Systematic Comparison of Hospital-Wide Standard and Model-Based Therapeutic Drug Monitoring of Vancomycin in Adults
Abstract
:1. Introduction
2. Materials and Methods
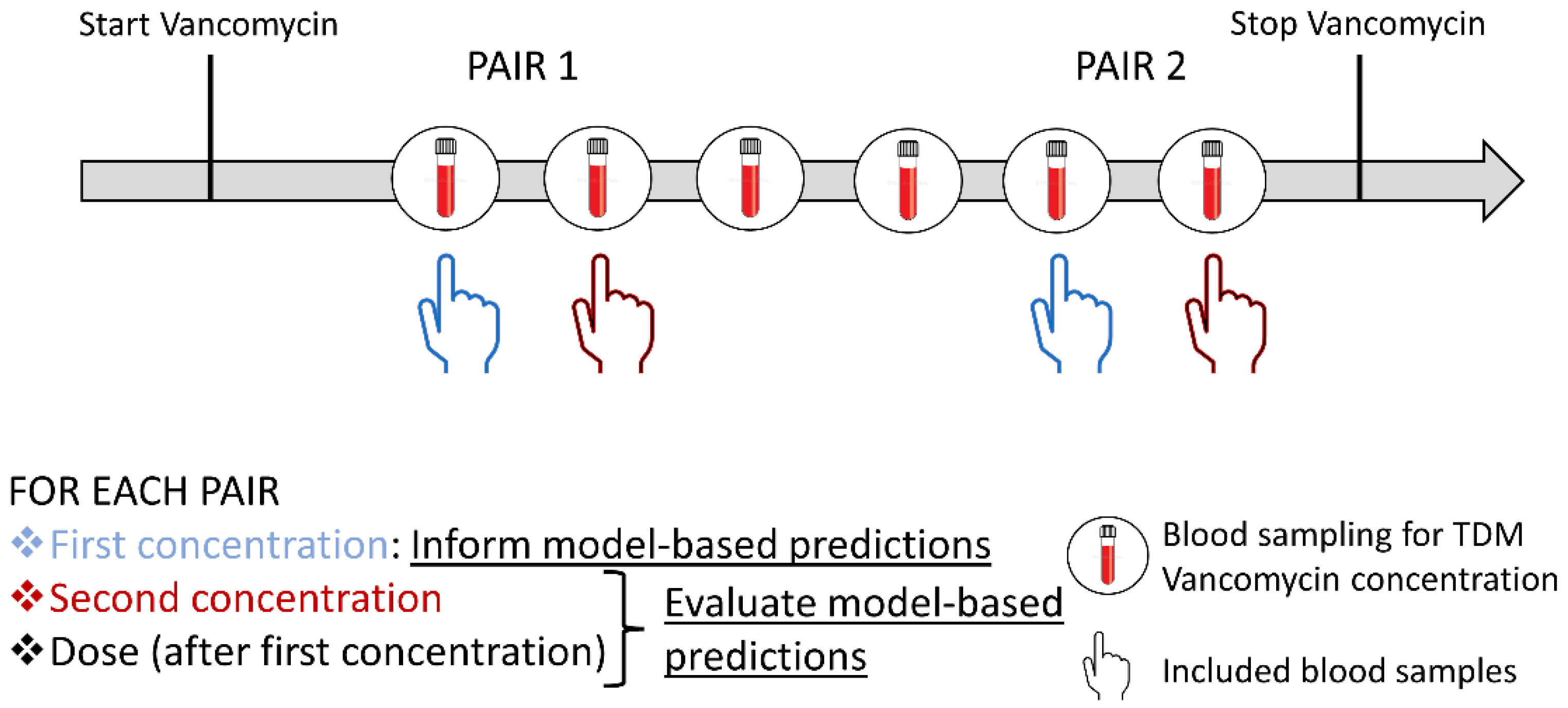
2.1. Study Design, Patients, and Data Collection
2.2. Bayesian Forecasting
2.3. Evaluation of the Model-Based Approaches
2.4. Statistics
3. Results
3.1. Clinical Data
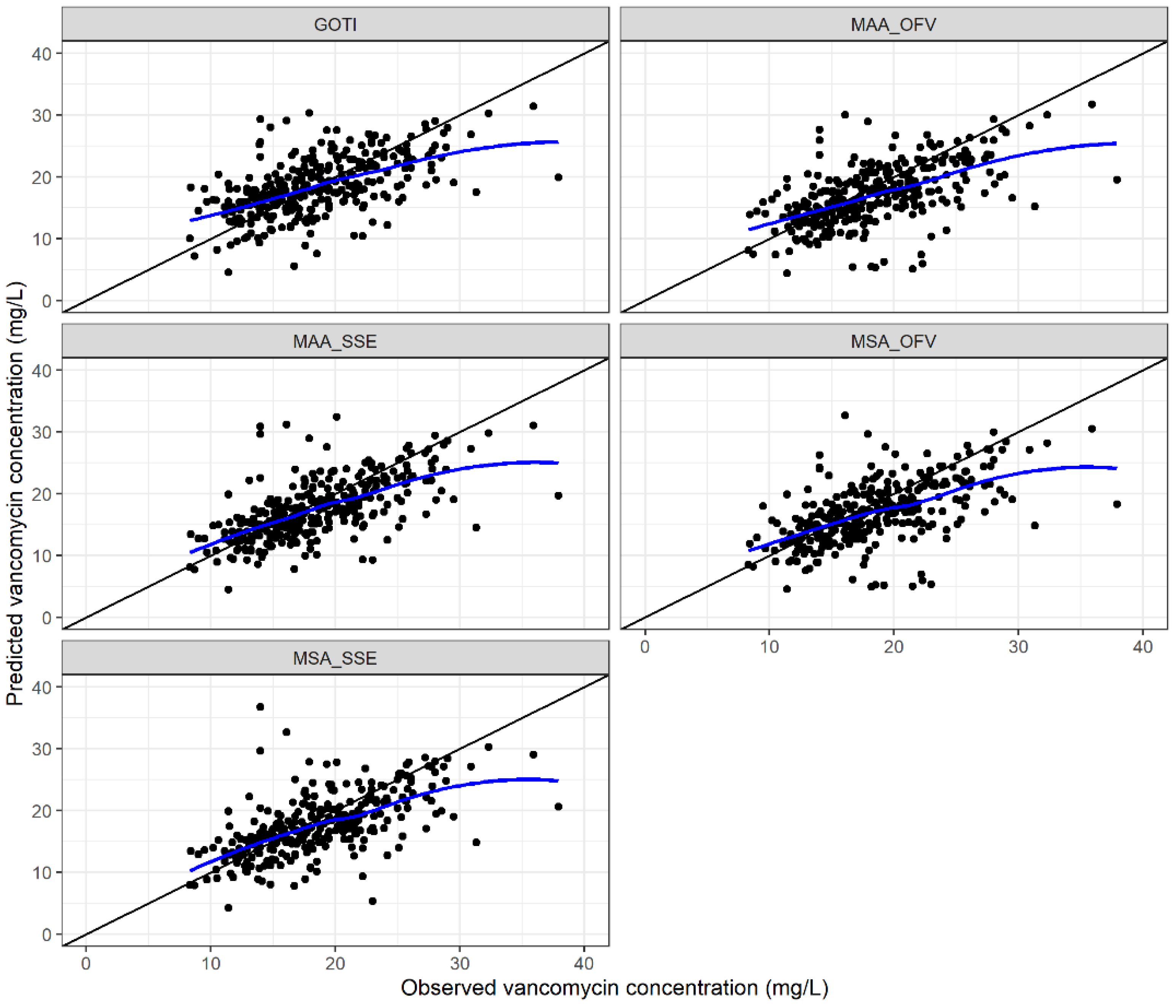
3.2. Evaluation of the Model-Based Approaches
3.2.1. Predictive Performance
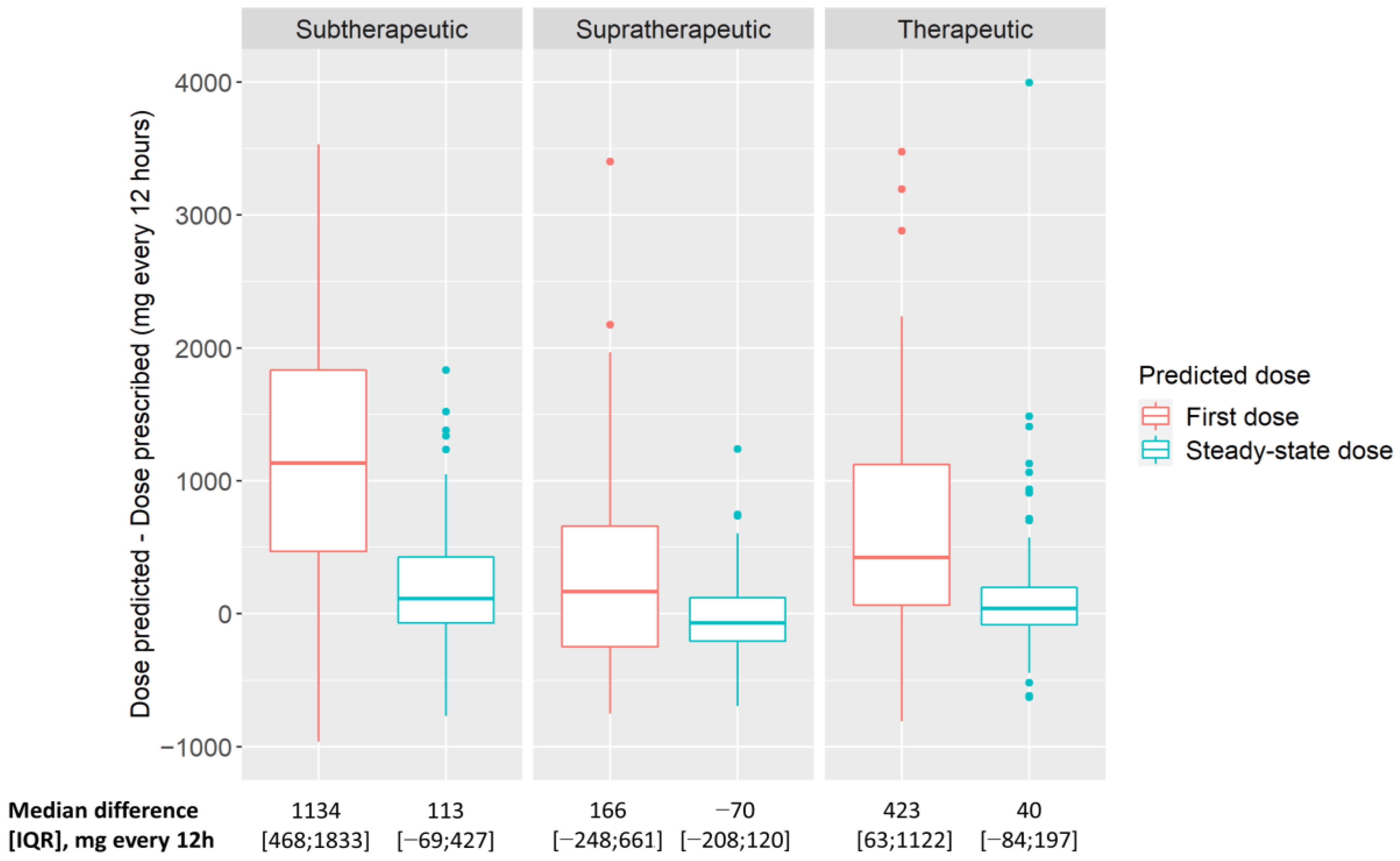
3.2.2. Model-Predicted Vancomycin Doses Compared with Standard TDM-Based Doses
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rybak, M.J. The pharmacokinetic and pharmacodynamic properties of vancomycin. Clin. Infect. Dis. 2006, 42 (Suppl. 1), S35–S39. [Google Scholar] [CrossRef] [PubMed]
- Rybak, M.J.; Le, J.; Lodise, T.P.; Levine, D.P.; Bradley, J.S.; Liu, C.; Mueller, B.A.; Pai, M.P.; Wong-Beringer, A.; Rotschafer, J.C.; et al. Therapeutic monitoring of vancomycin for serious methicillin-resistant Staphylococcus aureus infections: A revised consensus guideline and review by the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, the Pediatric Infectious Diseases Society, and the Society of Infectious Diseases Pharmacists. Am. J. Health Syst. Pharm. 2020, 77, 835–864. