Chemical vs. Physical Methods to Improve Dermal Drug Delivery: A Case Study with Nanoemulsions and Iontophoresis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Nanoemulsion Preparation
2.2.2. Droplet Size and Size Distribution Analysis
2.2.3. Determination of the Surface Charge of the Nanodroplets
2.2.4. pH Measurements
2.2.5. In Vitro Safety Assessment of the Nanoemulsions—Cytotoxicity Assay
Cell Line
Determination of the Cell Survival
2.2.6. In Vivo Assessment of the Irritation Potential of the Blank Nanoemulsions
- nanoemulsion without chemical penetration enhancers (F1);
- nanoemulsion with pinene as a chemical penetration enhancer (F1_PIN);
- nanoemulsion with eucalyptol as a chemical penetration enhancer (F1_EUC).
- trans-epidermal water loss (TEWL);
- erythema index (EI);
- degree of hydration of the stratum corneum (stratum corneum hydration—SCH).
2.2.7. In Vivo Penetration Study of Curcumin from Developed Curcumin-Loaded Nanoemulsions
- formulation with pinene (F1_PIN_CU);
- formulation with eucalyptol (F1_EUC_CU);
- formulation without monoterpenes (F1_CU).
2.2.8. Iontophoresis
Irritation Potential Assessment of the Proposed Iontophoresis Protocols
- continuous flow for 15 min (15-0);
- 3 min of continuous flow and 2 min pause (3-2; 5 cycles);
