Antiviral Activity of Flavonoids from Geopropolis of the Brazilian Jandaira Bee against Zika and Dengue Viruses
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
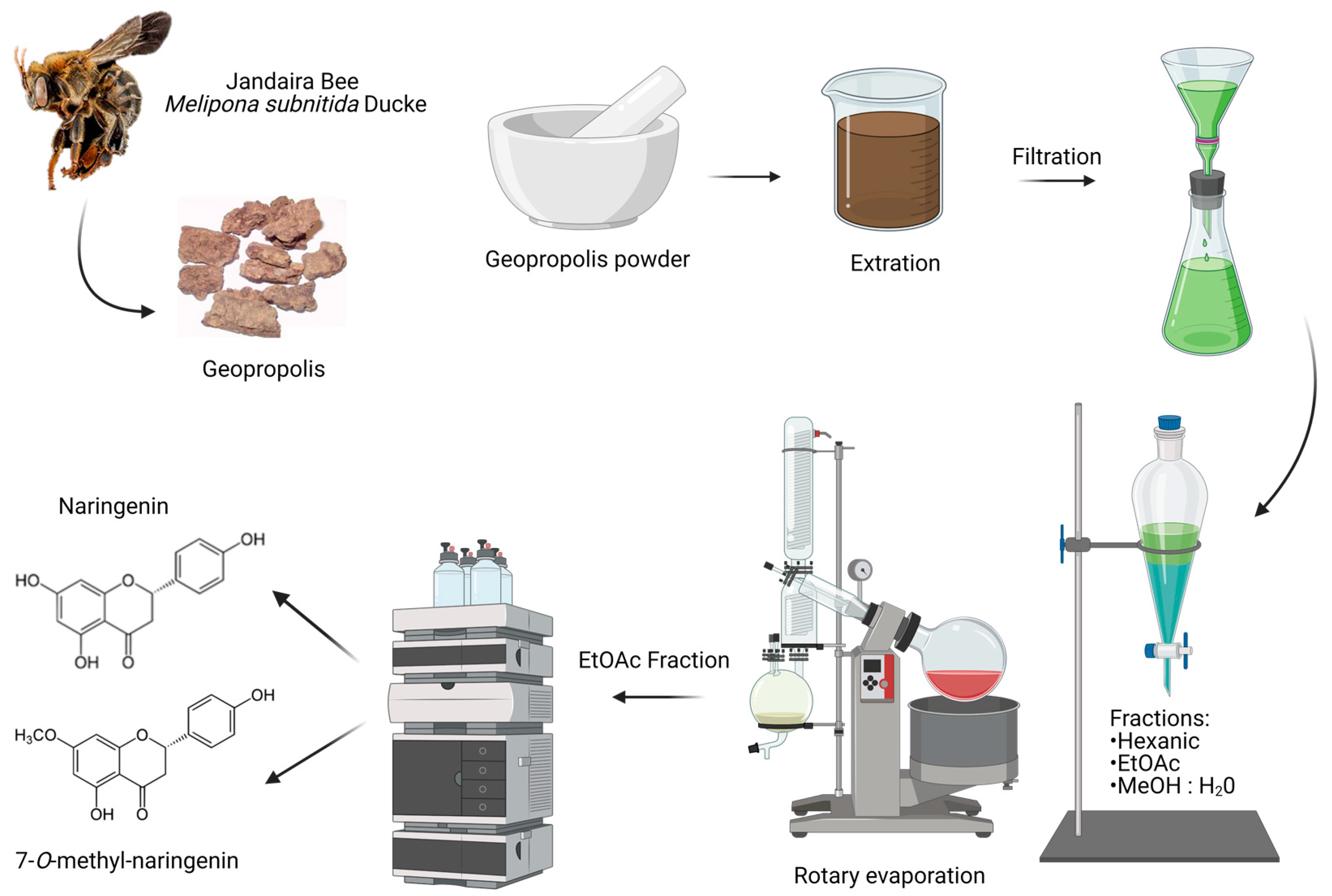
2.2. Geopropolis Sample and Flavonoid Isolation Process
2.3. Cells and Viruses
2.4. Cell Viability Assay
2.5. Antiviral Assay
2.6. Viral Titration
2.7. Statistical Analysis
3. Theoretical Methods
3.1. Molecular Docking
3.2. Enthalpy of Binding
3.3. Molecular Dynamics Simulation
3.4. Interaction Energy Analysis
4. Results
4.1. Identification of Flavonoids
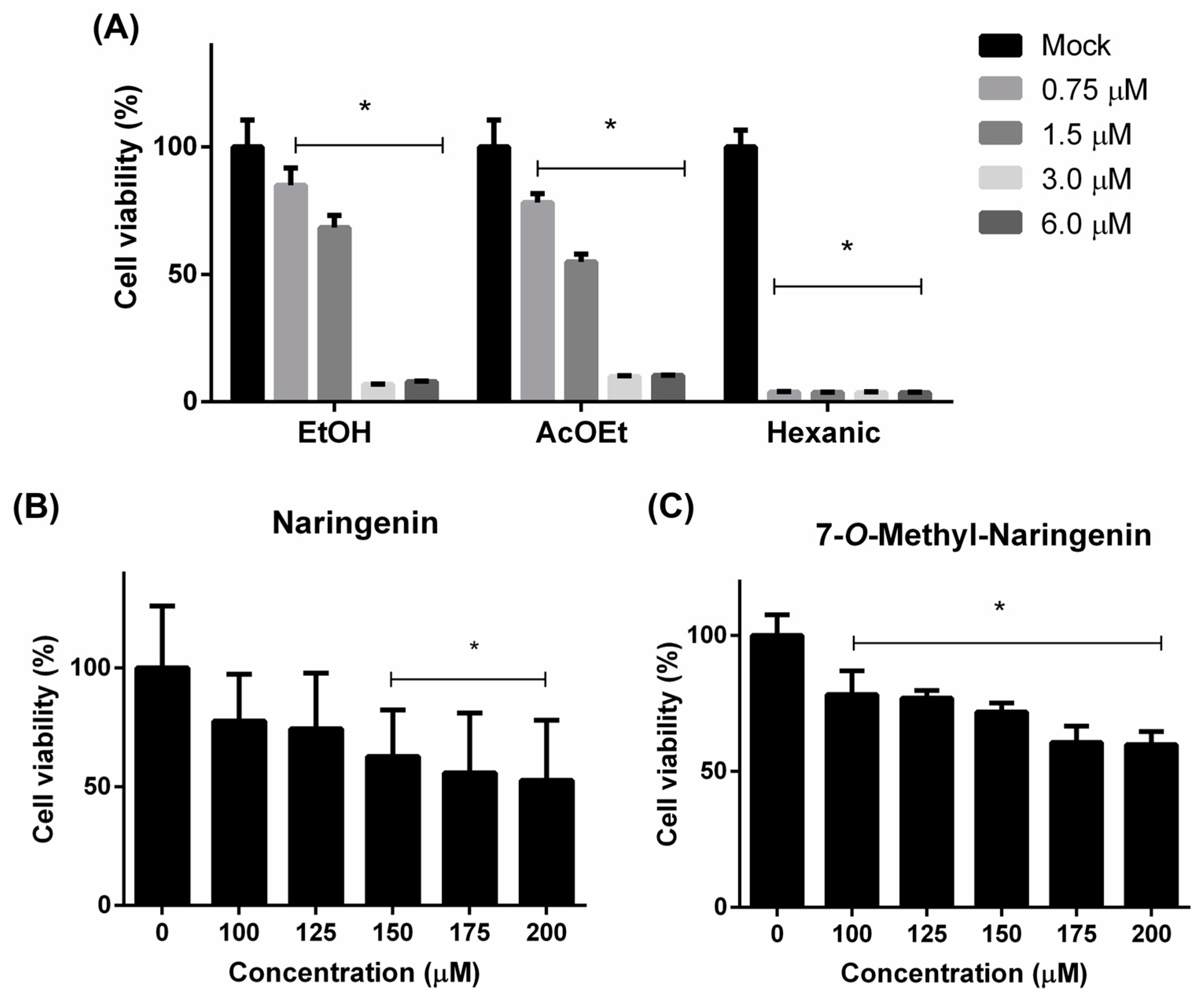