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rybak, M.; Lomaestro, B.; Rotschafer, J.C.; Moellering, R., Jr.; Craig, W.; Billeter, M.; Dalovisio, J.R.; Levine, D.P. Therapeutic monitoring of vancomycin in adult patients: A consensus review of the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, and the Society of Infectious Diseases Pharmacists. Am. J. Health Syst. Pharm. 2009, 66, 82–98. [Google Scholar] [CrossRef]
- Dalton, B.R.; Dersch-Mills, D.; Langevin, A.; Sabuda, D.; Rennert-May, E.; Greiner, T. Appropriateness of basing vancomycin dosing on area under the concentration-time curve. Am. J. Health. Syst. Pharm. 2019, 76, 1718–1721. [Google Scholar] [CrossRef]
- Wright, W.F.; Jorgensen, S.C.J.; Spellberg, B. Heaping the Pelion of Vancomycin on the Ossa of Methicillin-resistant Staphylococcus aureus: Back to Basics in Clinical Care and Guidelines. Clin. Infect. Dis. 2021, 72, e682–e684. [Google Scholar] [CrossRef]
- Dalton, B.R.; Rajakumar, I.; Langevin, A.; Ondro, C.; Sabuda, D.; Griener, T.P.; Dersch-Mills, D.; Rennert-May, E. Vancomycin area under the curve to minimum inhibitory concentration ratio predicting clinical outcome: A systematic review and meta-analysis with pooled sensitivity and specificity. Clin. Microbiol. Infect. 2020, 26, 436–446. [Google Scholar] [CrossRef] [PubMed]
