Modeling Blood–Brain Barrier Permeability to Solutes and Drugs In Vivo
Abstract
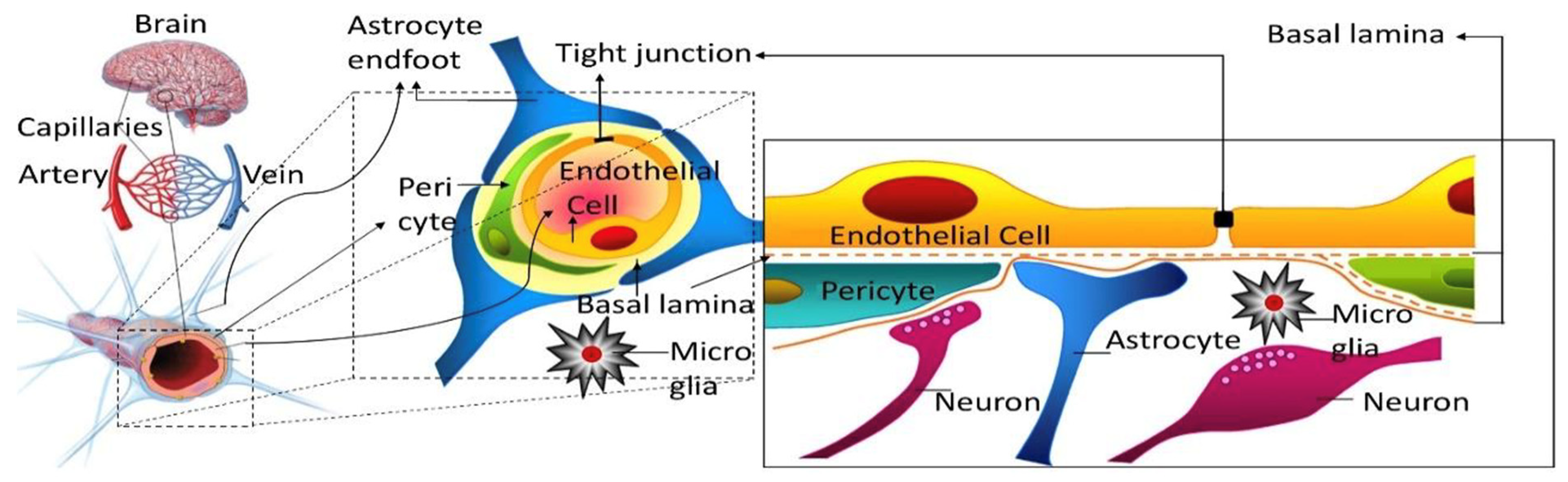
:1. Introduction
2. Models Assuming Unidirectional Brain Uptake
2.1. Brain Uptake Index (BUI)
2.2. In Situ Brain Perfusion
2.3. Intravenous Injection
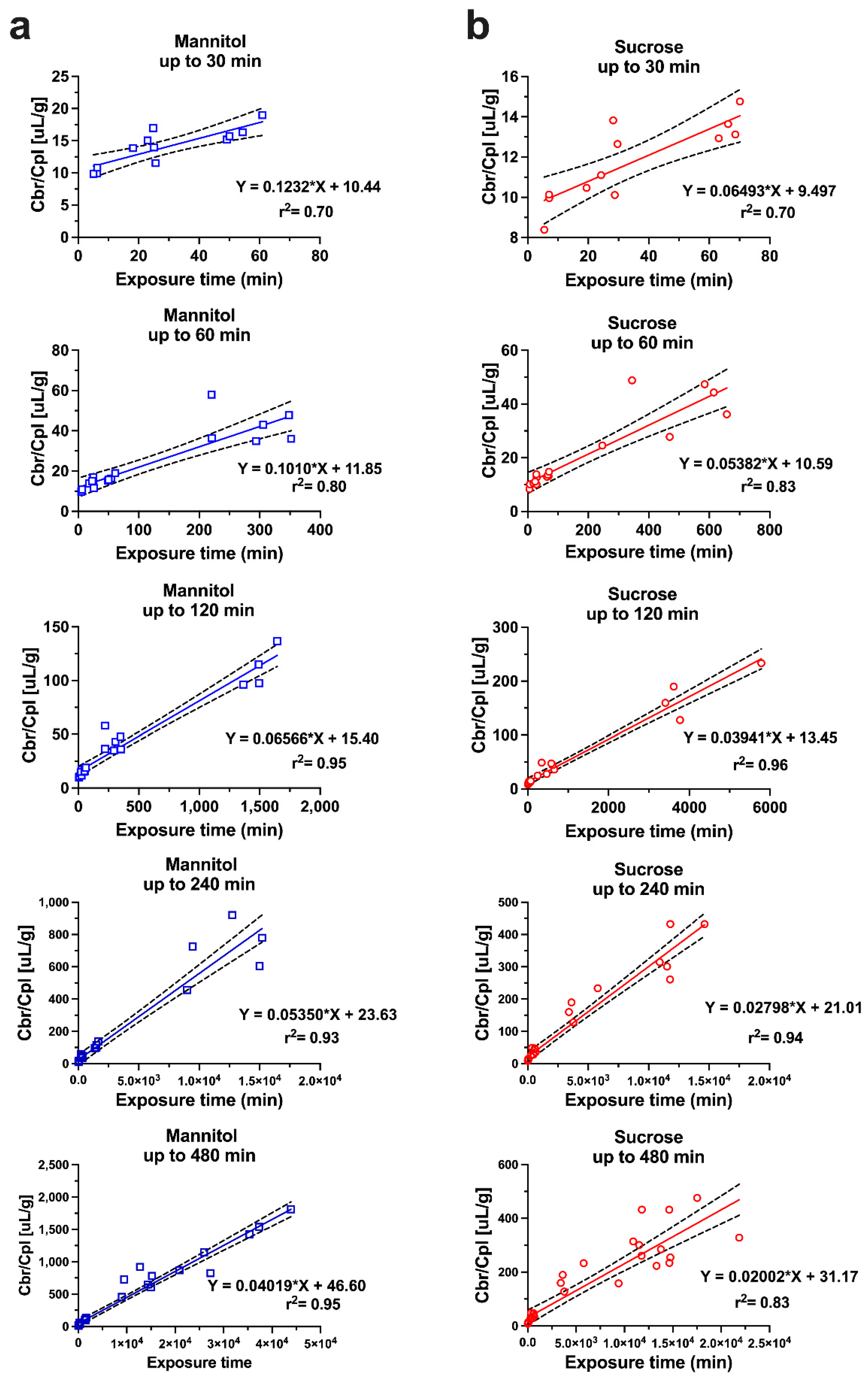
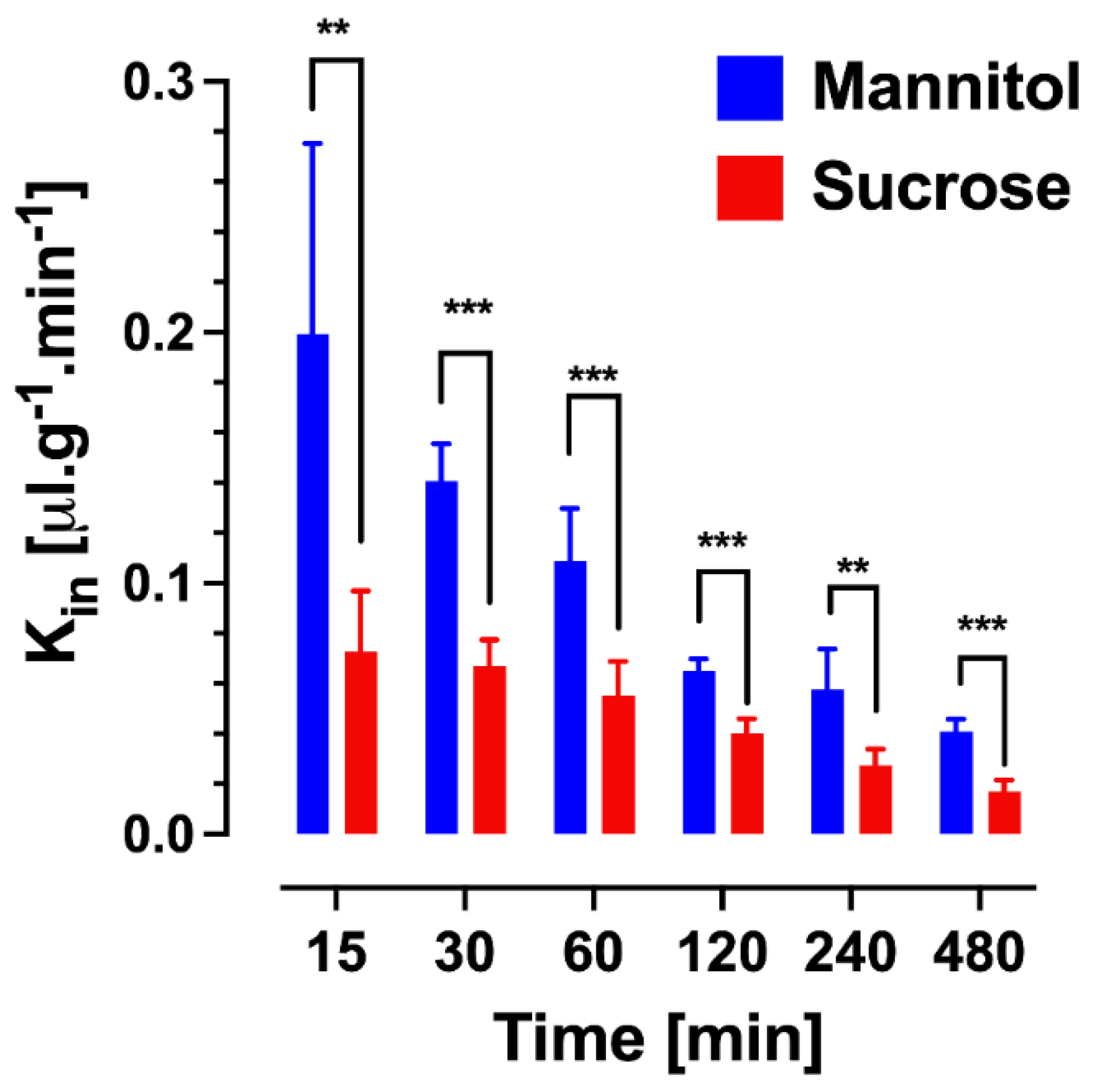
2.3.1. Multiple Time Point Analysis
2.3.2. Single Time Point Analysis
2.4. Caveats Associated with Unidirectional Uptake Models
2.5. PK Analysis in Brain Imaging Techniques
3. Compartmental Models of Brain Uptake
Choice of Permeability Markers
4. Extent of Brain Drug Exposure
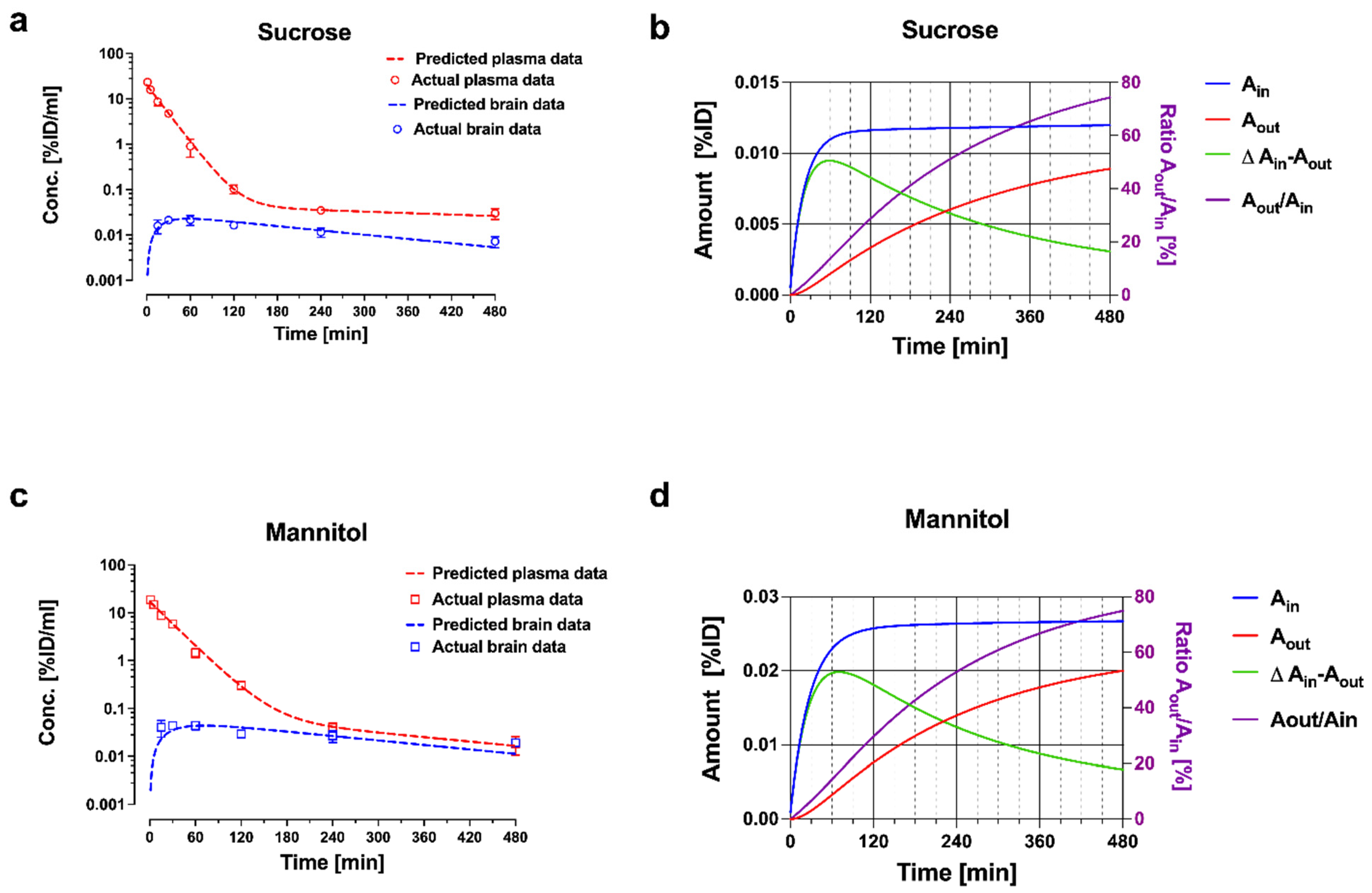
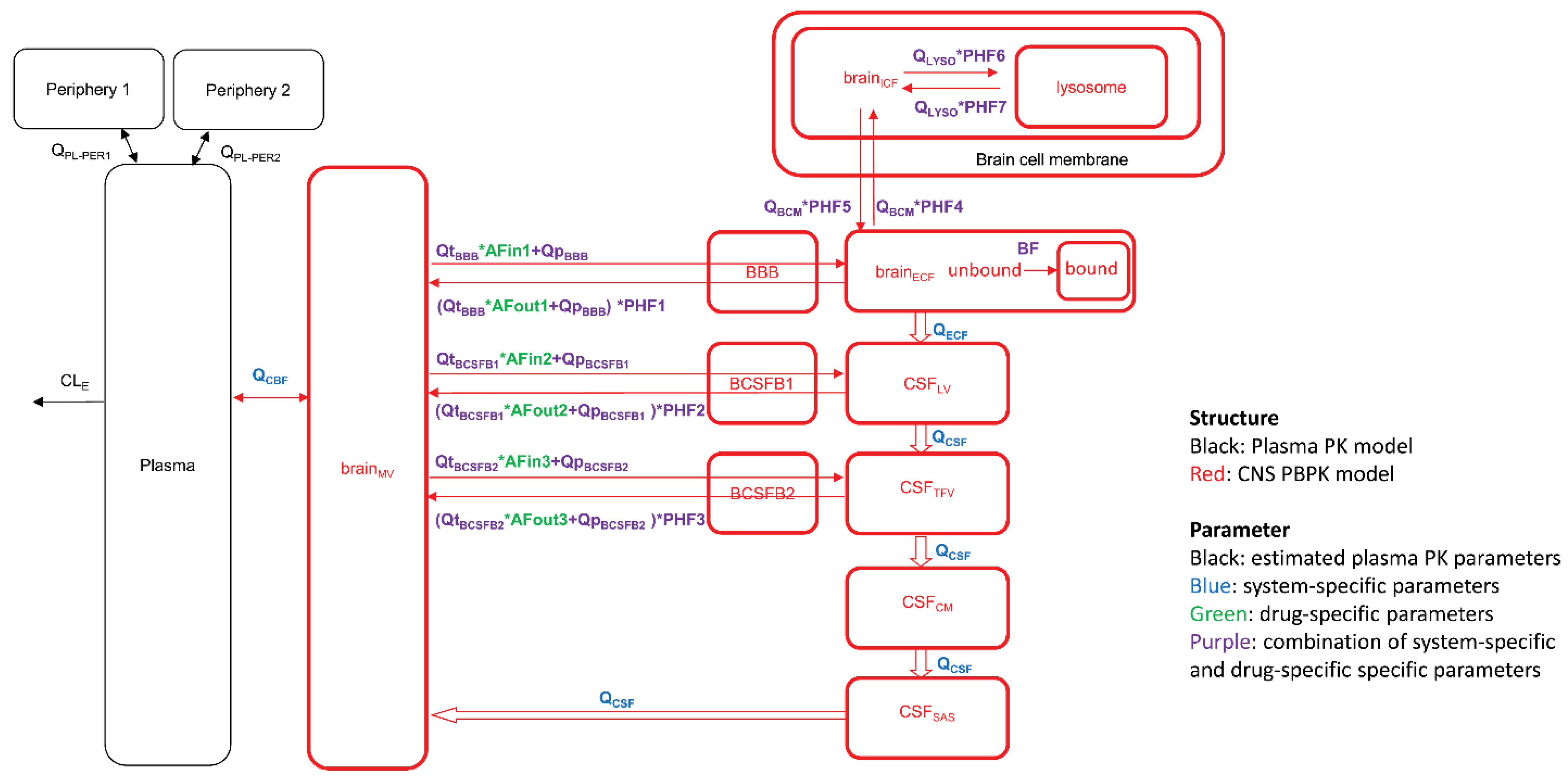
5. Physiologically Based Pharmacokinetic Models
6. Conclusions and Perspectives
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Saunders, N.R.; Dreifuss, J.J.; Dziegielewska, K.M.; Johansson, P.A.; Habgood, M.D.; Mollgard, K.; Bauer, H.C. The rights and wrongs of blood-brain barrier permeability studies: A walk through 100 years of history. Front. Neurosci. 2014, 8, 404. [Google Scholar] [CrossRef]
- Iadecola, C. The Neurovascular Unit Coming of Age: A Journey through Neurovascular Coupling in Health and Disease. Neuron 2017, 96, 17–42. [Google Scholar] [CrossRef] [PubMed]
- Abbott, N.J.; Patabendige, A.A.; Dolman, D.E.; Yusof, S.R.; Begley, D.J. Structure and function of the blood-brain barrier. Neurobiol. Dis. 2010, 37, 13–25. [Google Scholar] [CrossRef] [PubMed]
- Oldendorf, W.H. Clearance of Radiolabeled Substances by Brain after Arterial Injection Using a Diffusible Internal Standard. In Research Methods in Neurochemistry; Springer: Boston, MA, USA, 1981; Volume 5, pp. 91–112. [Google Scholar]
- Oldendorf, W.H. Measurement of brain uptake of radiolabeled substances using a tritiated water internal standard. Brain Res. 1970, 24, 372–376. [Google Scholar] [CrossRef]
- Pardridge, W.M.; Fierer, G. Blood-brain barrier transport of butanol and water relative to N-isopropyl-p-iodoamphetamine as the internal reference. J. Cereb. Blood Flow Metab. 1985, 5, 275–281. [Google Scholar] [CrossRef]
- Takasato, Y.; Rapoport, S.I.; Smith, Q.R. An in situ brain perfusion technique to study cerebrovascular transport in the rat. Am. J. Physiol. 1984, 247, H484–H493. [Google Scholar] [CrossRef]
- Pardridge, W.M. Blood-brain barrier carrier-mediated transport and brain metabolism of amino acids. Neurochem. Res. 1998, 23, 635–644. [Google Scholar] [CrossRef] [PubMed]