- 5 min of continuous flow and 1 min pause (5-1; 3 cycles).
In Vivo Penetration Study of Curcumin after the Application of Iontophoresis
2.2.9. LC-MS/MS Analysis
2.2.10. Statistical Analysis
3. Results and Discussion
3.1. In Vitro Characterization of the Selected Nanoemulsions
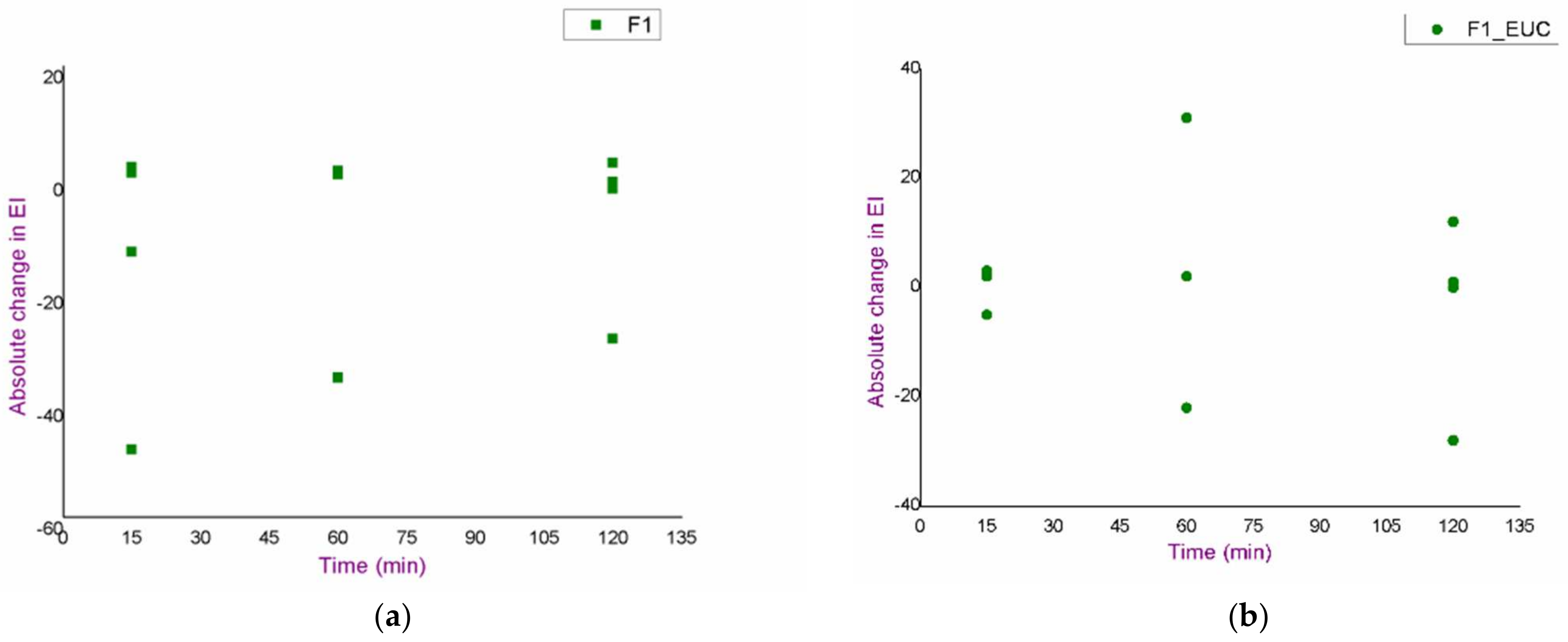
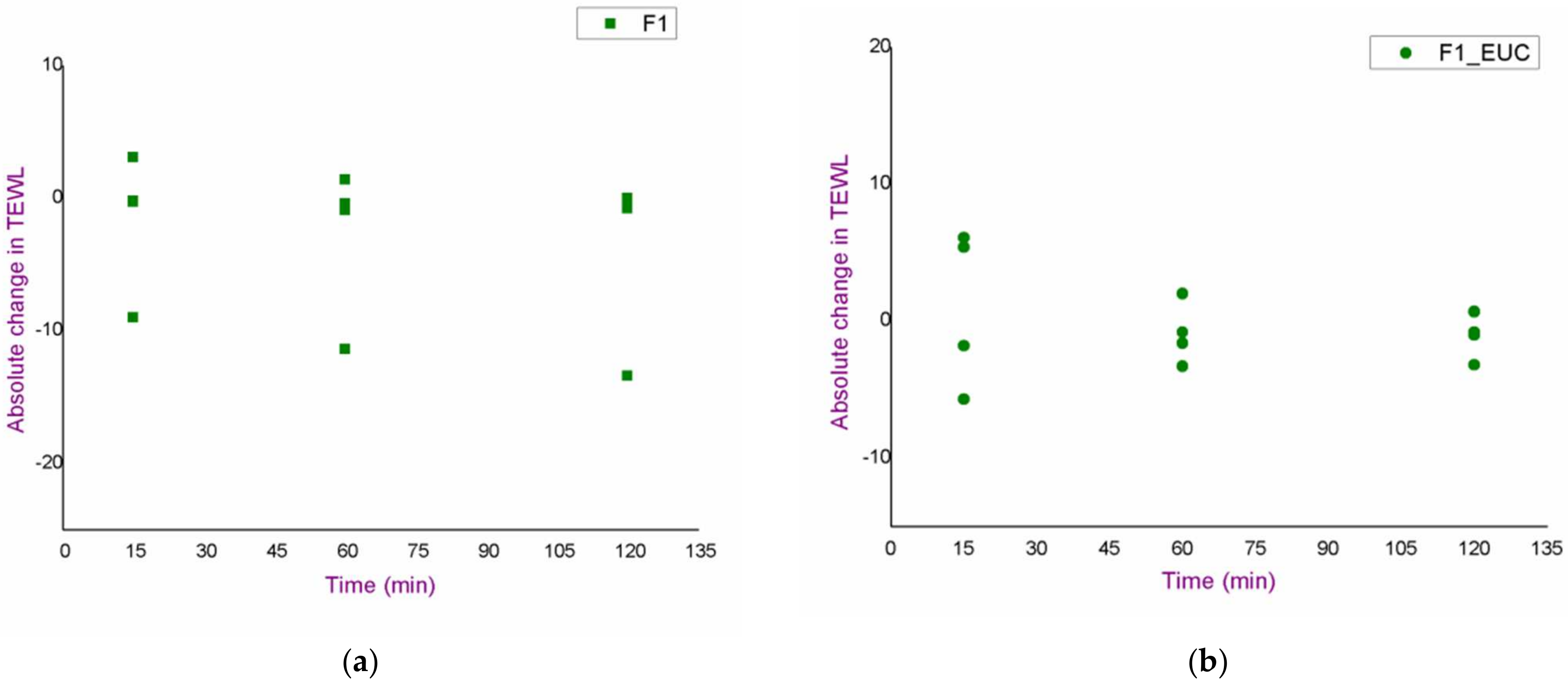
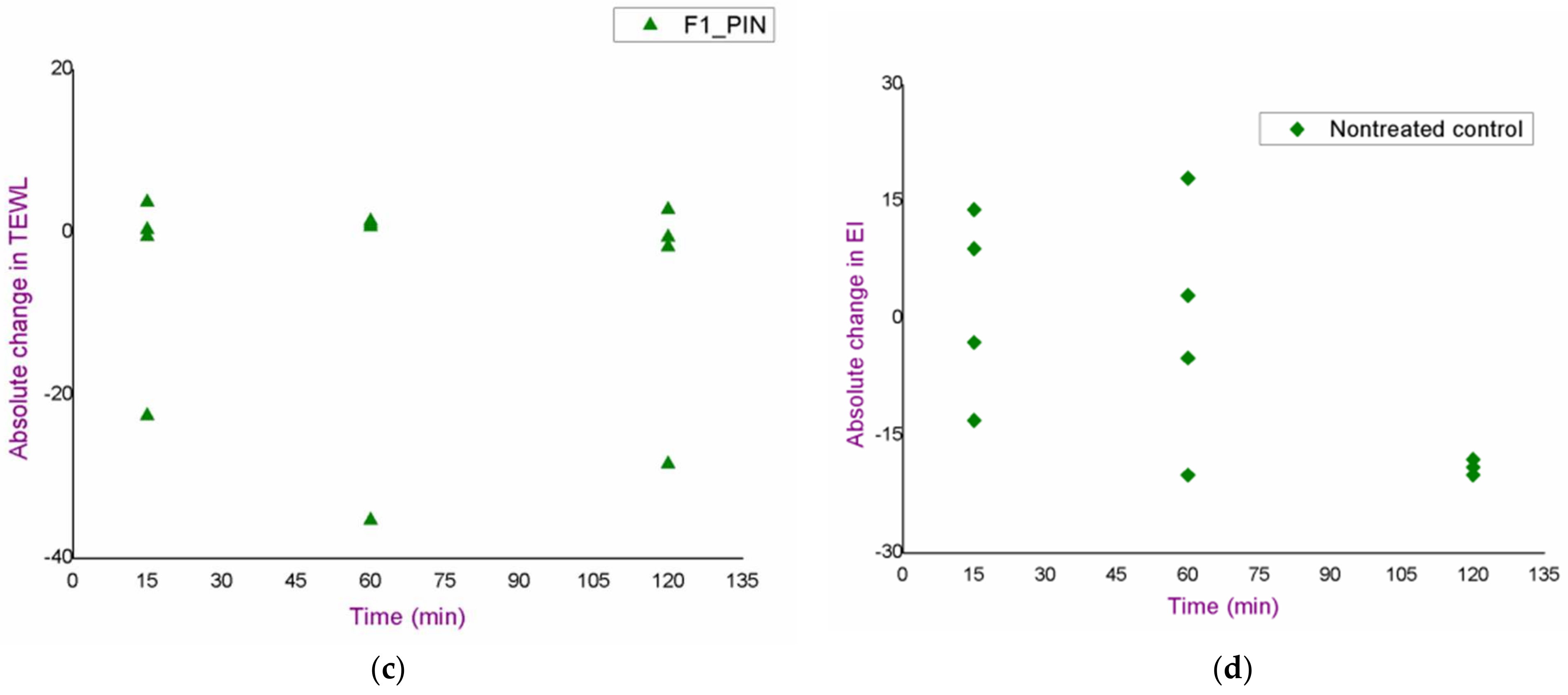
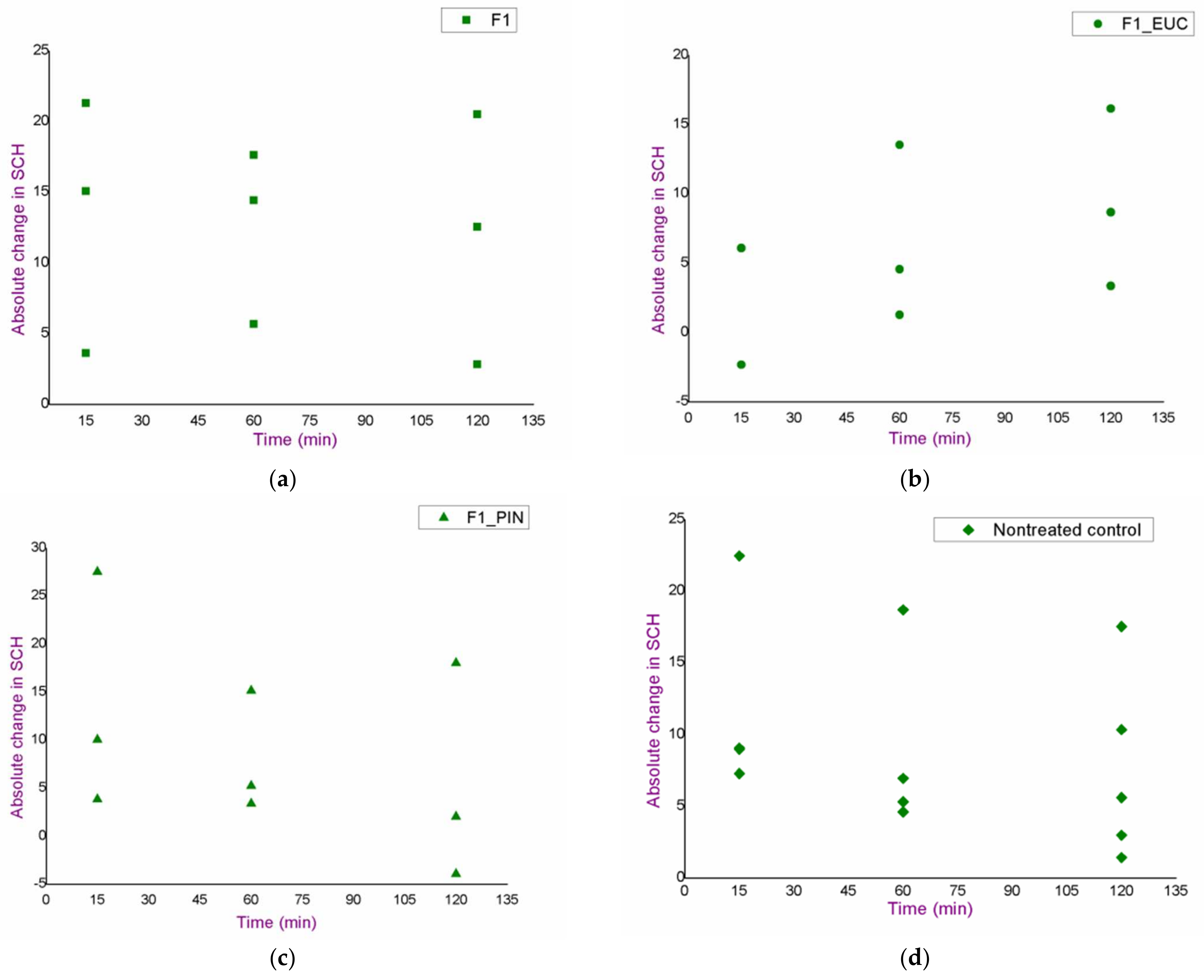
