemical species intercalated into layered double hydroxide: Structural investigation and biocompatibility assays. New J. Chem. 2020, 44, 10011–10021. [Google Scholar] [CrossRef]
- Cunha, V.R.R.; de Souza, R.B.; Martins, A.M.C.R.P.F.; Koh, I.H.J.; Constantino, V.R.L. Accessing the biocompatibility of layered double hydroxide by intramuscular implantation: Histological and microcirculation evaluation. Sci. Rep. 2016, 6, 30547. [Google Scholar] [CrossRef]
- Figueiredo, M.P.; Cunha, V.R.R.; Leroux, F.; Taviot-Gueho, C.; Nakamae, M.N.; Kang, Y.R.; Souza, R.B.; Martins, A.M.C.R.P.F.; Koh, I.H.J.; Constantino, V.R.L. Iron-based layered double hydroxide implants: Potential drug delivery carriers with tissue biointegration promotion and blood microcirculation preservation. ACS Omega 2018, 3, 18263–18274. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Jiang, Z.; Xu, C.; Gu, L. A review: Using nanoparticles to enhance absorption and bioavailability of phenolic compounds. Food Hydrocoll. 2015, 43, 153–164. [Google Scholar] [CrossRef]
- Ritger, P.L.; Peppas, N.A. A Simple Equation for Description of Solute Release I. Fickian and Non-Fickian Release from Non-Swellable Devices in the Form of Slabs, Spheres, Cylinders or Discs. J. Controll. Release 1987, 5, 23–36. [Google Scholar] [CrossRef]
- Repetto, G.; del Peso, A.; Zurita, J.L. Neutral red uptake assay for the estimation of cell viability/cytotoxicity. Nat. Protoc. 2008, 3, 1125–1131. [Google Scholar] [CrossRef] [PubMed]
- Dubuisson, D.; Dennis, S.G. The formalin test: A quantitative study of the analgesic effects of Morphine, meperidine, and brain stem stimulation in rats and cats. Pain 1977, 4, 161–174. [Google Scholar] [CrossRef]
- Griswold, D.E.; Marshall, P.J.; Webb, E.F.; Godfrey, R.; Newton Junior, J.; Dimartino, M.J.; Sarau, H.M.; Gleason, J.G.; Poste, G.; Hanna, N. Sk+f-86002: A structurally novel antiinflammatory agent that inhibits lipoxygenase-mediated and cyclooxygenase-mediated metabolism of arachidonic-acid. Biochem. Pharmacol. 1987, 7, 3463–3470. [Google Scholar] [CrossRef]
- Abbott, F.V.; Franklin, K.B.J.; Westbrook, F.R. The formalin test: Scoring properties of the first and second phases of the pain response in rats. Pain 1995, 60, 91–102. [Google Scholar] [CrossRef]
- Foley, K.M.; Inturrisi, C.E. Analgesic drug therapy in cancer pain: Principles and practice. Med. Clin. N. Am. 1987, 71, 207–232. [Google Scholar] [CrossRef]
