Evaluation of a Novel Dry Powder Surfactant Aerosol Delivery System for Use in Premature Infants Supported with Bubble CPAP
Abstract
:1. Introduction
2. Materials and Methods
2.1. B-YL: Trehalose Synthetic DP Surfactant Formulation
2.2. Low-Flow Aerosol Chamber (LFAC)
2.3. Aerosol Particle Sizing
2.4. Preterm Infant Nasal Airway and Lung Model
2.5. Bubble CPAP System and Nasal Airway Interfaces
2.6. LFAC Operational Compatibility with bCPAP
2.7. Nebulizer Efficiency and Delivered Dose Studies
2.8. Statistical Analysis
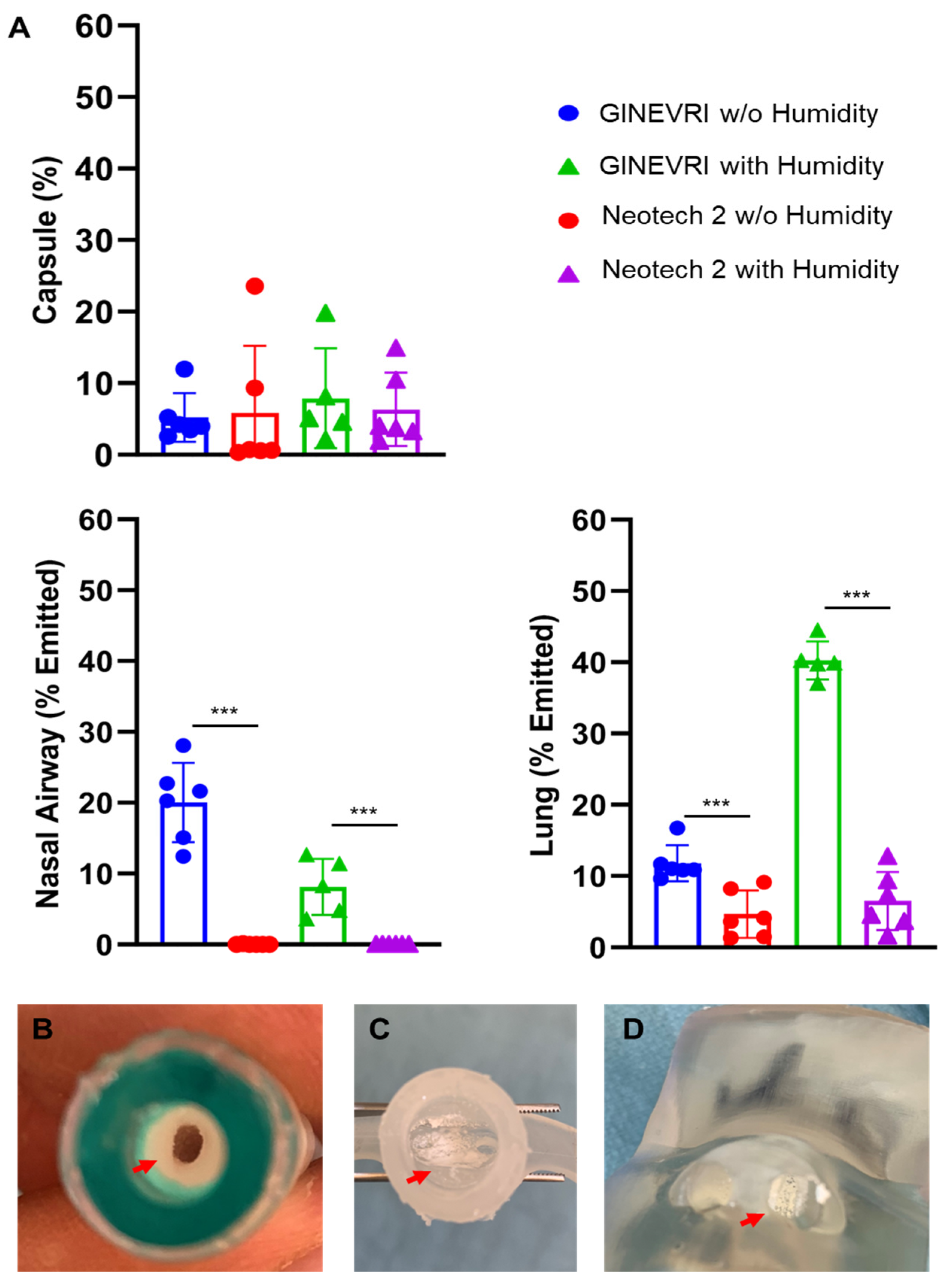
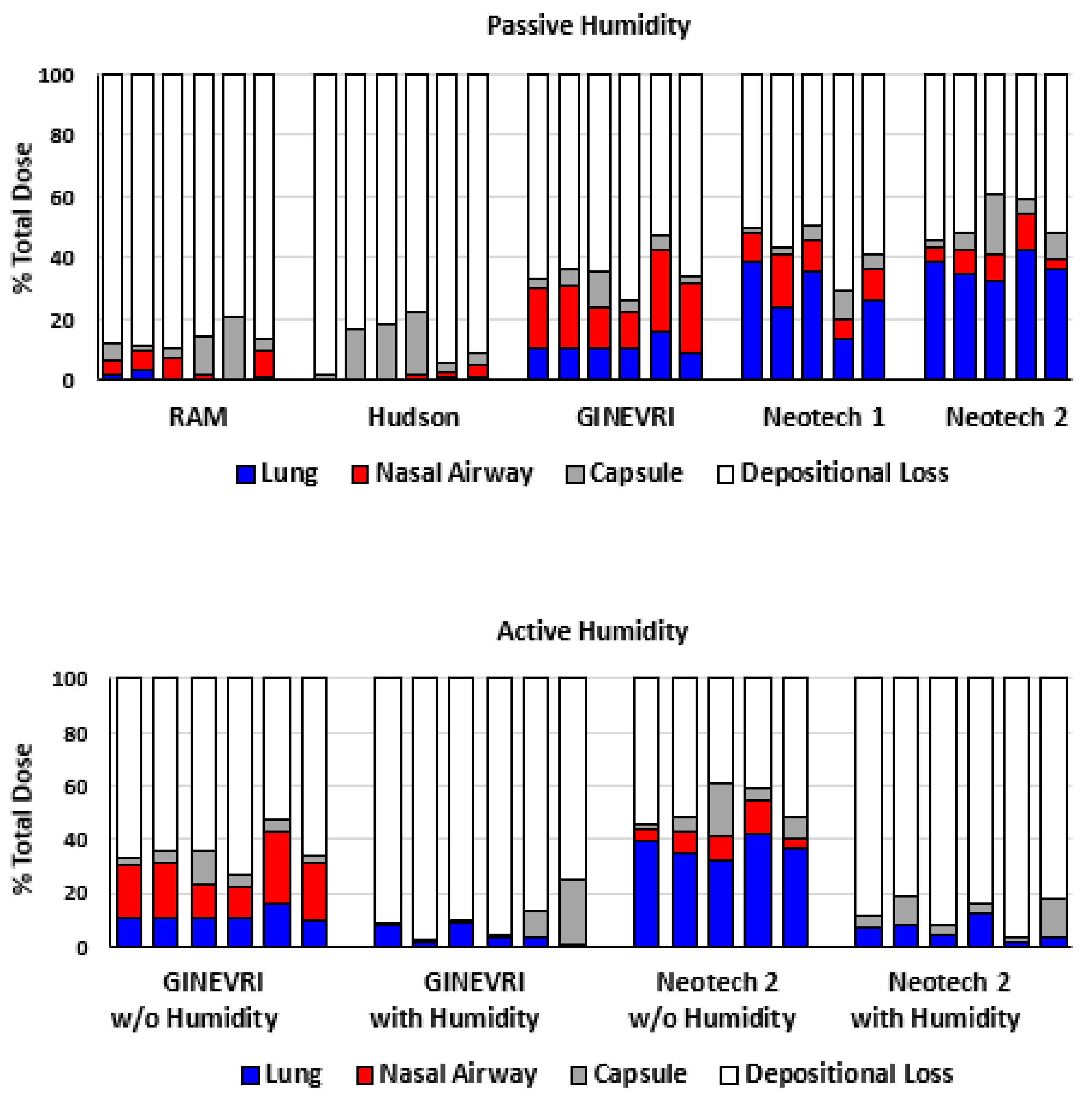
3. Results
3.1. Aerosol Particle Size Distribution
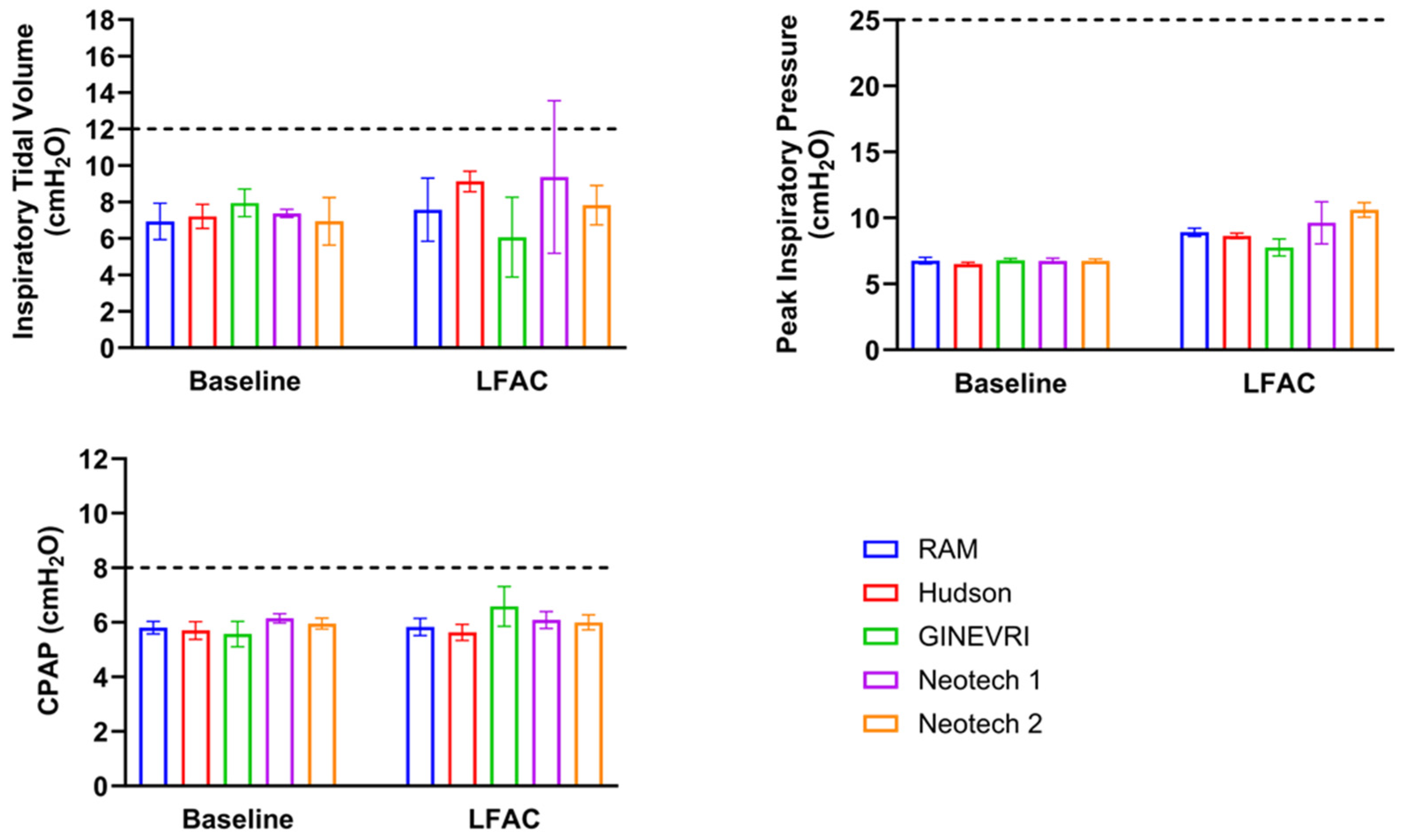
3.2. LFAC Operational Compatibility with bCPAP
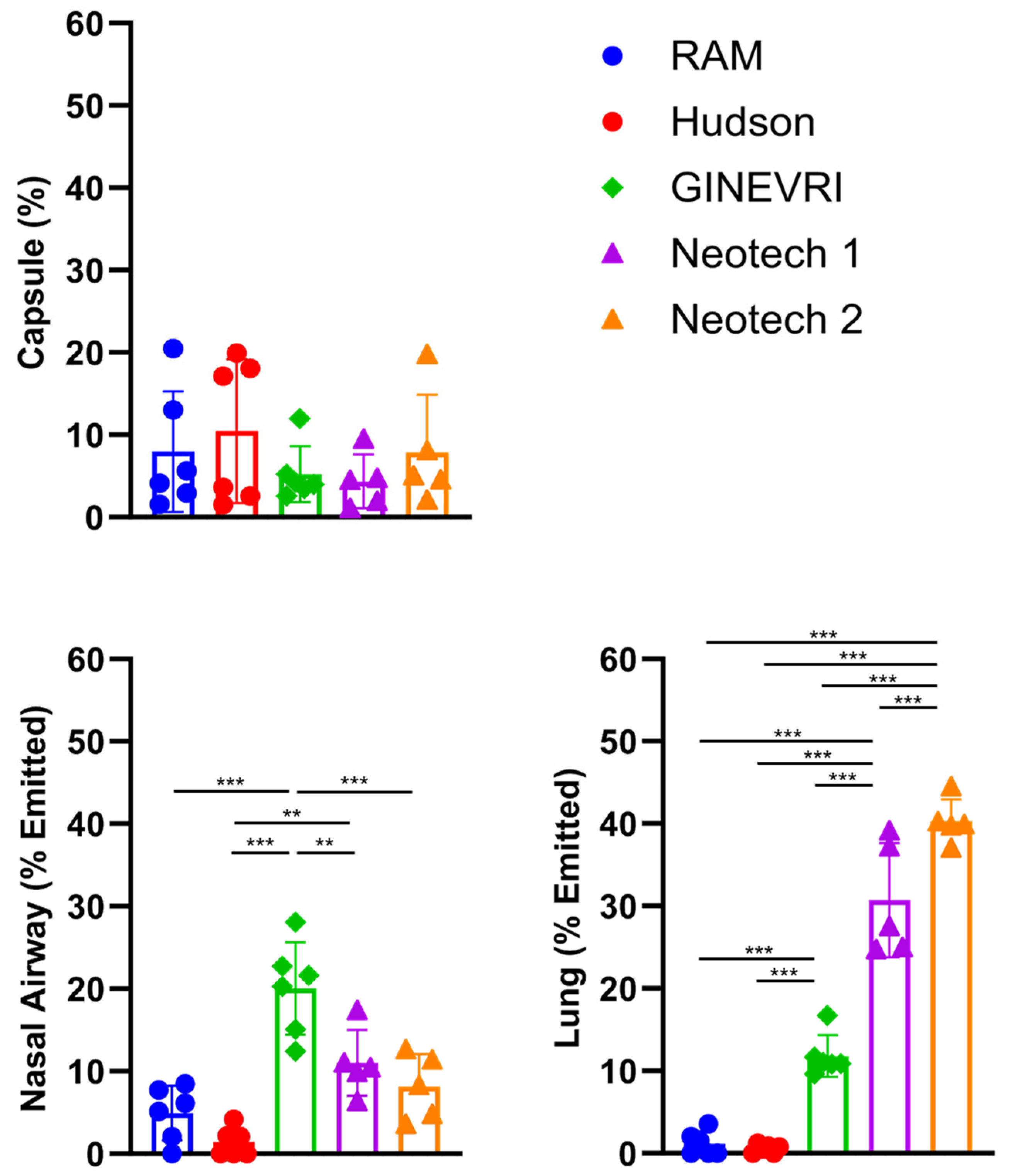
3.3. Nebulizer Efficiency and Delivered Dose
4. Discussion
4.1. Safety and Efficacy
4.2. Particle Size
4.3. Lung Dose
4.4. Nasal Prongs
4.5. Nebulizer
4.6. Humidification
4.7. Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lategan, I.; Price, C.; Rhoda, N.R.; Zar, H.J.; Tooke, L. Respiratory Interventions for preterm Infants in LMICs: A Prospective Study From Cape Town, South Africa. Front. Glob. Womens Health 2022, 3, 817817. [Google Scholar] [CrossRef]
- Poulain, F.R.; Clements, J.A. Pulmonary surfactant therapy. West. J. Med. 1995, 162, 43–50. [Google Scholar]
- Nouraeyan, N.; Lambrinakos-Raymond, A.; Leone, M.; Sant’Anna, G. Surfactant administration in neonates: A review of delivery methods. Can. J. Respir. Ther. 2014, 50, 91–95. [Google Scholar]
