Complexes of Ibuprofen Thiazolidin-4-One Derivatives with β-Cyclodextrin: Characterization and In Vivo Release Profile and Biological Evaluation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Solid β-CD Complexes Preparation
2.1.1. Materials
2.1.2. Synthesis of β-CD Complexes
2.2. Physico-Chemical Characterization of the β-CD Complexes
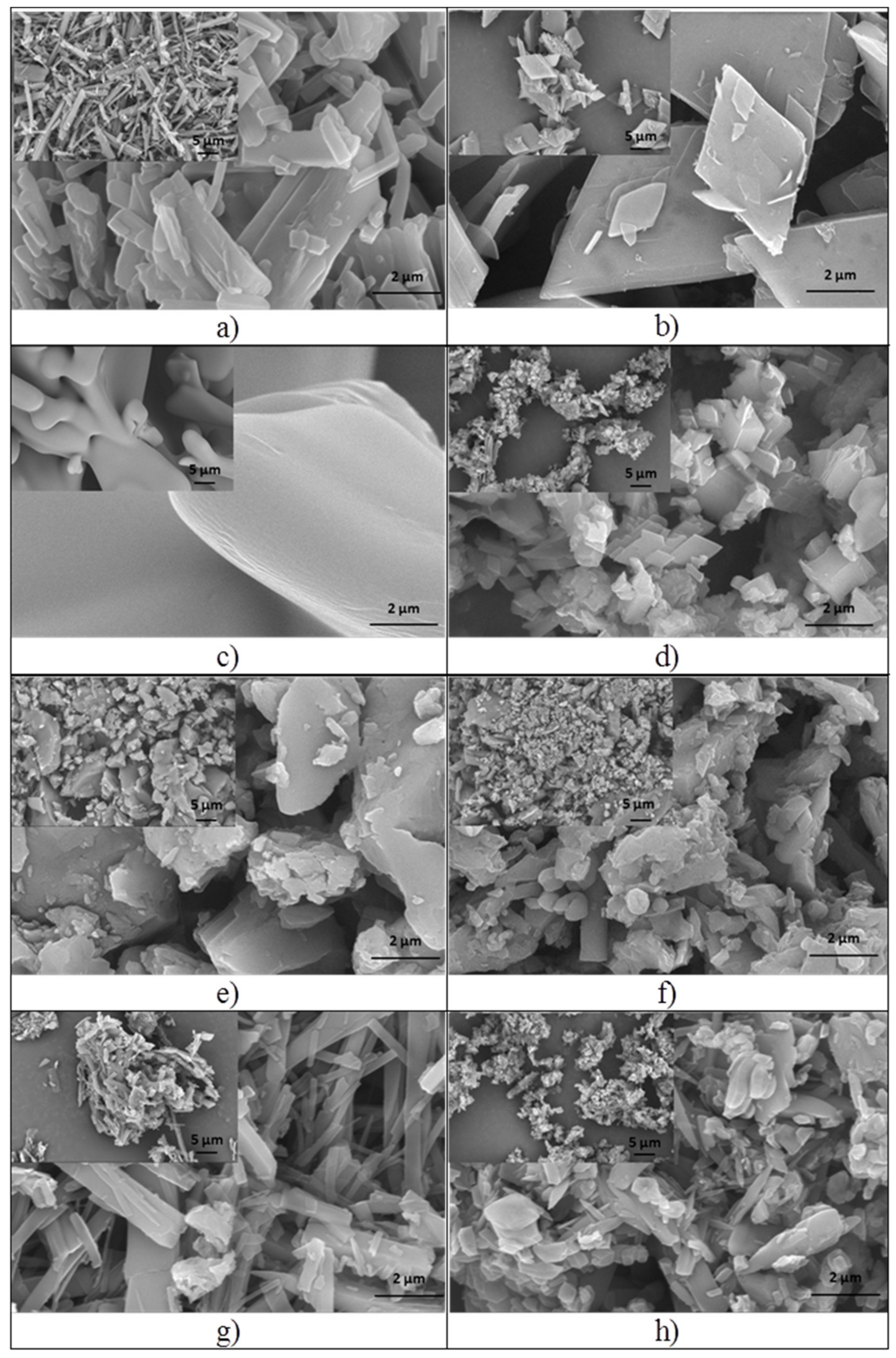
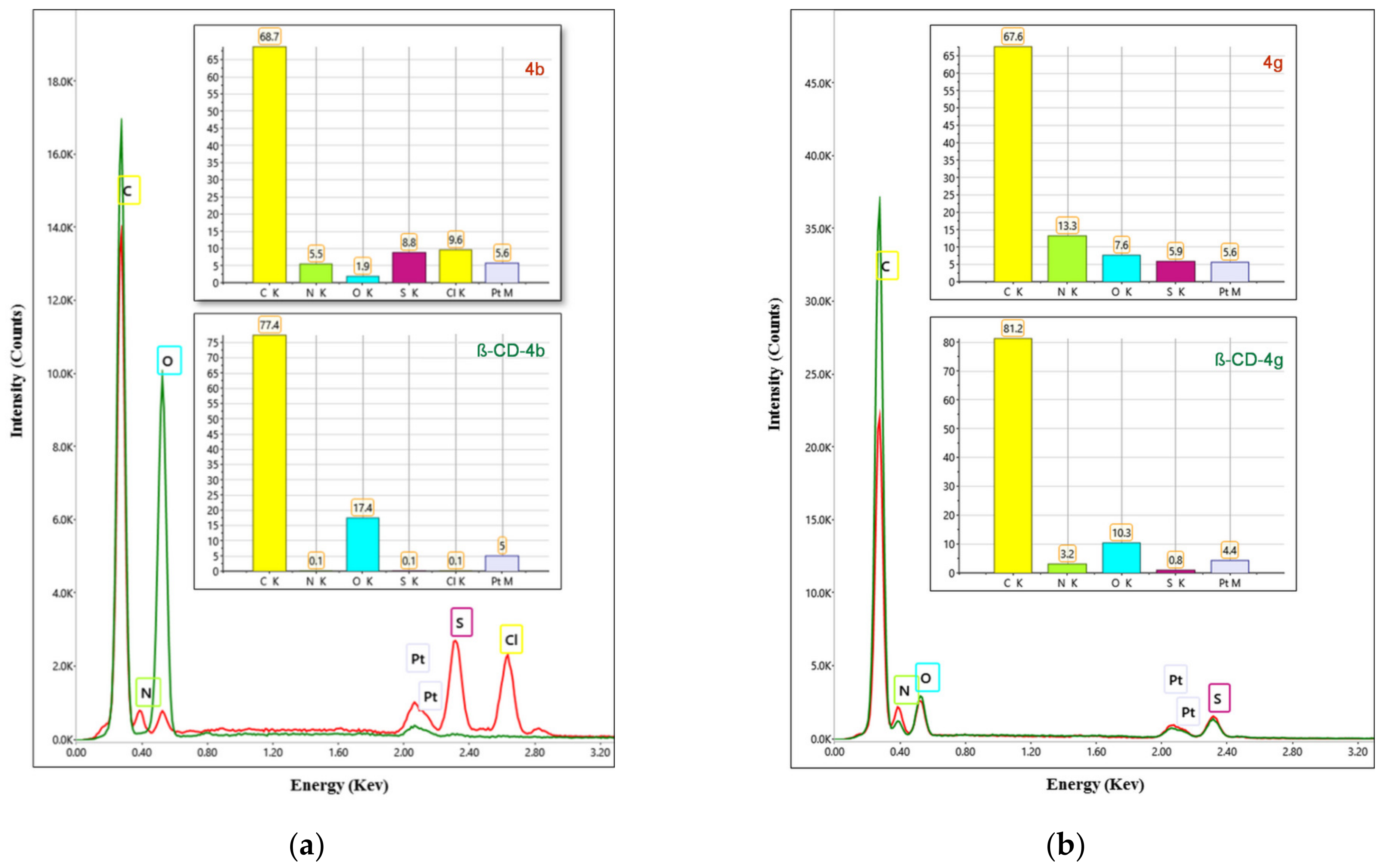
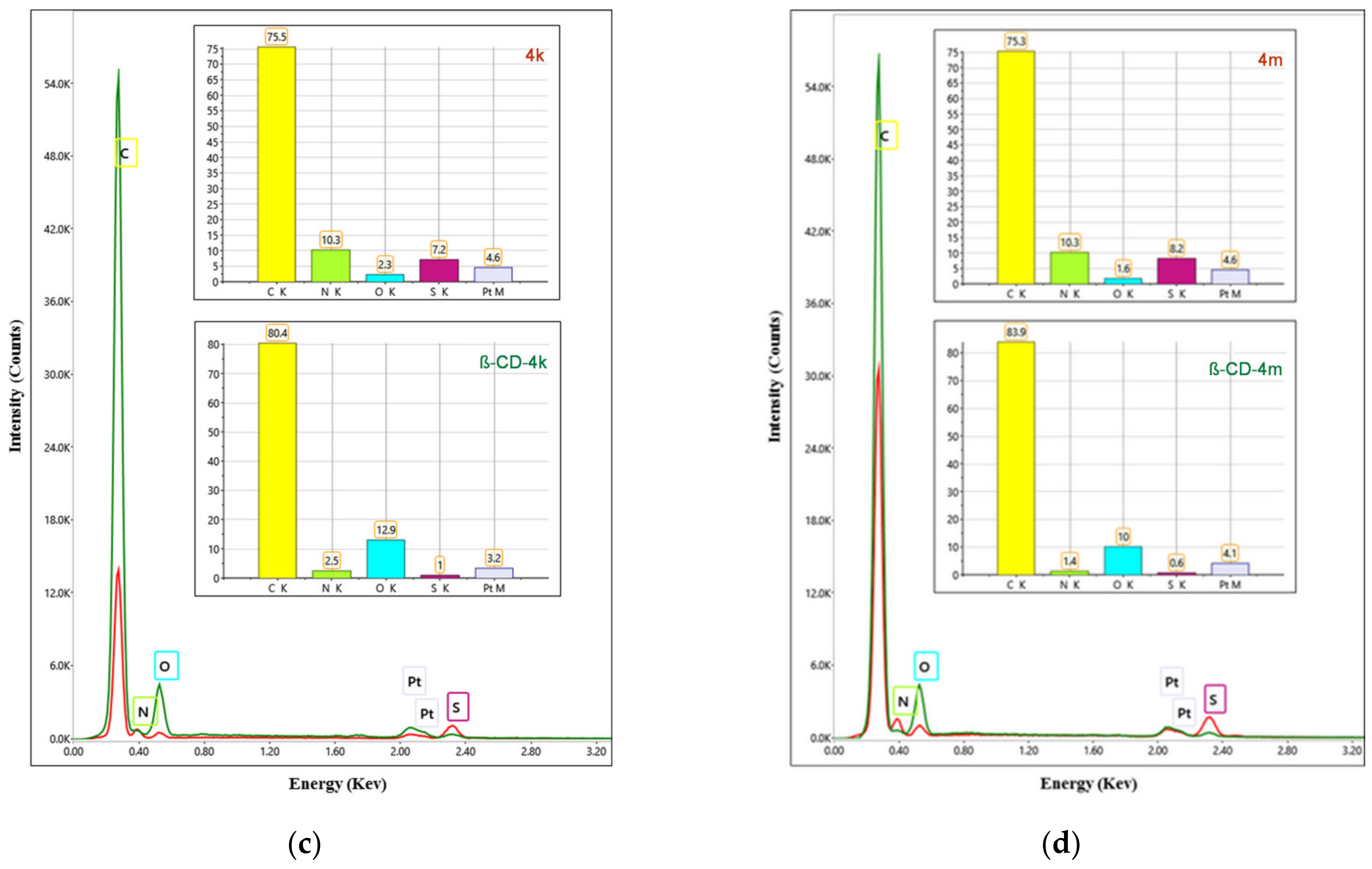
2.2.1. Scanning Electronic Microscopy and Energy Dispersive X-ray Analysis
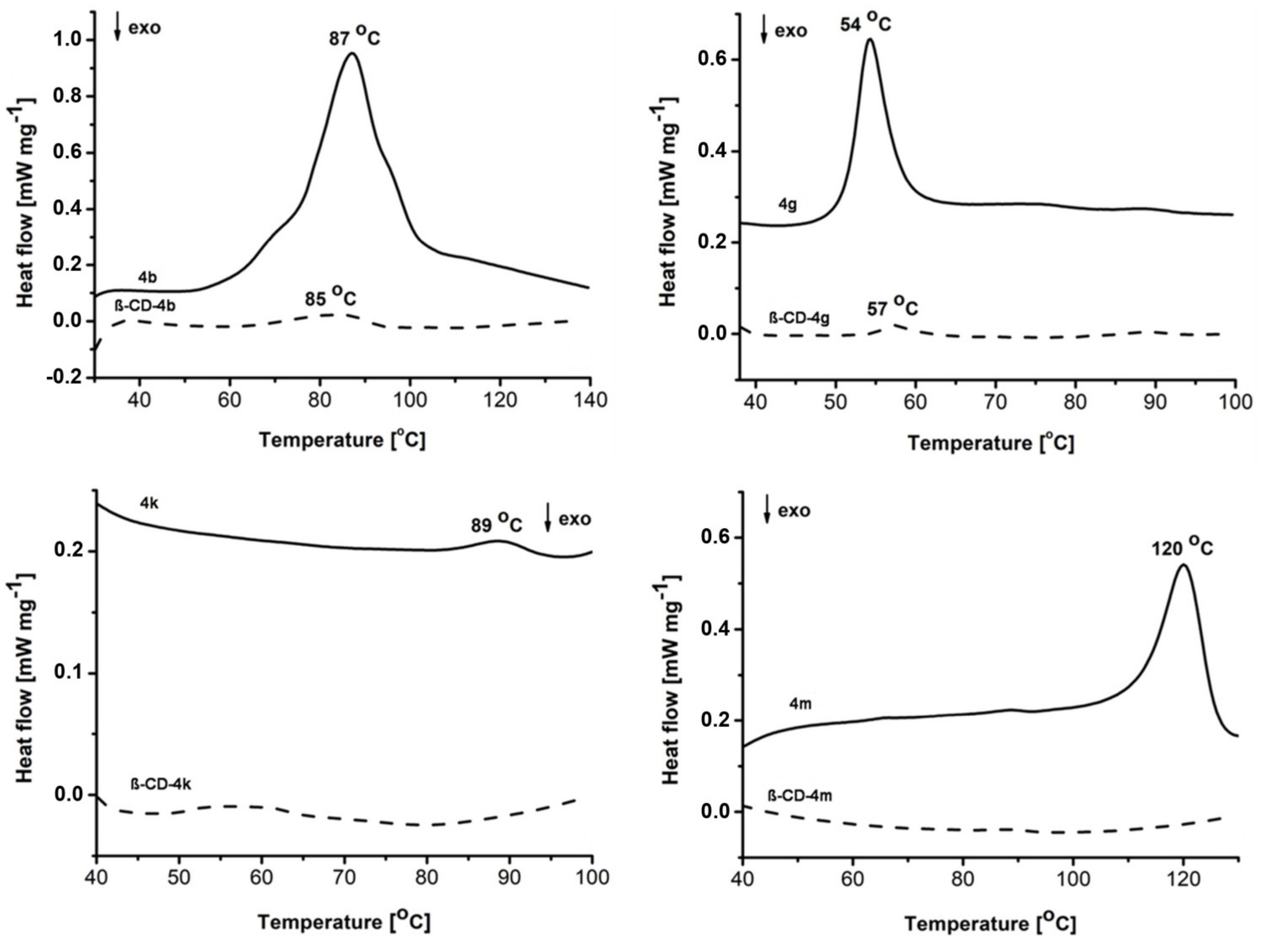
2.2.2. Differential Scanning Calorimetry
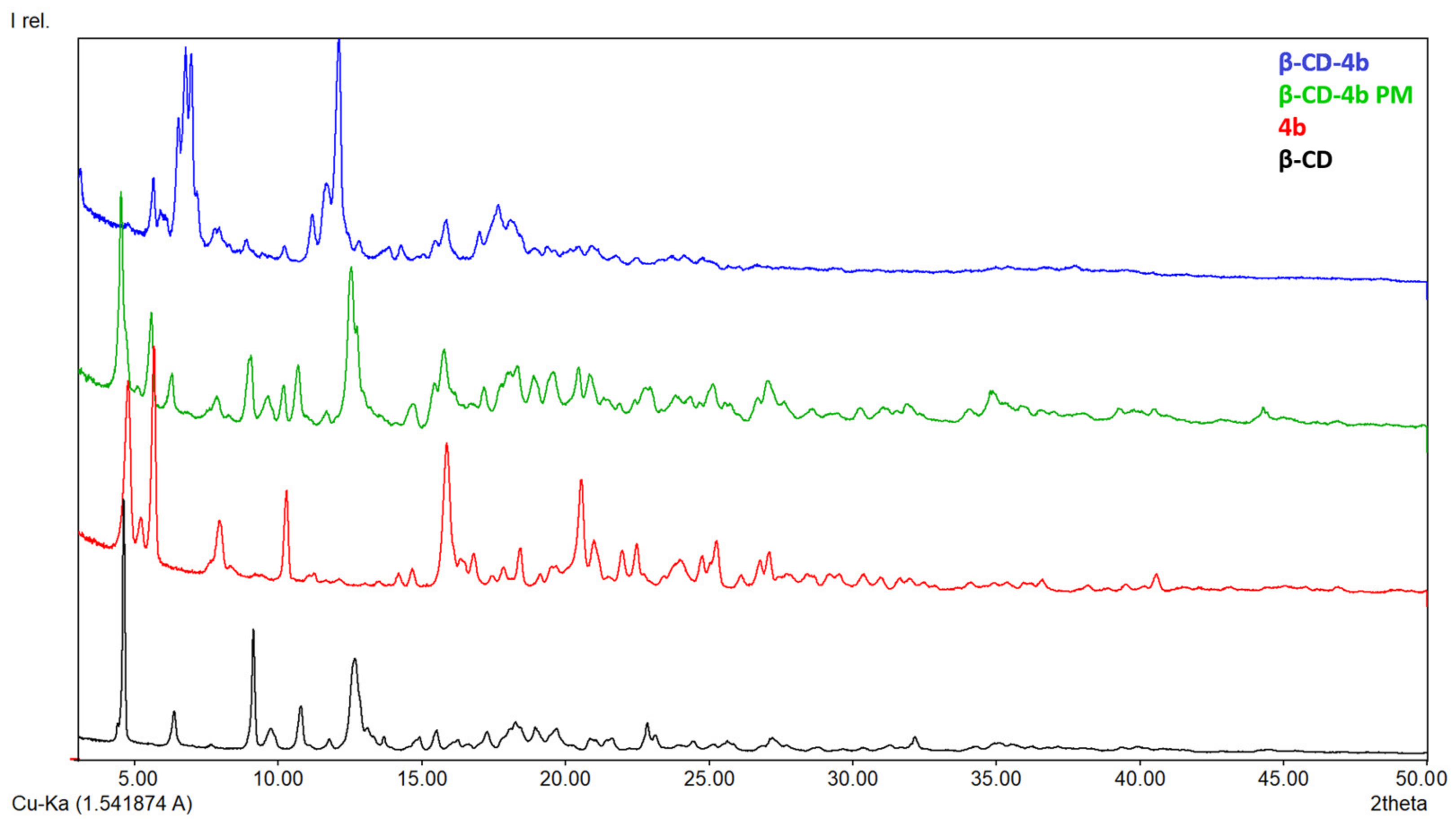
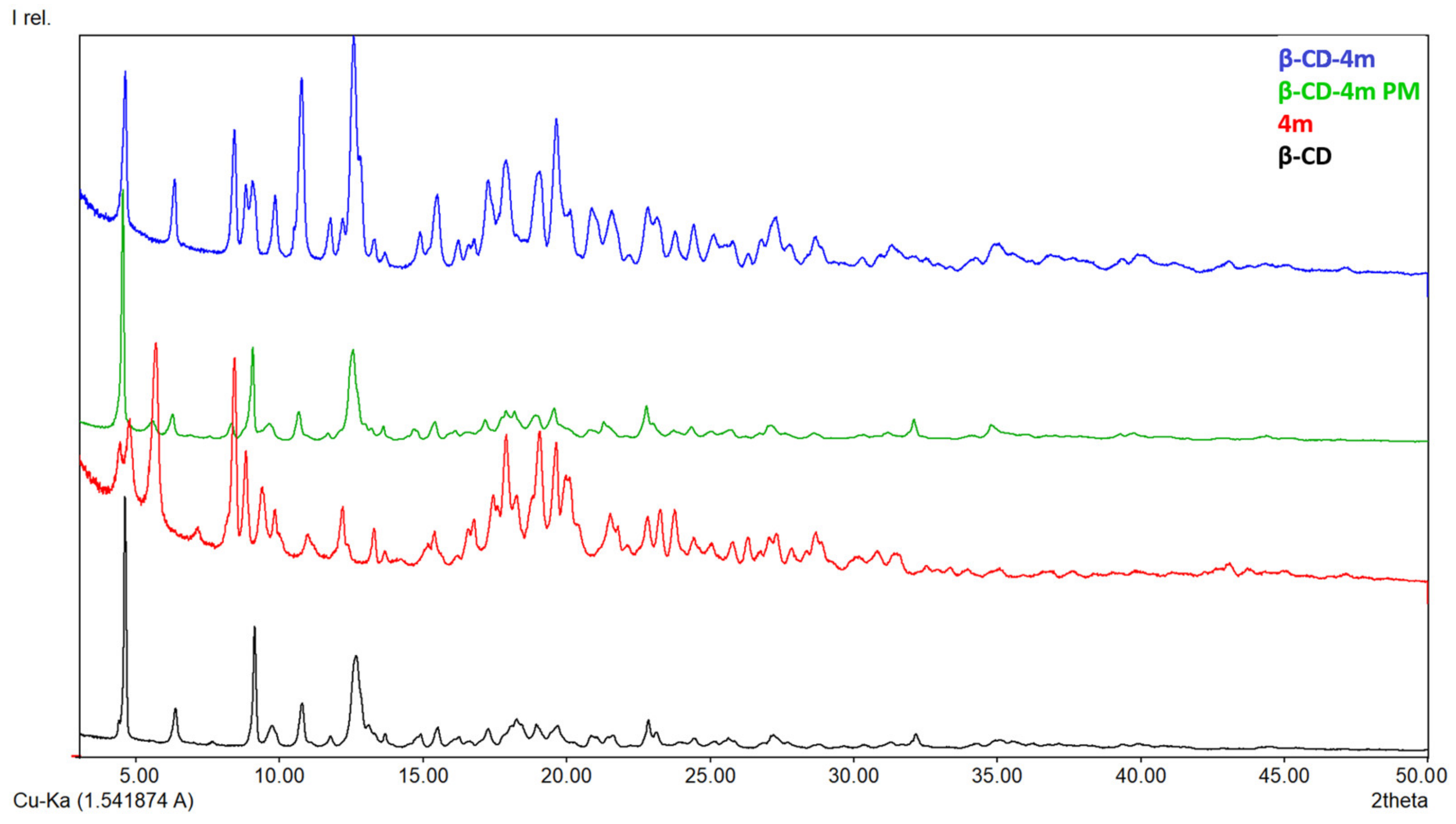
2.2.3. X-ray Diffraction
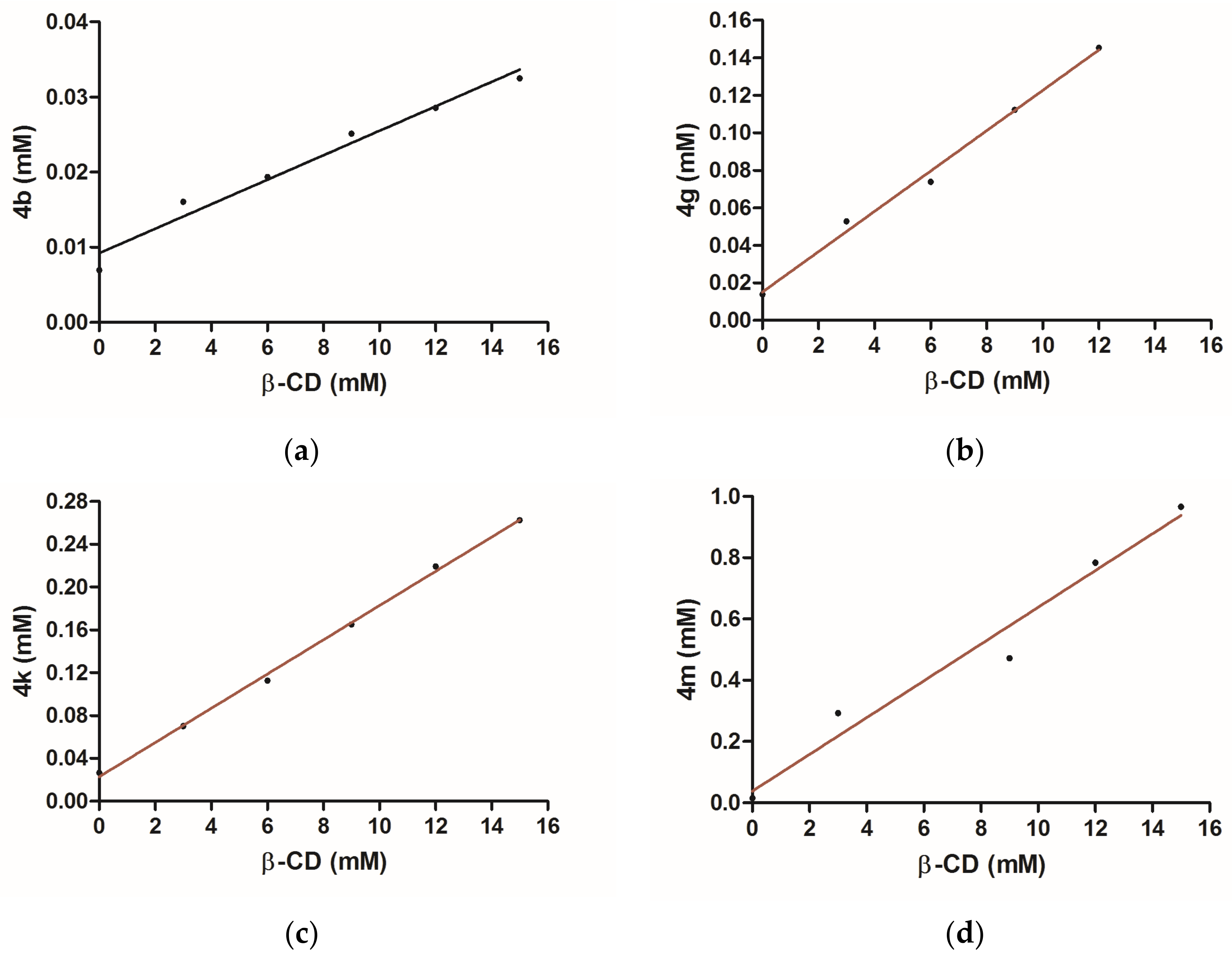
2.2.4. Phase Solubility Studies
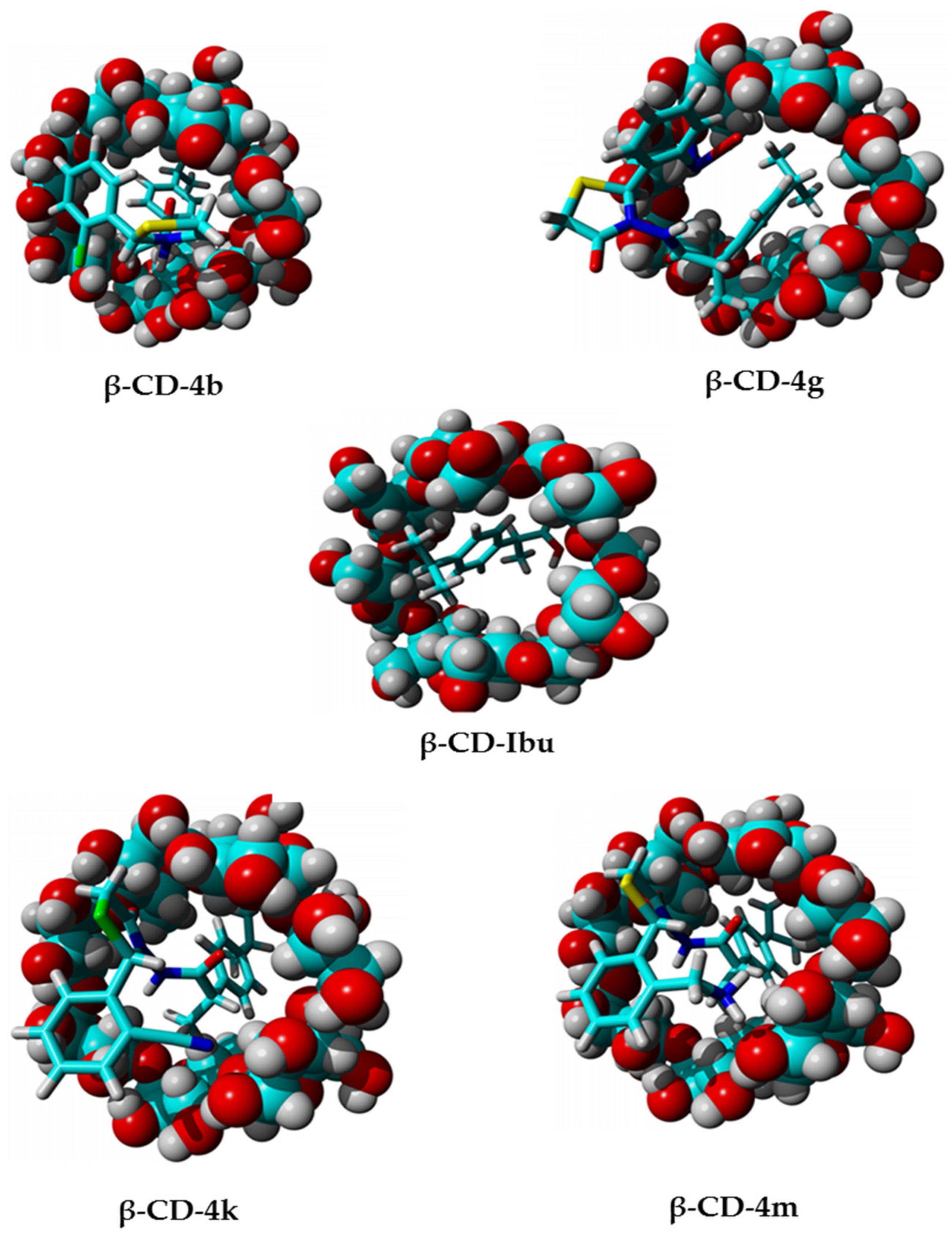
2.3. Molecular Docking Studies
2.4. In Vivo Biological Studies
2.4.1. Animals
2.4.2. Analgesic Tests
Tail Flick
Hot Plate
Writhing Test
Paw Formalin Test
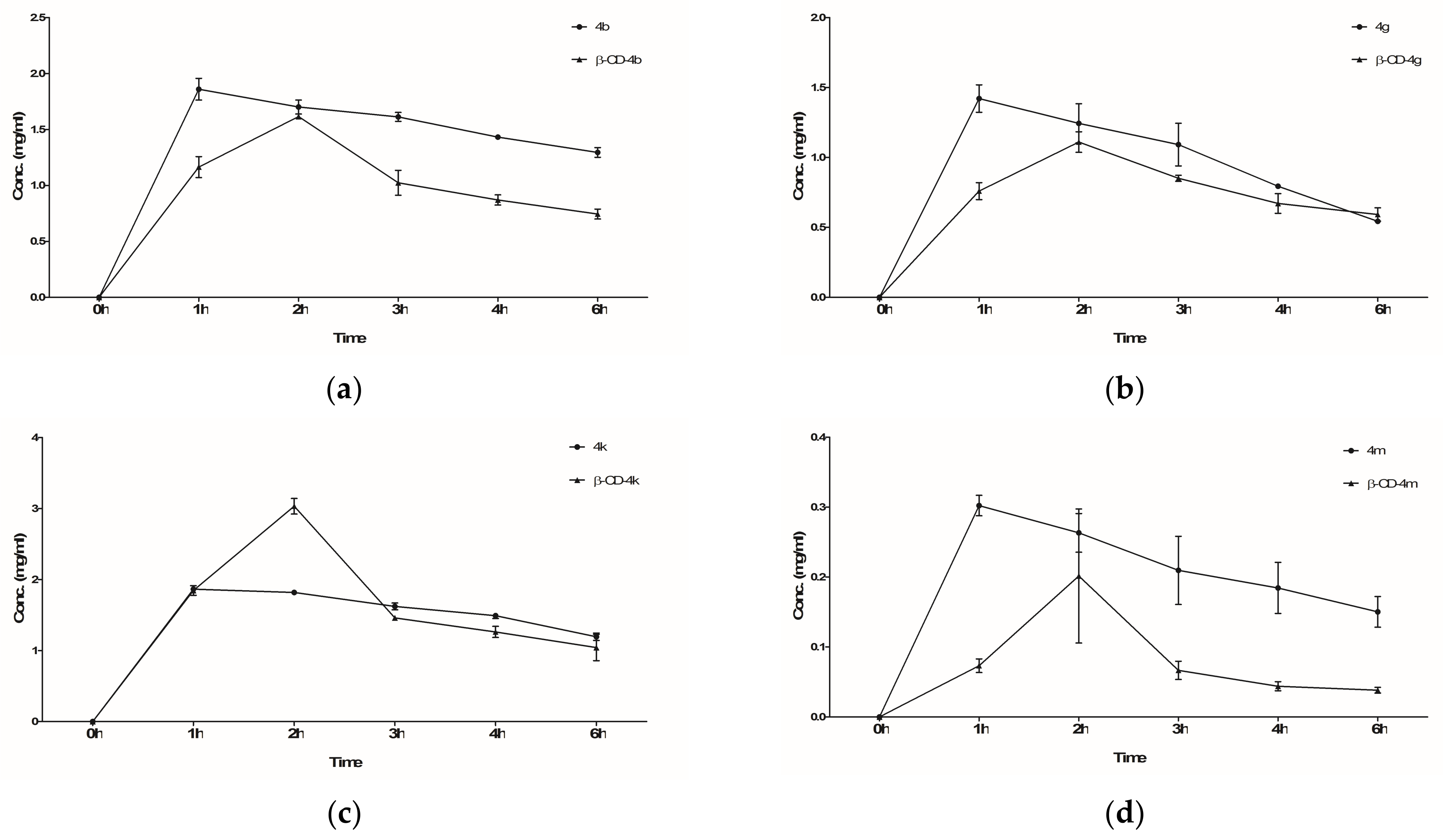
2.4.3. Evaluation of the Drug Release Profile
Experimental Design
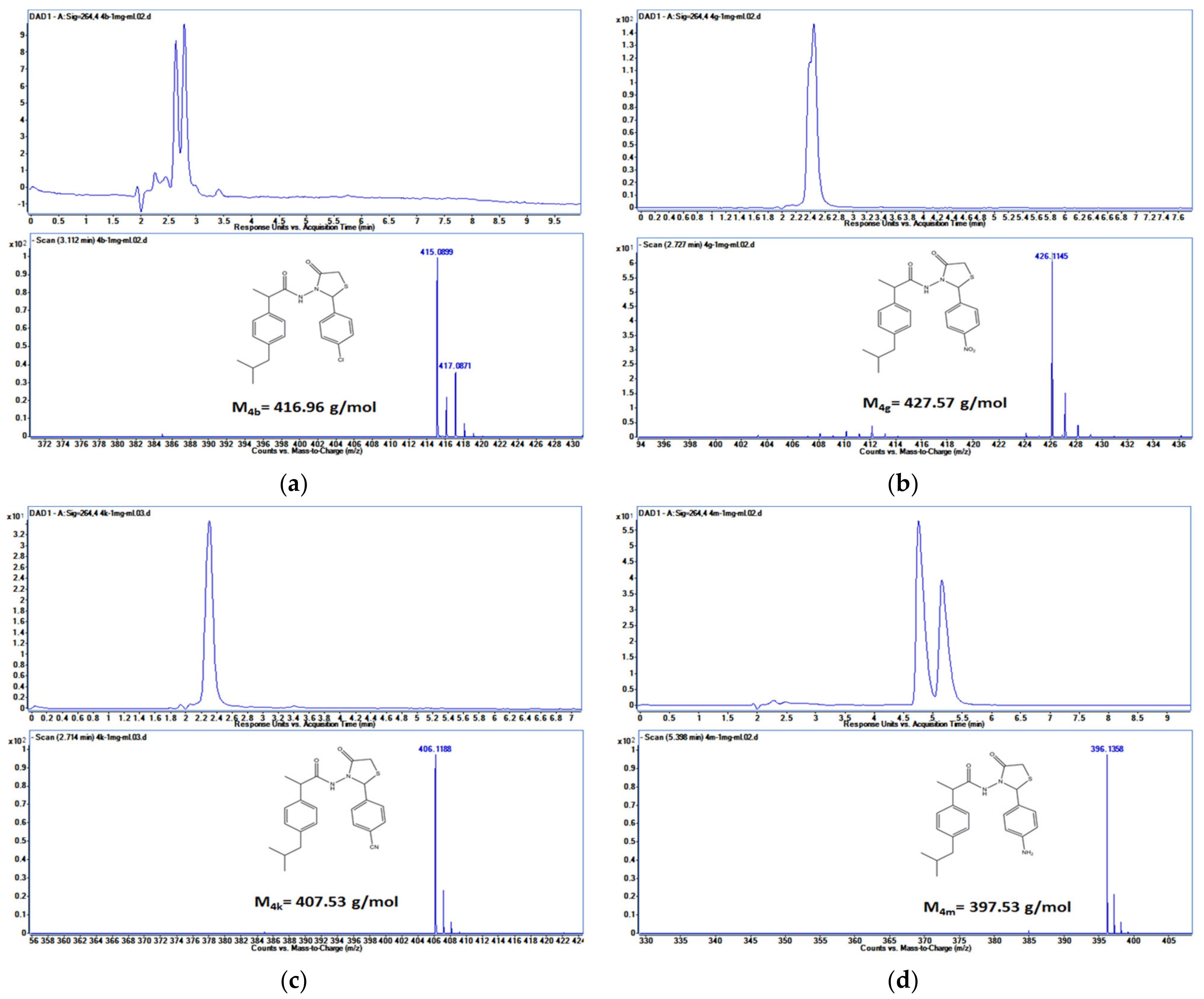
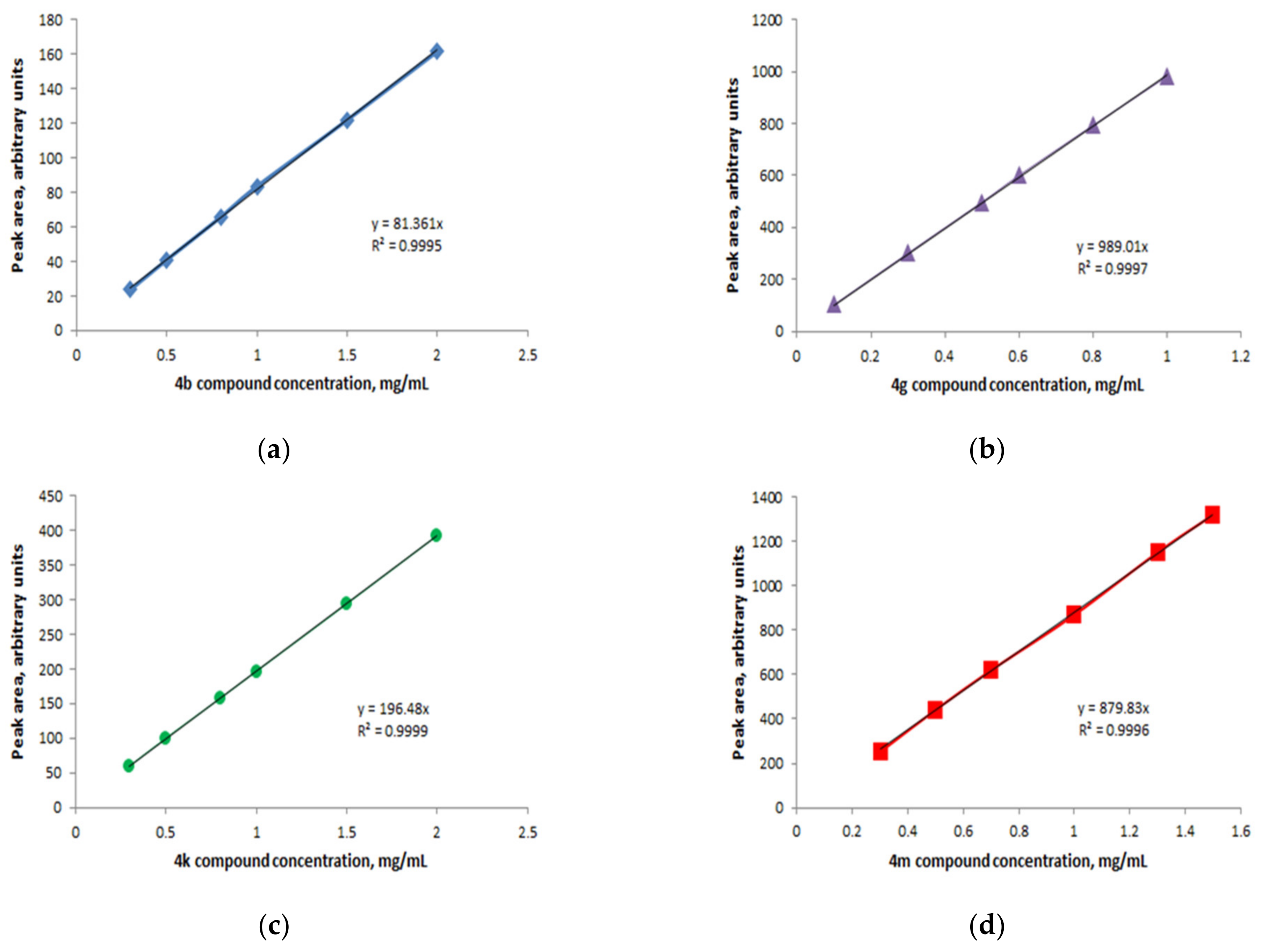
HPLC-ESI-MS Method
Preparation of Serial Dilutions
Blood Samples Preparation
3. Results and Discussions
3.1. Physico-Chemical Characterization of β-CD Complexes