4.2. Flavonoids’ Citotoxic Effects
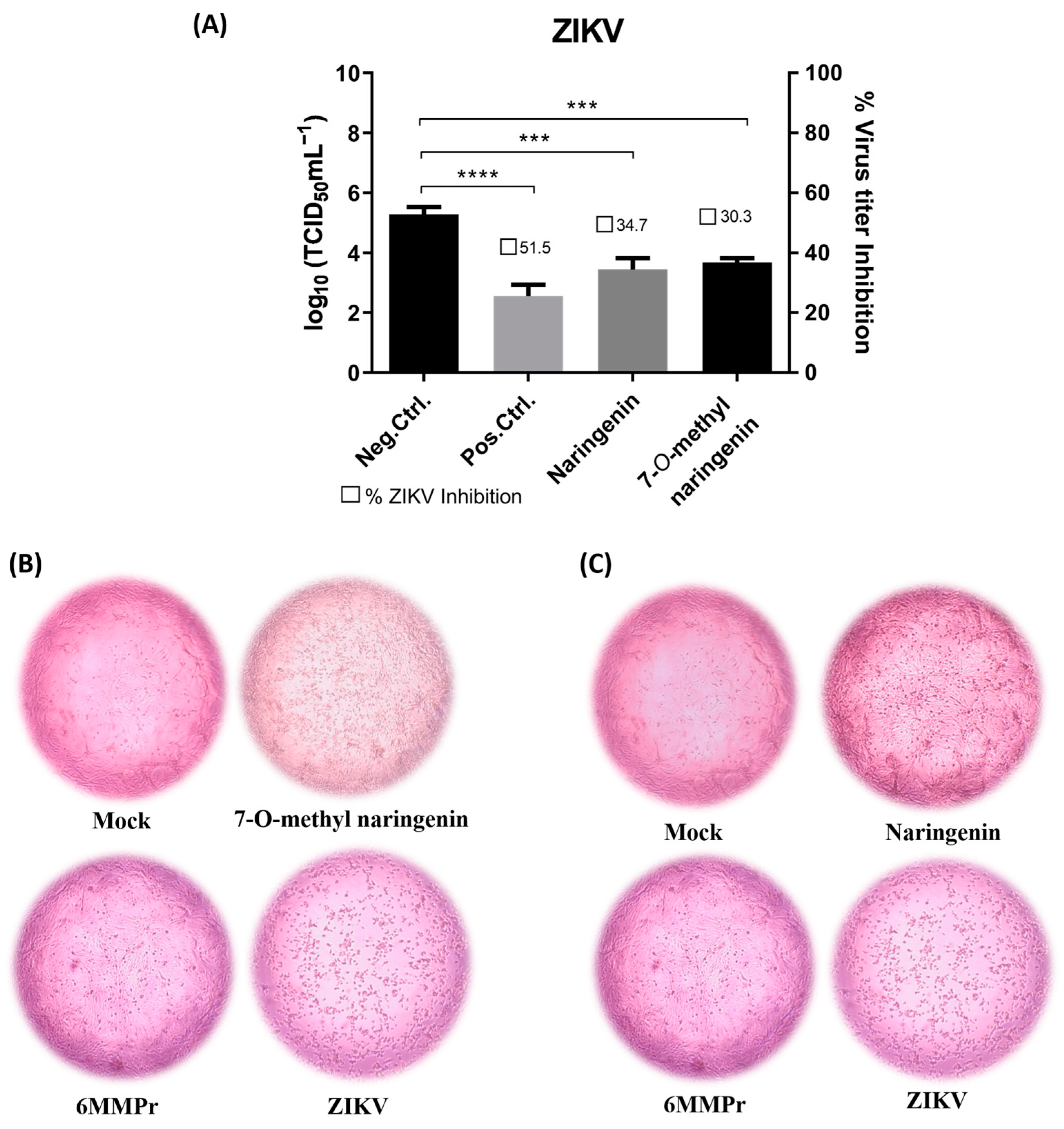
4.3. Post-Treatment Antiviral Activity of Flavonoids
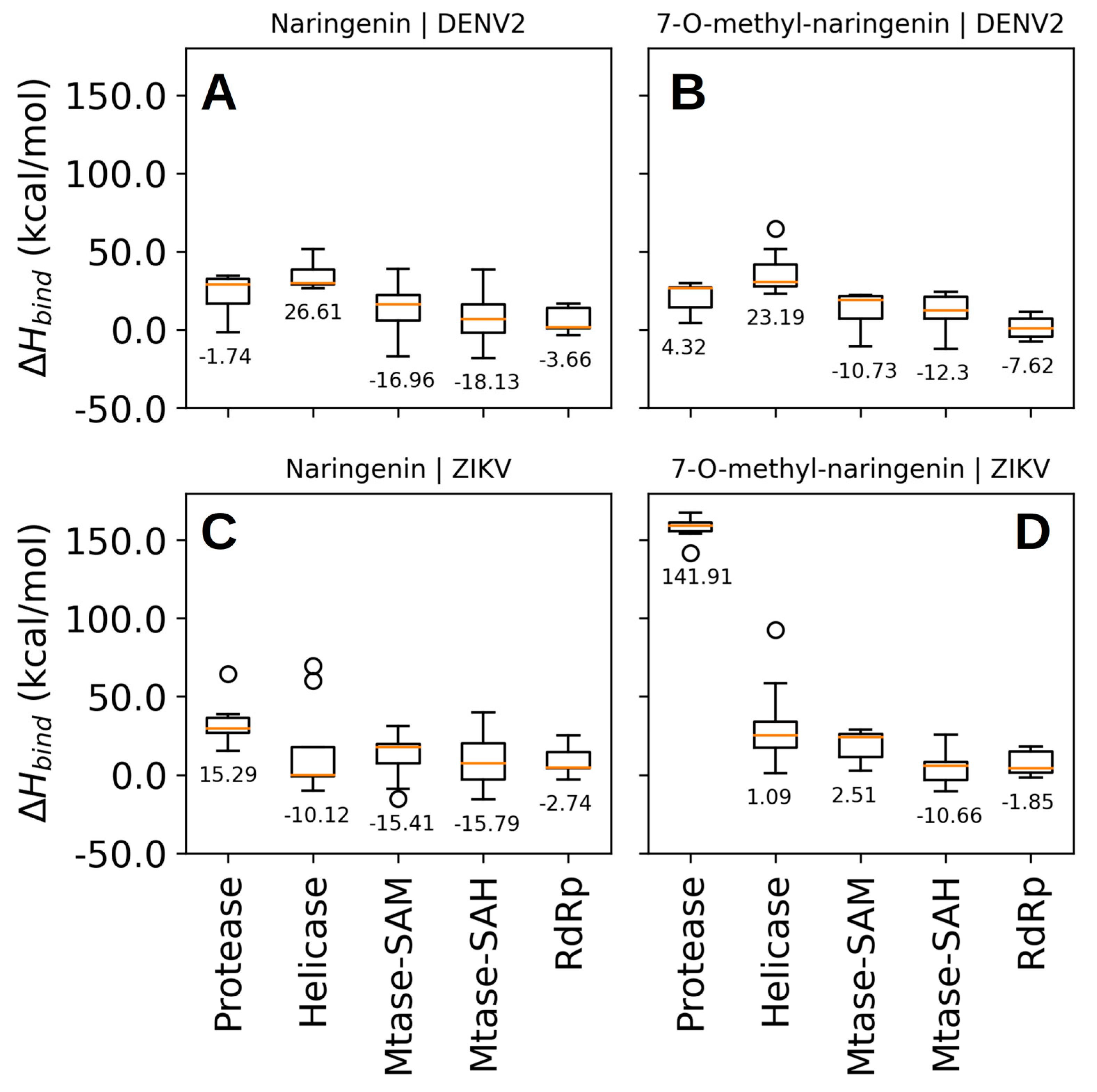
4.4. The Flavonoids Naringenin and 7-O-Methyl Naringenin Bind to the NS5-Methyltransferase of Both DENV and ZIKV
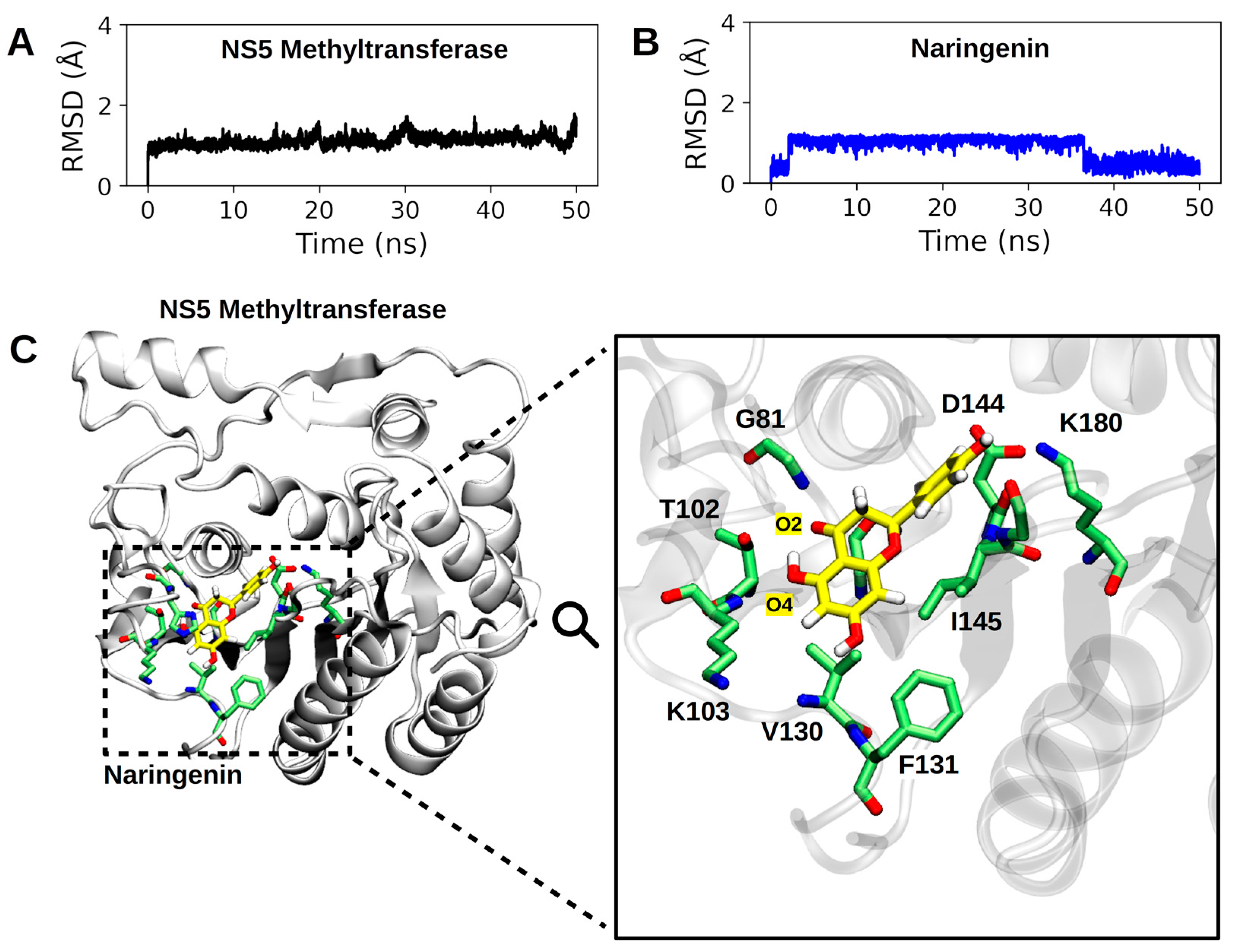
4.5. Molecular Dynamics Simulation Showed That Naringenin Forms a High Number of Non-Covalent Interactions at the Binding Site of NS5-Methyltransferase
5. Discussion
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zakaryan, H.; Arabyan, E.; Oo, A.; Zandi, K. Flavonoids: Promising natural compounds against viral infections. Arch. Virol. 