Natural Polymer-Based Hydrogels: From Polymer to Biomedical Applications
Abstract
:1. Introduction
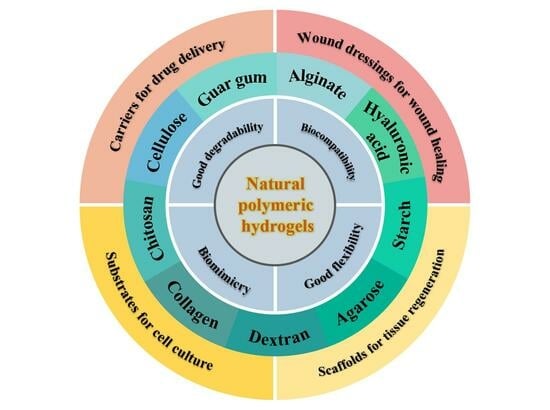
2. Natural Polymer-Based Hydrogels
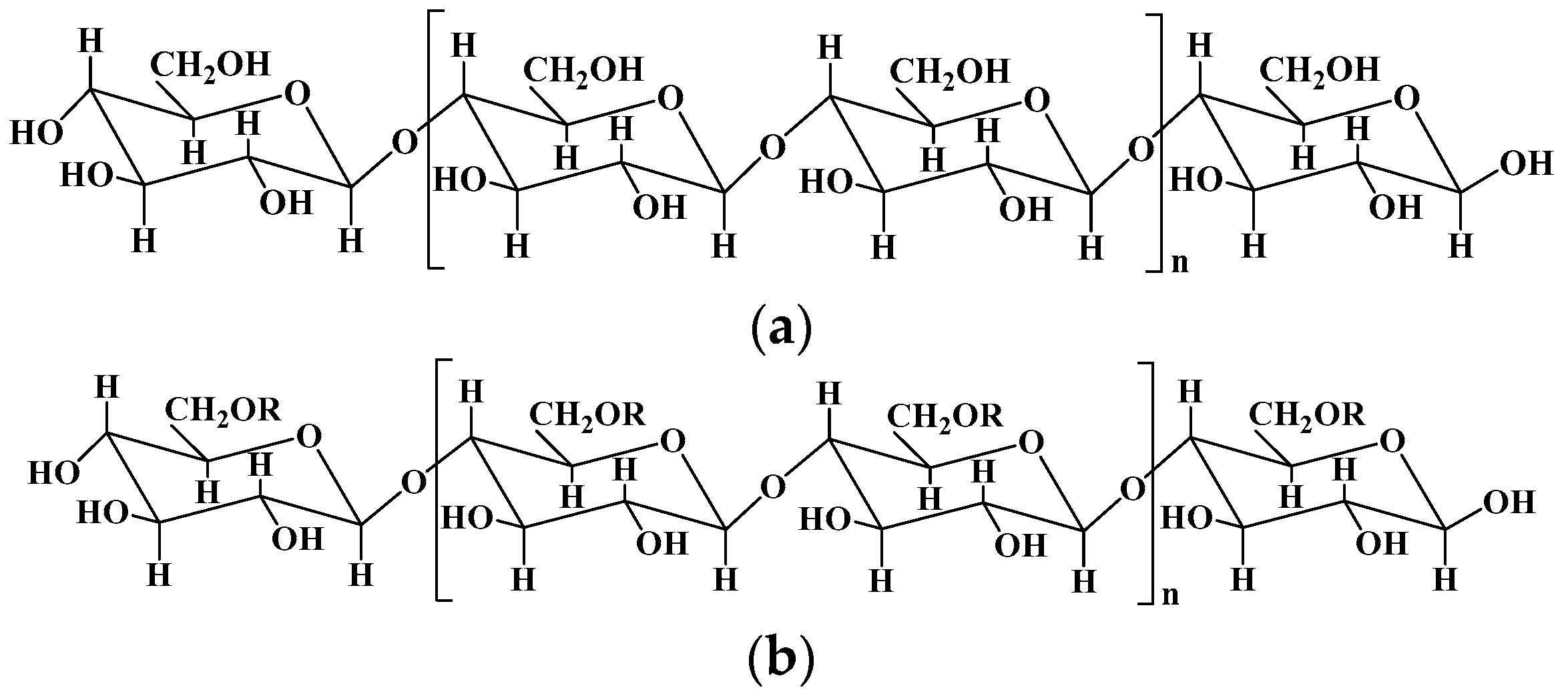
2.1. Cellulose and Cellulose-Based Hydrogels
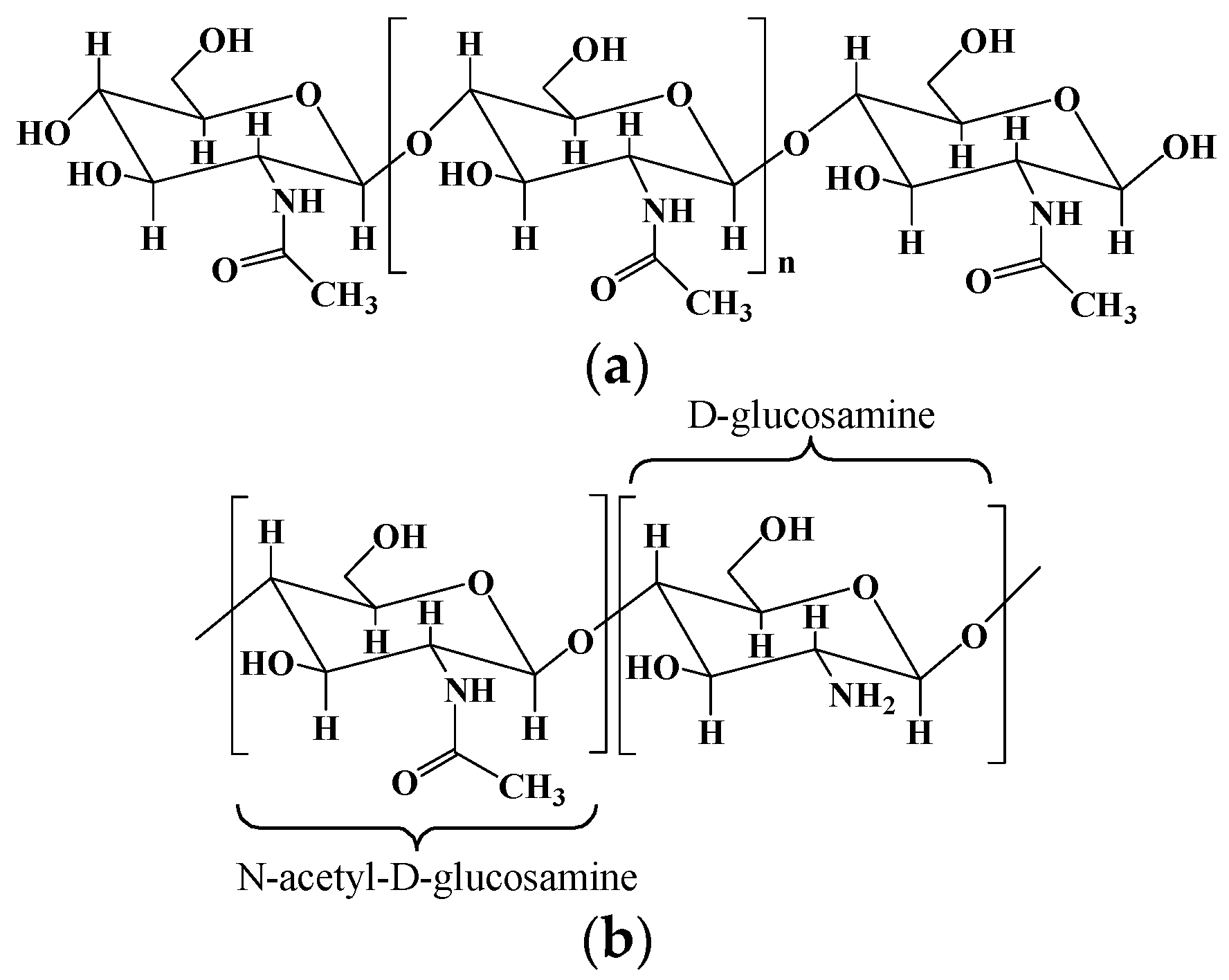
2.2. Chitosan and Chitosan-Based Hydrogels
2.3. Collagen/Gelatin and Collagen/Gelatin-Based Hydrogel
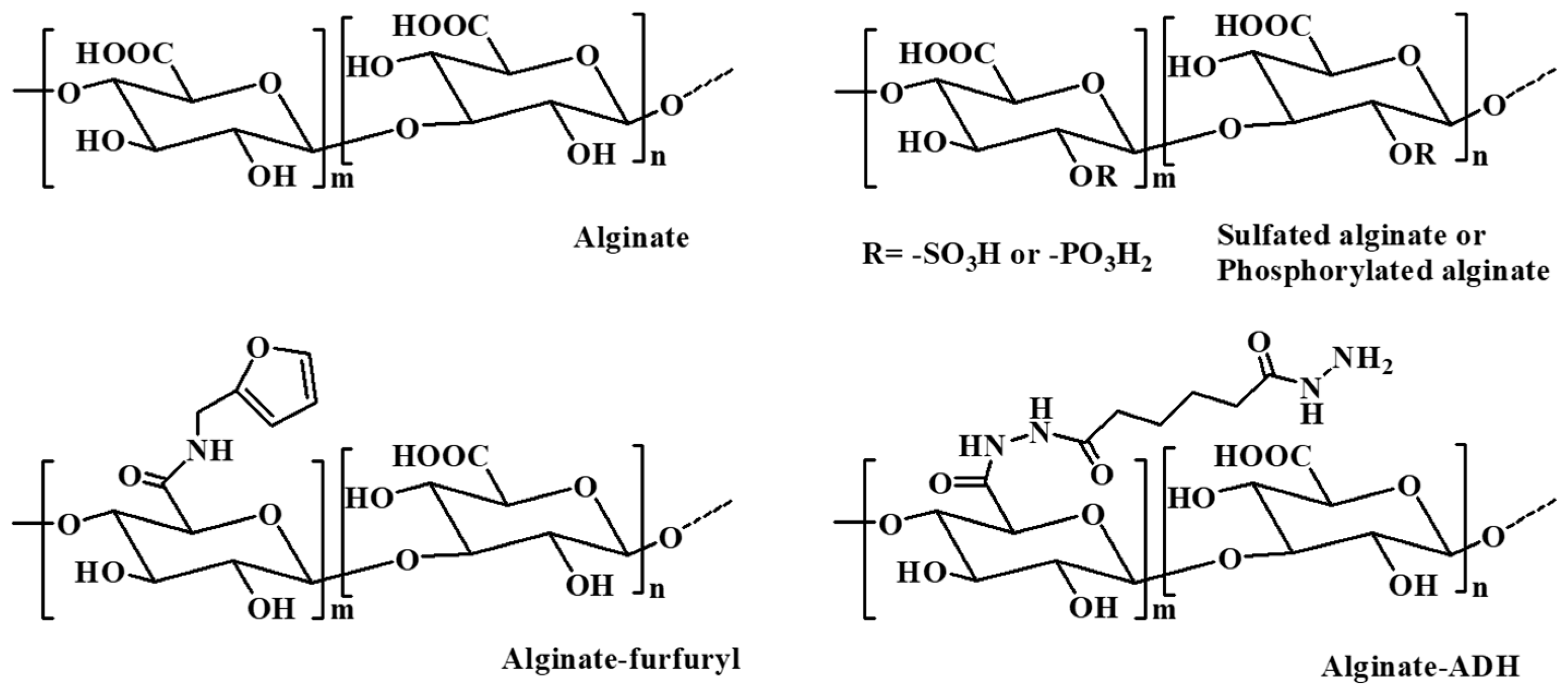
2.4. Alginate and Alginate-Based Hydrogel
2.5. Hyaluronic Acid and Hyaluronic Acid-Based Hydrogel
2.6. Starch and Starch-Based Hydrogel
2.7. Guar Gum and Guar Gum-Based Hydrogel
2.8. Agarose and Agarose-Based Hydrogel
2.9. Dextran and Dextran-Based Hydrogel
3. Natural Polymer-Based Hydrogels for Biomedical Applications
3.1. Drug Carriers for Drug Delivery
3.2. Wound Dressings for Wound Healing
3.3. Scaffolds for Regenerative Medicine
3.4. Other Biomedical Applications
4. Future Perspectives
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Klein, M.; Poverenov, E. Natural biopolymer-based hydrogels for use in food and agriculture. J. Sci. Food Agric. 2020, 100, 2337–2347. [Google Scholar] [CrossRef] [PubMed]
- Bao, Z.; Xian, C.; Yuan, Q.; Liu, G.; Wu, J. Natural polymer-based hydrogels with enhanced mechanical performances: Preparation, structure, and property. Adv. Healthc. Mater. 2019, 8, 1900670. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Huang, Y. Rational design of smart hydrogels for biomedical applications. Front. Chem. 2021, 8, 615665. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Sood, A.; Agrawal, G.; Thakur, S.; Thakur, V.K.; Tanaka, M.; Mishra, Y.K.; Christie, G.; Mostafavi, E.; Boukherroub, R.; et al. Polysaccharides, proteins, and synthetic polymers based multimodal hydrogels for various biomedical applications: A review. Int. J. Biol. Macromol. 2023, 247, 125606. [Google Scholar] [CrossRef] [PubMed]
- Calo, E.; Khutoryanskiy, V.V. Biomedical applications of hydrogels: A review of patents and commercial products. Eur. Polym. J. 2015, 65, 252–267. [Google Scholar] [CrossRef]
- Varaprasad, K.; Vimala, K.; Raghavendra, G.M.; Jayaramudu, T.; Sadiku, E.R.; Ramam, K. Chapter 10–Cell Encapsulation in Polymeric Self-Assembled Hydrogels. In Nanotechnology Applications for Tissue Engineering; Thomas, S., Grohens, Y., Ninan, N., Eds.; William Andrew Publishing: New York, NY, USA, 2015; pp. 149–171. [Google Scholar]
- Goswami, P.; O’Haire, T. 3–Developments in the use of green (biodegradable), recycled and biopolymer materials in technical nonwovens. In Advances in Technical Nonwovens; Kellie, G., Ed.; Woodhead Publishing: Cambridge, UK, 2016; pp. 97–114. [Google Scholar]
- Ghalia, M.A.; Dahman, Y. Chapter 6–Advanced nanobiomaterials in tissue engineering: Synthesis, properties, and applications. In Nanobiomaterials in Soft Tissue Engineering; Grumezescu, A.M., Ed.; William Andrew Publishing: Oxford, UK, 2016; pp. 141–172. [Google Scholar]
- Abdelraof, M.; Hasanin, M.S.; El-Saied, H. Ecofriendly green conversion of potato peel wastes to high productivity bacterial cellulose. Carbohydr. Polym. 2019, 211, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Qiu, P.; Wang, Y.; Wang, Y.; Zhou, J.; Zhang, B.; Zhang, L.; Gou, D. Chitosan-based hydrogel wound dressing: From mechanism to applications, a review. Int. J. Biol. Macromol. 2023, 244, 125250. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Zharkinbekov, Z.; Raziyeva, K.; Tabyldiyeva, L.; Berikova, K.; Zhumagul, D.; Temirkhanova, K.; Saparov, A. Chitosan-based biomaterials for tissue regeneration. Pharmaceutics 2023, 15, 807. [Google Scholar] [CrossRef]
- Miranda-Nieves, D.; Chaikof, E.L. Collagen and Elastin Biomaterials for the Fabrication of Engineered Living Tissues. ACS Biomater. Sci. Eng. 2017, 3, 694–711. [Google Scholar] [CrossRef]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and biomedical applications. Prog. Polym. Sci. 2012, 37, 106–126. [Google Scholar] [CrossRef]
- Hussain, Z.; Thu, H.E.; Katas, H.; Bukhari, S.N.A. Hyaluronic Acid-Based Biomaterials: A Versatile and Smart Approach to Tissue Regeneration and Treating Traumatic, Surgical, and Chronic Wounds. Polym. Rev. 2017, 57, 594–630. [Google Scholar] [CrossRef]
- Cui, C.; Jia, Y.; Sun, Q.; Yu, M.; Ji, N.; Dai, L.; Wang, Y.; Qin, Y.; Xiong, L. Recent advances in the preparation, characterization, and food application of starch-based hydrogels. Carbohydr. Polym. 2022, 291, 119624. [Google Scholar] [CrossRef] [PubMed]
- Verma, D.; Sharma, S.K. Recent advances in guar gum based drug delivery systems and their administrative routes. Int. J. Biol. Macromol. 2021, 181, 653–671. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.K.; Xu, Z.W.; Yang, X.; Li, M.; Yip, R.C.S.; Li, Y.Y.; Chen, H. Current application and modification strategy of marine polysaccharides in tissue regeneration: A review. Biomater. Adv. 2023, 154, 213580. [Google Scholar] [CrossRef] [PubMed]
- Heinze, T.; Liebert, T.; Heublein, B.; Hornig, S. Functional polymers based on dextran. In Polysaccharides II; Klemm, D., Ed.; Springer International Publishing: Cham, Switzerland, 2006; pp. 199–291. [Google Scholar]
- Hu, Q.; Lu, Y.; Luo, Y. Recent advances in dextran-based drug delivery systems: From fabrication strategies to applications. Carbohydr. Polym. 2021, 264, 117999. [Google Scholar] [CrossRef] [PubMed]
- Ahn, W.; Lee, J.H.; Kim, S.R.; Lee, J.; Lee, E.J. Designed protein- and peptide-based hydrogels for biomedical sciences. J. Mater. Chem. B 2021, 9, 1919–1940. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Tang, F.; Li, M.; Li, L. Facile synthesis of Ag@AgCl-contained cellulose hydrogels and their application. Colloids Surface A 2018, 553, 618–623. [Google Scholar] [CrossRef]
- Zou, P.; Yao, J.; Cui, Y.N.; Zhao, T.; Che, J.; Yang, M.; Li, Z.; Gao, C. Advances in cellulose-based hydrogels for biomedical engineering: A review summary. Gels 2022, 8, 364. [Google Scholar] [CrossRef]
- Rahman, A.; Wang, W.; Govindaraj, D.; Kang, S.; Vikesland, P.J. Recent advances in environmental science and engineering applications of cellulose nanocomposites. Crit. Rev. Environ. Sci. Technol. 2023, 53, 650–675. [Google Scholar] [CrossRef]