3.1.1. Scanning Electronic Microscopy and Energy Dispersive X-ray Analysis
3.1.2. Differential Scanning Calorimetry
3.1.3. X-ray Diffraction
3.1.4. Phase Solubility Studies
3.1.5. Molecular Docking
3.2. Analgesic Tests
3.2.1. Tail Flick
3.2.2. Hot Plate
3.2.3. Writhing Test
3.2.4. Paw Formalin Test
3.3. Drug Release Profile
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pereva, S.; Sarafska, T.; Bogdanova, S.; Spassov, T. Efficiency of “Cyclodextrin-Ibuprofen” Inclusion Complex Formation. J. Drug Deliv. Sci. Technol. 2016, 35, 34–39. [Google Scholar] [CrossRef]
- Sandilya, A.A.; Natarajan, U.; Priya, M.H. Molecular View into the Cyclodextrin Cavity: Structure and Hydration. ACS Omega 2020, 5, 25655–25667. [Google Scholar] [CrossRef]
- Raffaini, G.; Ganazzoli, F. Understanding Surface Interaction and Inclusion Complexes between Piroxicam and Native or Crosslinked β-Cyclodextrins: The Role of Drug Concentration. Molecules 2020, 25, 2848. [Google Scholar] [CrossRef]
- Miranda, G.M.; Santos, V.O.R.e.; Bessa, J.R.; Teles, Y.C.F.; Yahouédéhou, S.C.M.A.; Goncalves, M.S.; Ribeiro-Filho, J. Inclusion Complexes of Non-Steroidal Anti-Inflammatory Drugs with Cyclodextrins: A Systematic Review. Biomolecules 2021, 11, 361. [Google Scholar] [CrossRef]