2017, 162, 2539–2551. [Google Scholar] [CrossRef] [PubMed]
- Kaul, T.N.; Middleton, E., Jr.; Ogra, P.L. Antiviral effect of flavonoids on human viruses. J. Med. Virol. 1985, 15, 71–79. [Google Scholar] [CrossRef]
- Cataneo, A.H.D.; Kuczera, D.; Koishi, A.C.; Zanluca, C.; Silveira, G.F.; Arruda, T.B.D.; Bordignon, J. The citrus flavonoid naringenin impairs the in vitro infection of human cells by Zika virus. Sci. Rep. 2019, 9, 16348. [Google Scholar] [CrossRef]
- Frabasile, S.; Koishi, A.C.; Kuczera, D.; Silveira, G.F.; Verri, W.A.; Duarte dos Santos, C.N.; Bordignon, J. The citrus flavanone naringenin impairs dengue virus replication in human cells. Sci. Rep. 2017, 7, 41864. [Google Scholar] [CrossRef] [PubMed]
- da Silva, S.J.R.; de Magalhães, J.J.F.; Pena, L. Simultaneous circulation of DENV, CHIKV, ZIKV and SARS-CoV-2 in Brazil: An inconvenient truth. One Health 2021, 12, 100205. [Google Scholar] [CrossRef]
- Brazil Ministry of Health; Epidemiological Update. 2023. Available online: https://www.gov.br/saude/pt-br/centrais-de-conteudo/publicacoes/boletins/epidemiologicos/edicoes/2023/boletim-epidemiologico-volume-54-no-01/view (accessed on 17 March 2023).