- Reisine, T.; Law, S.F.; Blake, A.; Tallent, M. Molecular mechanisms of opiate receptor coupling to G proteins and effector systems. Ann. N. Y. Acad. Sci. 1996, 780, 168–175. [Google Scholar] [CrossRef] [PubMed]
- Ness, T.J.; Gebhart, G.F. Centrifugal modulation of the rat tail flick reflex evoked by graded noxious heating of the tail. Brain Res. 1986, 386, 41–52. [Google Scholar] [CrossRef]
- Yonathan, M.; Asres, K.; Assefa, A.; Bucar, F. In vivo anti-inflammatory and anti-nociceptive activities of Cheilanthes farinosa. J. Ethnopharmacol. 2006, 108, 462–470. [Google Scholar] [CrossRef]
- D’amour, F.E.; Blood, F.R.; Belden, D.A.J. Manual for laboratory work in Mammalian Physiology, 3rd ed.; Chicago Press: Chicago, IL, USA, 1965. [Google Scholar]
- Khan, A.I.; Lei, L.; Norquist, A.J.; O’Hare, D. Intercalation and controlled release of pharmaceutically active compounds from a layered double hydroxide. Chem. Commun. 2001, 22, 2342–2343. [Google Scholar] [CrossRef]
- Gordijo, C.R.; Barbosa, C.A.S.; Ferreira, A.M.C.; Constantino, V.R.L.; Silva, D.O. Immobilization of ibuprofen and copper-ibuprofen drugs on layered double hydroxide. J. Pharm. Sci. 2005, 94, 1135–1148. [Google Scholar] [CrossRef]
- Bonina, F.P.; Giannossi, M.L.; Medici, L.; Puglia, C.; Summa, V.; Tateo, F. Diclofenac-hydrotalcite: In vitro and in vivo release experiments. Appl. Clay Sci. 2008, 1, 165–171. [Google Scholar] [CrossRef]
- Rocha, M.A.; Petersen, P.A.; Teixeira-Neto, E.; Petrilli, H.M.; Leroux, F.; Taviot-Gueho, C.; Constantino, V.R.L. Layered double hydroxide and sulindac coiled and scrolled nanoassemblies for storage and drug release. RSC Adv. 2016, 6, 16419–16436. [Google Scholar] [CrossRef]
- Abbott, F.V.; Ocvirk, R.; Najafee, R.; Franklin, K.B.J. Improving the efficiency of the formalin test. Pain 1999, 83, 561–569. [Google Scholar] [CrossRef]
- Luceri, C.; Guglielmi, F.; Lodovici, M.; Giannini, L.; Messirini, L.; Dolara, P. Plant phenolic 4-Coumaric acid protects against intestinal inflammation in rats. Scand. J. Gastroenterol. 2004, 39, 1128–1133. [Google Scholar]
- Del Arco, M.; Fernández, A.; Martín, C.; Sayalero, M.L.; Rives, V. Solubility and release of fenamates intercalated in layered double hydroxide. Clay Miner. 2008, 43, 255–265. [Google Scholar] [CrossRef]
- Silion, M.; Hritcu, D.; Jaba, I.M.; Tamba, B.; Ionescu, D.; Mungiu, O.C.; Popa, I.M. In vitro and in vivo behavior of ketoprofen intercalated into layered double hydroxides. J. Mater. Sci. Mater. Med. 2010, 21, 3009–3018. [Google Scholar] [CrossRef] [PubMed]