- Oldendorf, W.H.; Hyman, S.; Braun, L.; Oldendorf, S.Z. Blood-brain barrier: Penetration of morphine, codeine, heroin, and methadone after carotid injection. Science 1972, 178, 984–986. [Google Scholar] [CrossRef] [PubMed]
- Zlokovic, B.V.; Lipovac, M.N.; Begley, D.J.; Davson, H.; Rakic, L. Transport of leucine-enkephalin across the blood-brain barrier in the perfused guinea pig brain. J. Neurochem. 1987, 49, 310–315. [Google Scholar] [CrossRef]
- Dagenais, C.; Rousselle, C.; Pollack, G.M.; Scherrmann, J.M. Development of an in situ mouse brain perfusion model and its application to mdr1a P-glycoprotein-deficient mice. J. Cereb. Blood Flow Metab. 2000, 20, 381–386. [Google Scholar] [CrossRef]
- Murakami, H.; Takanaga, H.; Matsuo, H.; Ohtani, H.; Sawada, Y. Comparison of blood-brain barrier permeability in mice and rats using in situ brain perfusion technique. Am. J. Physiol. Heart Circ. Physiol. 2000, 279, H1022–H1028. [Google Scholar] [CrossRef]
- Chowdhury, E.A.; Alqahtani, F.; Bhattacharya, R.; Mehvar, R.; Bickel, U. Simultaneous UPLC–MS/MS analysis of two stable isotope labeled versions of sucrose in mouse plasma and brain samples as markers of blood-brain barrier permeability and brain vascular space. J. Chromatogr. B 2018, 1073, 19–26. [Google Scholar] [CrossRef]
- Summerfield, S.G.; Yates, J.W.T.; Fairman, D.A. Free Drug Theory—No Longer Just a Hypothesis? Pharm. Res. 2022, 39, 213–222. [Google Scholar] [CrossRef]
- Blasberg, R.G.; Fenstermacher, J.D.; Patlak, C.S. Transport of alpha-aminoisobutyric acid across brain capillary and cellular membranes. J. Cereb. Blood Flow Metab. 1983, 3, 8–32. [Google Scholar] [CrossRef]
- Patlak, C.S.; Blasberg, R.G. Graphical evaluation of blood-to-brain transfer constants from multiple-time uptake data. Generalizations. J. Cereb. Blood Flow Metab. 1985, 5, 584–590. [Google Scholar] [CrossRef]
- Patlak, C.S.; Blasberg, R.G.; Fenstermacher, J.D. Graphical evaluation of blood-to-brain transfer constants from multiple-time uptake data. J. Cereb. Blood Flow Metab. 1983, 3, 1–7. [Google Scholar] [CrossRef]
- Smith, Q.R. A review of blood-brain barrier transport techniques. Methods Mol. Med. 2003, 89, 193–208. [Google Scholar]
- Noorani, B.; Chowdhury, E.A.; Alqahtani, F.; Sajib, M.S.; Ahn, Y.; Nozohouri, E.; Patel, D.; Mikelis, C.; Mehvar, R.; Bickel, U. A Semi-Physiological Three-Compartment Model Describes Brain Uptake Clearance and Efflux of Sucrose and Mannitol after IV Injection in Awake Mice. Pharm. Res. 2022, 39, 251–261. [Google Scholar] [CrossRef]