- Deosarkar, S.D.; Sawale, R.T.; Arsule, A.D.; Puyad, A.L. Volumetric and Ultraacoustic Properties of Sodium Salts of Ibuprofen/Diclofenac Drugs in Aqueous and Aqueous-β-Cyclodextrin Solution. J. Mol. Liq. 2020, 297, 111812. [Google Scholar] [CrossRef]
- Sharma, N.; Baldi, A. Exploring Versatile Applications of Cyclodextrins: An Overview. Drug Deliv. 2016, 23, 729–747. [Google Scholar] [CrossRef]
- Franco, P.; De Marco, I. Preparation of non-steroidal anti-inflammatory drug/β-cyclodextrin inclusion complexes by supercritical antisolvent process. J. CO2 Util. 2021, 44, 101397. [Google Scholar] [CrossRef]
- Jun, S.W.; Kim, M.-S.; Kim, J.-S.; Park, H.J.; Lee, S.; Woo, J.-S.; Hwang, S.-J. Preparation and characterization of simvastatin/hydroxypropyl-β-cyclodextrin inclusion complex using supercritical antisolvent (SAS) process. Eur. J. Pharm. Biopharm. 2007, 66, 413–421. [Google Scholar] [CrossRef]
- Korani, M.; Jamshidi, M. The Effect of Aqueous Extract of Trachyspermum Ammi Seeds and Ibuprofen on Inflammatory Gene Expression in the Cartilage Tissue of Rats with Collagen-Induced Arthritis. J. Inflamm. Res. 2020, 13, 133–139. [Google Scholar] [CrossRef]
- Gunaydin, C.; Bilge, S.S. Effects of Nonsteroidal Anti-Inflammatory Drugs at the Molecular Level. Eurasian J. Med. 2018, 50, 116–121. [Google Scholar] [CrossRef]
