3D Printed Osteoblast–Alginate/Collagen Hydrogels Promote Survival, Proliferation and Mineralization at Low Doses of Strontium Calcium Polyphosphate
Abstract
:1. Introduction
2. Materials and Methods
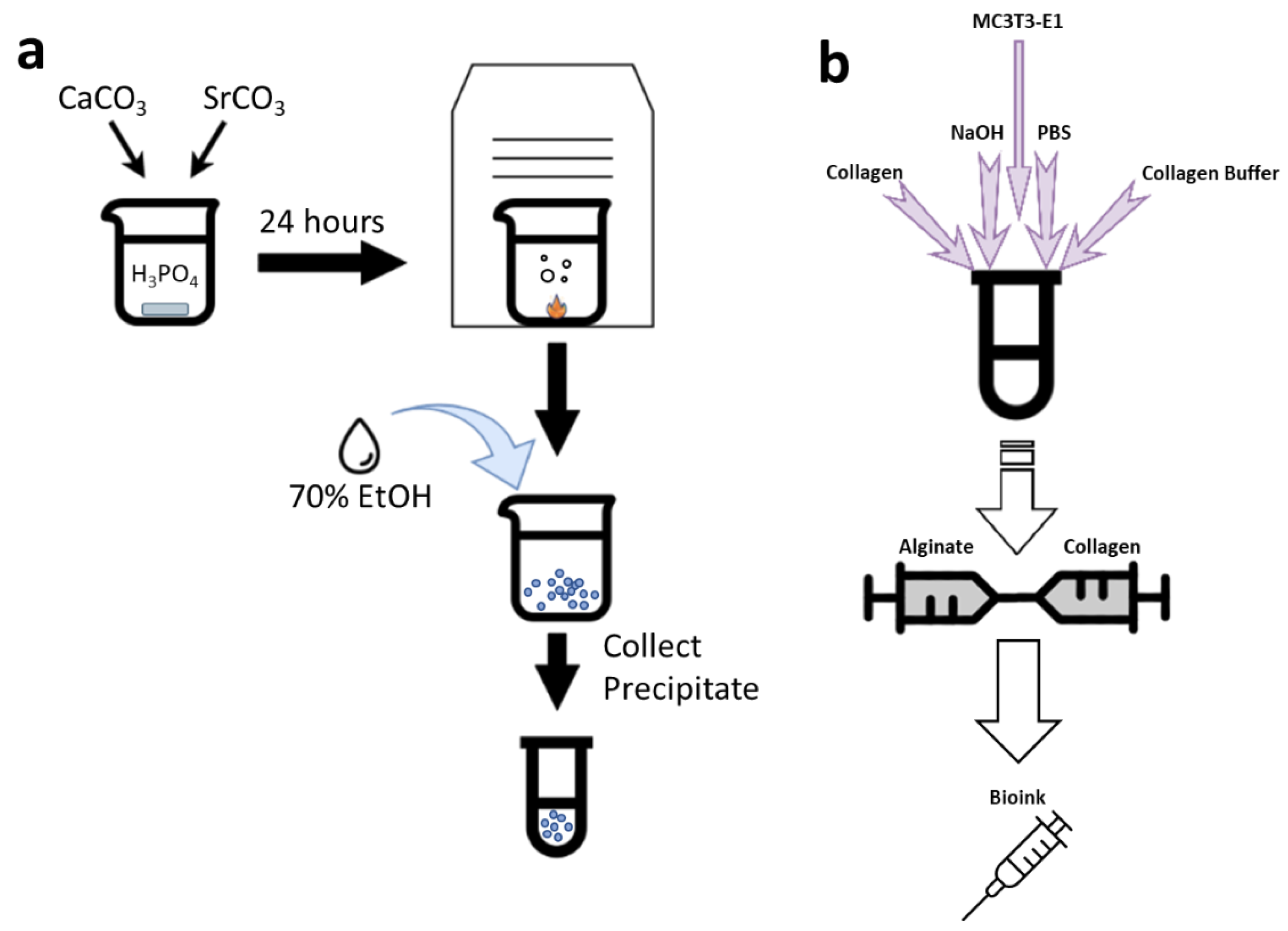
2.1. SCPP Synthesis
2.2. Cell Culture
2.3. SCPP Cytotoxicity
2.4. Cell Staining
2.5. Bioink Synthesis
2.6. 3D Printing
2.7. Scaffold Swelling, Degradation, and pH
2.8. Raman Spectroscopy and Imaging
2.9. Scanning Electron Microscopy
2.10. Immunofluorescence
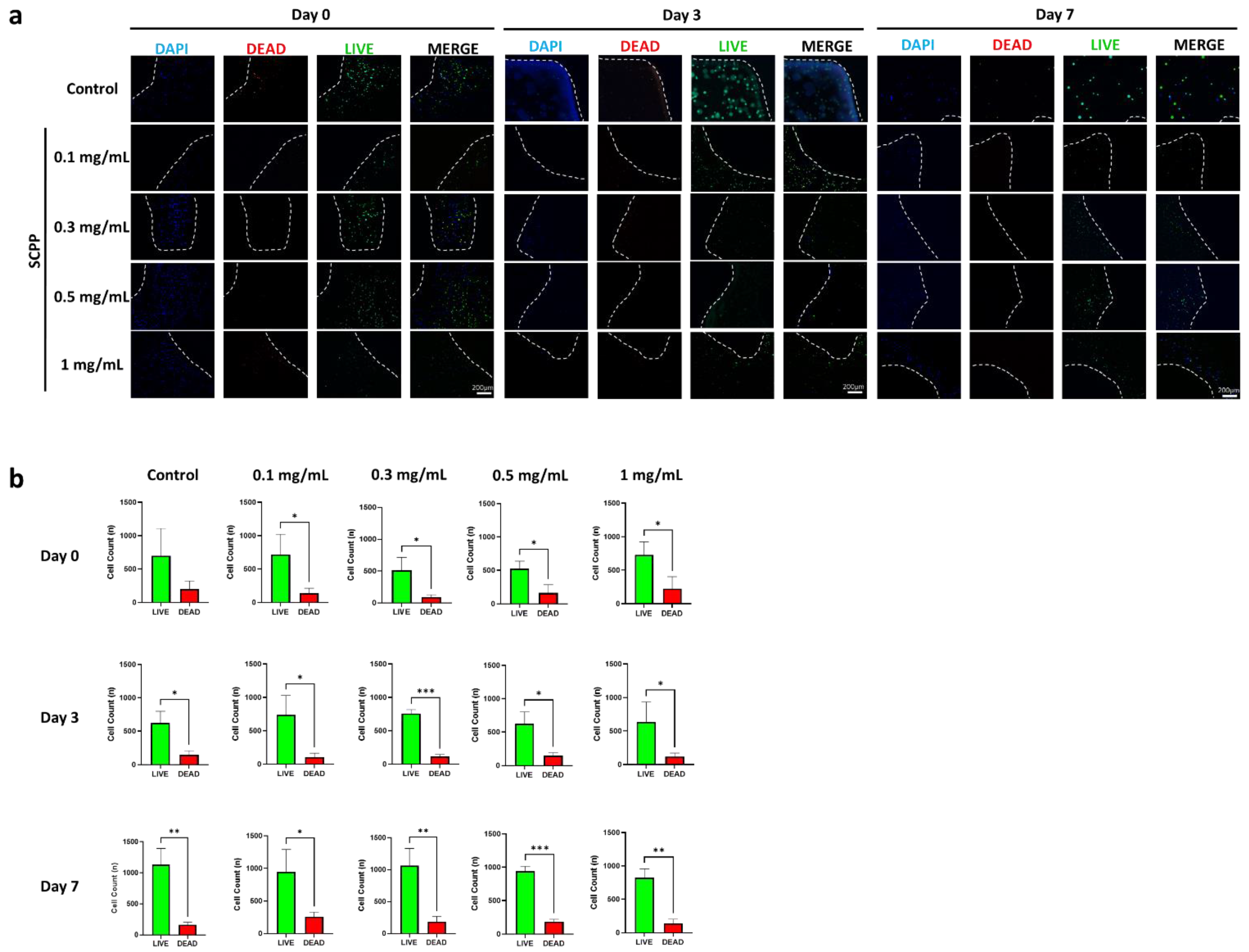
2.11. Scaffold Cell Viability
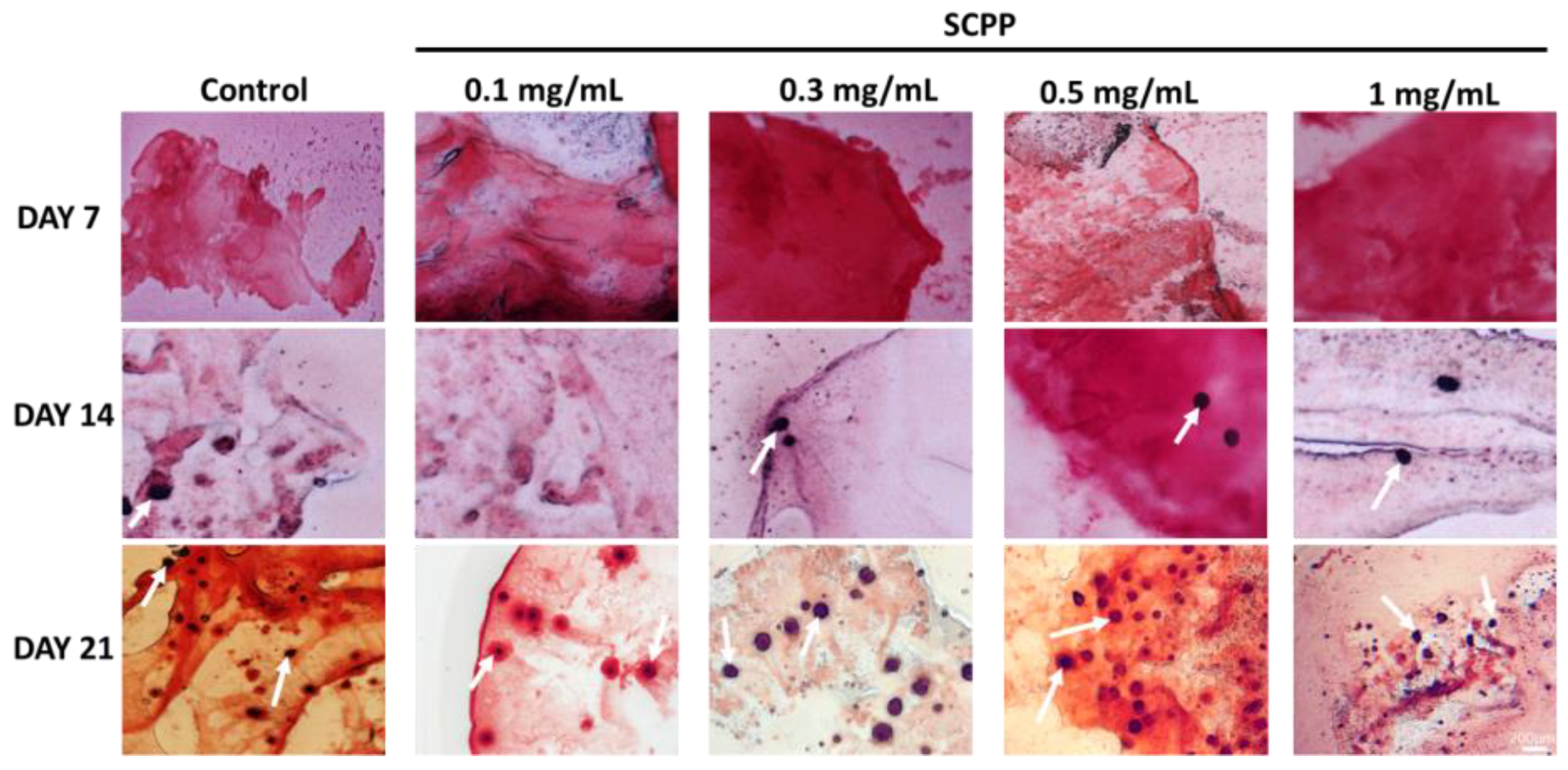
2.12. Alizarin Red Staining
2.13. Statistical Analyses
3. Results
3.1. Alginate/Collagen Scaffolds Buffer the Acidic SCPP
3.2. The Use of Scpp Does Not Change the Microscopic Structure or the Raman Spectra of the Scaffolds
3.3. Higher SCPP Concentrations Decrease Scaffold Swelling and Increase Degradation
3.4. Osteoblast Proliferation Is Not Affected in 1 mg/mL SCPP-Doped Scaffolds
3.5. Early Osteoblast Calcification Is Seen in 0.3, 0.5, and 1 mg/mL SCPP Doped Scaffolds
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hak, D.J.; Fitzpatrick, D.; Bishop, J.A.; Marsh, J.L.; Tilp, S.; Schnettler, R.; Simpson, H.; Alt, V. Delayed union and nonunions: Epidemiology, clinical issues, and financial aspects. Injury 2014, 45, S3–S7. [Google Scholar] [CrossRef] [PubMed]
- Marsell, R.; Einhorn, T.A. The biology of fracture healing. Injury 2011, 42, 551–555. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hadjiargyrou, M.; O’Keefe, R.J. The convergence of fracture repair and stem cells: Interplay of genes, aging, environmental factors and disease. J. Bone Miner. Res. 2014, 29, 2307–2322. [Google Scholar] [CrossRef]
- Maruyama, M.; Rhee, C.; Utsunomiya, T.; Zhang, N.; Ueno, M.; Yao, Z.; Goodman, S.B. Modulation of the inflammatory response and bone healing. Front. Endocrinol. 2020, 11, 386. [Google Scholar] [CrossRef] [PubMed]