- Tsutsuura, M.; Moriyama, H.; Kojima, N.; Mizukami, Y.; Tashiro, S.; Osa, S.; Enoki, Y.; Taguchi, K.; Oda, K.; Fujii, S.; et al. The monitoring of vancomycin: A systematic review and meta-analyses of area under the concentration-time curve-guided dosing and trough-guided dosing. BMC Infect. Dis. 2021, 21, 153. [Google Scholar] [CrossRef]
- Stewart, J.J.; Jorgensen, S.C.; Dresser, L.; Lau, T.T.; Gin, A.; Thirion, D.J.; Nishi, C.; Dalton, B. A Canadian perspective on the revised 2020 ASHP–IDSA–PIDS–SIDP guidelines for vancomycin AUC-based therapeutic drug monitoring for serious MRSA infections. Off. J. Assoc. Med. Microbiol. Infect. Dis. Can. 2021, 6, 3–9. [Google Scholar] [CrossRef]
- Stocker, S.L.; Carland, J.E.; Reuter, S.E.; Stacy, A.E.; Schaffer, A.L.; Stefani, M.; Lau, C.; Kirubakaran, R.; Yang, J.J.; Shen, C.F.J.; et al. Evaluation of a Pilot Vancomycin Precision Dosing Advisory Service on Target Exposure Attainment Using an Interrupted Time Series Analysis. Clin. Pharmacol. Ther. 2021, 109, 212–221. [Google Scholar] [CrossRef]
- Van Der Heggen, T.; Buyle, F.M.; Claus, B.; Somers, A.; Schelstraete, P.; De Paepe, P.; Vanhaesebrouck, S.; De Cock, P. Vancomycin dosing and therapeutic drug monitoring practices: Guidelines versus real-life. Int. J. Clin. Pharm. 2021, 43, 1394–1403. [Google Scholar] [CrossRef]
- Kantasiripitak, W.; Van Daele, R.; Gijsen, M.; Ferrante, M.; Spriet, I.; Dreesen, E. Software Tools for Model-Informed Precision Dosing: How Well Do They Satisfy the Needs? Front. Pharmacol. 2020, 11, 620. [Google Scholar] [CrossRef] [PubMed]