- Wu, A.; Mukhtar-Yola, M.; Luch, S.; John, S.; Adhikari, B.R.; Bakker, C.; Slusher, T.; Bjorklund, A.; Winter, J.; Ezeaka, C. Innovations and adaptations in neonatal and pediatric respiratory care for resource constrained settings. Front. Pediatr. 2022, 10, 954975. [Google Scholar] [CrossRef]
- Bancalari, E.; Jain, D. Bronchopulmonary Dysplasia: 50 Years after the Original Description. Neonatology 2019, 115, 384–391. [Google Scholar] [CrossRef]
- Mwatha, A.B.; Mahande, M.; Olomi, R.; John, B.; Philemon, R. Treatment outcomes of Pumani bubble-CPAP versus oxygen therapy among preterm babies presenting with respiratory distress at a tertiary hospital in Tanzania-Randomised trial. PLoS ONE 2020, 15, e0235031. [Google Scholar] [CrossRef]
- Abdallah, Y.; Mkony, M.; Noorani, M.; Moshiro, R.; Bakari, M.; Manji, K. CPAP failure in the management of preterm neonates with respiratory distress syndrome where surfactant is scarce. A prospective observational study. BMC Pediatr. 2023, 23, 211. [Google Scholar] [CrossRef]
- Dijk, P.H.; Heikamp, A.; Bambang Oetomo, S. Surfactant nebulisation: Lung function, surfactant distribution and pulmonary blood flow distribution in lung lavaged rabbits. Intensive Care Med. 1997, 23, 1070–1076. [Google Scholar] [CrossRef]
- Dijk, P.H.; Heikamp, A.; Bambang Oetomo, S. Surfactant nebulisation prevents the adverse effects of surfactant therapy on blood pressure and cerebral blood flow in rabbits with severe respiratory failure. Intensive Care Med. 1997, 23, 1077–1081. [Google Scholar] [CrossRef]