- Hasanin, M.S.; Mostafa, A.M.; Mwafy, E.A.; Darwesh, O.M. Eco-friendly cellulose nano fibers via first reported Egyptian Humicola fuscoatra Egyptia X4: Isolation and characterization. Environ. Nanotechnol. Monit. Manag. 2018, 10, 409–418. [Google Scholar] [CrossRef]
- Qi, Y.E.; Guo, Y.Z.; Liza, A.A.; Yang, G.H.; Sipponen, M.H.; Guo, J.Q.; Li, H.M. Nanocellulose: A review on preparation routes and applications in functional materials. Cellulose 2023, 30, 4115–4147. [Google Scholar] [CrossRef]
- Zhang, M.; Du, H.; Liu, K.; Nie, S.; Xu, T.; Zhang, X.; Si, C. Fabrication and applications of cellulose-based nanogenerators. Adv. Compos. Hybrid Mater. 2021, 4, 865–884. [Google Scholar] [CrossRef]
- Erfanian, E.; Moaref, R.; Ajdary, R.; Tam, K.C.; Rojas, O.J.; Kamkar, M.; Sundararaj, U. Electrochemically synthesized graphene/TEMPO-oxidized cellulose nanofibrils hydrogels: Highly conductive green inks for 3D printing of robust structured EMI shielding aerogels. Carbon 2023, 210, 118037. [Google Scholar] [CrossRef]
- Li, B.; Chen, Y.; Wu, W.; Cao, X.; Luo, Z. Copolymer-grafted cellulose nanocrystal induced nanocomposite hydrogels with enhanced strength, high elasticity and adhesiveness for flexible strain and pressure sensors. Carbohydr. Polym. 2023, 317, 121092. [Google Scholar] [CrossRef] [PubMed]
- Taheri, N.; Abdolmaleki, A.; Fashandi, H. Pyridinium-based ionic liquid/water mixture intended for efficient dissolution of cellulose, chitosan and chitin: The pivotal contribution of water. Carbohydr. Polym. 2018, 195, 413–419. [Google Scholar] [CrossRef]
- Wong, L.C.; Leh, C.P.; Goh, C.F. Designing cellulose hydrogels from non-woody biomass. Carbohydr. Polym. 2021, 264, 118036. [Google Scholar] [CrossRef] [PubMed]
- Ostlund, A.; Lundberg, D.; Nordstierna, L.; Holmberg, K.; Nyden, M. Dissolution and Gelation of Cellulose in TBAF/DMSO Solutions: The Roles of Fluoride Ions and Water. Biomacromolecules 2009, 10, 2401–2407. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wu, J.; Zhang, J.; He, J.S. 1-Allyl-3-methylimidazolium chloride room temperature ionic liquid: A new and powerful nonderivatizing solvent for cellulose. Macromolecules 2005, 38, 8272–8277. [Google Scholar] [CrossRef]
- Kang, H.; Liu, R.; Huang, Y. Cellulose-based gels. Macromol. Chem. Phys. 2016, 217, 1322–1334. [Google Scholar] [CrossRef]
- Shi, H.; Deng, Y.; Shi, Y. Cellulose-based stimuli-responsive anisotropic hydrogel for sensor applications. ACS Appl. Nano Mater. 2023, 6, 11524–11530. [Google Scholar] [CrossRef]
- Guilherme, M.R.; Aouada, F.A.; Fajardo, A.R.; Martins, A.F.; Paulino, A.T.; Davi, M.F.T.; Rubira, A.F.; Muniz, E.C. Superabsorbent hydrogels based on polysaccharides for application in agriculture as soil conditioner and nutrient carrier: A review. Eur. Polym. J. 2015, 72, 365–385. [Google Scholar] [CrossRef]
- Lu, Q.; Zhang, S.; Xiong, M.; Lin, F.; Tang, L.; Huang, B.; Chen, Y. One-pot construction of cellulose-gelatin supramolecular hydrogels with high strength and pH-responsive properties. Carbohydr. Polym. 2018, 196, 225–232. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Chen, S.; Wu, Z.; Han, Z.; Qu, X.; Jin, M.; Jia, Y.; Zhou, Z.; Wang, H. Bacterial cellulose hydrogel-based wearable thermo-electrochemical cells for continuous body heat harvest. Nano Energy 2023, 112, 108482. [Google Scholar] [CrossRef]
- Peers, S.; Montembault, A.; Ladaviere, C. Chitosan hydrogels for sustained drug delivery. J. Control. Release 2020, 326, 150–163. [Google Scholar] [CrossRef] [PubMed]
- Elsabee, M.Z.; Abdou, E.S. Chitosan based edible films and coatings: A review. Mater. Sci. Eng. C 2013, 33, 1819–1841. [Google Scholar] [CrossRef]
- Islam, M.M.; Shahruzzaman, M.; Biswas, S.; Sakib, M.N.; Rashid, T.U. Chitosan based bioactive materials in tissue engineering applications—A review. Bioact. Mater. 2020, 5, 164–183. [Google Scholar] [CrossRef] [PubMed]
- Troy, E.; Tilbury, M.A.; Power, A.M.; Wall, J.G. Nature-based biomaterials and their application in biomedicine. Polymers 2021, 13, 3321. [Google Scholar] [CrossRef] [PubMed]
- Dash, M.; Chiellini, F.; Ottenbrite, R.M.; Chiellini, E. Chitosan-A versatile semi-synthetic polymer in biomedical applications. Prog. Polym. Sci. 2011, 36, 981–1014. [Google Scholar] [CrossRef]
- Bhattarai, N.; Gunn, J.; Zhang, M. Chitosan-based hydrogels for controlled, localized drug delivery. Adv. Drug Deliv. Rev. 2010, 62, 83–99. [Google Scholar] [CrossRef]
- Thirupathi, K.; Raorane, C.J.; Ramkumar, V.; Ulagesan, S.; Santhamoorthy, M.; Raj, V.; Krishnakumar, G.S.; Phan, T.T.V.; Kim, S.C. Update on chitosan-based hydrogels: Preparation, characterization, and its antimicrobial and antibiofilm applications. Gels 2023, 9, 35. [Google Scholar] [CrossRef]
- Tian, B.; Liu, J. Smart stimuli-responsive chitosan hydrogel for drug delivery: A review. Int. J. Biol. Macromol. 2023, 235, 123902. [Google Scholar] [CrossRef]
- Xu, C.H.; Zhan, W.; Tang, X.Z.; Mo, F.; Fu, L.H.; Lin, B.F. Self-healing chitosan/vanillin hydrogels based on Schiff-base bond/hydrogen bond hybrid linkages. Polym. Test. 2018, 66, 155–163. [Google Scholar] [CrossRef]
- Lee, J.S.; Nah, H.; Moon, H.J.; Lee, S.J.; Heo, D.N.; Kwon, I.K. Controllable delivery system: A temperature and pH-responsive injectable hydrogel from succinylated chitosan. Appl. Surf. Sci. 2020, 528, 146812. [Google Scholar] [CrossRef]
- Lee, J.S.; Kim, H.S.; Nah, H.; Lee, S.J.; Moon, H.J.; Bang, J.B.; Lee, J.B.; Do, S.H.; Kwon, I.K.; Heo, D.N. The Effectiveness of Compartmentalized Bone Graft Sponges Made Using Complementary Bone Graft Materials and Succinylated Chitosan Hydrogels. Biomedicines 2021, 9, 1765. [Google Scholar] [CrossRef] [PubMed]
- Sukpaita, T.; Chirachanchai, S.; Pimkhaokham, A.; Ampornaramveth, R.S. Chitosan-Based Scaffold for Mineralized Tissues Regeneration. Mar. Drugs 2021, 19, 551. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Dong, Z.; Ding, Z.; Hu, Z.; Yu, D.; Hu, Y.; Abidi, N.; Li, W. Electroresponsive Homogeneous Polyelectrolyte Complex Hydrogels from Naturally Derived Polysaccharides. ACS Sustain. Chem. Eng. 2018, 6, 7052–7063. [Google Scholar] [CrossRef]