- Pranata, R.; Yonas, E.; Vania, R.; Prakoso, R. The Efficacy and Safety of Oral Paracetamol versus Oral Ibuprofen for Patent Ductus Arteriosus Closure in Preterm Neonates—A Systematic Review and Meta-Analysis. Indian Heart J. 2020, 72, 151–159. [Google Scholar] [CrossRef]
- Chen, T.; He, X.; Jiang, T.; Liu, W.; Li, Y.; Liu, P.; Liu, Z. Synthesis and Drug Delivery Properties of Ibuprofen-Cellulose Nanofibril System. Int. J. Biol. Macromol. 2021, 182, 931–937. [Google Scholar] [CrossRef]
- Statelova, M.; Holm, R.; Fotaki, N.; Reppas, C.; Vertzoni, M. Factors Affecting Successful Extrapolation of Ibuprofen Exposure from Adults to Pediatric Populations After Oral Administration of a Pediatric Aqueous Suspension. AAPS J. 2020, 22, 146. [Google Scholar] [CrossRef]
- Vasincu, I.-M.; Apotrosoaei, M.; Constantin, S.; Butnaru, M.; Vereștiuc, L.; Lupușoru, C.-E.; Buron, F.; Routier, S.; Lupașcu, D.; Taușer, R.-G.; et al. New Ibuprofen Derivatives with Thiazolidine-4-One Scaffold with Improved Pharmaco-Toxicological Profile. BMC Pharmacol. Toxicol. 2021, 22, 10. [Google Scholar] [CrossRef]
- Marangoci, N.; Timpu, D.; Corciova, A.; Mircea, C.; Petrovici, A.-R.; Nicolescu, A.; Ursu, E.-L.; Nastasa, V.; Bostanaru, A.-C.; Mares, M.; et al. β-Cyclodextrin as a Functional Excipient Used for Enhancing the Diminazene Aceturate Bioavailability. Pharmaceutics 2019, 11, 295. [Google Scholar] [CrossRef]
