Synthesis of Aliphatic Polyanhydrides with Controllable and Reproducible Molecular Weight
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Spectral Analysis
2.3. Molecular Weight Determination
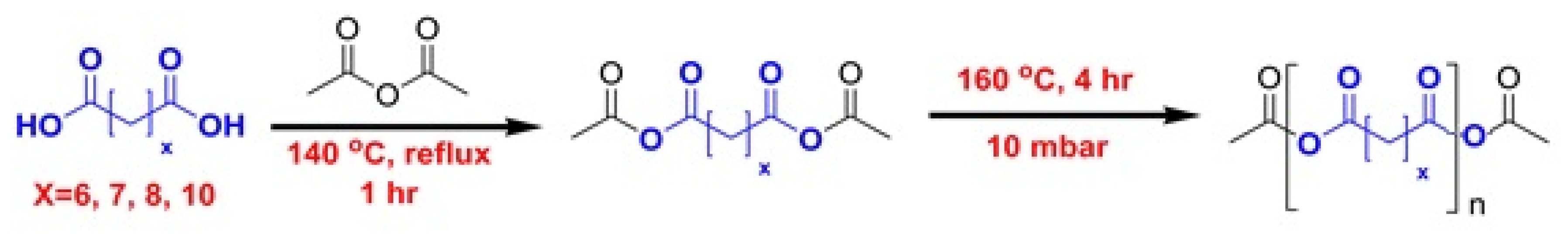
2.4. Synthesis of Polyanhydrides Using Acetic Anhydride
2.4.1. Synthesis of Poly(sebacic Acid)
2.4.2. Extension to Other Dicarboxylic Acids
2.5. Synthesis of Poly(sebacic Acid) Using Other Catalysts
2.6. Wafer Preparation of 50% Tmz, 50% Poly(Sebacic Acid), 2 Coating Layers
2.7. In Vitro Drug Release
2.8. HPLC Analysis for TMZ Release Study
3. Results and Discussion
3.1. Synthesis of Polyanhydrides
3.2. FTIR Study
3.3. NMR Study
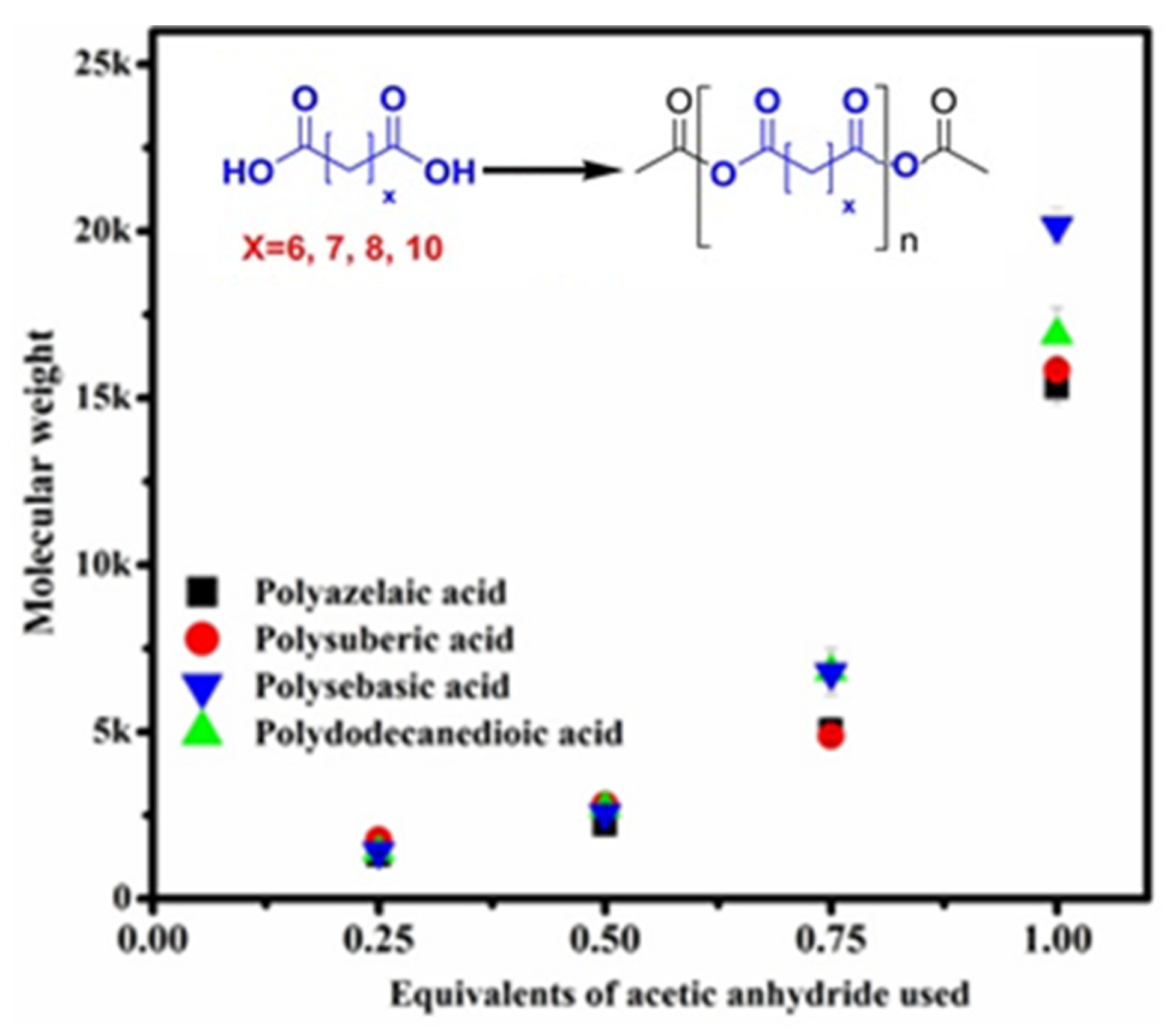
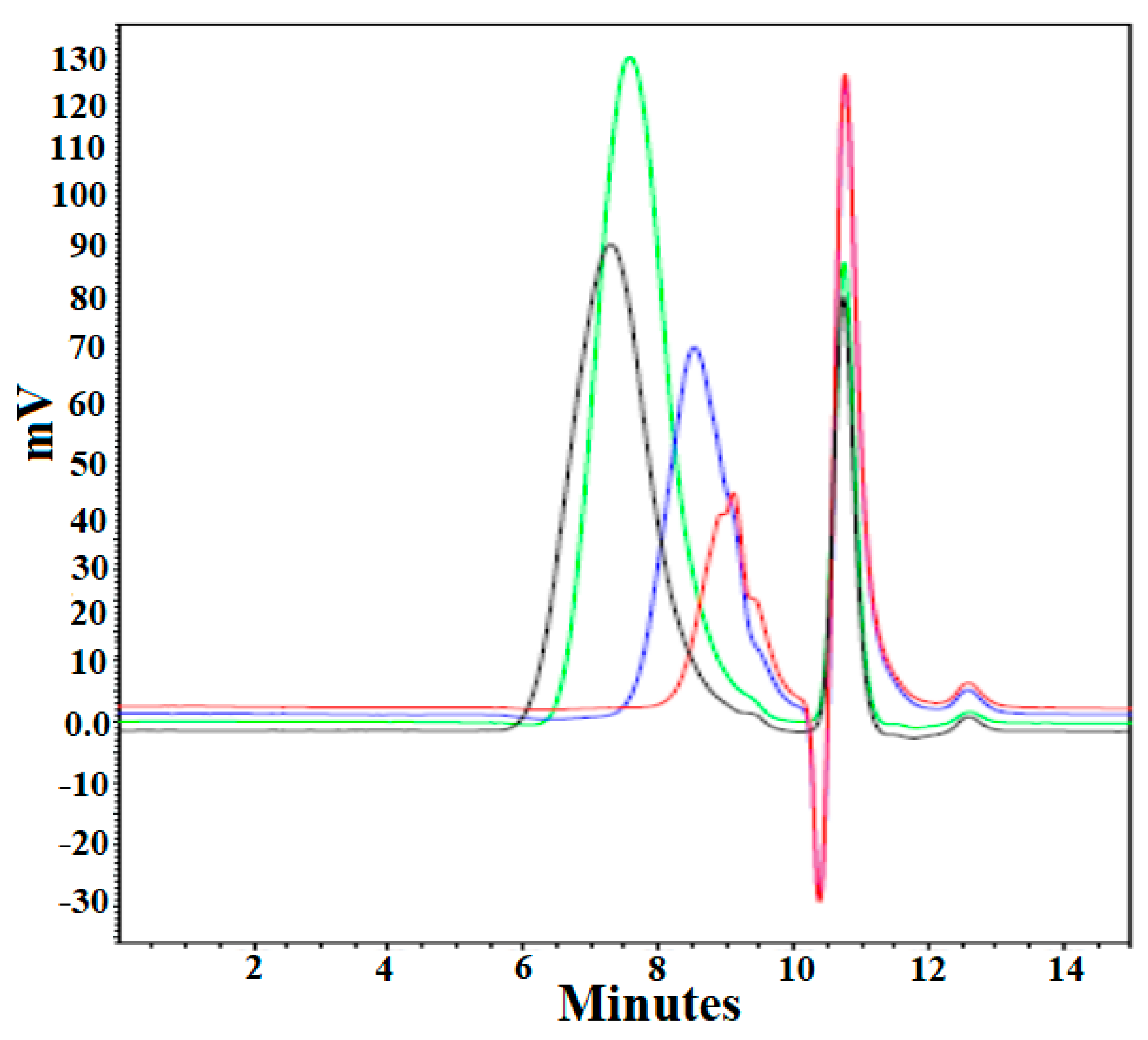
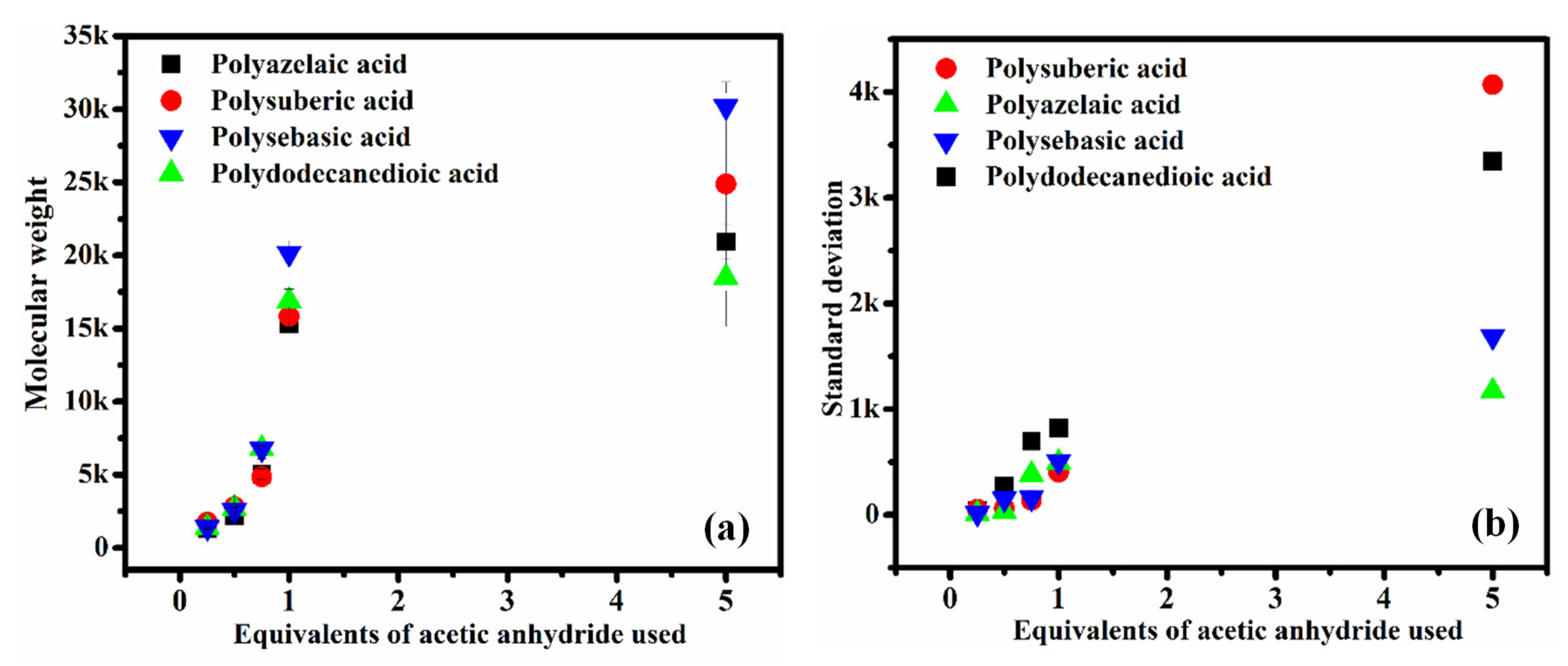
3.4. Molecular Weight Measurement by GPC
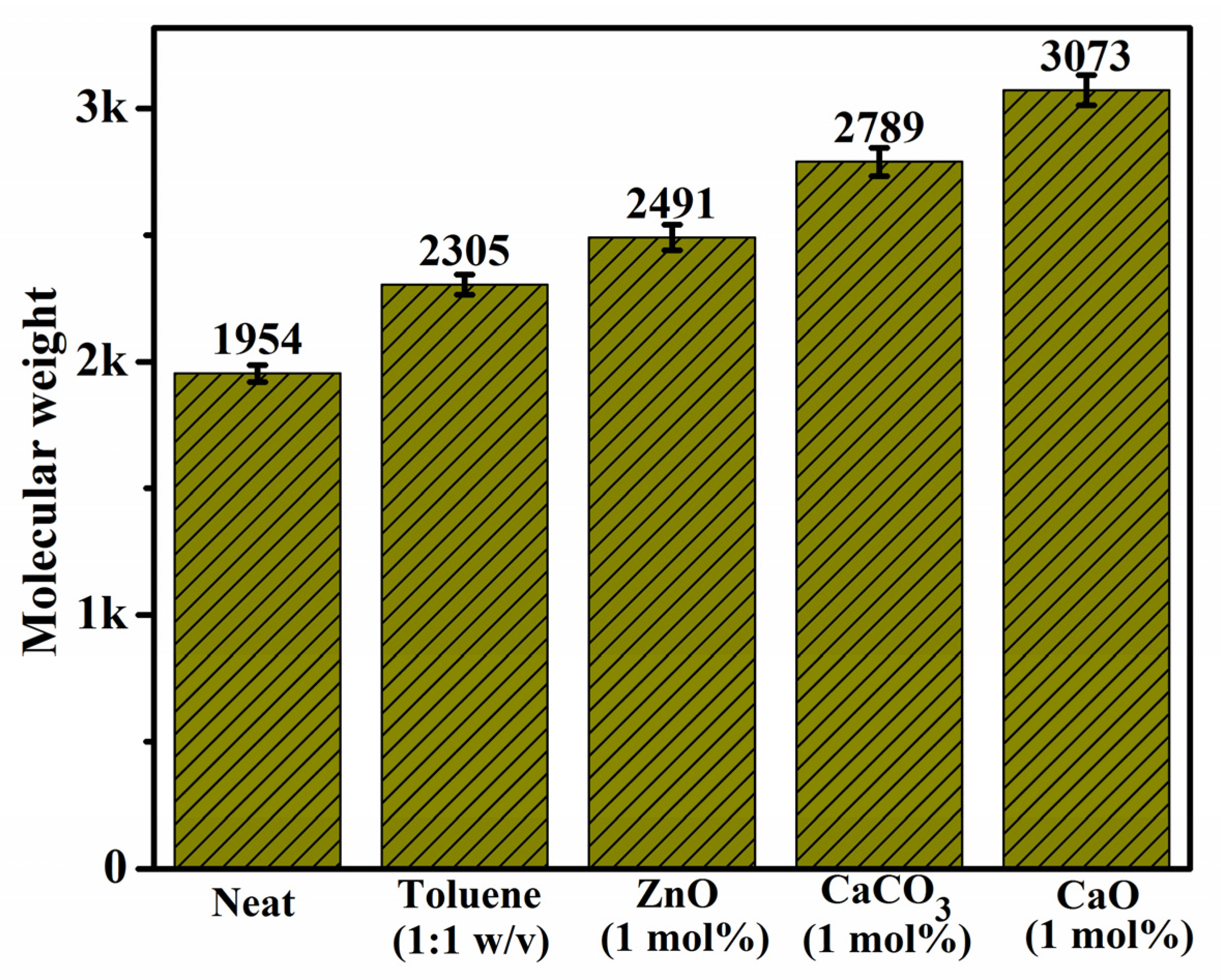
3.5. Effect of Inorganic Catalysts on Molecular Weight
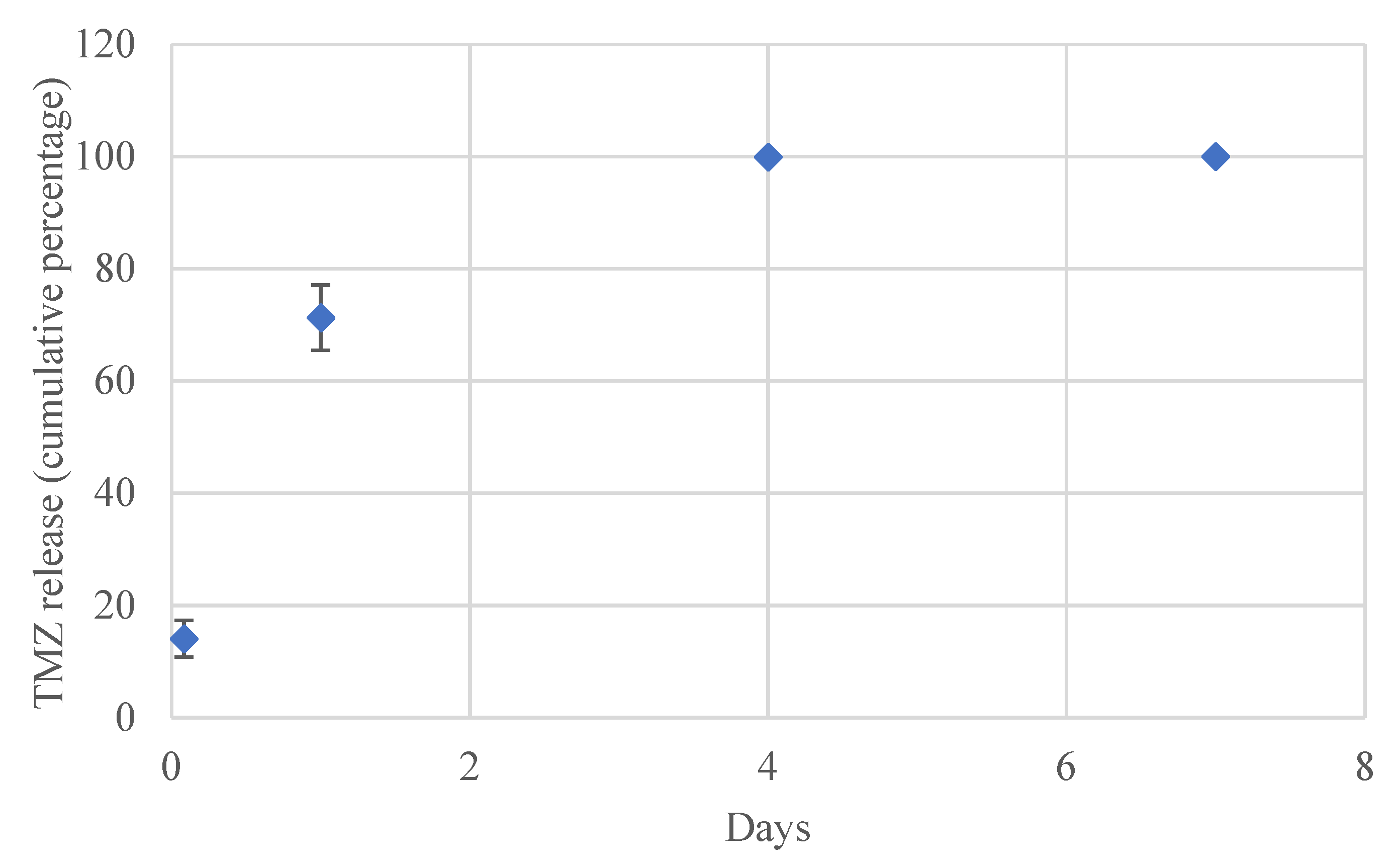
3.6. In Vitro Drug Release Studies
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