- Bianco, F.; Pasini, E.; Nutini, M.; Murgia, X.; Stoeckl, C.; Schlun, M.; Hetzer, U.; Bonelli, S.; Lombardini, M.; Milesi, I.; et al. Extended Pharmacopeial Characterization of Surfactant Aerosols Generated by a Customized eFlow Neos Nebulizer Delivered through Neonatal Nasal Prongs. Pharmaceutics 2020, 12, 319. [Google Scholar] [CrossRef]
- Bianco, F.; Salomone, F.; Milesi, I.; Murgia, X.; Bonelli, S.; Pasini, E.; Dellacà, R.; Ventura, M.L.; Pillow, J. Aerosol drug delivery to spontaneously-breathing preterm neonates: Lessons learned. Respir. Res. 2021, 22, 71. [Google Scholar] [CrossRef]
- Morley, C.J.; Bangham, A.D.; Miller, N.; Davis, J.A. Dry artificial lung surfactant and its effect on very premature babies. Lancet 1981, 1, 64–68. [Google Scholar] [CrossRef]
- Morley, C.J.; Greenough, A.; Miller, N.G.; Bangham, A.D.; Pool, J.; Wood, S.; South, M.; Davis, J.A.; Vyas, H. Randomized trial of artificial surfactant (ALEC) given at birth to babies from 23 to 34 weeks gestation. Early Hum. Dev. 1988, 17, 41–54. [Google Scholar] [CrossRef]
- Pohlmann, G.; Iwatschenko, P.; Koch, W.; Windt, H.; Rast, M.; de Abreu, M.G.; Taut, F.J.; De Muynck, C. A novel continuous powder aerosolizer (CPA) for inhalative administration of highly concentrated recombinant surfactant protein-C (rSP-C) surfactant to preterm neonates. J. Aerosol Med. Pulm. Drug Deliv. 2013, 26, 370–379. [Google Scholar] [CrossRef]
- Kamga Gninzeko, F.J.; Valentine, M.S.; Tho, C.K.; Chindal, S.R.; Boc, S.; Dhapare, S.; Momin, M.A.M.; Hassan, A.; Hindle, M.; Farkas, D.R.; et al. Excipient Enhanced Growth Aerosol Surfactant Replacement Therapy in an in Vivo Rat Lung Injury Model. J. Aerosol Med. Pulm. Drug Deliv. 2020, 33, 314–322. [Google Scholar] [CrossRef]