3.2. In Vivo Safety Study of the Blank Nanoemulsions
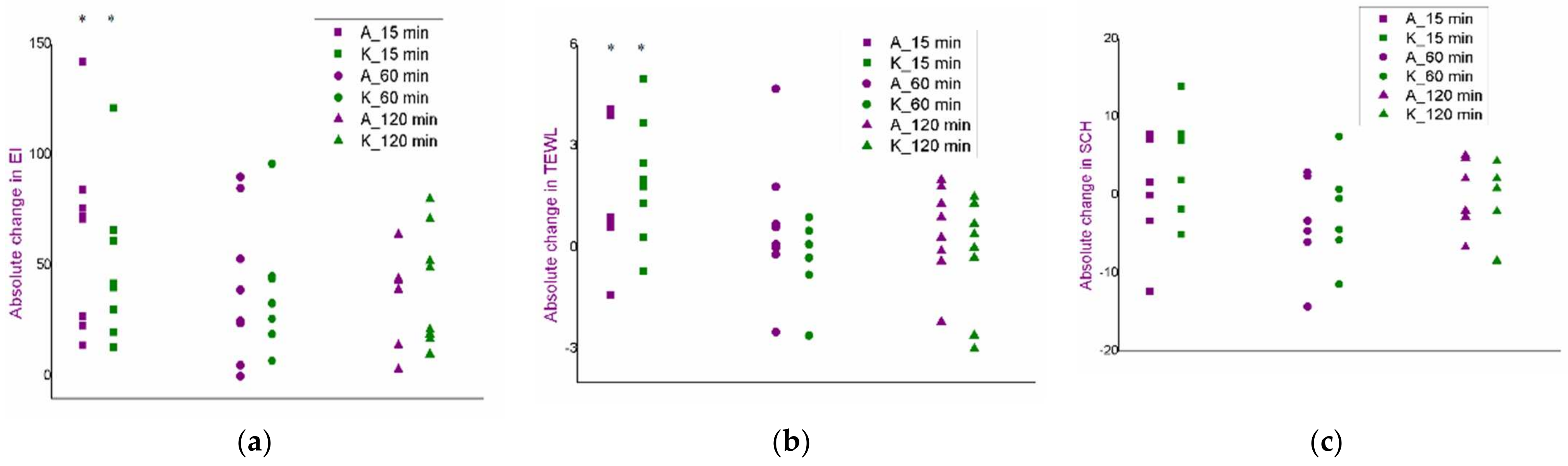
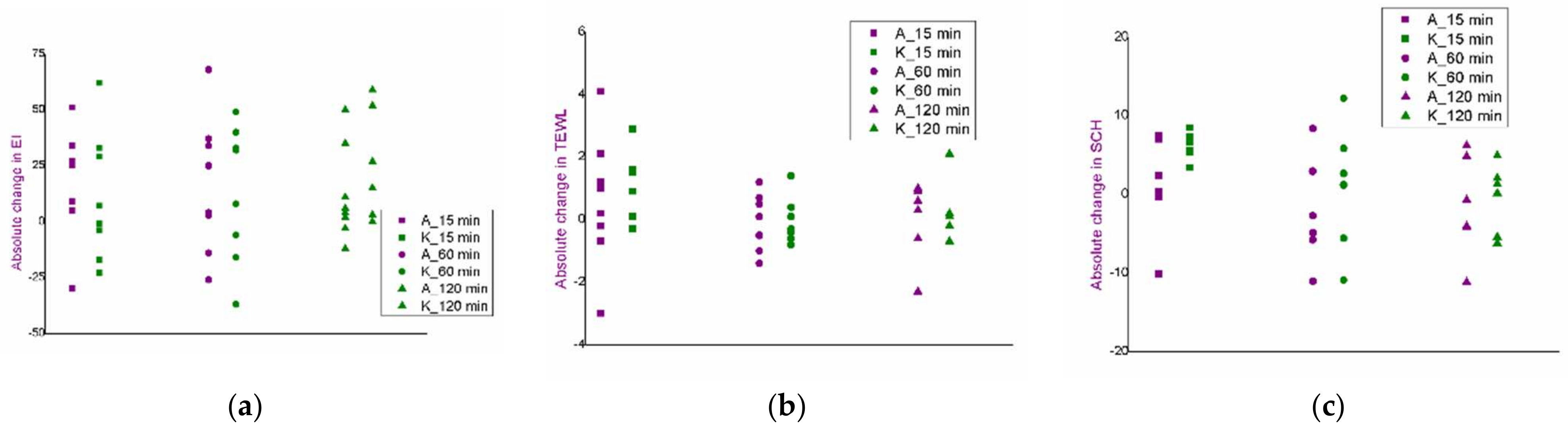
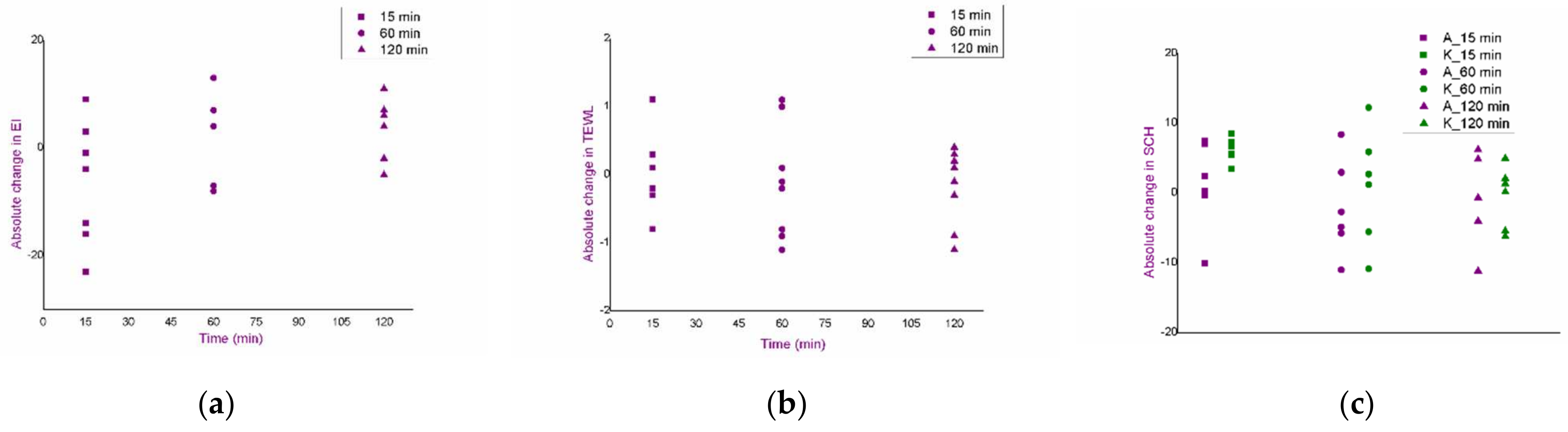
3.3. Selection of Experimental Conditions for the Iontophoresis: An In Vivo Safety Study
- continuous current flow for 15 min—15-0;
- discontinuous flow—5 min of flow with 1 min break (3 cycles)—5-1;
- discontinuous flow—3 min flow with 2 min break (5 cycles)—3-2.