Appendix A. Table of Contents
References
- Rosen, H.B.; Chang, J.; Wnek, G.E.; Linhardt, R.J.; Langer, R. Bioerodible polyanhydrides for controlled drug delivery. Biomaterials 1983, 4, 131–133. [Google Scholar] [CrossRef]
- Kumar, N.; Langer, R.S.; Domb, A.J. Polyanhydrides: An overview. Adv. Drug Deliv. Rev. 2002, 54, 889–910. [Google Scholar] [CrossRef]
- Göpferich, A.; Tessmar, J. Polyanhydride degradation and erosion. Adv. Drug Deliv. Rev. 2002, 54, 911–931. [Google Scholar] [CrossRef]
- Basu, A.; Domb, A.J. Recent Advances in Polyanhydride Based Biomaterials. Adv. Mater. 2018, 30, 1706815. [Google Scholar] [CrossRef] [PubMed]
- Arun, Y.; Ghosh, R.; Domb, A.J. Biodegradable Hydrophobic Injectable Polymers for Drug Delivery and Regenerative Medicine. Adv. Funct. Mater. 2021, 31, 2010284. [Google Scholar] [CrossRef]
- Champeaux, C.; Weller, J. Implantation of carmustine wafers (Gliadel®) for high-grade glioma treatment. A 9-year nationwide retrospective study. J. Neurooncol. 2020, 147, 159–169. [Google Scholar] [CrossRef] [PubMed]