- Biswas, S.; Mukherjee, P.K.; Kar, A.; Bannerjee, S.; Jana, S.N.; Haldar, P.K.; Sharma, N. Enhanced permeability and photoprotective potential of optimized p-Coumaric acid-phospholipid complex loaded gel against UVA mediated oxidative stress. J. Photochem. Photobiol. B 2021, 221, 112246. [Google Scholar] [CrossRef] [PubMed]
- Bal, S.S.; Leishangthem, G.D.; Sethi, R.S.; Singh, A. p-Coumaric acid ameliorates fipronil induced liver injury in mice through attenuation of structural changes, oxidative stress and inflammation. Pestic. Biochem. Physiol. 2022, 180, 104997. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.M.; Min, J.H.; Kim, J.H.; Choi, J.; Park, J.M.; Lee, J.; Goo, S.H.; Oh, J.H.; Kim, S.H.; Chun, W.; et al. Methyl p-hydroxycinnamate exerts anti-inflammatory effects in mouse models of lipopolysaccharide-induced ARDS. Mol. Med. Rep. 2022, 25, 37. [Google Scholar] [CrossRef]
- Ferreira, P.S.; Victorelli, F.D.; Rodero, C.F.; Fortunato, G.C.; Araújo, V.H.S.; Fonseca-Santos, B.; Bauab, T.M.; Van Dijck, P.; Chorilli, M. p-Coumaric acid loaded into liquid crystalline systems as a novel strategy to the treatment of vulvovaginal candidiasis. Int. J. Pharm. 2021, 603, 120658. [Google Scholar] [CrossRef] [PubMed]
- Saraji, M.; Ghani, M. Dissolvable layered double hydroxide coated magnetic nanoparticles for extraction followed by high performance liquid chromatography for the determination of phenolic acids in fruit juices. J. Chromatogr. A 2014, 366, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Soltani, R.; Shahvar, A.; Dinari, M.; Saraji, M. Environmentally-friendly and ultrasonic-assisted preparation of two-dimensional ultrathin Ni/Co-NO(3) layered double hydroxide nanosheet for micro solid-phase extraction of phenolic acids from fruit juices. Ultrason. Sonochem. 2018, 40, 395–401. [Google Scholar] [CrossRef]
- Choy, J.H.; Jung, J.S.; Oh, J.M.; Park, M.; Jeong, J.; Kang, Y.K.; Han, O.J. Layered double hydroxide as an efficient drug reservoir for folate derivatives. Biomaterials 2004, 25, 3059–3064. [Google Scholar] [CrossRef] [PubMed]