3.4. In Vivo Assessment of Curcumin Penetration through the Superficial Skin Layers after the Application of Curcumin-Loaded Nanoemulsions: Comparison of the Effect of Chemical Penetration Enhancers and Iontophoresis
- electromigration;
- electroosmosis;
- passive diffusion.
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ng, K.W.; Lau, W.M. Skin deep: The basics of human skin structure and drug penetration. In Percutaneous Penetration Enhancers: Chemical Methods in Penetration Enhancement, 1st ed.; Dragićević, N., Maibach, H.I., Eds.; Springer: Berlin/Heidelberg, Germany, 2015; pp. 2–11. [Google Scholar] [CrossRef]
- Santoro, R. Skin over the centuries. A short history of dermatology: Physiology, pathology and cosmetics. Med. Hist. 2017, 1, 94–102. [Google Scholar]
- Atkinson, J.P.; Maibach, H.I.; Dragićević, N. Targets in dermal and transdermal delivery and classification of penetration enhancement methods. In Percutaneous Penetration Enhancers: Chemical Methods in Penetration Enhancement, 1st ed.; Dragićević, N., Maibach, H.I., Eds.; Springer: Berlin/Heidelberg, Germany, 2015; pp. 93–108. [Google Scholar] [CrossRef]
- Brown, M.B.; Martin, G.P.; Jones, S.A.; Akomeah, F.K. Dermal and transdermal drug delivery systems: Current and future prospects. Drug Deliv. 2006, 13, 175–187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gupta, R.; Dwadasi, B.S.; Rai, B.; Mitragotri, S. Effect of chemical permeation enhancers on skin permeability: In silico screening using molecular dynamics simulations. Sci. Rep. 2019, 9, 1456. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marwah, H.; Garg, T.; Goyal, A.K.; Rath, G. Permeation enhancer strategies in transdermal drug delivery. Drug Deliv. 2016, 23, 564–578. [Google Scholar] [CrossRef] [PubMed]
- Lopes, L.B.; Garcia, M.T.J.; Bentley, M.V.L.B. Chemical penetration enhancers. Ther. Deliv. 2015, 6, 1053–1061. [Google Scholar] [CrossRef] [PubMed]
- Pham, Q.D.; Topgaard, D.; Sparr, E. Cyclic and linear monoterpenes in phospholipid membranes: Phase behavior, bilayer structure, and molecular dynamics. Langmuir 2015, 31, 11067–11077. [Google Scholar] [CrossRef] [PubMed]
- Ahad, A.; Aqil, M.; Kohli, K.; Chaudhary, H.; Sultana, Y.; Mujeeb, M.; Talegaonkar, S. Chemical penetration enhancers: A patent review. Expert Opin. Ther. Pat. 2009, 19, 969–988. [Google Scholar] [CrossRef]
- Lane, M.E. Skin penetration enhancers. Int. J. Pharm. 2013, 447, 12–21. [Google Scholar] [CrossRef]
- Nikolić, I.; Mitsou, E.; Pantelić, I.; Randjelović, D.; Marković, B.; Papadimitriou, V.; Xenakis, A.; Savić, S. Microstructure and biopharmaceutical performances of curcumin-loaded low-energy nanoemulsions containing eucalyptol and pinene: Terpenes’ role overcome penetration enhancement effect? Eur. J. Pharm. Sci. 2020, 142, 105135. [Google Scholar] [CrossRef]
- Zorec, B.; Préat, V.; Miklavčič, D.; Pavšelj, N. Active enhancement methods for intra-and transdermal drug delivery: A review. Slov. Med. J. 2013, 82, 339–356. [Google Scholar]
- Jadoul, A.; Bouwstra, J.; Preat, V. Effects of iontophoresis and electroporation on the stratum corneum: Review of the biophysical studies. Adv. Drug Deliv. Rev. 1999, 35, 89–105. [Google Scholar] [CrossRef]
- Fukuta, T.; Oshima, Y.; Michiue, K.; Tanaka, D.; Kogure, K. Non-invasive delivery of biological macromolecular drugs into the skin by iontophoresis and its application to psoriasis treatment. J. Control. Release 2020, 323, 323–332. [Google Scholar] [CrossRef]