- Walther, F.J.; Waring, A.J.; Otieno, M.; DiBlasi, R.M. Efficacy, dose-response, and aerosol delivery of dry powder synthetic lung surfactant treatment in surfactant-deficient rabbits and premature lambs. Respir. Res. 2022, 23, 78. [Google Scholar] [CrossRef]
- Walther, F.J.; Chan, H.; Smith, J.R.; Tauber, M.; Waring, A.J. Aerosol, chemical and physical properties of dry powder synthetic lung surfactant for noninvasive treatment of neonatal respiratory distress syndrome. Sci. Rep. 2021, 11, 16439. [Google Scholar] [CrossRef]
- Latzin, P.; Roth, S.; Thamrin, C.; Hutten, G.J.; Pramana, I.; Kuehni, C.E.; Casaulta, C.; Nelle, M.; Riedel, T.; Frey, U. Lung volume, breathing pattern and ventilation inhomogeneity in preterm and term infants. PLoS ONE 2009, 4, e4635. [Google Scholar] [CrossRef]
- McCann, E.M.; Goldman, S.L.; Brady, J.P. Pulmonary function in the sick newborn infant. Pediatr. Res. 1987, 21, 313–325. [Google Scholar] [CrossRef]
- Poli, J.A.; Richardson, C.P.; DiBlasi, R.M. Volume Oscillations Delivered to a Lung Model Using 4 Different Bubble CPAP Systems. Respir. Care. 2015, 60, 371–381. [Google Scholar] [CrossRef]
- Won, A.; Suarez-Rebling, D.; Baker, A.L.; Burke, T.F.; Nelson, B.D. Bubble CPAP devices for infants and children in resource-limited settings: Review of the literature. Paediatr. Int. Child. Health 2019, 39, 168–176. [Google Scholar] [CrossRef] [PubMed]