- Ewing, J.R.; Brown, S.L.; Lu, M.; Panda, S.; Ding, G.; Knight, R.A.; Cao, Y.; Jiang, Q.; Nagaraja, T.N.; Churchman, J.L.; et al. Model selection in magnetic resonance imaging measurements of vascular permeability: Gadomer in a 9L model of rat cerebral tumor. J. Cereb. Blood Flow Metab. 2006, 26, 310–320. [Google Scholar] [CrossRef]
- Karakatsanis, N.A.; Zhou, Y.; Lodge, M.A.; Casey, M.E.; Wahl, R.L.; Zaidi, H.; Rahmim, A. Generalized whole-body Patlak parametric imaging for enhanced quantification in clinical PET. Phys. Med. Biol. 2015, 60, 8643–8673. [Google Scholar] [CrossRef]
- Tofts, P.S.; Brix, G.; Buckley, D.L.; Evelhoch, J.L.; Henderson, E.; Knopp, M.V.; Larsson, H.B.; Lee, T.Y.; Mayr, N.A.; Parker, G.J.; et al. Estimating kinetic parameters from dynamic contrast-enhanced T(1)-weighted MRI of a diffusable tracer: Standardized quantities and symbols. J. Magn. Reson. Imaging 1999, 10, 223–232. [Google Scholar] [CrossRef]
- Logan, J.; Fowler, J.S.; Volkow, N.D.; Wolf, A.P.; Dewey, S.L.; Schlyer, D.J.; MacGregor, R.R.; Hitzemann, R.; Bendriem, B.; Gatley, S.J.; et al. Graphical analysis of reversible radioligand binding from time-activity measurements applied to [N-11C-methyl]-(-)-cocaine PET studies in human subjects. J. Cereb. Blood Flow Metab. 1990, 10, 740–747. [Google Scholar] [CrossRef]
- Logan, J.; Alexoff, D.; Fowler, J.S. The use of alternative forms of graphical analysis to balance bias and precision in PET images. J. Cereb. Blood Flow Metab. 2011, 31, 535–546. [Google Scholar] [CrossRef]
- Absinta, M.; Sati, P.; Reich, D.S. Advanced MRI and staging of multiple sclerosis lesions. Nat. Rev. Neurol. 2016, 12, 358–368. [Google Scholar] [CrossRef]
- Guo, Y.; Lebel, R.M.; Zhu, Y.; Lingala, S.G.; Shiroishi, M.S.; Law, M.; Nayak, K. High-resolution whole-brain DCE-MRI using constrained reconstruction: Prospective clinical evaluation in brain tumor patients. Med. Phys. 2016, 43, 2013. [Google Scholar] [CrossRef]
- Candelario-Jalil, E.; Dijkhuizen, R.M.; Magnus, T. Neuroinflammation, Stroke, Blood-Brain Barrier Dysfunction, and Imaging Modalities. Stroke 2022, 53, 1473–1486. [Google Scholar] [CrossRef]
- Canjels, L.P.W.; Jansen, J.F.A.; van den Kerkhof, M.; Alers, R.J.; Poser, B.A.; Wiggins, C.J.; Schiffer, V.; van de Ven, V.; Rouhl, R.P.W.; Palm, W.M.; et al. 7T dynamic contrast-enhanced MRI for the detection of subtle blood-brain barrier leakage. J. Neuroimaging 2021, 31, 902–911. [Google Scholar] [CrossRef]