- Gratieri, T.; Kalia, Y.N. Iontophoresis: Basic Principles. In Percutaneous Penetration Enhancers: Chemical Methods in Penetration Enhancement, 1st ed.; Dragićević, N., Maibach., H.I., Eds.; Springer: Berlin/Heidelberg, Germany, 2015; pp. 61–76. [Google Scholar] [CrossRef]
- Nikolic, I.; Lunter, D.J.; Randjelovic, D.; Zugic, A.; Tadic, V.; Markovic, B.; Cekic, N.; Zivkovic, L.; Topalovic, D.; Spremo-Potparevic, B.; et al. Curcumin-loaded low-energy nanoemulsions as a prototype of multifunctional vehicles for different administration routes: Physicochemical and in vitro peculiarities important for dermal application. Int. J. Pharm. 2018, 550, 333–346. [Google Scholar] [CrossRef]
- Liu, C.H.; Chang, F.Y.; Hung, D.K. Terpene microemulsions for transdermal curcumin delivery: Effects of terpenes and cosurfactants. Colloids Surf. B Biointerfaces 2011, 82, 63–70. [Google Scholar] [CrossRef]
- De Leo, V.; Di Gioia, S.; Milano, F.; Fini, P.; Comparelli, R.; Mancini, E.; Agostiano, A.; Conese, M.; Catucci, L. Eudragit s100 entrapped liposome for curcumin delivery: Anti-oxidative effect in Caco-2 cells. Coatings 2020, 10, 114. [Google Scholar] [CrossRef] [Green Version]
- De Leo, V.; Milano, F.; Agostiano, A.; Catucci, L. Recent advancements in polymer/liposome assembly for drug delivery: From surface modifications to hybrid vesicles. Polymers 2021, 13, 1027. [Google Scholar] [CrossRef]
- Theochari, I.; Mitsou, E.; Nikolic, I.; Ilic, T.; Dobricic, V.; Pletsa, V.; Savic, S.; Xenakis, A.; Papadimitriou, V. Colloidal nanodispersions for the topical delivery of Ibuprofen: Structure, dynamics and bioperformances. J. Mol. Liq. 2021, 334, 116021. [Google Scholar] [CrossRef]
- Savić, V.; Ilić, T.; Nikolić, I.; Marković, B.; Čalija, B.; Cekić, N.; Savić, S. Tacrolimus-loaded lecithin-based nanostructured lipid carrier and nanoemulsion with propylene glycol monocaprylate as a liquid lipid: Formulation characterization and assessment of dermal delivery compared to referent ointment. Int. J. Pharm. 2019, 569, 118624. [Google Scholar] [CrossRef]
- NIST-NCL Joint Assay Protocol, PCC-1: Measuring the Size of Nanoparticles in Aqueous Media Using Batch-Mode Dynamic Light Scattering. 2010. Available online: https://ncl.cancer.gov/sites/default/files/protocols/NCL_Method_PCC-1.pdf (accessed on 28 March 2022).
- Aslantürk, Ö.S. In vitro cytotoxicity and cell viability assays: Principles, advantages, and disadvantages. In Genotoxicity: A Predictable Risk to Our Actual World; Larramendy, M.L., Soloneski, S., Eds.; InTechOpen: London, UK, 2018; pp. 1–17. [Google Scholar] [CrossRef] [Green Version]
- Stockert, J.C.; Horobin, R.W.; Colombo, L.L.; Blázquez-Castro, A. Tetrazolium salts and formazan products in Cell Biology: Viability assessment, fluorescence imaging, and labelling perspectives. Acta Histochem. 2018, 120, 159–167. [Google Scholar] [CrossRef] [Green Version]
- Berardesca, E.; Loden, M.; Serup, J.; Masson, P.; Rodrigues, L.M. The revised EEMCO guidance for the in vivo measurement of water in the skin. Skin Res. Technol. 2018, 24, 351–358. [Google Scholar] [CrossRef]
- Rogiers, V. EEMCO guidance for the assessment of transepidermal water loss in cosmetic sciences. Ski. Pharmocol. Physiol. 2001, 14, 117–128. [Google Scholar] [CrossRef]
- Fullerton, A.; Fisher, T.; Lahti, A.; Wilhelm, K.-P.; Takiwaki, H.; Serup, J. Guidelines for measurement of skin colour and erythema. Contact Derm. 1996, 35, 1–10. [Google Scholar] [CrossRef]
- Committee for Medicinal Products for Human Use. Draft Guideline on Quality and Equivalence of Topical Products. CHMP/QWP/708282/2018. Available online: https://www.ema.europa.eu/en/quality-equivalence-topical-products#current-version-section (accessed on 28 March 2022).