- Mazela, J.; Chmura, K.; Kulza, M.; Henderson, C.; Gregory, T.J.; Moskal, A.; Sosnowski, T.R.; Florek, E.; Kramer, L.; Keszler, M. Aerosolized albuterol sulfate delivery under neonatal ventilatory conditions: In vitro evaluation of a novel ventilator circuit patient interface connector. J. Aerosol Med. Pulm. Drug Deliv. 2014, 27, 58–65. [Google Scholar] [CrossRef]
- Björklund, L.J.; Ingimarsson, J.; Curstedt, T.; John, J.; Robertson, B.; Werner, O.; Vilstrup, C.T. Manual ventilation with a few large breaths at birth compromises the therapeutic effect of subsequent surfactant replacement in immature lambs. Pediatr. Res. 1997, 42, 348–355. [Google Scholar] [CrossRef] [PubMed]
- Wada, K.; Jobe, A.H.; Ikegami, M. Tidal volume effects on surfactant treatment responses with the initiation of ventilation in preterm lambs. J. Appl. Physiol. 1997, 83, 1054–1061. [Google Scholar] [CrossRef] [PubMed]
- van Vonderen, J.J.; Hooper, S.B.; Krabbe, V.B.; Siew, M.L.; Te Pas, A.B. Monitoring tidal volumes in preterm infants at birth: Mask versus endotracheal ventilation. Arch. Dis. Childhood. Fetal Neonatal Ed. 2015, 100, F43–F46. [Google Scholar] [CrossRef]
- Morley, C.J.; Davis, P.G.; Doyle, L.W.; Brion, L.P.; Hascoet, J.M.; Carlin, J.B.; COIN Trial Investigators. Nasal CPAP or intubation at birth for very preterm infants. N. Engl. J. Med. 2008, 358, 700–708. [Google Scholar] [CrossRef]
- Rao, S.; Edmonda, K.; Bahl, R. Target product profile: Aerosolized surfactant for neonatal respiratory distress. Bull. World Health Organ. 2023, 101, 341–345. [Google Scholar] [CrossRef]