- Ambrogi, V.; Fardella, G.; Grandolini, G.; Nocchetti, M.; Perioli, L. Effect of hydrotalcite-like compounds on the aqueous solubility of some poorly water-soluble drugs. J. Pharm. Sci. 2003, 92, 1407–1418. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.P.; Niebert, M.; Porazik, K.; Walker, T.L.; Cooper, H.M.; Middelberg, A.P.J.; Gray, P.P.; Bartlett, P.F.; Lu, G.Q. Subcellular compartment targeting of layered double hydroxide nanoparticles. J. Controll. Release 2008, 130, 86–94. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; He, L.; Li, T.; Yin, J.; Yin, Y.; Guan, G. Antioxidant and Anti-Inflammatory Effects of Different Zinc Sources on Diquat-Induced Oxidant Stress in a Piglet Model. BioMed. Res. Int. 2020, 2020, 3464068. [Google Scholar] [CrossRef] [PubMed]
- Santos, H.O.; Teixeira, F.J.; Schoenfeld, B.J. Dietary vs. pharmacological doses of zinc: A clinical review. Clin. Nutr. 2020, 39, 1345–1353. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Kang, P.; Liu, Y.; Na, Y.; Hu, Y.; Jin, X.; Cao, X.; Qi, Y.; Ramesh, T.; Wang, X. Zinc oxide nanoparticles synthesised from the Vernonia amygdalina shows the anti-inflammatory and antinociceptive activities in the mice model. Artif. Cells Nanomed. Biotechnol. 2020, 48, 1068–1078. [Google Scholar] [CrossRef] [PubMed]


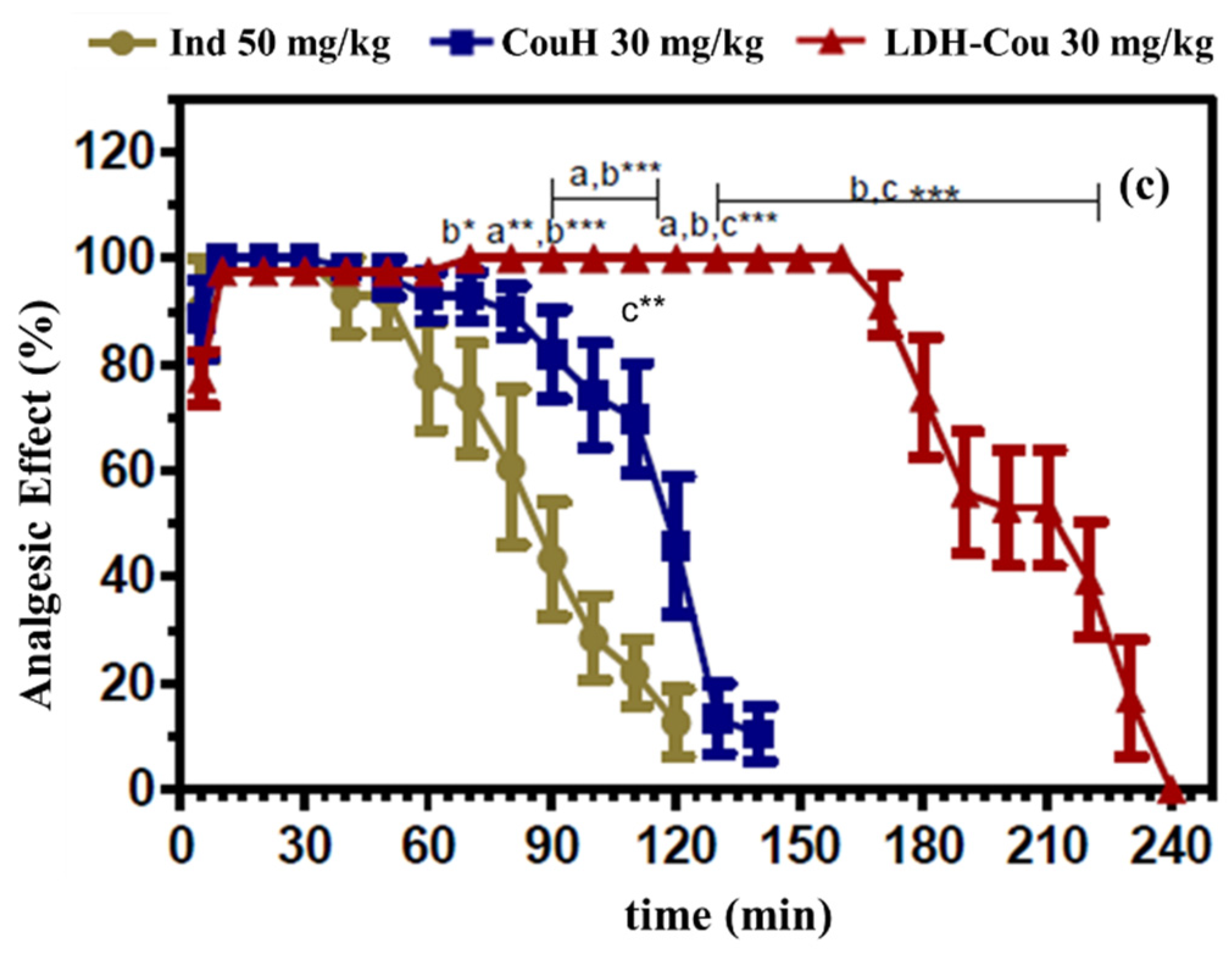
| Samples | AUC0-240 | Trec (min) |
|---|---|---|
| Ind—50 mg/kg | 106.0 (92.0–114.5) d,f,g *** | 120 (90–130) d,g *** |
| CouH—10 mg/kg | 53.5 (33.5–54.5) a *** | 50 (40–60) a *** |
| LDH-Cou—10 mg/kg | 74.5 (52.5–84.0) b ***; c ** | 70 (60–80) b,c *** |
| CouH—20 mg/kg | 111.0 (97.0–117.0) e *** | 110 (100–120) e *** |
| LDH-Cou—20 mg/kg | 194.0 (190.0–201.0) | 190 (190–210) |
| CouH—30 mg/kg | 142.5 (110.0–146.0) h *** | 130 (120–140) h *** |
| LDH-Cou—30 mg/kg | 220.0 (210.5–242.0) | 220 (210–230) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guilherme, V.A.; Cunha, V.R.R.; de Paula, E.; de Araujo, D.R.; Constantino, V.R.L. Anti-Inflammatory and Analgesic Evaluation of a Phytochemical Intercalated into Layered Double Hydroxide. Pharmaceutics 2022, 14, 934. https://doi.org/10.3390/pharmaceutics14050934
Guilherme VA, Cunha VRR, de Paula E, de Araujo DR, Constantino VRL. Anti-Inflammatory and Analgesic Evaluation of a Phytochemical Intercalated into Layered Double Hydroxide. Pharmaceutics. 2022; 14(5):934. https://doi.org/10.3390/pharmaceutics14050934
Chicago/Turabian StyleGuilherme, Viviane A., Vanessa R. R. Cunha, Eneida de Paula, Daniele R. de Araujo, and Vera R. L. Constantino. 2022. "Anti-Inflammatory and Analgesic Evaluation of a Phytochemical Intercalated into Layered Double Hydroxide" Pharmaceutics 14, no. 5: 934. https://doi.org/10.3390/pharmaceutics14050934