- Turner, R.B.; Kojiro, K.; Shephard, E.A.; Won, R.; Chang, E.; Chan, D.; Elbarbry, F. Review and Validation of Bayesian Dose-Optimizing Software and Equations for Calculation of the Vancomycin Area under the Curve in Critically Ill Patients. Pharmacotherapy 2018, 38, 1174–1183. [Google Scholar] [CrossRef] [PubMed]
- Cunio, C.B.; Uster, D.W.; Carland, J.E.; Buscher, H.; Liu, Z.; Brett, J.; Stefani, M.; Jones, G.R.D.; Day, R.O.; Wicha, S.G.; et al. Towards precision dosing of vancomycin in critically ill patients: An evaluation of the predictive performance of pharmacometric models in ICU patients. Clin. Microbiol. Infect. 2020, 27, 783-e7. [Google Scholar] [CrossRef]
- Broeker, A.; Nardecchia, M.; Klinker, K.P.; Derendorf, H.; Day, R.O.; Marriott, D.J.; Carland, J.E.; Stocker, S.L.; Wicha, S.G. Towards precision dosing of vancomycin: A systematic evaluation of pharmacometric models for Bayesian forecasting. Clin. Microbiol. Infect. 2019, 25, e1281–e1286. [Google Scholar] [CrossRef] [PubMed]
- Uster, D.W.; Stocker, S.L.; Carland, J.E.; Brett, J.; Marriott, D.J.E.; Day, R.O.; Wicha, S.G. A Model Averaging/Selection Approach Improves the Predictive Performance of Model-Informed Precision Dosing: Vancomycin as a Case Study. Clin. Pharmacol. Ther. 2021, 109, 175–183. [Google Scholar] [CrossRef]
- Available online: https://www.randomizer.org/ (accessed on 17 March 2022).