- Pinsky, M.R.; Hrehocik, D.; Culpepper, J.A.; Snyder, J.V. Flow resistance of expiratory positive-pressure systems. Chest 1988, 94, 788–791. [Google Scholar] [CrossRef]
- Clark, A.R. Essentials for aerosol delivery to term and pre-term infants. Ann. Transl. Med. 2021, 9, 594. [Google Scholar] [CrossRef]
- Amirav, I.; Luder, A.S.; Halamish, A.; Raviv, D.; Kimmel, R.; Waisman, D.; Newhouse, M.T. Design of aerosol face masks for children using computerized 3D face analysis. J. Aerosol Med. Pulm. Drug Deliv. 2014, 27, 272–278. [Google Scholar] [CrossRef]
- Gregory, T.J.; Irshad, H.; Chand, R.; Kuehl, P.J. Deposition of Aerosolized Lucinactant in Nonhuman Primates. J. Aerosol Med. Pulm. Drug Deliv. 2020, 33, 21–33. [Google Scholar] [CrossRef] [PubMed]
- DiBlasi, R.M.; Kajimoto, M.; Poli, J.A.; Deutsch, G.; Pfeiffer, J.; Zimmerman, J.; Crotwell, D.N.; Malone, P.; Fink, J.B.; Ringer, C.; et al. Breath-Synchronized Nebulized Surfactant in a Porcine Model of Acute Respiratory Distress Syndrome. Crit. Care Explor. 2021, 3, e0338. [Google Scholar] [CrossRef] [PubMed]
- DiBlasi, R.M.; Micheletti, K.J.; Zimmerman, J.D.; Poli, J.A.; Fink, J.B.; Kajimoto, M. Physiologic Effects of Instilled and Aerosolized Surfactant Using a Breath-Synchronized Nebulizer on Surfactant-Deficient Rabbits. Pharmaceutics 2021, 13, 1580. [Google Scholar] [CrossRef] [PubMed]
- Jorch, G.; Hartl, H.; Roth, B.; Kribs, A.; Gortner, L.; Schaible, T.; Hennecke, K.H.; Poets, C. Surfactant aerosol treatment of respiratory distress syndrome in spontaneously breathing premature infants. Pediatr. Pulmonol. 1997, 24, 222–224. [Google Scholar] [CrossRef]