- Zhang, D.; Wu, X.; Chen, J.; Lin, K. The development of collagen based composite scaffolds for bone regeneration. Bioact. Mater. 2018, 3, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Valentino, C.; Vigani, B.; Zucca, G.; Ruggeri, M.; Boselli, C.; Cornaglia, A.I.; Malavasi, L.; Sandri, G.; Rossi, S. Formulation development of collagen/chitosan-based porous scaffolds for skin wounds repair and regeneration. Int. J. Biol. Macromol. 2023, 242, 125000. [Google Scholar] [CrossRef] [PubMed]
- Gajbhiye, S.; Wairkar, S. Collagen fabricated delivery systems for wound healing: A new roadmap. Biomater. Adv. 2022, 142, 213152. [Google Scholar] [CrossRef]
- Copes, F.; Pien, N.; Van Vlierberghe, S.; Boccafoschi, F.; Mantovani, D. Collagen-Based Tissue Engineering Strategies for Vascular Medicine. Front. Bioeng. Biotechnol. 2019, 7, 166. [Google Scholar] [CrossRef]
- Montalbano, G.; Toumpaniari, S.; Popov, A.; Duan, P.; Chen, J.; Dalgarno, K.; Scott, W.E., 3rd; Ferreira, A.M. Synthesis of bioinspired collagen/alginate/fibrin based hydrogels for soft tissue engineering. Mater. Sci. Eng. C-Mater. 2018, 91, 236–246. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, A.M.; Gentile, P.; Chiono, V.; Ciardelli, G. Collagen for bone tissue regeneration. Acta Biomater. 2012, 8, 3191–3200. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Fan, D.; Shen, S.; Ma, X. An M2 macrophage-polarized anti-inflammatory hydrogel combined with mild heat stimulation for regulating chronic inflammation and impaired angiogenesis of diabetic wounds. Chem. Eng. J. 2022, 433, 133859. [Google Scholar] [CrossRef]
- Akilbekova, D.; Shaimerdenova, M.; Adilov, S.; Berillo, D. Biocompatible scaffolds based on natural polymers for regenerative medicine. Int. J. Biol. Macromol. 2018, 114, 324–333. [Google Scholar] [CrossRef] [PubMed]
- Guillen-Carvajal, K.; Valdez-Salas, B.; Beltran-Partida, E.; Salomon-Carlos, J.; Cheng, N. Chitosan, Gelatin, and Collagen Hydrogels for Bone Regeneration. Polymers 2023, 15, 2762. [Google Scholar] [CrossRef]
- Bohidar, H.B.; Jena, S.S. Kinetics of sol-gel transition in thermoreversible gelation of gelatin. J. Chem. Phys. 1993, 98, 8970–8977. [Google Scholar] [CrossRef]
- Smith, M.L.; Heitfeld, K.; Slone, C.; Vaia, R.A. Autonomic Hydrogels through Postfunctionalization of Gelatin. Chem. Mater. 2012, 24, 3074–3080. [Google Scholar] [CrossRef]
- Mad-Ali, S.; Benjakul, S.; Prodpran, T.; Maqsood, S. Characteristics and gelling properties of gelatin from goat skin as affected by drying methods. J. Food Sci. Technol. 2017, 54, 1646–1654. [Google Scholar] [CrossRef]
- Scherzer, T.; Beckert, A.; Langguth, H.; Rummel, S.; Mehnert, R. Electron beam curing of methacrylated gelatin. 1. Dependence of the degree of crosslinking on the irradiation dose. J. Appl. Polym. Sci. 1997, 63, 1303–1312. [Google Scholar] [CrossRef]
- Kurian, A.G.; Singh, R.K.; Patel, K.D.; Lee, J.H.; Kim, H.W. Multifunctional GelMA platforms with nanomaterials for advanced tissue therapeutics. Bioact. Mater. 2022, 8, 267–295. [Google Scholar] [CrossRef]
- Bupphathong, S.; Quiroz, C.; Huang, W.; Chung, P.F.; Tao, H.Y.; Lin, C.H. Gelatin Methacrylate Hydrogel for Tissue Engineering Applications—A Review on Material Modifications. Pharmaceuticals 2022, 15, 171. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.; Luo, Y.; Guo, Y.; Zhou, Y.; Liao, X.; Li, D.; Lai, X.; Liu, Y. Development of alginate-based hydrogels: Crosslinking strategies and biomedical applications. Int. J. Biol. Macromol. 2023, 239, 124275. [Google Scholar] [CrossRef] [PubMed]
- Silva, R.; Singh, R.; Sarker, B.; Papageorgiou, D.G.; Juhasz-Bortuzzo, J.A.; Roether, J.A.; Cicha, I.; Kaschta, J.; Schubert, D.W.; Chrissafis, K.; et al. Hydrogel matrices based on elastin and alginate for tissue engineering applications. Int. J. Biol. Macromol. 2018, 114, 614–625. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Xu, Z.; Wang, J.; Pei, X.; Chen, J.; Wan, Q. Alginate-based biomaterial-mediated regulation of macrophages in bone tissue engineering. Int. J. Biol. Macromol. 2023, 230, 123246. [Google Scholar] [CrossRef] [PubMed]
- Tonnesen, H.H.; Karlsen, J. Alginate in drug delivery systems. Drug Dev. Ind. Pharm. 2002, 28, 621–630. [Google Scholar] [CrossRef] [PubMed]
- Chandel, A.K.S.; Shimizu, A.; Hasegawa, K.; Ito, T. Advancement of Biomaterial-Based Postoperative Adhesion Barriers. Macromol. Biosci. 2021, 21, 2000395. [Google Scholar] [CrossRef] [PubMed]
- Ohta, S.; Toda, T.; Inagaki, F.; Omichi, K.; Shimizu, A.; Kokudo, N.; Hasegawa, K.; Ito, T. The Prevention of Hepatectomy-Induced Adhesions by Bilayer Sponge Composed of Ultrapure Alginate. J. Surg. Res. 2019, 242, 286–295. [Google Scholar] [CrossRef] [PubMed]
- Chandel, A.K.S.; Ohta, S.; Taniguchi, M.; Yoshida, H.; Tanaka, D.; Omichi, K.; Shimizu, A.; Isaji, M.; Hasegawa, K.; Ito, T. Balance of antiperitoneal adhesion, hemostasis, and operability of compressed bilayer ultrapure alginate sponges. Biomater. Adv. 2022, 137, 212825. [Google Scholar]
- Li, G.; Zhang, G.; Sun, R.; Wong, C.P. Mechanical strengthened alginate/polyacrylamide hydrogel crosslinked by barium and ferric dual ions. J. Mater. Sci. 2017, 52, 8538–8545. [Google Scholar] [CrossRef]
- Wang, Q.Q.; Liu, Y.; Zhang, C.J.; Zhang, C.; Zhu, P. Alginate/gelatin blended hydrogel fibers cross-linked by Ca2+ and oxidized starch: Preparation and properties. Mater. Sci. Eng. C-Mater. 2019, 99, 1469–1476. [Google Scholar] [CrossRef]
- Kim, M.; Cha, C. Graft Architecture Guided Simultaneous Control of Degradation and Mechanical Properties of In Situ Forming and Fast Dissolving Polyaspartamide Hydrogels. Biomacromolecules 2020, 21, 3693–3703. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.Y.; Bouhadir, K.H.; Mooney, D.J. Controlled degradation of hydrogels using multi-functional cross-linking molecules. Biomaterials 2004, 25, 2461–2466. [Google Scholar] [CrossRef] [PubMed]
- Chalanqui, M.J.; Pentlavalli, S.; McCrudden, C.; Chambers, P.; Ziminska, M.; Dunne, N.; McCarthy, H.O. Influence of alginate backbone on efficacy of thermo-responsive alginate-g-P (NIPAAm) hydrogel as a vehicle for sustained and controlled gene delivery. Mater. Sci. Eng. C-Mater. 2019, 95, 409–421. [Google Scholar] [CrossRef] [PubMed]