- Wicha, S.G.; Kees, M.G.; Solms, A.; Minichmayr, I.K.; Kratzer, A.; Kloft, C. TDMx: A novel web-based open-access support tool for optimising antimicrobial dosing regimens in clinical routine. Int. J. Antimicrob. Agents 2015, 45, 442–444. [Google Scholar] [CrossRef]
- Goti, V.; Chaturvedula, A.; Fossler, M.J.; Mok, S.; Jacob, J.T. Hospitalized Patients with and without Hemodialysis Have Markedly Different Vancomycin Pharmacokinetics: A Population Pharmacokinetic Model-Based Analysis. Ther. Drug Monit. 2018, 40, 212–221. [Google Scholar] [CrossRef]
- Adane, E.D.; Herald, M.; Koura, F. Pharmacokinetics of vancomycin in extremely obese patients with suspected or confirmed Staphylococcus aureus infections. Pharmacotherapy 2015, 35, 127–139. [Google Scholar] [CrossRef]
- Mangin, O.; Urien, S.; Mainardi, J.L.; Fagon, J.Y.; Faisy, C. Vancomycin pharmacokinetic and pharmacodynamic models for critically ill patients with post-sternotomy mediastinitis. Clin. Pharmacokinet. 2014, 53, 849–861. [Google Scholar] [CrossRef]
- Medellín-Garibay, S.E.; Ortiz-Martín, B.; Rueda-Naharro, A.; García, B.; Romano-Moreno, S.; Barcia, E. Pharmacokinetics of vancomycin and dosing recommendations for trauma patients. J. Antimicrob. Chemother. 2016, 71, 471–479. [Google Scholar] [CrossRef] [Green Version]
- Revilla, N.; Martín-Suárez, A.; Pérez, M.P.; González, F.M.; Fernández de Gatta Mdel, M. Vancomycin dosing assessment in intensive care unit patients based on a population pharmacokinetic/pharmacodynamic simulation. Br. J. Clin. Pharmacol. 2010, 70, 201–212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roberts, J.A.; Taccone, F.S.; Udy, A.A.; Vincent, J.L.; Jacobs, F.; Lipman, J. Vancomycin dosing in critically ill patients: Robust methods for improved continuous-infusion regimens. Antimicrob. Agents Chemother. 2011, 55, 2704–2709. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thomson, A.H.; Staatz, C.E.; Tobin, C.M.; Gall, M.; Lovering, A.M. Development and evaluation of vancomycin dosage guidelines designed to achieve new target concentrations. J. Antimicrob. Chemother. 2009, 63, 1050–1057. [Google Scholar] [CrossRef]
- Watson, P.F.; Petrie, A. Method agreement analysis: A review of correct methodology. Theriogenology 2010, 73, 1167–1179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koo, T.K.; Li, M.Y. A Guideline of Selecting and Reporting Intraclass Correlation Coefficients for Reliability Research. J. Chiropr. Med. 2016, 15, 155–163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heus, A.; Uster, D.W.; Grootaert, V.; Vermeulen, N.; Somers, A.; In’t Veld, D.H.; Wicha, S.G.; De Cock, P.A. Model-informed precision dosing of vancomycin via continuous infusion: A clinical fit-for-purpose evaluation of published PK models. Int. J. Antimicrob. Agents 2022, 59, 106579. [Google Scholar] [CrossRef]