- Dimitriou, R.; Jones, E.; McGonagle, D.; Giannoudis, P.V. Bone regeneration: Current concepts and future directions. BMC Med. 2011, 9, 66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tharakan, S.; Khondkar, S.; Ilyas, A. Bioprinting of stem cells in multimaterial scaffolds and their applications in bone tissue engineering. Sensors 2021, 21, 7477. [Google Scholar] [CrossRef]
- Syed, A.H.; Baghaie, A.; Ilyas, A. Automated Image Processing Based 3D Printed Scaffolds for Critical Size Bone Fracture Treatment. In Proceedings of the 2022 IEEE Long Island Systems, Applications and Technology Conference (LISAT), Old Westbury, NY, USA, 6 May 2022; pp. 1–6. [Google Scholar] [CrossRef]
- Varanasi, V.G.; Ilyas, A.; Kramer, P.R.; Azimaie, T.; Aswath, P.B.; Cebe, T. In Vivo Live 3D Printing of Regenerative Bone Healing Scaffolds for Rapid Fracture Healing. U.S. Patent 10,442,182 B2, 15 October 2019. [Google Scholar]
- Arif, Z.U.; Khalid, M.Y.; Ahmed, W.; Arshad, H. A review on four-dimensional bioprinting in pursuit of advanced tissue engineering applications. Bioprinting 2022, e00203. [Google Scholar] [CrossRef]
- Arif, Z.U.; Khalid, M.Y.; Zolfagharian, A.; Bodaghi, M. 4D bioprinting of smart polymers for biomedical applications: Recent progress, challenges, and future perspectives. React. Funct. Polym. 2022, 105374. [Google Scholar] [CrossRef]
- Khalid, M.Y.; Arif, Z.U.; Ahmed, W.; Umer, R.; Zolfagharian, A.; Bodaghi, M. 4D printing: Technological developments in robotics applications. Sens. Actuators A Phys. 2022, 343, 113670. [Google Scholar] [CrossRef]
- Khalid, M.Y.; Arif, Z.U.; Ahmed, W. 4D Printing: Technological and Manufacturing Renaissance. Macromol. Mater. Eng. 2022, 2200003. [Google Scholar] [CrossRef]
- Khalid, M.Y.; Arif, Z.U.; Noroozi, R.; Zolfagharian, A.; Bodaghi, M. 4D printing of shape memory polymer composites: A review on fabrication techniques, applications, and future perspectives. J. Manuf. Process. 2022, 81, 759–797. [Google Scholar] [CrossRef]