- Shi, G.; Ding, Y.; Zhang, X.; Wu, L.; He, F.; Ni, C. Drug release behavior of poly (lactic-glycolic acid) grafting from sodium alginate (ALG-g-PLGA) prepared by direct polycondensation. J. Biomater. Sci.-Polym. Ed. 2015, 26, 1152–1162. [Google Scholar] [CrossRef] [PubMed]
- Anitha, A.; Fletcher, N.L.; Houston, Z.H.; Thurecht, K.J.; Grondahl, L. Evaluation of the in vivo fate of ultrapure alginate in a BALB/c mouse model. Carbohydr. Polym. 2021, 262, 117947. [Google Scholar]
- Hay, I.D.; Rehman, Z.U.; Ghafoor, A.; Rehm, B.H.A. Bacterial biosynthesis of alginates. J. Chem. Technol. Biotechnol. 2010, 85, 752–759. [Google Scholar] [CrossRef]
- Alshamkhani, A.; Duncan, R. Radioiodination of alginate via covalently-bound tyrosinamide allows monitoring of tis fate in vivo. J. Bioact. Compat. Polym. 1995, 10, 4–13. [Google Scholar] [CrossRef]
- Bouhadir, K.H.; Lee, K.Y.; Alsberg, E.; Damm, K.L.; Anderson, K.W.; Mooney, D.J. Degradation of partially oxidized alginate and its potential application for tissue engineering. Biotechnol. Prog. 2001, 17, 945–950. [Google Scholar] [CrossRef]
- Leor, J.; Tuvia, S.; Guetta, V.; Manczur, F.; Castel, D.; Willenz, U.; Petneházy, Ö.; Landa, N.; Feinberg, M.S.; Konen, E.; et al. Intracoronary Injection of In Situ Forming Alginate Hydrogel Reverses Left Ventricular Remodeling After Myocardial Infarction in Swine. J. Am. Coll. Cardiol. 2009, 54, 1014–1023. [Google Scholar] [CrossRef]
- Landa, N.; Miller, L.; Feinberg, M.S.; Holbova, R.; Shachar, M.; Freeman, I.; Cohen, S.; Leor, J. Effect of injectable alginate implant on cardiac remodeling and function after recent and old infarcts in rat. Circulation 2008, 117, 1388–1396. [Google Scholar] [CrossRef]
- Follin, B.; Juhl, M.; Cohen, S.; Pedersen, A.E.; Gad, M.; Kastrup, J.; Ekblond, A. Human adipose-derived stromal cells in a clinically applicable injectable alginate hydrogel: Phenotypic and immunomodulatory evaluation. Cytotherapy 2015, 17, 1104–1118. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.H.; Kim, S.; Park, J.P.; Shin, M.; Kim, K.; Ryu, J.H.; Lee, H. Dynamic Bonds between Boronic Acid and Alginate: Hydrogels with Stretchable, Self-Healing, Stimuli-Responsive, Remoldable, and Adhesive Properties. Biomacromolecules 2018, 19, 2053–2061. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.Y.; Chen, Y.; Zhao, L.; Zhang, J.H.; Luo, H. Constructions and Properties of Physically Cross-Linked Hydrogels Based on Natural Polymers. Polym. Rev. 2022, 63, 574–612. [Google Scholar] [CrossRef]
- Hu, R.; Zheng, H.; Cao, J.; Davoudi, Z.; Wang, Q. Self-Assembled Hyaluronic Acid Nanoparticles for pH-Sensitive Release of Doxorubicin: Synthesis and In Vitro Characterization. J. Biomed. Nanotechnol. 2017, 13, 1058–1068. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Liu, Y.; Zheng, C.; Huo, Q.; Liu, X. Development of novel hyaluronic acid/human-like collagen bio-composite membranes: A facile “surface modification-assembly” approach. Int. J. Biol. Macromol. 2021, 193, 378–386. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Song, L.; Zou, Y.; Sun, D.; Wang, L.; Yu, Z.; Guo, J. Role of Hyaluronic Acids and Potential as Regenerative Biomaterials in Wound Healing. ACS Appl. Bio Mater. 2021, 4, 311–324. [Google Scholar] [CrossRef] [PubMed]
- Jensen, G.; Holloway, J.L.; Stabenfeldt, S.E. Hyaluronic Acid Biomaterials for Central Nervous System Regenerative Medicine. Cells 2020, 9, 2113. [Google Scholar] [CrossRef] [PubMed]
- Gottschalk, J.; Assmann, M.; Kuballa, J.; Elling, L. Repetitive Synthesis of High-Molecular-Weight Hyaluronic Acid with Immobilized Enzyme Cascades. ChemSusChem 2022, 15, e202101071. [Google Scholar] [CrossRef]
- Zhu, Q.; Jiang, M.; Liu, Q.; Yan, S.; Feng, L.; Lan, Y.; Shan, G.; Xue, W.; Guo, R. Enhanced healing activity of burn wound infection by a dextran-HA hydrogel enriched with sanguinarine. Biomater. Sci. 2018, 6, 2472–2486. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, J.; Cui, W.; Fang, Y.; Li, L.; Ji, S.; Mao, D.; Ke, T.; Yao, X.; Ding, D.; et al. Composite Hydrogel Modified by IGF-1C Domain Improves Stem Cell Therapy for Limb Ischemia. ACS Appl. Mater. Interfaces 2018, 10, 4481–4493. [Google Scholar] [CrossRef]
- Hosseinzadeh, B.; Ahmadi, M. Degradable hydrogels: Design mechanisms and versatile applications. Mater. Today Sustain. 2023, 23, 100468. [Google Scholar] [CrossRef]
- Chung, E.J.; Choi, J.S.; Shin, J.; Cho, H.N.; Kim, S.; Park, J.Y.; Lee, Y.S.; Kim, Y.I.; Wu, H.G.; Cho, S.W.; et al. Prevention of irradiation-induced damage to salivary glands by local delivery of adipose-derived stem cells via hyaluronic acid-based hydrogels. J. Ind. Eng. Chem. 2020, 90, 47–57. [Google Scholar] [CrossRef]
- Kadokawa, J.; Shoji, T.; Yamamoto, K. Preparation of supramolecular network materials by means of amylose helical assemblies. Polymer 2018, 140, 73–79. [Google Scholar] [CrossRef]
- Qamruzzaman, M.; Ahmed, F.; Mondal, M.I.H. An Overview on Starch-Based Sustainable Hydrogels: Potential Applications and Aspects. J. Polym. Environ. 2021, 30, 19–50. [Google Scholar] [CrossRef]
- Chen, X.; Ren, Y.; Cai, Y.; Huang, X.; Zhou, L.; Ai, Y.; Jiang, B. Interactions between exogenous free fatty acids and maize starches varying in amylose content at high heating temperatures. Food Hydrocoll. 2023, 143, 108855. [Google Scholar] [CrossRef]
- Guo, P.; Li, Y.; An, J.; Shen, S.; Dou, H. Study on structure-function of starch by asymmetrical flow field-flow fractionation coupled with multiple detectors: A review. Carbohydr. Polym. 2019, 226, 115330. [Google Scholar] [CrossRef] [PubMed]
- Biduski, B.; da Silva, W.M.F.; Colussi, R.; El Halal, S.L.D.; Lim, L.T.; Dias, A.R.G.; Zavareze, E.D. Starch hydrogels: The influence of the amylose content and gelatinization method. Int. J. Biol. Macromol. 2018, 113, 443–449. [Google Scholar] [CrossRef]
- Olad, A.; Doustdar, F.; Gharekhani, H. Starch-based semi-IPN hydrogel nanocomposite integrated with clinoptilolite: Preparation and swelling kinetic study. Carbohydr. Polym. 2018, 200, 516–528. [Google Scholar] [CrossRef]