- Ohno, K.; Pettigrew, K.D.; Rapoport, S.I. Lower limits of cerebrovascular permeability to nonelectrolytes in the conscious rat. Am. J. Physiol. 1978, 235, H299–H307. [Google Scholar] [CrossRef]
- Nicholson, C.; Hrabetova, S. Brain Extracellular Space: The Final Frontier of Neuroscience. Biophys. J. 2017, 113, 2133–2142. [Google Scholar] [CrossRef]
- Cserr, H.F.; Cooper, D.N.; Suri, P.K.; Patlak, C.S. Efflux of radiolabeled polyethylene glycols and albumin from rat brain. Am. J. Physiol. 1981, 240, F319–F328. [Google Scholar] [CrossRef]
- Groothuis, D.R.; Vavra, M.W.; Schlageter, K.E.; Kang, E.W.; Itskovich, A.C.; Hertzler, S.; Allen, C.V.; Lipton, H.L. Efflux of drugs and solutes from brain: The interactive roles of diffusional transcapillary transport, bulk flow and capillary transporters. J. Cereb. Blood Flow Metab. 2007, 27, 43–56. [Google Scholar] [CrossRef] [PubMed]
- Hladky, S.B.; Barrand, M.A. Elimination of substances from the brain parenchyma: Efflux via perivascular pathways and via the blood-brain barrier. Fluids Barriers CNS 2018, 15, 30. [Google Scholar] [CrossRef] [PubMed]
- Fenstermacher, J.D.; Patlak, C.S.; Blasberg, R.G. Transport of material between brain extracellular fluid, brain cells and blood. Fed. Proc. 1974, 33, 2070–2074. [Google Scholar]
- Collins, J.M.; Dedrick, R.L. Distributed model for drug delivery to CSF and brain tissue. Am. J. Physiol. 1983, 245, R303–R310. [Google Scholar] [CrossRef] [PubMed]
- De Lange, E.C.; Danhof, M.; de Boer, A.G.; Breimer, D.D. Critical factors of intracerebral microdialysis as a technique to determine the pharmacokinetics of drugs in rat brain. Brain Res. 1994, 666, 1–8. [Google Scholar] [CrossRef]
- Elmquist, W.F.; Sawchuk, R.J. Application of microdialysis in pharmacokinetic studies. Pharm. Res. 1997, 14, 267–288. [Google Scholar] [CrossRef] [PubMed]
- Hammarlund-Udenaes, M.; Paalzow, L.K.; de Lange, E.C. Drug equilibration across the blood-brain barrier--pharmacokinetic considerations based on the microdialysis method. Pharm. Res. 1997, 14, 128–134. [Google Scholar] [CrossRef]
- Alqahtani, F.; Chowdhury, E.A.; Bhattacharya, R.; Noorani, B.; Mehvar, R.; Bickel, U. Brain Uptake of [13C] and [14C]Sucrose Quantified by Microdialysis and Whole Tissue Analysis in Mice. Drug Metab. Dispos. 2018, 46, 1514–1518. [Google Scholar] [CrossRef] [PubMed]