- Agumadu, V.C.; Ramphul, K. Zika Virus: A review of literature. Cureus 2018, 10, 282–289. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.; Ortiz, K.; Ansari, A.; Gershwin, M.E. The Zika outbreak of the 21st century. J. Autoimmun. 2016, 68, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Sukhralia, S.; Verma, M.; Gopirajan, S.; Dhanaraj, P.S.; Lal, R.; Mehla, N.; Kant, C.R. From dengue to Zika: The wide spread of mosquito-borne arboviruses. Eur. J. Clin. Microbiol. Infect. Dis. 2019, 38, 3–14. [Google Scholar] [CrossRef]
- Barrows, N.J.; Campos, R.K.; Liao, K.C.; Prasanth, K.R.; Soto-Acosta, R.; Yeh, S.C.; Garcia-Blanco, M.A. Biochemistry and molecular biology of flaviviruses. Chem. Rev. 2018, 118, 4448–4482. [Google Scholar] [CrossRef]
- Pan American Health Organization (PAHO). United States; Epidemiological Update 2016. Available online: https://www.paho.org/hq/dmdocuments/2016/2016-jan-17-cha-epi-update-zika-virus.pdf (accessed on 17 March 2023).
- World Health Organization (WHO). Zika Epidemiology Update—February 2022. Available online: https://www.who.int/publications/m/item/zika-epidemiology-update---february-2022 (accessed on 17 March 2023).
- Sharma, V.; Sharma, M.; Dhull, D.; Sharma, Y.; Kaushik, S.; Kaushik, S. Zika virus: An emerging challenge to public health worldwide. Can. J. Microbiol. 2020, 66, 87–98. [Google Scholar] [CrossRef]
- Balmaseda, A.; Hammond, S.N.; Pérez, L.; Tellez, Y.; Saborío, S.I.; Mercado, J.C.; Harris, E. Serotype-specific differences in clinical manifestations of dengue. Am. J. Trop. Med. Hyg. 2006, 74, 449–456. [Google Scholar] [CrossRef] [PubMed]
- Fares, R.C.; Souza, K.P.; Añez, G.; Rios, M. Epidemiological scenario of dengue in Brazil. BioMed Res. Int. 2015, 2015, 321873. [Google Scholar] [CrossRef] [PubMed]
- Guzman, M.G.; Halstead, S.B.; Artsob, H.; Buchy, P.; Farrar, J.; Gubler, D.J.; Peeling, R.W. Dengue: A continuing global threat. Nat. Rev. Microbiol. 2010, 12, S7–S16. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, S.; Gething, P.W.; Brady, O.J.; Messina, J.P.; Farlow, A.W.; Moyes, C.L.; Hay, S.I. The global distribution and burden of dengue. Nature 2013, 496, 504–507. [Google Scholar] [CrossRef]
- Brady, O.J.; Gething, P.W.; Bhatt, S.; Messina, J.P.; Brownstein, J.S.; Hoen, A.G.; Moyes, C.L.; Farlow, A.W.; Scott, T.W.; Hay, S.I. Refining the global spatial limits of dengue virus transmission by evidence-based consensus. PLoS Negl. Trop. Dis. 2012, 6, e1760. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.K.; Bhattacharjee, S. Dengue virus: Epidemiology; biology; and disease aetiology. Can. J. Microbiol. 2021, 67, 687–702. [Google Scholar] [CrossRef] [PubMed]
- López-Montenegro, L.E.; Pulecio-Montoya, A.M.; Marcillo-Hernández, G.A. Dengue Cases in Colombia: Mathematical Forecasts for 2018–2022. MEDICC Rev. 2019, 21, 38–45. [Google Scholar]
- López, M.S.; Jordan, D.I.; Blatter, E.; Walker, E.; Gómez, A.A.; Müller, G.V.; Estallo, E.L. Dengue emergence in the temperate Argentinian province of Santa Fe; 2009–2020. Sci. Data 2021, 8, 134. [Google Scholar] [CrossRef]
- Barzon, L.; Gobbi, F.; Capelli, G.; Montarsi, F.; Martini, S.; Riccetti, S.; Lazzarini, L. Autochthonous dengue outbreak in Italy 2020: Clinical; virological and entomological findings. J. Travel Med. 2021, 28, 130. [Google Scholar] [CrossRef]
- Harapan, H.; Ryan, M.; Yohan, B.; Abidin, R.S.; Nainu, F.; Rakib, A.; Sasmono, R.T. COVID-19 and dengue: Double punches for dengue-endemic countries in Asia. Rev. Med. Virol. 2021, 31, 2161. [Google Scholar] [CrossRef]
- Norshidah, H.; Vignesh, R.; Lai, N.S. Updates on dengue vaccine and antiviral: Where are we heading? Molecules 2021, 26, 6768. [Google Scholar] [CrossRef] [PubMed]
- Coudeville, L.; Baurin, N.; L’Azou, M.; Guy, B. Potential impact of dengue vaccination: Insights from two large-scale phase III trials with a tetravalent dengue vaccine. Vaccine 2016, 34, 6426–6435. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.H.; Tsai, Y.T.; Wang, S.F.; Wang, W.H.; Chen, Y.H. Dengue vaccine: An update. Expert Rev. Anti-Infect. Ther. 2021, 19, 1495–1502. [Google Scholar] [CrossRef] [PubMed]