- Papaioannou, T.G.; Manolesou, D.; Dimakakos, E.; Tsoucalas, G.; Vavuranakis, M.; Tousoulis, D. 3D bioprinting methods and techniques: Applications on artificial blood vessel fabrication. Acta Cardiol. Sin. 2019, 35, 284. [Google Scholar]
- Arif, Z.U.; Khalid, M.Y.; Noroozi, R.; Sadeghianmaryan, A.; Jalalvand, M.; Hossain, M. Recent advances in 3D-printed polylactide and polycaprolactone-based biomaterials for tissue engineering applications. Int. J. Biol. Macromol. 2022, 218, 930–968. [Google Scholar] [CrossRef]
- Noroozi, R.; Shamekhi, M.A.; Mahmoudi, R.; Zolfagharian, A.; Asgari, F.; Mousavizadeh, A.; Bodaghi, M.; Hadi, A.; Haghighipour, N. In vitro static and dynamic cell culture study of novel bone scaffolds based on 3D-printed PLA and cell-laden alginate hydrogel. Biomed. Mater. 2022, 17, 045024. [Google Scholar] [CrossRef] [PubMed]
- Vazquez, O.R.; Avila, I.O.; Díaz, J.C.S.; Hernandez, E. An overview of mechanical tests for polymeric biomaterial scaffolds used in tissue engineering. J. Res. Updat. Polym. Sci. 2015, 4, 168–178. [Google Scholar]
- Noroozi, R.; Tatar, F.; Zolfagharian, A.; Brighenti, R.; Shamekhi, M.A.; Rastgoo, A.; Hadi, A.; Bodaghi, M. Additively manufactured multi-morphology bone-like porous scaffolds: Experiments and micro-computed tomography-based finite element modeling approaches. Int. J. Bioprinting 2022, 8, 40–53. [Google Scholar] [CrossRef] [PubMed]
- Khalid, M.Y.; Al Rashid, A.; Arif, Z.U.; Ahmed, W.; Arshad, H. Recent advances in nanocellulose-based different biomaterials: Types, properties, and emerging applications. J. Mater. Res. Technol. 2021, 14, 2601–2623. [Google Scholar] [CrossRef]
- Arif, Z.U.; Khalid, M.Y.; Sheikh, M.F.; Zolfagharian, A.; Bodaghi, M. Biopolymeric sustainable materials and their emerging applications. J. Environ. Chem. Eng. 2022, 10, 108159. [Google Scholar] [CrossRef]