- Flink, R.C.; van Kaam, A.H.; de Jongh, F.H. A humidifier in the invasive mode during noninvasive respiratory support could increase condensation and thereby impair airway patency. Acta Paediatr. 2018, 107, 1888–1892. [Google Scholar] [CrossRef]
- Herrod, S.K.; Stevenson, A.; Vaucher, Y.E.; Lambert, S.R.; Isenberg, S.J.; Yap, V.L.; Ezeaka, V.C.; Carlo, W.A. Oxygen management among infants in neonatal units in sub-Saharan Africa: A cross-sectional survey. J. Perinatol. 2021, 41, 2631–2638. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
DiBlasi, R.M.; Crandall, C.N.; Engberg, R.J.; Bijlani, K.; Ledee, D.; Kajimoto, M.; Walther, F.J. Evaluation of a Novel Dry Powder Surfactant Aerosol Delivery System for Use in Premature Infants Supported with Bubble CPAP. Pharmaceutics 2023, 15, 2368. https://doi.org/10.3390/pharmaceutics15102368
DiBlasi RM, Crandall CN, Engberg RJ, Bijlani K, Ledee D, Kajimoto M, Walther FJ. Evaluation of a Novel Dry Powder Surfactant Aerosol Delivery System for Use in Premature Infants Supported with Bubble CPAP. Pharmaceutics. 2023; 15(10):2368. https://doi.org/10.3390/pharmaceutics15102368
Chicago/Turabian StyleDiBlasi, Robert M., Coral N. Crandall, Rebecca J. Engberg, Kunal Bijlani, Dolena Ledee, Masaki Kajimoto, and Frans J. Walther. 2023. "Evaluation of a Novel Dry Powder Surfactant Aerosol Delivery System for Use in Premature Infants Supported with Bubble CPAP" Pharmaceutics 15, no. 10: 2368. https://doi.org/10.3390/pharmaceutics15102368