- Pereva, S.; Nikolova, V.; Sarafska, T.; Angelova, S.; Spassov, T.; Dudev, T. Inclusion complexes of ibuprofen and β-cyclodextrin: Supramolecular structure and stability. J. Mol. Struct. 2020, 1205, 127575. [Google Scholar] [CrossRef]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the Speed and Accuracy of Docking with a New Scoring Function, Efficient Optimization, and Multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef]
- Krieger, E.; Vriend, G. YASARA View—Molecular Graphics for All Devices—From Smartphones to Workstations. Bioinformatics 2014, 30, 2981–2982. [Google Scholar] [CrossRef]
- Alexa, T.; Luca, A.; Dondas, A.; Bohotin, C.R. Preconditioning with Cobalt Chloride Modifies Pain Perception in Mice. Exp. Ther. Med. 2015, 9, 1465–1469. [Google Scholar] [CrossRef]
- Tamba, B.I.; Petreus, T.; Constantin, M.-M.L.; Rezus, C.; Floria, M.; Rezus, E. Heavy Metal Trace Elements Induced Antinociception in an Experimental Mouse Model. Rev. Chim. 2015, 66, 976–982. [Google Scholar]
- Alexa, T.; Marza, A.; Voloseniuc, T.; Tamba, B. Enhanced Analgesic Effects of Tramadol and Common Trace Element Coadministration in Mice: Coadministration of Tramadol and Trace Elements in Mice. J. Neurosci. Res. 2015, 93, 1534–1541. [Google Scholar] [CrossRef] [PubMed]