- Hu, T.; Lo, A.C. Collagen–Alginate Composite Hydrogel: Application in Tissue Engineering and Biomedical Sciences. Polymers 2021, 13, 1852. [Google Scholar] [CrossRef]
- Kang, S.W.; Cha, B.H.; Park, H.; Park, K.S.; Lee, K.Y.; Lee, S.H. The effect of conjugating RGD into 3D alginate hydrogels on adipogenic differentiation of human adipose-derived stromal cells. Macromol. Biosci. 2011, 11, 673–679. [Google Scholar] [CrossRef]
- Li, Y.; Liu, Y.; Li, R.; Bai, H.; Zhu, Z.; Zhu, L.; Zhu, C.; Che, Z.; Liu, H.; Wang, J. Collagen-based biomaterials for bone tissue engineering. Mater. Des. 2021, 210, 110049. [Google Scholar] [CrossRef]
- Luo, X.; Zhang, S.; Luo, B.; Li, H. Engineering collagen fiber templates with oriented nanoarchitecture and concerns on osteoblast behaviors. Int. J. Biol. Macromol. 2021, 185, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, T.L.; Little, D. Synthetic scaffolds for musculoskeletal tissue engineering: Cellular responses to fiber parameters. NPJ Regen. Med. 2019, 4, 15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wan, B.; Wang, R.; Sun, Y.; Cao, J.; Wang, H.; Guo, J.; Chen, D. Building osteogenic microenvironments with strontium-substituted calcium phosphate ceramics. Front. Bioeng. Biotechnol. 2020, 8, 591467. [Google Scholar] [CrossRef]
- Yan, M.-D.; Ou, Y.-J.; Lin, Y.-J.; Liu, R.-M.; Fang, Y.; Wu, W.-L.; Zhou, L.; Yao, X.; Chen, J. Does the incorporation of strontium into calcium phosphate improve bone repair? A meta-analysis. BMC Oral Health 2022, 22, 62. [Google Scholar] [CrossRef]
- Qiu, K.; Zhao, X.J.; Wan, C.X.; Zhao, C.S.; Chen, Y.W. Effect of strontium ions on the growth of ROS17/2.8 cells on porous calcium polyphosphate scaffolds. Biomaterials 2006, 27, 1277–1286. [Google Scholar] [CrossRef]
- Tian, M.; Chen, F.; Song, W.; Song, Y.; Chen, Y.; Wan, C.; Yu, X.; Zhang, X. In vivo study of porous strontium-doped calcium polyphosphate scaffolds for bone substitute applications. J. Mater. Sci. Mater. Med. 2009, 20, 1505–1512. [Google Scholar] [CrossRef]