- Brem, H.; Gabikian, P. Biodegradable polymer implants to treat brain tumors. J. Control. Release 2001, 74, 63–67. [Google Scholar] [CrossRef]
- Darling, R.; Senapati, S.; Christiansen, J.; Liu, L.; Ramer-Tait, A.E.; Narasimhan, B.; Wannemuehler, M. Polyanhydride Nanoparticles Induce Low Inflammatory Dendritic Cell Activation Resulting in CD8+ T Cell Memory and Delayed Tumor Progression. Int. J. Nanomed. 2020, 15, 6579–6592. [Google Scholar] [CrossRef]
- Zada, M.H.; Kubek, M.; Khan, W.; Kumar, A.; Domb, A.J. Dispersible hydrolytically sensitive nanoparticles for nasal delivery of thyrotropin releasing hormone (TRH). J. Control. Release 2019, 295, 278–289. [Google Scholar] [CrossRef]
- Krasko, M.Y.; Golenser, J.; Nyska, A.; Nyska, M.; Brin, Y.S.; Domb, A.J. Gentamicin extended release from an injectable polymeric implant. J. Control. Release 2007, 117, 90–96. [Google Scholar] [CrossRef]
- Ramot, Y.; Steiner, M.; Amouyal, N.; Lavie, Y.; Klaiman, G.; Domb, A.J.; Nyska, A.; Hagigit, T. Treatment of contaminated radial fracture in Sprague-Dawley rats by application of a degradable polymer releasing gentamicin. J. Toxicol. Pathol. 2021, 34, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Brenza, T.M.; Schlichtmann, B.W.; Bhargavan, B.; Vela Ramirez, J.E.; Nelson, R.D.; Panthani, M.G.; McMillan, J.M.; Kalyanaraman, B.; Gendelman, H.E.; Anantharam, V.; et al. Biodegradable polyanhydride-based nanomedicines for blood to brain drug delivery. J. Biomed. Mater. Res. A 2018, 106, 2881–2890. [Google Scholar] [CrossRef] [PubMed]
- Jaszcz, K. Effect of Basic Factors of Preparation on Characteristics, Hydrolytic Degradation, and Drug Release from Poly(ester-anhydride) Microspheres. Int. J. Polym. Mater. 2014, 63, 97–106. [Google Scholar] [CrossRef]
- Korhonen, H.; Hakala, R.A.; Helminen, A.O.; Seppala, J.V. Synthesis and Hydrolysis Behaviour of Poly(ester anhydrides) from Polylactone Precursors Containing Alkenyl Moieties. Macromol. Biosci. 2006, 6, 496–505. [Google Scholar] [CrossRef]
- de Ronde, B.M.; Carbone, A.L.; Uhrich, K. Storage stability study of salicylate-based Poly(anhydride-esters). Polym. Degrad. Stab. 2010, 95, 1778–1782. [Google Scholar] [CrossRef] [Green Version]