- Miah, M.K.; Chowdhury, E.A.; Bickel, U.; Mehvar, R. Evaluation of [14C] and [13C]Sucrose as Blood-Brain Barrier Permeability Markers. J. Pharm. Sci. 2017, 106, 1659–1669. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, E.A.; Noorani, B.; Alqahtani, F.; Bhalerao, A.; Raut, S.; Sivandzade, F.; Cucullo, L. Understanding the brain uptake and permeability of small molecules through the BBB: A technical overview. J. Cereb. Blood Flow Metab. 2021, 41, 1797–1820. [Google Scholar] [CrossRef]
- Saunders, N.R.; Dziegielewska, K.M.; Mollgard, K.; Habgood, M.D. Markers for blood-brain barrier integrity: How appropriate is Evans blue in the twenty-first century and what are the alternatives? Front. Neurosci. 2015, 9, 385. [Google Scholar] [CrossRef] [PubMed]
- Preston, E.; Haas, N. Defining the lower limits of blood-brain barrier permeability: Factors affecting the magnitude and interpretation of permeability-area products. J. Neurosci. Res. 1986, 16, 709–719. [Google Scholar] [CrossRef]
- Noorani, B.; Chowdhury, E.A.; Alqahtani, F.; Ahn, Y.; Patel, D.; Al-Ahmad, A.; Mehvar, R.; Bickel, U. LC-MS/MS-based in vitro and in vivo investigation of blood-brain barrier integrity by simultaneous quantitation of mannitol and sucrose. Fluids Barriers CNS 2020, 17, 61. [Google Scholar] [CrossRef]
- Shaik, I.H.; Miah, M.K.; Bickel, U.; Mehvar, R. Effects of short-term portacaval anastomosis on the peripheral and brain disposition of the blood-brain barrier permeability marker sodium fluorescein in rats. Brain Res. 2013, 1531, 84–93. [Google Scholar] [CrossRef] [PubMed]
- Hawkins, B.T.; Ocheltree, S.M.; Norwood, K.M.; Egleton, R.D. Decreased blood-brain barrier permeability to fluorescein in streptozotocin-treated rats. Neurosci. Lett. 2007, 411, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Johnson, D.R.; Finch, R.A.; Sartorelli, A.C.; Miller, D.W.; Elmquist, W.F. Transport of fluorescein in MDCKII-MRP1 transfected cells and mrp1-knockout mice. Biochem. Biophys. Res. Commun. 2001, 284, 863–869. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Slattengren, T.; de Lange, E.C.M.; Smith, D.E.; Hammarlund-Udenaes, M. Revisiting atenolol as a low passive permeability marker. Fluids Barriers CNS 2017, 14, 30. [Google Scholar] [CrossRef]
- Gupta, A.; Chatelain, P.; Massingham, R.; Jonsson, E.N.; Hammarlund-Udenaes, M. Brain distribution of cetirizine enantiomers: Comparison of three different tissue-to-plasma partition coefficients: K(p), K(p,u), and K(p,uu). Drug Metab. Dispos. 2006, 34, 318–323. [Google Scholar] [CrossRef]