- Khondkar, S.; Tharakan, S.; Badran, A.; Hadjiargyrou, M.; Ilyas, A. Controlled Biodegradation and Swelling of Strontium-doped Alginate/Collagen Scaffolds for Bone Tissue Engineering. In Proceedings of the 2022 44th Annual International Conference of the IEEE Engineering in Medicine & Biology Society (EMBC), Glasgow, UK, 11–15 July 2022; pp. 1561–1564. [Google Scholar]
- Zhang, Z.; Lai, Q.; Li, Y.; Xu, C.; Tang, X.; Ci, J.; Sun, S.; Xu, B.; Li, Y. Acidic pH environment induces autophagy in osteoblasts. Sci. Rep. 2017, 7, 46161. [Google Scholar] [CrossRef]
- Galow, A.-M.; Rebl, A.; Koczan, D.; Bonk, S.M.; Baumann, W.; Gimsa, J. Increased osteoblast viability at alkaline pH in vitro provides a new perspective on bone regeneration. Biochem. Biophys. Rep. 2017, 10, 17–25. [Google Scholar] [CrossRef]
- Samorezov, J.E.; Morlock, C.M.; Alsberg, E. Dual ionic and photo-crosslinked alginate hydrogels for micropatterned spatial control of material properties and cell behavior. Bioconjugate Chem. 2015, 26, 1339–1347. [Google Scholar] [CrossRef]
- Mørch, Ý.A.; Donati, I.; Strand, B.L.; Skjak-Braek, G. Effect of Ca2+, Ba2+, and Sr2+ on alginate microbeads. Biomacromolecules 2006, 7, 1471–1480. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Fernandez, T.; Tenorio, A.J.; Campbell, K.T.; Silva, E.A.; Leach, J.K. Evaluation of Alginate-Based Bioinks for 3D Bioprinting, Mesenchymal Stromal Cell Osteogenesis, and Application for Patient-Specific Bone Grafts. bioRxiv 2020. [Google Scholar] [CrossRef]
- Kamata, H.; Li, X.; Chung, U.i.; Sakai, T. Design of hydrogels for biomedical applications. Adv. Healthc. Mater. 2015, 4, 2360–2374. [Google Scholar] [CrossRef] [PubMed]