- Krasko, M.Y.; Shikanov, A.; Ezra, A.; Domb, A.J. Poly(ester anhydride)s prepared by the insertion of ricinoleic acid into poly(sebacic acid). J. Polym. Sci. Part A Polym. Chem. 2003, 41, 1059–1069. [Google Scholar] [CrossRef]
- Krasko, M.Y.; Domb, A.J. Hydrolytic Degradation of Ricinoleic-Sebacic-Ester-Anhydride Copolymers. Biomacromolecules 2005, 6, 1877–1884. [Google Scholar] [CrossRef]
- Poetz, K.L.; Shipp, D.A. Polyanhydrides: Synthesis, Properties, and Applications. Aust. J. Chem. 2016, 69, 1223–1239. [Google Scholar] [CrossRef]
- Zada, M.H.; Basu, A.; Hagigit, T.; Schlinger, R.; Grishko, M.; Kraminsky, A.; Hanuka, E.; Domb, A.J. Stable polyanhydride synthesized from sebacic acid and ricinoleic acid. J. Control. Release 2017, 257, 156–162. [Google Scholar] [CrossRef]
- Eameema, M.; Duvvuri, L.S.; Khan, W.; Domb, A.J. Polyanhydrides. In Natural and Synthetic Biomedical Polymers; Elsevier: Amsterdam, The Netherlands, 2014; pp. 181–192. [Google Scholar] [CrossRef]