- Maniglia, B.C.; Lima, D.C.; Matta, M.D.; Le-Bail, P.; Le-Bail, A.; Augusto, P.E.D. Hydrogels based on ozonated cassava starch: Effect of ozone processing and gelatinization conditions on enhancing 3D-printing applications. Int. J. Biol. Macromol. 2019, 138, 1087–1097. [Google Scholar] [CrossRef]
- Mendes, J.F.; Paschoalin, R.T.; Carmona, V.B.; Sena Neto, A.R.; Marques, A.C.P.; Marconcini, M.; Mattoso, L.H.C.; Medeiros, E.S.; Oliveira, J.E. Biodegradable polymer blends based on corn starch and thermoplastic chitosan processed by extrusion. Carbohydr. Polym. 2016, 137, 452–458. [Google Scholar] [CrossRef]
- Moghadam, M.; Dorraji, M.S.S.; Dodangeh, F.; Ashjari, H.R.; Mousavi, S.N.; Rasoulifard, M.H. Design of a new light curable starch-based hydrogel drug delivery system to improve the release rate of quercetin as a poorly water-soluble drug. Eur. J. Pharm. Sci. 2022, 174, 106191. [Google Scholar] [CrossRef] [PubMed]
- Zarbab, A.; Sajjad, A.; Rasul, A.; Jabeen, F.; Iqbal, M.J. Synthesis and characterization of Guar gum based biopolymeric hydrogels as carrier materials for controlled delivery of methotrexate to treat colon cancer. Saudi J. Biol. Sci. 2023, 30, 103731. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Liu, C.J.; Chen, W. Facile Access to Guar Gum Based Supramolecular Hydrogels with Rapid Self-Healing Ability and Multistimuli Responsive Gel-Sol Transitions. J. Agric. Food Chem. 2019, 67, 746–752. [Google Scholar] [CrossRef] [PubMed]
- Prabaharan, M.; Sathya Seeli, D. 10–Progress of guar gum-based biomaterials as drug delivery carriers. In Natural Biopolymers in Drug Delivery and Tissue Engineering; Jayakumar, R., Murali, V.P., Eds.; Woodhead Publishing Series in Biomaterials: Cambridge, UK, 2023; pp. 2239–2255. [Google Scholar]
- Pak, S.; Chen, F. Functional Enhancement of Guar Gum−Based Hydrogel by Polydopamine and Nanocellulose. Foods 2023, 12, 1304. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Sun, X.Y.; Wang, J.; Zhang, Y.L.; Dong, M.N.; Bu, T.; Li, L.H.; Liu, Y.N.; Wang, L. Multifunctional Injectable Hydrogel Dressings for Effectively Accelerating Wound Healing: Enhancing Biomineralization Strategy. Adv. Funct. Mater. 2021, 31, 2100093. [Google Scholar] [CrossRef]
- Rao, Z.L.; Liu, S.M.; Wu, R.Y.; Wang, G.L.; Sun, Z.X.; Bai, L.J.; Wang, W.X.; Chen, H.; Yang, H.W.; Wei, D.L.; et al. Fabrication of dual network self-healing alginate/guar gum hydrogels based on polydopamine-type microcapsules from mesoporous silica nanoparticles. Int. J. Biol. Macromol. 2019, 129, 916–926. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Afgan, S.; Deepak; Kumar, A.; Kumar, R. L-Alanine induced thermally stable self-healing guar gum hydrogel as potential drug vehicle for sustained release of hydrophilic drug. Mater. Sci. Eng. C-Mater. 2019, 99, 1384–1391. [Google Scholar] [CrossRef] [PubMed]
- Madhavikutty, A.S.; Chandel, A.K.S.; Tsai, C.C.; Inagaki, N.F.; Ohta, S.; Ito, T. pH responsive cationic guar gum-borate self-healing hydrogels for muco-adhesion. Sci. Technol. Adv. Mater. 2023, 24, 2175586. [Google Scholar] [CrossRef]
- Moghaddam, R.H.; Dadfarnia, S.; Shabani, A.M.H.; Moghaddam, Z.H.; Tavakol, M. Electron beam irradiation synthesis of porous and non-porous pectin based hydrogels for a tetracycline drug delivery system. Mater. Sci. Eng. C-Mater. 2019, 102, 391–404. [Google Scholar] [CrossRef]
- Sun, R.; Åhlén, M.; Tai, C.W.; Bajnóczi, É.; de Kleijne, F.; Ferraz, N.; Persson, I.; Stromme, M.; Cheung, O. Highly Porous Amorphous Calcium Phosphate for Drug Delivery and Bio-Medical Applications. Nanomaterials 2020, 10, 20. [Google Scholar] [CrossRef]
- Yazdi, M.K.; Taghizadeh, A.; Taghizadeh, M.; Stadler, F.J.; Farokhi, M.; Mottaghitalab, F.; Zarrintaj, P.; Ramsey, J.D.; Seidi, F.; Saeb, M.R.; et al. Agarose-based biomaterials for advanced drug delivery. J. Control. Release 2020, 326, 523–543. [Google Scholar] [CrossRef] [PubMed]
- Arnott, S.; Fulmer, A.; Scott, W.E.; Dea, I.C.M.; Moorhouse, R.; Rees, D.A. The agarose double helix and its function in agarose gel structure. J. Mol. Biol. 1974, 90, 269–284. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Wang, J.L.; Ye, J.; Zhao, P.; Xiao, M.T. Oxyalkylation modification as a promising method for preparing low-melting-point agarose. Int. J. Biol. Macromol. 2018, 117, 696–703. [Google Scholar] [CrossRef] [PubMed]
- Forget, A.; Pique, R.A.; Ahmadi, V.; Lüdeke, S.; Shastri, V.P. Mechanically Tailored Agarose Hydrogels through Molecular Alloying with β-Sheet Polysaccharides. Macromol. Rapid Commum. 2015, 36, 196–203. [Google Scholar] [CrossRef]
- Beaumont, M.; Tran, R.; Vera, G.; Niedrist, D.; Rousset, A.; Pierre, R.; Shastri, V.P.; Forget, A. Hydrogel-Forming Algae Polysaccharides: From Seaweed to Biomedical Applications. Biomacromolecules 2021, 22, 1027–1052. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.; Jeong, D.; Kim, S.; Kim, Y.; Jung, S. Cyclodextrin functionalized agarose gel with low gelling temperature for controlled drug delivery systems. Carbohydr. Polym. 2019, 222, 115011. [Google Scholar] [CrossRef]
- Zhao, J.; Marczynski, M.; Henkel, M.; Lieleg, O. Agarose-based hydrogels with tunable, charge-selective permeability properties. J. Appl. Polym. Sci. 2023, 140, e54303. [Google Scholar] [CrossRef]
- Wang, Y.C.; Wang, F.; Wang, R.Y.; Tian, C.; Hua, X.T.; Zhao, P.; Xia, Q.Y. Human-derived cytokine functionalized sericin/agarose composite gel material with cell proliferation-promoting activity fabricated using genetically engineered silk for medical application. Mater. Design 2022, 224, 111362. [Google Scholar] [CrossRef]
- Karimi, T.; Mottaghitalab, F.; Keshvari, H.; Farokhi, M. Carboxymethyl chitosan/sodium carboxymethyl cellulose/agarose hydrogel dressings containing silk fibroin/polydopamine nanoparticles for antibiotic delivery. J. Drug Deliv. Sci. Technol. 2023, 80, 104134. [Google Scholar] [CrossRef]
- Varshosaz, J. Dextran conjugates in drug delivery. Expert Opin. Drug Deliv. 2012, 9, 509–523. [Google Scholar] [CrossRef]
- Jeanes, A.; Haynes, W.C.; Wilham, C.A.; Rankin, J.C.; Melvin, E.H.; Austin, M.J.; Cluskey, J.E.; Fisher, B.E.; Tsuchiya, H.M.; Rist, C.E. Characterization and Classification of Dextrans from Ninety-six Strains of Bacteria1b. J. Am. Chem. Soc. 1954, 76, 5041–5052. [Google Scholar] [CrossRef]