- Guilherme, V.A.; Cunha, V.R.R.; de Paula, E.; de Araujo, D.R.; Constantino, V.R.L. Anti-Inflammatory and Analgesic Evaluation of a Phytochemical Intercalated into Layered Double Hydroxide. Pharmaceutics 2022, 14, 934. [Google Scholar] [CrossRef]
- de Oliveira, D.R.; Maia, R.C.; de Carvalho França, P.R.; Fernandes, P.D.; Barbosa, G.; Lima, L.M.; Fraga, C.A.M. 2-Arylpropionic Acid Pyrazolamides as Cannabinoid CB2 Receptor Inverse Agonists Endowed with Anti-Inflammatory Properties. Pharmaceuticals 2022, 15, 1519. [Google Scholar] [CrossRef]
- Koshiishi, I.; Mamura, Y.; Liu, J.; Imanari, T. Evaluation of an Acidic Deproteinization for the Measurement of Ascorbate and Dehydroascorbate in Plasma Samples. Clin. Chem. 1998, 44, 863–868. [Google Scholar] [CrossRef]
- Lerdkanchanaporn, S.; Dollimore, D. A Thermal Analysis Study of Ibuprofen. J. Therm. Anal. 1997, 49, 879–886. [Google Scholar] [CrossRef]
- Romero, A.J.; Rhodes, C.T. Stereochemical Aspects of the Molecular Pharmaceutics of Ibuprofen. J. Pharm. Pharmacol. 1993, 45, 258–262. [Google Scholar] [CrossRef]
- Corciovă, A.; Ciobanu, C.F.; Poiată, A.; Nicolescu, A.; Drobotă, M.; Pinteala, T.; Fifere, A.; Marangoci, N.L.; Mircea, C. Inclusion Complexes of Hesperidin with Hydroxypropyl-β-Cyclodextrin. Physico-Chemical Characterization and Biological Assessment. Dig. J. Nanomater. Biostruct. 2014, 9, 1623–1637. [Google Scholar]