- Domb, A.J.; Maniar, M.J. Absorbable biopolymers derived from dimer fatty acids. J. Polym. Sci. Part A Polym. Chem. 1993, 31, 1275–1285. [Google Scholar] [CrossRef]
- Zada, M.H.; Basu, A.; Hagigit, T.; Schlinger, R.; Grishko, M.; Kraminsky, A.; Hanuka, E.; Domb, A. Alternating Poly(ester-anhydride) by Insertion Polycondensation. J. Biomacromol. 2016, 17, 2253–2259. [Google Scholar] [CrossRef] [PubMed]
- Hakala, R.A.; Korhonen, H.; Meretoja, V.V.; Seppälä, J.V. Photo-cross-linked biodegradable poly (ester anhydride) networks prepared from alkenylsuccinic anhydride functionalized poly (ε-caprolactone) precursors. Biomacromolecules 2011, 12, 2806–2814. [Google Scholar] [CrossRef] [PubMed]
- Niewolik, D.; Krukiewicz, K.; Cwynar, B.B.; Ruszkowski, P.; Jaszcz, K. Novel polymeric derivatives of betulin with anticancer activity. RSC Adv. 2019, 9, 20892–20900. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kelly, S.M.; Mitra, A.; Mathur, S.; Narasimhan, B. Synthesis and Characterization of Rapidly Degrading Polyanhydrides as Vaccine Adjuvants. ACS Biomater. Sci. Eng. 2020, 6, 265–276. [Google Scholar] [CrossRef] [Green Version]