- Huang, S.; Huang, G. Preparation and drug delivery of dextran-drug complex. Drug Deliv. 2019, 26, 252–261. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.; Zang, M.; Zhang, Y.; Chen, Y.; Du, J.; Yan, A.; Gu, J.; Li, Y.; Wei, S.; Xu, J.; et al. A bioresponsive diselenide-functionalized hydrogel with cascade catalytic activities for enhanced local starvation- and hypoxia-activated melanoma therapy. Acta Biomater. 2023, 167, 182–194. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Jiao, W.; Chen, Z.; Wang, C.; Song, X.; Ma, L.; Tang, Z.; Yan, W.; Xie, H.; Yuan, B.; et al. Injectable multifunctional chitosan/dextran-based hydrogel accelerates wound healing in combined radiation and burn injury. Carbohydr. Polym. 2023, 316, 121024. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Li, H.; Yin, C.; Tang, F. Research progress in the application of in situ hydrogel system in tumor treatment. Drug Deliv. 2020, 27, 460–468. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Wang, Y.; Li, Q.; Yu, C.; Chu, W. Natural Polymer-based Stimuli-responsive Hydrogels. Curr. Med. Chem. 2020, 27, 2631–2657. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, S.; Hui, P.; Kan, C.W. Thermoresponsive Hydrogels and Their Biomedical Applications: Special Insight into Their Applications in Textile Based Transdermal Therapy. Polymers 2018, 10, 480. [Google Scholar] [CrossRef] [PubMed]
- Hamedi, H.; Moradi, S.; Hudson, S.M.; Tonelli, A.E. Chitosan based hydrogels and their applications for drug delivery in wound dressings: A review. Carbohydr. Polym. 2018, 199, 445–460. [Google Scholar] [CrossRef]
- Lin, S.H.; Huang, A.P.H.; Hsu, S.H. Injectable, Micellar Chitosan Self-Healing Hydrogel for Asynchronous Dual-Drug Delivery to Treat Stroke Rats. Adv. Funct. Mater. 2023, 2303853. [Google Scholar] [CrossRef]
- Leach, D.G.; Dharmaraj, N.; Piotrowski, S.L.; Lopez-Silva, T.L.; Lei, Y.L.; Sikora, A.G.; Young, S.; Hartgerink, J.D. STINGel: Controlled release of a cyclic dinucleotide for enhanced cancer immunotherapy. Biomaterials 2018, 163, 67–75. [Google Scholar] [CrossRef]
- Chen, Y.; Yan, X.T.; Zhao, J.A.; Feng, H.Y.; Li, P.W.; Tong, Z.R.; Yang, Z.M.; Li, S.D.; Yang, J.Y.; Jin, S.H. Preparation of the chitosan/poly(glutamic acid)/alginate polyelectrolyte complexing hydrogel and study on its drug releasing property. Carbohydr. Polym. 2018, 191, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Mukhopadhyay, R.D.; Das, G.; Ajayaghosh, A. Stepwise control of host-guest interaction using a coordination polymer gel. Nat. Commun. 2018, 9, 1987. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Zhang, H.; Dai, Y.; Zhu, H.; Hong, G.; Zhu, C.; Qian, X.; Chai, R.; Gao, X.; Zhao, Y. Magnetic Hydrogel Microrobots Delivery System for Deafness Prevention. Adv. Funct. Mater. 2023, 33, 2303011. [Google Scholar] [CrossRef]
- Kesavan, M.P.; Ayyanaar, S.; Vijayakumar, V.; Raja, J.D.; Annaraj, J.; Sakthipandi, K.; Rajesh, J. Magnetic iron oxide nanoparticles (MIONs) cross-linked natural polymer-based hybrid gel beads: Controlled nano anti-TB drug delivery application. J. Biomed. Mater. Res. A 2018, 106, 1039–1050. [Google Scholar] [CrossRef] [PubMed]
- Qiao, S.; Ouyang, H.; Zheng, X.; Qi, C.; Ma, L. Magnetically actuated hydrogel-based capsule microrobots for intravascular targeted drug delivery. J. Mater. Chem. B 2023, 11, 6095–6105. [Google Scholar] [CrossRef] [PubMed]
- Javanbakht, S.; Namazi, H. Doxorubicin loaded carboxymethyl cellulose/graphene quantum dot nanocomposite hydrogel films as a potential anticancer drug delivery system. Mater. Sci. Eng. C-Mater. 2018, 87, 50–59. [Google Scholar] [CrossRef] [PubMed]
- Pooresmaeil, M.; Namazi, H. Folic acid-modified photoluminescent dialdehyde carboxymethyl cellulose crosslinked bionanogels for pH-controlled and tumor-targeted co-drug delivery. Int. J. Biol. Macromol. 2022, 200, 247–262. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Wang, Y.J.; Luo, G.S.; Dai, Y.Y. In situ preparation of magnetic Fe3O4-chitosan nanoparticles for lipase immobilization by cross-linking and oxidation in aqueous solution. Bioresour. Technol. 2009, 100, 3459–3464. [Google Scholar] [CrossRef]
- Ristic, B.Z.; Milenkovic, M.M.; Dakic, I.R.; Todorovic-Markovic, B.M.; Milosavljevic, M.S.; Budimir, M.D.; Paunovic, V.G.; Dramicanin, M.D.; Markovic, Z.M.; Trajkovic, V.S. Photodynamic antibacterial effect of graphene quantum dots. Biomaterials 2014, 35, 4428–4435. [Google Scholar] [CrossRef]
- Zhou, W.; Hu, Z.; Wei, J.; Dai, H.; Chen, Y.; Liu, S.; Duan, Z.; Xie, F.; Zhang, W.; Guo, R. Quantum dots-hydrogel composites for biomedical applications. Chin. Chem. Lett. 2022, 33, 1245–1253. [Google Scholar] [CrossRef]
- Zhao, L.; Niu, L.; Liang, H.; Tan, H.; Liu, C.; Zhu, F. pH and Glucose Dual-Responsive Injectable Hydrogels with Insulin and Fibroblasts as Bioactive Dressings for Diabetic Wound Healing. ACS Appl. Mater. Interfaces 2017, 9, 37563–37574. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; He, J.; Guo, B. Functional Hydrogels as Wound Dressing to Enhance Wound Healing. ACS Nano 2021, 15, 12687–12722. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.J.; Lee, G.H.; Chou, C.W.; Chen, Y.P.; Wu, T.H.; Lin, H.R. Stimulation of wound healing by PU/hydrogel composites containing fibroblast growth factor-2. J. Mater. Chem. B 2015, 3, 1931–1941. [Google Scholar] [CrossRef] [PubMed]
- Futrega, K.; King, M.; Lott, W.B.; Doran, M.R. Treating the whole not the hole: Necessary coupling of technologies for diabetic foot ulcer treatment. Trends Mol. Med. 2014, 20, 137–142. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Kang, H.F.; Bielec, M.; Wu, X.P.; Cheng, Q.; Wei, W.Y.; Dai, H.L. Influence of different divalent ions cross-linking sodium alginate-polyacrylamide hydrogels on antibacterial properties and wound healing. Carbohydr. Polym. 2018, 197, 292–304. [Google Scholar] [CrossRef] [PubMed]