- García, L.L.; Padilla, L.; Castaño, J.C. Inhibitors compounds of the flavivirus replication process. Virol. J. 2017, 14, 95. [Google Scholar] [CrossRef] [PubMed]
- Pereira, D.S.; Holanda-neto, J.P.D.; Oliveira, M.S.D.; Pereira, N.S.; Maracajá, P.B.; Souza, A.P.D.S. Phytotoxic potential of the Geopropolis extracts of the jandaira stingless bee (Melipona subnitida) in weeds. Rev. Caatinga 2017, 30, 876–884. [Google Scholar] [CrossRef]
- Camara, J.Q.; de Sousa, A.H.; de Vasconcelos, W.E.; da Silveira Maia, P.H.; de Almeida, J.C.; Borges, P.M. Estudos de meliponíneos; com ênfase a Melipona subnitida D. no município de Jandaíra; RN. Rev. De Biol. E Ciências Da Terra 2004, 4, 1–20. [Google Scholar]
- de Sousa, D.M.N.; Olinda, R.G.; Martins, C.G.; Abrantes, M.R.; Coelho, W.A.C.; da Silva, J.B.A.; Batista, J.S. Phytochemical screening; in vitro toxicity and evaluation of antioxidant and antibacterial activities of jandaíra bee’s geopropolis. Acta Vet. Bras. 2015, 9, 134–140. [Google Scholar]
- Coelho, G.R.; Mendonça, R.Z.; Vilar, K.D.S.; Figueiredo, C.A.; Badari, J.C.; Taniwaki, N.; Negri, G. Antiviral action of hydromethanolic extract of geopropolis from Scaptotrigona postica against antiherpes simplex virus (HSV-1). Evid.-Based Complement. Altern. Med. 2015, 2015, 296086. [Google Scholar] [CrossRef]
- Hoffmann, H.H.; Palese, P.; Shaw, M.L. Modulation of influenza virus replication by alteration of sodium ion transport and protein kinase C activity. Antivir. Res. 2008, 80, 124–134. [Google Scholar] [CrossRef]
- de Carvalho, O.V.; Félix, D.M.; de Mendonça, L.R.; de Araújo, C.M.C.S.; de Oliveira Franca, R.F.; Cordeiro, M.T.; Pena, L.J. The thiopurine nucleoside analogue 6-methylmercaptopurine riboside (6MMPr) effectively blocks Zika virus replication. Int. J. Antimicrob. Agentes 2017, 50, 718–725. [Google Scholar] [CrossRef]
- Reed, L.J.; Muench, H. A simple method of estimating fifty per cent endpoints. Am. J. Epidemiol. 1938, 27, 493–497. [Google Scholar] [CrossRef]
- Xu, T.; Sampath, A.; Chao, A.; Wen, D.; Nanao, M.; Chene, P.; Lescar, J. Structure of the Dengue virus helicase/nucleoside triphosphatase catalytic domain at a resolution of 2.4 A. J. Virol. 2005, 79, 10278–10288. [Google Scholar] [CrossRef] [PubMed]
- Noble, C.G.; Seh, C.C.; Chao, A.T.; Shi, P.Y. Ligand-bound structures of the dengue virus protease reveal the active conformation. J. Virol. 2012, 86, 438–446. [Google Scholar] [CrossRef]
- Egloff, M.P.; Decroly, E.; Malet, H.; Selisko, B.; Benarroch, D.; Ferron, F.; Canard, B. Structural and functional analysis of methylation and 5′-RNA sequence requirements of short capped RNAs by the methyltransferase domain of dengue virus NS5. J. Mol. Biol. 2007, 372, 723–736. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.P.; Noble, C.G.; Seh, C.C.; Soh, T.S.; El Sahili, A.; Chan, G.K.Y.; Yokokawa, F. Potent allosteric dengue virus NS5 polymerase inhibitors: Mechanism of action and resistance profiling. PLoS Pathog. 2016, 12, e1005737. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Li, Y.; Jin, X.; Li, Y.; Guo, F.; Jin, T. Molecular mechanism of divalent-metal-induced activation of NS3 helicase and insights into Zika virus inhibitor design. Nucleic Acids Res. 2016, 44, 10505–10514. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, Z.; Phoo, W.W.; Loh, Y.R.; Li, R.; Yang, H.Y.; Kang, C. Structural insights into the inhibition of Zika virus NS2B-NS3 protease by a small-molecule inhibitor. Structure 2018, 26, 555–564. [Google Scholar] [CrossRef]
- Duan, W.; Song, H.; Wang, H.; Chai, Y.; Su, C.; Qi, J.; Gao, G.F. The crystal structure of Zika virus NS 5 reveals conserved drug targets. EMBO J. 2017, 36, 919–933. [Google Scholar] [CrossRef]
- Gharbi-Ayachi, A.; Santhanakrishnan, S.; Wong, Y.H.; Chan, K.W.; Tan, S.T.; Bates, R.W.; Lescar, J. Non-nucleoside inhibitors of Zika virus RNA-dependent RNA polymerase. J. Virol. 2020, 94, e00794-20. [Google Scholar] [CrossRef]