- Hammarlund-Udenaes, M.; Friden, M.; Syvanen, S.; Gupta, A. On the rate and extent of drug delivery to the brain. Pharm. Res. 2008, 25, 1737–1750. [Google Scholar] [CrossRef]
- Becker, S.; Liu, X. Evaluation of the utility of brain slice methods to study brain penetration. Drug Metab. Dispos. 2006, 34, 855–861. [Google Scholar] [CrossRef] [PubMed]
- Tunblad, K.; Jonsson, E.N.; Hammarlund-Udenaes, M. Morphine blood-brain barrier transport is influenced by probenecid co-administration. Pharm. Res. 2003, 20, 618–623. [Google Scholar] [CrossRef] [PubMed]
- Bickel, U.; Schumacher, O.P.; Kang, Y.S.; Voigt, K. Poor permeability of morphine 3-glucuronide and morphine 6-glucuronide through the blood-brain barrier in the rat. J. Pharmacol. Exp. Ther. 1996, 278, 107–113. [Google Scholar] [PubMed]
- Stain-Texier, F.; Boschi, G.; Sandouk, P.; Scherrmann, J.M. Elevated concentrations of morphine 6-beta-D-glucuronide in brain extracellular fluid despite low blood-brain barrier permeability. Br. J. Pharmacol. 1999, 128, 917–924. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Kang, Y.S.; Bickel, U.; Pardridge, W.M. Blood-brain barrier permeability to morphine-6-glucuronide is markedly reduced compared with morphine. Drug Metab. Dispos. 1997, 25, 768–771. [Google Scholar] [PubMed]
- Xie, R.; Bouw, M.R.; Hammarlund-Udenaes, M. Modelling of the blood-brain barrier transport of morphine-3-glucuronide studied using microdialysis in the rat: Involvement of probenecid-sensitive transport. Br. J. Pharmacol. 2000, 131, 1784–1792. [Google Scholar] [CrossRef]
- Bostrom, E.; Simonsson, U.; Hammarlund-Udenaes, M. In vivo blood-brain barrier transport of oxycodone in the rat—Indications for active influx and implications for PK/PD. Drug Metab. Dispos. 2006, 34, 1624–1631. [Google Scholar] [CrossRef] [PubMed]
- Xie, R.; Hammarlund-Udenaes, M. Blood-brain barrier equilibration of codeine in rats studied with microdialysis. Pharm. Res. 1998, 15, 570–575. [Google Scholar] [CrossRef]
- Tunblad, K.; Hammarlund-Udenaes, M.; Jonsson, E.N. Influence of probenecid on the delivery of morphine-6-glucuronide to the brain. Eur. J. Pharm. Sci. 2005, 24, 49–57. [Google Scholar] [CrossRef]
- Yang, J.; Reilly, B.G.; Davis, T.P.; Ronaldson, P.T. Modulation of Opioid Transport at the Blood-Brain Barrier by Altered ATP-Binding Cassette (ABC) Transporter Expression and Activity. Pharmaceutics 2018, 10, 192. [Google Scholar] [CrossRef]