- Qiao, L.; Liang, Y.; Chen, J.; Huang, Y.; Alsareii, S.A.; Alamri, A.M.; Harraz, F.A.; Guo, B. Antibacterial conductive self-healing hydrogel wound dressing with dual dynamic bonds promotes infected wound healing. Bioact. Mater. 2023, 30, 129–141. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Zhang, C.; Deng, D.; Gu, Y.; Wang, H.; Zhong, Q. Multiple Stimuli-Responsive MXene-Based Hydrogel as Intelligent Drug Delivery Carriers for Deep Chronic Wound Healing. Small 2022, 18, 2104368. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Chen, Z.; Chen, X.; Feng, K.; Hu, T.; Huang, B.; Tang, J.; Wang, G.; Liu, S.; Yang, G.; et al. Double-network cellulose-based hybrid hydrogels with favourable biocompatibility and antibacterial activity for wound healing. Carbohydr. Polym. 2023, 319, 121193. [Google Scholar] [CrossRef]
- Zhang, W.; Zha, K.; Xiong, Y.; Hu, W.; Chen, L.; Lin, Z.; Yu, C.; Zhou, W.; Cao, F.; Hu, H.; et al. Glucose-responsive, antioxidative HA-PBA-FA/EN106 hydrogel enhanced diabetic wound healing through modulation of FEM1b-FNIP1 axis and promoting angiogenesis. Bioact. Mater. 2023, 30, 29–45. [Google Scholar] [CrossRef]
- Kong, L.; Wu, Z.; Zhao, H.; Cui, H.; Shen, J.; Chang, J.; Li, H.; He, Y. Bioactive Injectable Hydrogels Containing Desferrioxamine and Bioglass for Diabetic Wound Healing. ACS Appl. Mater. Interfaces 2018, 10, 30103–30114. [Google Scholar] [CrossRef]
- Liu, K.; Kang, Y.; Dong, X.; Li, Q.; Wang, Y.; Wu, X.; Yang, X.; Chen, Z.; Dai, H. A simple yet effective hydrogel dressing for advanced microenvironmental management of diabetic wounds with intrinsic regulation. Chem. Eng. J. 2023, 470, 143987. [Google Scholar] [CrossRef]
- Guebitz, G.M.; Nyanhongo, G.S. Enzymes as Green Catalysts and Interactive Biomolecules in Wound Dressing Hydrogels. Trends Biotechnol. 2018, 36, 1040–1053. [Google Scholar] [CrossRef] [PubMed]
- Seliktar, D. Designing Cell-Compatible Hydrogels for Biomedical Applications. Science 2012, 336, 1124–1128. [Google Scholar] [CrossRef] [PubMed]
- Kutluk, H.; Bastounis, E.E.; Constantinou, I. Integration of Extracellular Matrices into Organ-on-Chip Systems. Adv. Healthc. Mater. 2023, 12, 2203256. [Google Scholar] [CrossRef] [PubMed]
- Rockwood, D.N.; Preda, R.C.; Yücel, T.; Wang, X.; Lovett, M.L.; Kaplan, D.L. Materials fabrication from Bombyx mori silk fibroin. Nat. Protoc. 2011, 6, 1612. [Google Scholar] [CrossRef] [PubMed]
- Ifkovits, J.L.; Tous, E.; Minakawa, M.; Morita, M.; Robb, J.D.; Koomalsingh, K.J.; Gorman, J.H., III; Gorman, R.C.; Burdick, J.A. Injectable hydrogel properties influence infarct expansion and extent of postinfarction left ventricular remodeling in an ovine model. Proc. Natl. Acad. Sci. USA 2010, 107, 11507–11512. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.; Fang, R.; Zhang, S.; Shi, X.; Wang, Z.; Chen, X.; Yang, J.; Hou, X.; Nie, Y.; Li, Y.; et al. Self-cross-linkable hydrogels composed of partially oxidized alginate and gelatin for myocardial infarction repair. J. Bioact. Compat. Polym. 2013, 28, 126–140. [Google Scholar] [CrossRef]
- Li, G.C.; Zhao, Y.X.; Zhang, L.Z.; Gao, M.; Kong, Y.; Yang, Y.M. Preparation of graphene oxide/polyacrylamide composite hydrogel and its effect on Schwann cells attachment and proliferation. Colloids Surf. B Biointerfaces 2016, 143, 547–556. [Google Scholar] [CrossRef]
- Chen, Q.; Li, J.; Han, F.; Meng, Q.; Wang, H.; Wei, Q.; Li, Z.; Li, F.; Xie, E.; Qin, X.; et al. A Multifunctional Composite Hydrogel That Rescues the ROS Microenvironment and Guides the Immune Response for Repair of Osteoporotic Bone Defects. Adv. Funct. Mater. 2022, 32, 2201067. [Google Scholar] [CrossRef]
- Hasani-Sadrabadi, M.M.; Sarrion, P.; Pouraghaei, S.; Chau, Y.; Ansari, S.; Li, S.; Aghaloo, T.; Moshaverinia, A. An engineered cell-laden adhesive hydrogel promotes craniofacial bone tissue regeneration in rats. Sci. Transl. Med. 2020, 12, eaay6853. [Google Scholar] [CrossRef]
- Deshmukh, D.V.; Reichert, P.; Zvick, J.; Labouesse, C.; Kunzli, V.; Dudaryeva, O.; Bar-Nur, O.; Tibbitt, M.W.; Dual, J. Continuous Production of Acoustically Patterned Cells Within Hydrogel Fibers for Musculoskeletal Tissue Engineering. Adv. Funct. Mater. 2022, 32, 2113038. [Google Scholar] [CrossRef]
- Luo, Z.; Tang, G.; Ravanbakhsh, H.; Li, W.; Wang, M.; Kuang, X.; Garciamendez-Mijares, C.E.; Lian, L.; Yi, S.; Liao, J.; et al. Vertical Extrusion Cryo(bio)printing for Anisotropic Tissue Manufacturing. Adv. Mater. 2022, 34, 2108931. [Google Scholar] [CrossRef]
- Zlotnick, H.M.; Clark, A.T.; Gullbrand, S.E.; Carey, J.L.; Cheng, X.M.; Mauck, R.L. Magneto-Driven Gradients of Diamagnetic Objects for Engineering Complex Tissues. Adv. Mater. 2020, 32, 19. [Google Scholar] [CrossRef] [PubMed]
- Andrews, F.H.; Singh, A.R.; Joshi, S.; Smith, C.A.; Morales, G.A.; Garlich, J.R.; Durden, D.L.; Kutateladze, T.G. Dual-activity PI3K-BRD4 inhibitor for the orthogonal inhibition of MYC to block tumor growth and metastasis. Proc. Natl. Acad. Sci. USA 2017, 114, E1072–E1080. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Yang, M.; Li, S.; Zhu, J. Surface plasmon resonance imaging validation of small molecule drugs binding on target protein microarrays. Appl. Surf. Sci. 2018, 450, 328–335. [Google Scholar] [CrossRef]
- Wang, Y.; Xiao, Y.; Long, S.; Fan, Y.; Zhang, X. Role of N-Cadherin in a Niche-Mimicking Microenvironment for Chondrogenesis of Mesenchymal Stem Cells In Vitro. ACS Biomater. Sci. Eng. 2020, 6, 3491–3501. [Google Scholar] [CrossRef]
- Karamikamkar, S.; Behzadfar, E.; Cheung, K.C. A novel approach to producing uniform 3-D tumor spheroid constructs using ultrasound treatment. Biomed. Microdevices 2018, 20, 27. [Google Scholar] [CrossRef]
- Xu, J.; Shamul, J.G.; Staten, N.A.; White, A.M.; Jiang, B.; He, X. Bioinspired 3D Culture in Nanoliter Hyaluronic Acid-Rich Core-Shell Hydrogel Microcapsules Isolates Highly Pluripotent Human iPSCs. Small 2021, 17, 2102219. [Google Scholar] [CrossRef]
- Hadden, W.J.; Young, J.L.; Holle, A.W.; McFetridge, M.L.; Kim, D.Y.; Wijesinghe, P.; Taylor-Weiner, H.; Wen, J.H.; Lee, A.R.; Bieback, K.; et al. Stem cell migration and mechanotransduction on linear stiffness gradient hydrogels. Proc. Natl. Acad. Sci. USA 2017, 114, 5647–5652. [Google Scholar] [CrossRef]
- Goodrich, R.; Tai, Y.; Ye, Z.; Yin, Y.; Nam, J. A Magneto-Responsive Hydrogel System for the Dynamic Mechano-Modulation of Stem Cell Niche. Adv. Funct. Mater. 2023, 33, 2211288. [Google Scholar] [CrossRef]