- Fredholm, Y.C.; Karpukhina, N.; Brauer, D.S.; Jones, J.R.; Law, R.V.; Hill, R.G. Influence of strontium for calcium substitution in bioactive glasses on degradation, ion release and apatite formation. J. R. Soc. Interface 2012, 9, 880–889. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Otto, T.; Patka, P.; Haarman, H.T.M. Closed fracture healing: A rat model. Eur. Surg. Res. 1995, 27, 277–284. [Google Scholar] [CrossRef]
- Blair, H.C.; Larrouture, Q.C.; Li, Y.; Lin, H.; Beer-Stoltz, D.; Liu, L.; Tuan, R.S.; Robinson, L.J.; Schlesinger, P.H.; Nelson, D.J. Osteoblast differentiation and bone matrix formation in vivo and in vitro. Tissue Eng. Part B Rev. 2017, 23, 268–280. [Google Scholar] [CrossRef] [Green Version]
- Blaeser, A.; Duarte Campos, D.F.; Puster, U.; Richtering, W.; Stevens, M.M.; Fischer, H. Controlling shear stress in 3D bioprinting is a key factor to balance printing resolution and stem cell integrity. Adv. Healthc. Mater. 2016, 5, 326–333. [Google Scholar] [CrossRef] [PubMed]
- Taymour, R.; Kilian, D.; Ahlfeld, T.; Gelinsky, M.; Lode, A. 3D bioprinting of hepatocytes: Core–shell structured co-cultures with fibroblasts for enhanced functionality. Sci. Rep. 2021, 11, 5130. [Google Scholar] [CrossRef]
- Sahoo, D.R.; Biswal, T. Alginate and its application to tissue engineering. SN Appl. Sci. 2021, 3, 30. [Google Scholar] [CrossRef]
- Neves, M.I.; Moroni, L.; Barrias, C.C. Modulating alginate hydrogels for improved biological performance as cellular 3D microenvironments. Front. Bioeng. Biotechnol. 2020, 8, 665. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, Z.; Li, Y.; Wang, Y.; Yao, M.; Zhang, K.; Chen, Z.; Yue, H.; Shi, J.; Guan, F. Sodium alginate/collagen hydrogel loaded with human umbilical cord mesenchymal stem cells promotes wound healing and skin remodeling. Cell Tissue Res. 2021, 383, 809–821. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Xiao, W.; Tang, Y.; Li, K.; Fan, H. Biomimetic interpenetrating polymer network hydrogels based on methacrylated alginate and collagen for 3D pre-osteoblast spreading and osteogenic differentiation. Soft Matter 2012, 8, 2398–2404. [Google Scholar] [CrossRef]