- Dolinsky, T.J.; Nielsen, J.E.; McCammon, J.A.; Baker, N.A. PDB2PQR: An automated pipeline for the setup of Poisson–Boltzmann electrostatics calculations. Nucleic Acids Res. 2004, 32, W665–W667. [Google Scholar] [CrossRef]
- Cornell, W.D.; Cieplak, P.; Bayly, C.I.; Kollman, P.A. Application of RESP charges to calculate conformational energies, hydrogen bond energies, and free energies of solvation. J. Am. Chem. Soc. 2002, 115, 9620–9631. [Google Scholar] [CrossRef]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef]
- Stewart, J.J.P. MOPAC2016, Stewart Computational Chemistry; OpenMOPAC: Colorado Springs, CO, USA, 2016. [Google Scholar]
- Phillips, J.C.; Braun, R.; Wang, W.; Gumbart, J.; Tajkhorshid, E.; Villa, E.; Schulten, K. Scalable molecular dynamics with NAMD. J. Comput. Chem. 2005, 26, 1781–1802. [Google Scholar] [CrossRef] [PubMed]
- Tian, C.; Kasavajhala, K.; Belfon, K.A.; Raguette, L.; Huang, H.; Migues, A.N.; Simmerling, C. ff19SB: Amino-acid-specific protein backbone parameters trained against quantum mechanics energy surfaces in solution. J. Chem. Theory Comput. 2019, 16, 528–552. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Greene, D.A.; Xiao, L.; Qi, R.; Luo, R. Recent developments and applications of the MMPBSA method. Front. Mol. Biosci. 2018, 4, 87. [Google Scholar] [CrossRef]
- Evangelista-Falcon, W.; Denhez, C.; Baena-Moncada, A.; Ponce-Vargas, M. Revisiting the Sweet Taste Receptor T1R2-T1R3 through Molecular Dynamics Simulations Coupled with a Noncovalent Interactions Analysis. J. Phys. Chem. B 2023, 127, 1110–1119. [Google Scholar] [CrossRef]
- Miller, B.R., III; McGee, T.D., Jr.; Swails, J.M.; Homeyer, N.; Gohlke, H.; Roitberg, A.E. MMPBSA. py: An efficient program for end-state free energy calculations. J. Chem. Theory Comput. 2012, 8, 3314–3321. [Google Scholar] [CrossRef]
- Silva, T.M.S.; De Souza, S.A.; Dias, T.L.M.F.; Silva, T.M.G.; Falcão, R.A.; Moreira, M.S.A.; Camara, C.A. Chemical composition, antinociceptive and free radical-scavenging activities of geopropolis from Melipona subnitida Ducke (Hymenoptera: Apidae: Meliponini). Sociobiology 2014, 61, 560–565. [Google Scholar] [CrossRef]
- de Souza, S.A.; da Silva, T.M.G.; da Silva, E.M.S.; Camara, C.A.; Silva, T.M.S. Characterisation of phenolic compounds by UPLC-QTOF-MS/MS of geopropolis from the stingless bee Melipona subnitida (jandaíra). Phytochem. Anal. 2018, 29, 549–558. [Google Scholar] [CrossRef] [PubMed]
- de Sousa-Fontoura, D.M.; Olinda, R.G.; Viana, G.A.; de Costa, F.M.K.M.; Batista, J.S.; Serrano, R.M.; Silva, T.M. Wound healing activity and chemical composition of geopropolis from Melipona subnitida. Rev. Bras. De Farmacogn. 2020, 30, 367–373. [Google Scholar] [CrossRef]
- Zandi, K.; Boon-Teong, T.; Sam, S.S.; Wong, P.F.; Mustafa, M.R.; AbuBakar, S. In vitro antiviral activity of fisetin, rutin and naringenin against dengue virus type-2. J. Med. Plants Res. 2011, 5, 5534–5539. [Google Scholar] [CrossRef]
- Lani, R.; Hassandarvish, P.; Shu, M.H.; Phoon, W.H.; Chu, J.J.H.; Higgs, S.; Zandi, K. Antiviral activity of selected flavonoids against Chikungunya virus. Antivir. Res. 2016, 133, 50–61. [Google Scholar] [CrossRef]
- Sajitha Lulu, S.; Thabitha, A.; Vino, S.; Mohana Priya, A.; Rout, M. Naringenin and quercetin–potential anti-HCV agents for NS2 protease targets. Nat. Prod. Res. 2016, 30, 464–468. [Google Scholar] [CrossRef]
- Kwon, D.H.; Ji, J.H.; Yim, S.H.; Kim, B.S.; Choi, H.J. Suppression of influenza B virus replication by sakuranetin and mode of its action. Phytother. Res. 2018, 32, 2475–2479. [Google Scholar] [CrossRef]
- Choi, H.J. In vitro antiviral activity of sakuranetin against human rhinovirus 3. Osong Public Health Res. Perspect. 2017, 8, 415. [Google Scholar] [CrossRef]
- Tutunchi, H.; Naeini, F.; Ostadrahimi, A.; Hosseinzadeh-Attar, M.J. Naringenin, a flavanone with antiviral and anti-inflammatory effects: A promising treatment strategy against COVID-19. Phytother. Res. 2020, 34, 3137–3147. [Google Scholar] [CrossRef]
- Lim, P.Y.; Keating, J.A.; Hoover, S.; Striker, R.; Bernard, K.A. A thiopurine drug inhibits West Nile virus production in cell culture, but not in mice. PLoS ONE 2011, 6, e26697. [Google Scholar] [CrossRef]