- Okada, A.; Kariya, M.; Irie, K.; Okada, Y.; Hiramoto, N.; Hashimoto, H.; Kajioka, R.; Maruyama, C.; Kasai, H.; Hamori, M.; et al. Population Pharmacokinetics of Vancomycin in Patients Undergoing Allogeneic Hematopoietic Stem-Cell Transplantation. J. Clin. Pharmacol. 2018, 58, 1140–1149. [Google Scholar] [CrossRef]
- Colin, P.J.; Allegaert, K.; Thomson, A.H.; Touw, D.J.; Dolton, M.; de Hoog, M.; Roberts, J.A.; Adane, E.D.; Yamamoto, M.; Santos-Buelga, D.; et al. Vancomycin Pharmacokinetics Throughout Life: Results from a Pooled Population Analysis and Evaluation of Current Dosing Recommendations. Clin. Pharmacokinet. 2019, 58, 767–780. [Google Scholar] [CrossRef] [Green Version]
- Gastmans, H.; Dreesen, E.; Dia, N.; Desmet, S.; Lagrou, K.; Peetermans, W. Model-based TDM of vancomycin: A retrospective comparison with routine TDM-based dosing. In Oral Presentation at the European Congress of Clinical Microbiology and Infectious Diseases; Abstract/Presentation Number: 1258/O0302; ESCMID Library: Lisbon, Portugal, May 2022. [Google Scholar]

| Per Patient | |||
|---|---|---|---|
| All (n = 154) | Intermittent (n = 95) | Continuous (n = 59) | |
| Male, n (%) | 103 (66.9) | 68 (71.6) | 35 (59.3) |
| Caucasian, n (%)/Afro-American, n (%) | 149 (96.8)/5 (3.2) | 90 (94.7)/5 (5.3) | 59 (100) |
| Age (years), median [IQR] | 63 [53; 72] | 63 [55; 74] | 60 [49; 68] |
| Weight (kg), median [IQR] | 76 [64; 94] | 77 [63; 95] | 74 [65; 94] |
| Diabetes mellitus (type I, type II, and corticosteroid-induced), n (%) | 43 (27.9) | 32 (33.7) | 11 (16.8) |
| Intensive care unit, n (%) | 60 (39) | 30 (31.6) | 30 (50.8) |
| In-hospital mortality, n (%) | 43 (27.9) | 17 (17.3) | 26 (44.1) |
| Reason for Hospital Admission (n= 154) | |||
| Surgical, n (%) | 54 (35.1) | 45 (47.4) | 9 (15.3) |
| Medical, n (%) | 44 (28.6) | 23 (24.2) | 21 (35.6) |
| Emergency, n (%) | 51 (33.1) | 26 (27.4) | 25 (42.4) |
| Others, n (%) | 5 (3.2) | 1 (1.1) | 4 (6.8) |
| Focus of the Infection (n = 154) | |||
| Respiratory, n (%) | 21 (13.6) | 10 (10.5) | 11 (18.6) |
| Gastrointestinal, n (%) | 7 (4.5) | 5 (5.3) | 2 (3.4) |
| Endocarditis, n (%) | 6 (3.9) | 3 (3.2) | 3 (5.1) |
| Urinary, n (%) | 3 (1.9) | 3 (3.2) | 0 (0) |
| Skin and soft tissue, n (%) | 19 (12.3) | 16 16.8) | 3 (5.1) |
| Bone and joint, n (%) | 24 (15.6) | 20 (21.1) | 4 (6.8) |
| Catheter-related, n (%) | 18 (11.7) | 9 (9.5) | 9 (15.3) |
| Abdominal, n (%) | 15 (9.7) | 9 (9.5) | 6 (10.2) |
| Postoperative, n (%) | 10 (6.5) | 7 (7.4) | 3 (5.1) |
| Neutropenic fever, n (%) | 25 (16.2) | 8 (8.4) | 17 (28.8) |
| Other, n (%) | 6 (3.9) | 5 (5.3) | 1 (1.7) |
| Clinical and Biochemical Data on the Day of the First Vancomycin Concentration Measurement | |||
| All (n = 308) | Intermittent (n = 190) | Continuous (n = 118) | |
| Serum creatinine (mg/dL), median [IQR] | 0.83 [0.64; 1.22] | 0.82 [0.61; 1.17] | 0.84 [0.67; 1.34] |