- Jia, J.; Richards, D.J.; Pollard, S.; Tan, Y.; Rodriguez, J.; Visconti, R.P.; Trusk, T.C.; Yost, M.J.; Yao, H.; Markwald, R.R. Engineering alginate as bioink for bioprinting. Acta Biomater. 2014, 10, 4323–4331. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matthews, B.G.; Naot, D.; Callon, K.E.; Musson, D.S.; Locklin, R.; Hulley, P.A.; Grey, A.; Cornish, J. Enhanced osteoblastogenesis in three-dimensional collagen gels. BoneKEy Rep. 2014. [Google Scholar] [CrossRef] [Green Version]
- Manavitehrani, I.; Fathi, A.; Wang, Y.; Maitz, P.K.; Mirmohseni, F.; Cheng, T.L.; Peacock, L.; Little, D.G.; Schindeler, A.; Dehghani, F. Fabrication of a biodegradable implant with tunable characteristics for bone implant applications. Biomacromolecules 2017, 18, 1736–1746. [Google Scholar] [CrossRef]
- Perez, R.A.; Kim, M.; Kim, T.-H.; Kim, J.-H.; Lee, J.H.; Park, J.-H.; Knowles, J.C.; Kim, H.-W. Utilizing core–shell fibrous collagen-alginate hydrogel cell delivery system for bone tissue engineering. Tissue Eng. Part A 2014, 20, 103–114. [Google Scholar] [CrossRef] [Green Version]
- Schmid, T.; Messmer, A.; Yeo, B.-S.; Zhang, W.; Zenobi, R. Towards chemical analysis of nanostructures in biofilms II: Tip-enhanced Raman spectroscopy of alginates. Anal. Bioanal. Chem. 2008, 391, 1907–1916. [Google Scholar] [CrossRef] [Green Version]
- Bergholt, M.S.; Serio, A.; Albro, M.B. Raman spectroscopy: Guiding light for the extracellular matrix. Front. Bioeng. Biotechnol. 2019, 7, 303. [Google Scholar] [CrossRef]
- Im, J.; Choi, C.H.; Mun, F.; Lee, J.; Kim, H.; Jung, W.-K.; Jang, C.H.; Kim, G. A polycaprolactone/fish collagen/alginate biocomposite supplemented with phlorotannin for hard tissue regeneration. RSC Adv. 2017, 7, 2009–2018. [Google Scholar] [CrossRef] [Green Version]