- Alexander, C.; Prajith, N.U.; Priyanka, P.V.; Nithyakumar, A.; Arockia Samy, N. Dinuclear platinum (II) complexes of imidazophenanthroline-based bridging ligands as potential anticancer agents: Synthesis, characterization, and in vitro cytotoxicity studies. JBIC J. Biol. Inorg. Chem. 2019, 24, 405–418. [Google Scholar] [CrossRef]
- Amna, U.; Wahyuningsih, P.; Saidi, N.; Nasution, R. Evaluation of cytotoxic activity from Temurui (Murraya koenigii [Linn.] Spreng) leaf extracts against HeLa cell line using MTT assay. J. Adv. Pharm. Technol. Res. 2019, 10, 51. [Google Scholar] [CrossRef] [PubMed]
- Salehi, B.; Fokou, P.V.T.; Sharifi-Rad, M.; Zucca, P.; Pezzani, R.; Martins, N.; Sharifi-Rad, J. The therapeutic potential of naringenin: A review of clinical trials. Pharmaceuticals 2019, 12, 11. [Google Scholar] [CrossRef]
- Bennett, J.E.; Dolin, R.; Blaser, M.J. Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases E-Book; Elsevier Health Sciences: Edinburgh, UK, 2019. [Google Scholar] [CrossRef]
- Fatriansyah, J.F.; Rizqillah, R.K.; Yandi, M.Y. Molecular docking and molecular dynamics simulation of fisetin, galangin, hesperetin, hesperidin, myricetin, and naringenin against polymerase of dengue virus. J. Trop. Med. 2022, 2022, 7254990. [Google Scholar] [CrossRef]
- de Sousa, L.R.F.; Wu, H.; Nebo, L.; Fernandes, J.B.; Kiefer, W.; Kanitz, M.; Vieira, P.C. Flavonoids as noncompetitive inhibitors of Dengue virus NS2B-NS3 protease: Inhibition kinetics and docking studies. Bioorganic Med. Chem. 2015, 23, 466–470. [Google Scholar] [CrossRef]
- Pattnaik, A.; Palermo, N.; Sahoo, B.R.; Yuan, Z.; Hu, D.; Annamalai, A.S.; Xiang, S.H. Discovery of a non-nucleoside RNA polymerase inhibitor for blocking Zika virus replication through in silico screening. Antivir. Res. 2018, 151, 78–86. [Google Scholar] [CrossRef] [PubMed]



| Organism | PDB | Protein | Resolution (Å) | Reference |
|---|---|---|---|---|
| DENV-2 | 2BMF | NS3-helicase | 2.41 | [35] |
| 3U1I | NS3-protease | 2.30 | [36] | |
| 2P41 | NS5-methyltransferase | 1.80 | [37] | |
| 5K5M | NS5-RdRp | 2.00 | [38] | |
| ZIKV | 5K8I | NS3-helicase | 1.69 | [39] |
| 5YOF | NS3-protease | 1.51 | [40] | |
| 5WZ2 | NS5-methyltransferase | 2.60 | [41] | |
| 6LD2 | NS5-RdRp | 1.40 | [42] |
| Sample | Cytotoxicity a | |
|---|---|---|
| CC50 (µM/mL) | CC20 (µM/mL) | |
| EtOH | 2.47 | 0.641 |
| AcOEt | 2.29 | 0.293 |
| Hexanic | 10.30 | 18.76 |
| Naringenin | 241.6 | 86.43 |
| 7-O-methyl naringenin | 240.4 | 105.2 |
| Positive control (6-MMPr) | 86.00 | 17.88 |
| d# CC20 (μM/mL) | a# CC50 (μM/mL) | b# IC50 (μM/mL) | c# SI | |
|---|---|---|---|---|
| Naringenin | 86.43 | 241.6 | 58.89 | 4.1 |
| 7-O-methyl naringenin | 105.2 | 240.4 | 12.80 | 18.7 |
| 6-MMPr | 60.5 | 291 | 24.5 | 11.9 |
| d# CC20 (μM/mL) | a# CC50 (μM/mL) | b# IC50 (μM/mL) | c# SI | |
|---|---|---|---|---|
| Naringenin | 86.43 | 241.6 | 42.91 | 5.6 |
| 7-O-methyl naringenin | 105.2 | 240.4 | 30.95 | 7.7 |
| 6-MMPr | 60.5 | 291 | 18.15 | 16.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, P.G.d.; Chaves, E.J.F.; Silva, T.M.S.; Rocha, G.B.; Dantas, W.M.; Oliveira, R.N.d.; Pena, L.J. Antiviral Activity of Flavonoids from Geopropolis of the Brazilian Jandaira Bee against Zika and Dengue Viruses. Pharmaceutics 2023, 15, 2494. https://doi.org/10.3390/pharmaceutics15102494
Silva PGd, Chaves EJF, Silva TMS, Rocha GB, Dantas WM, Oliveira RNd, Pena LJ. Antiviral Activity of Flavonoids from Geopropolis of the Brazilian Jandaira Bee against Zika and Dengue Viruses. Pharmaceutics. 2023; 15(10):2494. https://doi.org/10.3390/pharmaceutics15102494
Chicago/Turabian StyleSilva, Poliana Gomes da, Elton José Ferreira Chaves, Tania Maria Sarmento Silva, Gerd Bruno Rocha, Willyenne Marília Dantas, Ronaldo Nascimento de Oliveira, and Lindomar José Pena. 2023. "Antiviral Activity of Flavonoids from Geopropolis of the Brazilian Jandaira Bee against Zika and Dengue Viruses" Pharmaceutics 15, no. 10: 2494. https://doi.org/10.3390/pharmaceutics15102494