- Varganici, C.-D.; Marangoci, N.; Rosu, L.; Barbu-Mic, C.; Rosu, D.; Pinteala, M.; Simionescu, B.C. TGA/DTA–FTIR–MS Coupling as Analytical Tool for Confirming Inclusion Complexes Occurrence in Supramolecular Host–Guest Architectures. J. Anal. Appl. Pyrolysis 2015, 115, 132–142. [Google Scholar] [CrossRef]
- Mukne, A.P.; Nagarsenker, M.S. Triamterene-beta-cyclodextrin systems: Preparation, characterization and in vivo evaluation. AAPS PharmSciTech 2004, 5, 19. [Google Scholar] [CrossRef]
- Marangoci, N.; Farcas, A.; Pinteala, M.; Harabagiu, V.; Simionescu, B.C.; Sukhanova, T.; Bronnikov, S.; Grigoryev, A.; Gubanova, G.; Perminova, M.; et al. Polyrotaxanes composed of β-cyclodextrin and polydimethylsiloxanes: Synthesis, morphology and thermal behavior. High Perform. Polym. 2007, 20, 251–266. [Google Scholar] [CrossRef]
- Higuchi, T.; Connors, K.A. Phase Solubility Techniques. Adv. Anal. Chem. Instrum. 1965, 4, 117–212. [Google Scholar]
- Saokham, P.; Muankaew, C.; Jansook, P.; Loftsson, T. Solubility of Cyclodextrins and Drug/Cyclodextrin Complexes. Molecules 2018, 23, 1161. [Google Scholar] [CrossRef] [PubMed]
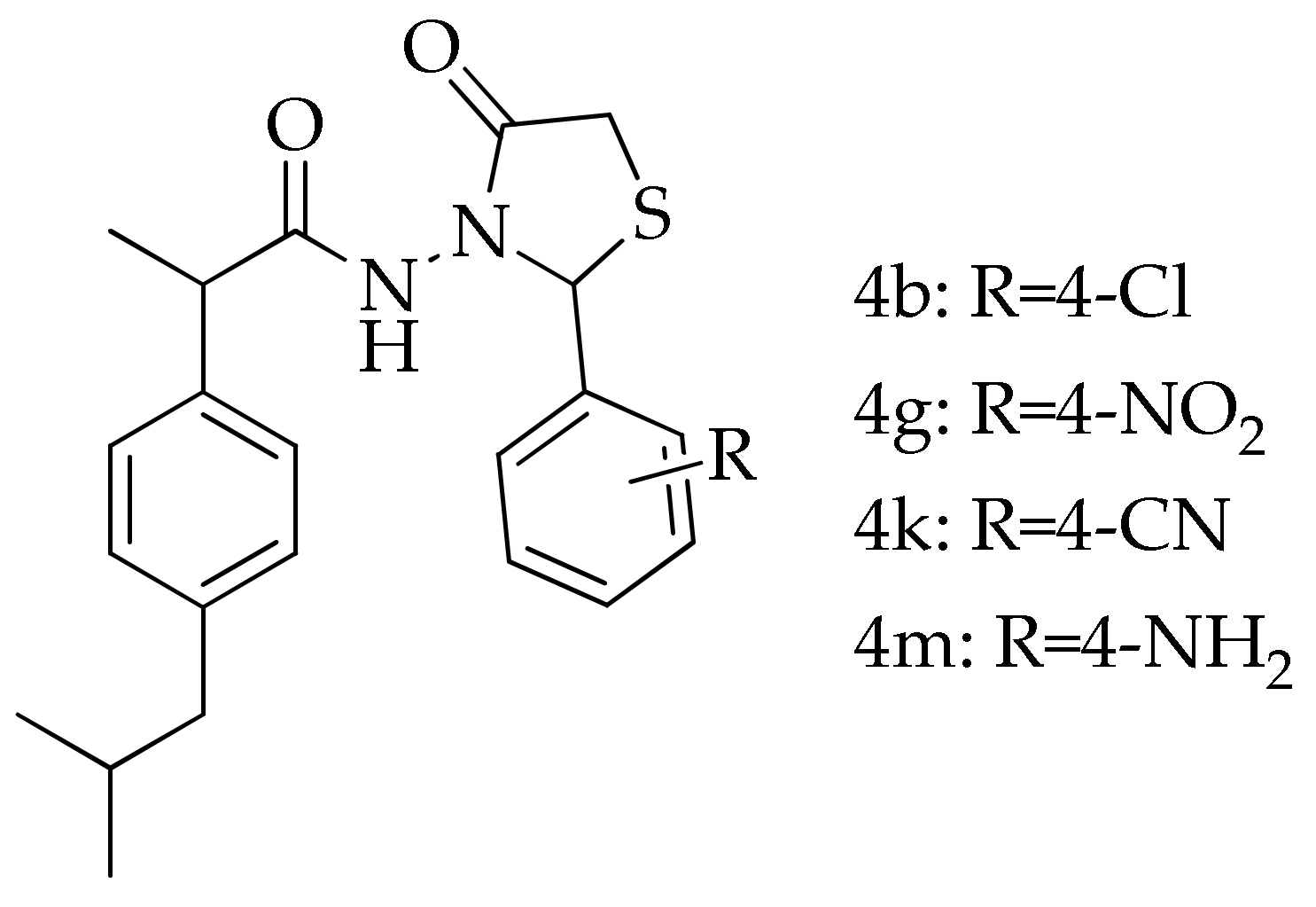






| Compound/Complexes | Dose/Equivalent Dose (mg/kg b.w.) |
|---|---|
| 4b/β-CD-4b | 81.25/302.41 |
| 4g/β-CD-4g | 91.00/332.55 |
| 4k/β-CD-4k | 78.25/296.17 |