- Naahidi, S.; Jafari, M.; Logan, M.; Wang, Y.; Yuan, Y.; Bae, H.; Dixon, B.; Chen, P. Biocompatibility of hydrogel-based scaffolds for tissue engineering applications. Biotechnol. Adv. 2017, 35, 530–544. [Google Scholar] [CrossRef]
- Komori, T. Regulation of bone development and maintenance by Runx2. Front. Biosci. Landmark 2008, 13, 898–903. [Google Scholar] [CrossRef] [PubMed]
- Miller, S.C.; Halloran, B.P.; DeLuca, H.F.; Jee, W.S. Studies on the role of vitamin D in early skeletal development, mineralization, and growth in rats. Calcif. Tissue Int. 1983, 35, 455–460. [Google Scholar] [CrossRef] [PubMed]
- Marycz, K.; Meissner, J.M.; Grzesiak, J.; Śmieszek, A.; Kasprzyk, I. Sphingosine-1-phosphate enhance osteogenic activity of multipotent stromal cells cultured in biodegradable 3D alginate hydrogels. J. Biomater. Tissue Eng. 2016, 6, 85–97. [Google Scholar] [CrossRef]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tharakan, S.; Khondkar, S.; Lee, S.; Ahn, S.; Mathew, C.; Gresita, A.; Hadjiargyrou, M.; Ilyas, A. 3D Printed Osteoblast–Alginate/Collagen Hydrogels Promote Survival, Proliferation and Mineralization at Low Doses of Strontium Calcium Polyphosphate. Pharmaceutics 2023, 15, 11. https://doi.org/10.3390/pharmaceutics15010011
Tharakan S, Khondkar S, Lee S, Ahn S, Mathew C, Gresita A, Hadjiargyrou M, Ilyas A. 3D Printed Osteoblast–Alginate/Collagen Hydrogels Promote Survival, Proliferation and Mineralization at Low Doses of Strontium Calcium Polyphosphate. Pharmaceutics. 2023; 15(1):11. https://doi.org/10.3390/pharmaceutics15010011
Chicago/Turabian StyleTharakan, Shebin, Shams Khondkar, Sally Lee, Serin Ahn, Chris Mathew, Andrei Gresita, Michael Hadjiargyrou, and Azhar Ilyas. 2023. "3D Printed Osteoblast–Alginate/Collagen Hydrogels Promote Survival, Proliferation and Mineralization at Low Doses of Strontium Calcium Polyphosphate" Pharmaceutics 15, no. 1: 11. https://doi.org/10.3390/pharmaceutics15010011