- Sharma, R.A.; Gescher, A.J.; Steward, W.P. Curcumin: The story so far. Eur. J. Cancer. 2015, 41, 1955–1968. [Google Scholar] [CrossRef]
- Jacobs, C.; Müller, R.H. Production and characterisation of a budesonide nanosuspension for pulmonary administration. Pharm. Res. 2002, 19, 189–194. [Google Scholar] [CrossRef]
- Som, I.; Bhatia, K.; Yasir, M. Status of surfactants as penetration enhancers in transdermal drug delivery. J. Pharm. Bioallied Sci. 2012, 4, 2. [Google Scholar]
- Shaker, D.S.; Ishak, R.A.; Ghoneim, A.; Elhuoni, M.A. Nanoemulsion: A review on mechanisms for the transdermal delivery of hydrophobic and hydrophilic drugs. Sci. Pharm. 2019, 87, 17. [Google Scholar] [CrossRef] [Green Version]
- Guy, R.H.; Kalia, Y.N.; Delgado-Charro, M.B.; Merino, V.; López, A.; Marro, D. Iontophoresis: Electrorepulsion and electroosmosis. J. Control. Release. 2000, 64, 129–132. [Google Scholar] [CrossRef]
- Baspinar, Y. Penetration and release studies of positive and negatively charged nanoemulsions—Is there a benefit to a positive charge? Int. J. Pharm. 2012, 430, 247–252. [Google Scholar] [CrossRef]
- Wu, X. Disposition of charged nanoparticles after their topical application to the skin. Skin Pharmacol. Physiol. 2010, 23, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Tønnesen, H.H.; Másson, M.; Loftsson, T. Studies of curcumin and curcuminoids. XXVII. Cyclodextrin complexation: Solubility, chemical and photochemical stability. Int. J. Pharm. 2002, 244, 127–135. [Google Scholar] [CrossRef]
- Dalmolin, L.F.; Lopez, R.F. Nanoemulsion as a platform for iontophoretic delivery of lipophilic drugs in skin tumors. Pharmaceutics 2018, 10, 214. [Google Scholar] [CrossRef] [Green Version]





| Formulation | MCT | Eucalyptol | Pinene | Curcumin | Soybean Lecithin | Polysorbate 80 | Water |
|---|---|---|---|---|---|---|---|
| F1 | 10 | / | / | / | 1 | 9 | 80 |
| F1_EUC | 5 | 5 | / | ||||
| F1_PIN | 5 | / | 5 | ||||
| F1_CU | 10 | / | / | 0.3 | 79.7 | ||
| F1_EUC_CU | 5 | 5 | / | ||||
| F1_PIN_CU | 5 | / | 5 |
| Formulation | pH | Zeta Potential (mV) |
|---|---|---|
| F1 | 4.78 ± 0.04 | −33.07 ± 0.95 |
| F1_EUC | 6.25 ± 0.03 | −21.17 ± 1.17 |
| F1_PIN | 4.65 ± 0.01 | −39.50 ± 1.30 |
| F1_CU | 5.36 ± 0.03 | −30.33 ± 1.81 |
| F1_EUC_CU | 6.23 ± 0.01 | −31.10 ± 0.10 |
| F1_PIN_CU | 5.80 ± 0.03 | −22.27 ± 0.06 |
| Formulation | Cumulative Amount of Curcumin That Penetrated through the Stratum Corneum per Unit Area (μg/cm2) |
|---|---|
| F1_CU | 30.62 ± 2.62 |
| F1_EUC_CU | 34.24 ± 5.68 # |
| F1_PIN_CU | 21.61 ± 4.01 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nikolić, I.; Simić, M.; Pantelić, I.; Stojanović, G.; Antić Stanković, J.; Marković, B.; Savić, S. Chemical vs. Physical Methods to Improve Dermal Drug Delivery: A Case Study with Nanoemulsions and Iontophoresis. Pharmaceutics 2022, 14, 1144. https://doi.org/10.3390/pharmaceutics14061144
Nikolić I, Simić M, Pantelić I, Stojanović G, Antić Stanković J, Marković B, Savić S. Chemical vs. Physical Methods to Improve Dermal Drug Delivery: A Case Study with Nanoemulsions and Iontophoresis. Pharmaceutics. 2022; 14(6):1144. https://doi.org/10.3390/pharmaceutics14061144
Chicago/Turabian StyleNikolić, Ines, Mitar Simić, Ivana Pantelić, Goran Stojanović, Jelena Antić Stanković, Bojan Marković, and Snežana Savić. 2022. "Chemical vs. Physical Methods to Improve Dermal Drug Delivery: A Case Study with Nanoemulsions and Iontophoresis" Pharmaceutics 14, no. 6: 1144. https://doi.org/10.3390/pharmaceutics14061144