| eGFR CKD-EPI (mL/min/1.73 m2), median [IQR] | 87 [59; 104] | 86 [61; 102] | 88 [54.5; 107] |
| eCrCl CG (mL/min), median [IQR] | 93 [58; 132] | 90.2 [60; 132] | 98 [51; 129] |
| Serum albumin (g/L) a, median [IQR], n | 31.2 [27.8; 34.6], 175 | 30.5 [26.8; 34.3], 62 | 31.9 [29; 34; 8], 113 |
| Serum urea nitrogen (mg/dL), median [IQR] | 31 [21; 55] | 28 [20; 45] | 39 [24; 77] |
| SOFA score b, median [IQR], n | 11 [7; 15], 120 | 8 [5; 12], 60 | 15 [11; 18], 60 |
| Intermittent hemodialysis, n (%) | 3 (1.0) | 0 (0) | 3 (2.5) |
| Intermittent peritoneal dialysis, n (%) | 2 (0.6) | 2 (1.1) | 0 (0) |
| Continuous veno-venous hemofiltration, n (%) | 18 (5.8) | 5 (2.6) | 13 (11) |
| Use of furosemide, n (%) | 45 (14.6) | 30 (15.8) | 15 (12.7) |
| Vancomycin trough Concentrations during Intermittent Infusion (n = 190). | |
|---|---|
| First concentration (mg/L), median [IQR] | 15.0 [12; 17.7] |
| Second concentration (mg/L), median [IQR] | 15.7 [13.7; 18.3] |
| Vancomycin Concentrations during Continuous Infusion (n = 118) | |
| First concentration (mg/L), median [IQR] | 21.3 [17.4; 23.5] |
| Second concentration (mg/L), median [IQR] | 22.1 [19.3; 25;5] |
| Exposure at Second Concentration (n = 308) | |
| Therapeutic exposure a, n (%) | 148 (48.1) |
| Supratherapeutic exposure b, n (%) | 94 (30.5) |
| Subtherapeutic exposure c, n (%) | 66 (21.4) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gastmans, H.; Dreesen, E.; Wicha, S.G.; Dia, N.; Spreuwers, E.; Dompas, A.; Allegaert, K.; Desmet, S.; Lagrou, K.; Peetermans, W.E.; et al. Systematic Comparison of Hospital-Wide Standard and Model-Based Therapeutic Drug Monitoring of Vancomycin in Adults. Pharmaceutics 2022, 14, 1459. https://doi.org/10.3390/pharmaceutics14071459
Gastmans H, Dreesen E, Wicha SG, Dia N, Spreuwers E, Dompas A, Allegaert K, Desmet S, Lagrou K, Peetermans WE, et al. Systematic Comparison of Hospital-Wide Standard and Model-Based Therapeutic Drug Monitoring of Vancomycin in Adults. Pharmaceutics. 2022; 14(7):1459. https://doi.org/10.3390/pharmaceutics14071459
Chicago/Turabian StyleGastmans, Heleen, Erwin Dreesen, Sebastian G. Wicha, Nada Dia, Ellen Spreuwers, Annabel Dompas, Karel Allegaert, Stefanie Desmet, Katrien Lagrou, Willy E. Peetermans, and et al. 2022. "Systematic Comparison of Hospital-Wide Standard and Model-Based Therapeutic Drug Monitoring of Vancomycin in Adults" Pharmaceutics 14, no. 7: 1459. https://doi.org/10.3390/pharmaceutics14071459
APA StyleGastmans, H., Dreesen, E., Wicha, S. G., Dia, N., Spreuwers, E., Dompas, A., Allegaert, K., Desmet, S., Lagrou, K., Peetermans, W. E., Debaveye, Y., Spriet, I., & Gijsen, M. (2022). Systematic Comparison of Hospital-Wide Standard and Model-Based Therapeutic Drug Monitoring of Vancomycin in Adults. Pharmaceutics, 14(7), 1459. https://doi.org/10.3390/pharmaceutics14071459