- Chu, I.M.; Liu, T.H.; Chen, Y.R. Preparation and characterization of sustained release system based on polyanhydride microspheres with core/shell-like structures. J. Polym. Res. 2019, 26, 1–12. [Google Scholar] [CrossRef]
- Gutiérrez, M.; Sierra, C.; Morantes, M.A.; Herrera, A.P. Synthesis of (azelaic-co-dodecanedioic) polyanhydride by microwave technique. J. Phys. Conf. Ser. 2016, 687, 012049. [Google Scholar] [CrossRef]
- Wafa, E.I.; Geary, S.M.; Ross, K.A.; Goodman, J.T.; Narasimhan, B.; Salem, A.K. Single Dose of a Polyanhydride Particle-Based Vaccine Generates Potent Antigen-Specific Antitumor Immune Responses. J. Pharmacol. Exp. Ther. 2019, 370, 855–863. [Google Scholar] [CrossRef]
- Domb, A.J.; Langer, R.S. Polyanhydrides. I. Preparation of high molecular weight polyanhydrides. J. Polym. Sci. Part A Polym. Chem. 1987, 25, 3373–3386. [Google Scholar] [CrossRef]
- Ickowicz, D.E.; Abtew, E.; Khan, W.; Golovanevski, L.; Steinman, N.; Weiniger, C.F.; Domb, A.J. Poly(ester-anhydride) for controlled delivery of hydrophilic drugs. J. Bioact. Compat. Polym. 2016, 31, 127–139. [Google Scholar] [CrossRef]
- Albertsson, A.C.; Lundmark, S. Synthesis, characterization and degradation of aliphatic polyanhydrides. Br. Polym. J. 1990, 23, 205–212. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghosh, R.; Arun, Y.; Siman, P.; Domb, A.J. Synthesis of Aliphatic Polyanhydrides with Controllable and Reproducible Molecular Weight. Pharmaceutics 2022, 14, 1403. https://doi.org/10.3390/pharmaceutics14071403
Ghosh R, Arun Y, Siman P, Domb AJ. Synthesis of Aliphatic Polyanhydrides with Controllable and Reproducible Molecular Weight. Pharmaceutics. 2022; 14(7):1403. https://doi.org/10.3390/pharmaceutics14071403
Chicago/Turabian StyleGhosh, Radhakanta, Yuvaraj Arun, Peter Siman, and Abraham J. Domb. 2022. "Synthesis of Aliphatic Polyanhydrides with Controllable and Reproducible Molecular Weight" Pharmaceutics 14, no. 7: 1403. https://doi.org/10.3390/pharmaceutics14071403