- Bischoff, K.B.; Dedrick, R.L. Thiopental pharmacokinetics. J. Pharm. Sci. 1968, 57, 1346–1351. [Google Scholar] [CrossRef]
- Teorell, T. Kinetics of distribution of substances administered to the body. I. The extravascular modes of administration. Arch. Int. Pharmacodyn. Ther. 1937, 57, 205–225. [Google Scholar]
- Uchida, Y.; Ohtsuki, S.; Katsukura, Y.; Ikeda, C.; Suzuki, T.; Kamiie, J.; Terasaki, T. Quantitative Targeted Absolute Proteomics of Human Blood-Brain Barrier Transporters and Receptors. J. Neurochem. 2011, 117, 333–345. [Google Scholar] [CrossRef]
- Huttunen, K.M.; Terasaki, T.; Urtti, A.; Montaser, A.B.; Uchida, Y. Pharmacoproteomics of Brain Barrier Transporters and Substrate Design for the Brain Targeted Drug Delivery. Pharm. Res. 2022, 39, 1363–1392. [Google Scholar] [CrossRef]
- Sugano, K. Lost in modelling and simulation? ADMET DMPK 2021, 9, 75–109. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Valitalo, P.A.; Huntjens, D.R.; Proost, J.H.; Vermeulen, A.; Krauwinkel, W.; Beukers, M.W.; van den Berg, D.J.; Hartman, R.; Wong, Y.C.; et al. Predicting Drug Concentration-Time Profiles in Multiple CNS Compartments Using a Comprehensive Physiologically-Based Pharmacokinetic Model. CPT Pharmacomet. Syst. Pharmacol. 2017, 6, 765–777. [Google Scholar] [CrossRef]
- De Lange, E.C. The mastermind approach to CNS drug therapy: Translational prediction of human brain distribution, target site kinetics, and therapeutic effects. Fluids Barriers CNS 2013, 10, 12. [Google Scholar] [CrossRef]
- Ball, K.; Bouzom, F.; Scherrmann, J.M.; Walther, B.; Decleves, X. Development of a physiologically based pharmacokinetic model for the rat central nervous system and determination of an in vitro-in vivo scaling methodology for the blood-brain barrier permeability of two transporter substrates, morphine and oxycodone. J. Pharm. Sci. 2012, 101, 4277–4292. [Google Scholar] [CrossRef]
- Vendel, E.; Rottschafer, V.; de Lange, E.C.M. The need for mathematical modelling of spatial drug distribution within the brain. Fluids Barriers CNS 2019, 16, 12. [Google Scholar] [CrossRef]
- Baxter, L.T.; Zhu, H.; Mackensen, D.G.; Jain, R.K. Physiologically based pharmacokinetic model for specific and nonspecific monoclonal antibodies and fragments in normal tissues and human tumor xenografts in nude mice. Cancer Res. 1994, 54, 1517–1528. [Google Scholar]