| 4m/β-CD-4m | 82.50/318.04 |
| Ibu-Na | 68.75 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vasincu, I.M.; Apotrosoaei, M.; Lupascu, F.; Iacob, A.-T.; Giusca, S.-E.; Caruntu, I.-D.; Marangoci, N.-L.; Petrovici, A.R.; Stanciu, G.D.; Tamba, B.-I.; et al. Complexes of Ibuprofen Thiazolidin-4-One Derivatives with β-Cyclodextrin: Characterization and In Vivo Release Profile and Biological Evaluation. Pharmaceutics 2023, 15, 2492. https://doi.org/10.3390/pharmaceutics15102492
Vasincu IM, Apotrosoaei M, Lupascu F, Iacob A-T, Giusca S-E, Caruntu I-D, Marangoci N-L, Petrovici AR, Stanciu GD, Tamba B-I, et al. Complexes of Ibuprofen Thiazolidin-4-One Derivatives with β-Cyclodextrin: Characterization and In Vivo Release Profile and Biological Evaluation. Pharmaceutics. 2023; 15(10):2492. https://doi.org/10.3390/pharmaceutics15102492
Chicago/Turabian StyleVasincu, Ioana Mirela, Maria Apotrosoaei, Florentina Lupascu, Andreea-Teodora Iacob, Simona-Eliza Giusca, Irina-Draga Caruntu, Narcisa-Laura Marangoci, Anca Roxana Petrovici, Gabriela Dumitrita Stanciu, Bogdan-Ionel Tamba, and et al. 2023. "Complexes of Ibuprofen Thiazolidin-4-One Derivatives with β-Cyclodextrin: Characterization and In Vivo Release Profile and Biological Evaluation" Pharmaceutics 15, no. 10: 2492. https://doi.org/10.3390/pharmaceutics15102492