- Ferl, G.Z.; Wu, A.M.; DiStefano, J.J., III. A predictive model of therapeutic monoclonal antibody dynamics and regulation by the neonatal Fc receptor (FcRn). Ann. Biomed. Eng. 2005, 33, 1640–1652. [Google Scholar] [CrossRef]
- Garg, A.; Balthasar, J.P. Physiologically-based pharmacokinetic (PBPK) model to predict IgG tissue kinetics in wild-type and FcRn-knockout mice. J. Pharmacokinet. Pharmacodyn. 2007, 34, 687–709. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.Y.; Wu, S.; Chowdhury, E.A.; Shah, D.K. Towards a translational physiologically-based pharmacokinetic (PBPK) model for receptor-mediated transcytosis of anti-transferrin receptor monoclonal antibodies in the central nervous system. J. Pharmacokinet. Pharmacodyn. 2022, 49, 337–362. [Google Scholar] [CrossRef] [PubMed]
- Gadkar, K.; Yadav, D.B.; Zuchero, J.Y.; Couch, J.A.; Kanodia, J.; Kenrick, M.K.; Atwal, J.K.; Dennis, M.S.; Prabhu, S.; Watts, R.J.; et al. Mathematical PKPD and safety model of bispecific TfR/BACE1 antibodies for the optimization of antibody uptake in brain. Eur. J. Pharm. Biopharm. 2016, 101, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Pardridge, W.M.; Chou, T. Mathematical Models of Blood-Brain Barrier Transport of Monoclonal Antibodies Targeting the Transferrin Receptor and the Insulin Receptor. Pharmaceuticals 2021, 14, 535. [Google Scholar] [CrossRef]

| [13C12] Sucrose | [13C6] Mannitol | ||||||
|---|---|---|---|---|---|---|---|
| Parameters | Units | Value | SE | CV % | Value | SE | CV% |
| V1 | mL | 4.97 a | 0.326 | 6.56 | 6.06 | 0.34 | 5. 6 |
| V2 | mL | 14.1 b | 8.28 | 58.6 | 3.22 | 0.63 | 19.7 |
| Ve (fixed) | mL/g | 0.2 | 0.2 | ||||
| CL10 | mL/min | 0.226 c | 0.011 | 4.84 | 0.212 | 0.008 | 3.86 |
| CL12 | mL/min | 0.019 d | 0.005 | 23.2 | 0.010 | 0.001 | 9.94 |
| CL13/Wbrain | µL/(min × g) | 0.068 e | 0.005 | 7.73 | 0.146 | 0.020 | 9.64 |
| CL31/Wbrain | µL/(min × g) | 0.693 f | 0.106 | 15.4 | 0.881 | 0.20 | 22.5 |
| Wbrain (fixed) | g | 0.4 | 0.4 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bickel, U. Modeling Blood–Brain Barrier Permeability to Solutes and Drugs In Vivo. Pharmaceutics 2022, 14, 1696. https://doi.org/10.3390/pharmaceutics14081696
Bickel U. Modeling Blood–Brain Barrier Permeability to Solutes and Drugs In Vivo. Pharmaceutics. 2022; 14(8):1696. https://doi.org/10.3390/pharmaceutics14081696
Chicago/Turabian StyleBickel, Ulrich. 2022. "Modeling Blood–Brain Barrier Permeability to Solutes and Drugs In Vivo" Pharmaceutics 14, no. 8: 1696. https://doi.org/10.3390/pharmaceutics14081696