- Jeon, O.; Lee, K.; Alsberg, E. Spatial Micropatterning of Growth Factors in 3D Hydrogels for Location-Specific Regulation of Cellular Behaviors. Small 2018, 14, 1800579. [Google Scholar] [CrossRef] [PubMed]
- Deangelis, P.L.; Oatman, L.C.; Gay, D.F. Rapid chemoenzymatic synthesis of monodisperse hyaluronan oligosaccharides with immobilized enzyme reactors. J. Biol. Chem. 2003, 278, 35199–35203. [Google Scholar] [CrossRef] [PubMed]
- Cong, H.P.; Wang, P.; Yu, S.H. Stretchable and Self-Healing Graphene Oxide–Polymer Composite Hydrogels: A Dual-Network Design. Chem. Mater. 2013, 25, 3357–3362. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, M.; Jiang, H.; Shi, J.; Li, F.; Xia, Y.; Zhang, G.; Li, H. Bio-inspired layered chitosan/graphene oxide nanocomposite hydrogels with high strength and pH-driven shape memory effect. Carbohydr. Polym. 2017, 177, 116–125. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.W.; Zeng, M.; Chen, J.B.; Wang, Y.Q.; Xu, Q.Y. Multi-structural network design and mechanical properties of graphene oxide filled chitosan-based hydrogel nanocomposites. Mater. Design 2018, 148, 104–114. [Google Scholar] [CrossRef]
- Gong, J.P.; Katsuyama, Y.; Kurokawa, T.; Osada, Y. Double-network hydrogels with extremely high mechanical strength. Adv. Mater. 2003, 15, 1155–1158. [Google Scholar] [CrossRef]
- Han, C.; Zhang, H.; Wu, Y.; He, X.; Chen, X. Dual-crosslinked hyaluronan hydrogels with rapid gelation and high injectability for stem cell protection. Sci. Rep. 2020, 10, 14997. [Google Scholar] [CrossRef]
- Li, D.; Chen, K.; Tang, H.; Hu, S.; Xin, L.; Jing, X.; He, Q.; Wang, S.; Song, J.; Mei, L.; et al. A Logic-Based Diagnostic and Therapeutic Hydrogel with Multistimuli Responsiveness to Orchestrate Diabetic Bone Regeneration. Adv. Mater. 2022, 34, 2108430. [Google Scholar] [CrossRef]
- Sun, W.; Zhao, X.; Webb, E.; Xu, G.; Zhang, W.; Wang, Y. Advances in metal-organic framework-based hydrogel materials: Preparation, properties and applications. J. Mater. Chem. A 2023, 11, 2092–2127. [Google Scholar] [CrossRef]
- Lim, J.Y.C.; Goh, L.; Otake, K.-i.; Goh, S.S.; Loh, X.J.; Kitagawa, S. Biomedically-relevant metal organic framework-hydrogel composites. Biomater. Sci. 2023, 11, 2661–2677. [Google Scholar] [CrossRef]
- Li, M.; Huang, W.; Tang, B.; Fang, Q.; Ling, X.; Lv, A. Characterizations and n-Hexane Vapor Adsorption of a Series of MOF/Alginates. Ind. Eng. Chem. Res. 2020, 59, 18835–18843. [Google Scholar] [CrossRef]
- Yao, X.; Zhu, G.; Zhu, P.; Ma, J.; Chen, W.; Liu, Z.; Kong, T. Omniphobic ZIF-8@Hydrogel Membrane by Microfluidic-Emulsion-Templating Method for Wound Healing. Adv. Funct. Mater. 2020, 30, 1909389. [Google Scholar] [CrossRef]
- Wang, Y.; Qi, W.; Mao, Z.; Wang, J.; Zhao, R.C.; Chen, H. rPDAs doped antibacterial MOF-hydrogel: Bio-inspired synergistic whole-process wound healing. Mater. Today Nano 2023, 23, 100363. [Google Scholar] [CrossRef]
- Chimene, D.; Kaunas, R.; Gaharwar, A.K. Hydrogel Bioink Reinforcement for Additive Manufacturing: A Focused Review of Emerging Strategies. Adv. Mater. 2020, 32, 1902026. [Google Scholar] [CrossRef] [PubMed]
- Hull, S.M.; Brunel, L.G.; Heilshorn, S.C. 3D Bioprinting of Cell-Laden Hydrogels for Improved Biological Functionality. Adv. Mater. 2022, 34, 2103691. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Xu, J.; Fei, Z.; Dai, H.; Fan, Q.; Yang, Q.; Chen, Y.; Wang, B.; Wang, C. 3D Printing Scaffold Vaccine for Antitumor Immunity. Adv. Mater. 2021, 33, 2106768. [Google Scholar] [CrossRef]
- Gong, J.; Schuurmans, C.C.L.; Genderen, A.M.V.; Cao, X.; Li, W.; Cheng, F.; He, J.J.; Lopez, A.; Huerta, V.; Manriquez, J.; et al. Complexation-induced resolution enhancement of 3D-printed hydrogel constructs. Nat. Commun. 2020, 11, 1267. [Google Scholar] [CrossRef]
- Anandakrishnan, N.; Ye, H.; Guo, Z.; Chen, Z.; Mentkowski, K.I.; Lang, J.K.; Rajabian, N.; Andreadis, S.T.; Ma, Z.; Spernyak, J.A.; et al. Fast Stereolithography Printing of Large-Scale Biocompatible Hydrogel Models. Adv. Healthc. Mater. 2021, 10, 2002103. [Google Scholar] [CrossRef]
- Agarwal, T.; Hann, S.Y.; Chiesa, I.; Cui, H.; Celikkin, N.; Micalizzi, S.; Barbetta, A.; Costantini, M.; Esworthy, T.; Zhang, L.G.; et al. 4D printing in biomedical applications: Emerging trends and technologies. J. Mater. Chem. B 2021, 9, 7608–7632. [Google Scholar] [CrossRef]
- Champeau, M.; Heinze, D.A.; Viana, T.N.; de Souza, E.R.; Chinellato, A.C.; Titotto, S. 4D Printing of Hydrogels: A Review. Adv. Funct. Mater. 2020, 30, 1910606. [Google Scholar] [CrossRef]
- You, D.; Chen, G.; Liu, C.; Ye, X.; Wang, S.; Dong, M.; Sun, M.; He, J.; Yu, X.; Ye, G.; et al. 4D Printing of Multi-Responsive Membrane for Accelerated In Vivo Bone Healing Via Remote Regulation of Stem Cell Fate. Adv. Funct. Mater. 2021, 31, 2103920. [Google Scholar] [CrossRef]



| Natural Polymer | Chemical Structures | Preparation and Processing | Ref. |
|---|---|---|---|
| Cellulose | Composed of β (1-4)-glycosidic-linked glucose units | 1. Lignocelluloses purification by chemical treatment. 2. Biological method depending on microbial enzymes. 3. Bacterial cellulose produced by certain types of bacteria. | [9] |
| Chitosan | Poly-(β-1-4) N-acetyl-D-glucosamine | Derived from chitin by partial deacetylation though chemical or enzymatic hydrolysis. | [10,11] |
| Collagen | A helical fibrous protein formed by three peptide chains | 1. Extracted and purified from various animal sources by chemical and enzyme treatment. 2. Recombinant collagen produced by recombinant technology and biosynthesis. | [12] |
| Alginate | Consisting of α-L glucuronate and β-D mannuronate repeating units | 1. Extraction from brown algae (Phaeophyceae) by treatment with aqueous alkali solutions. 2. Bacterial biosynthesis from Azotobacter and Pseudomonas. | [13] |
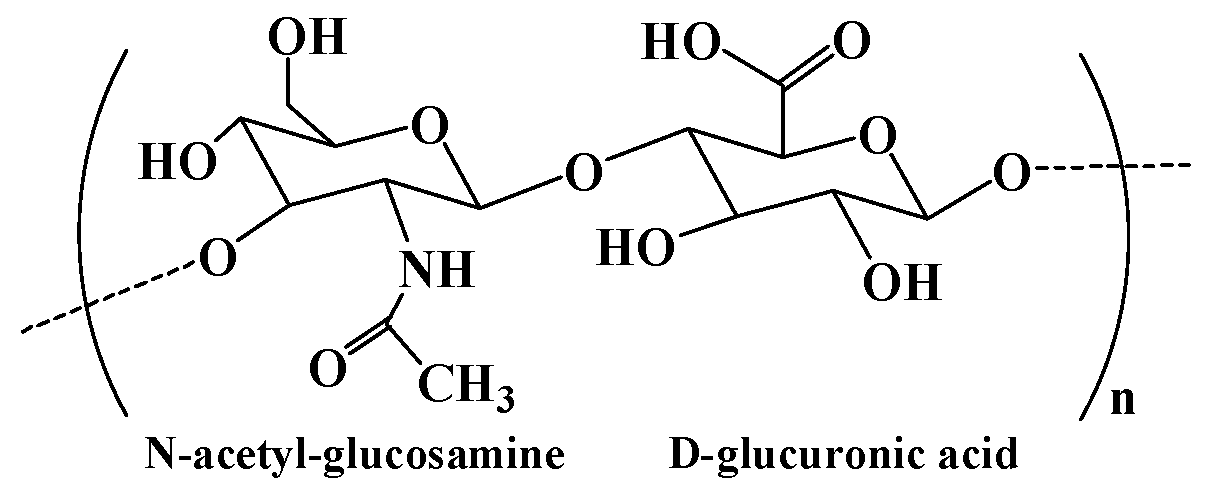
| Hyaluronic acid | Consisting of N-acetyl-glucosamine and D-glucuronic acid residues | 1. Extraction from animal tissues. 2. Microbial fermentation using pathogenic bacteria and nonpathogenic bacteria. 3. Enzymatic polymerization of UDP-sugar monomers. | [14] |
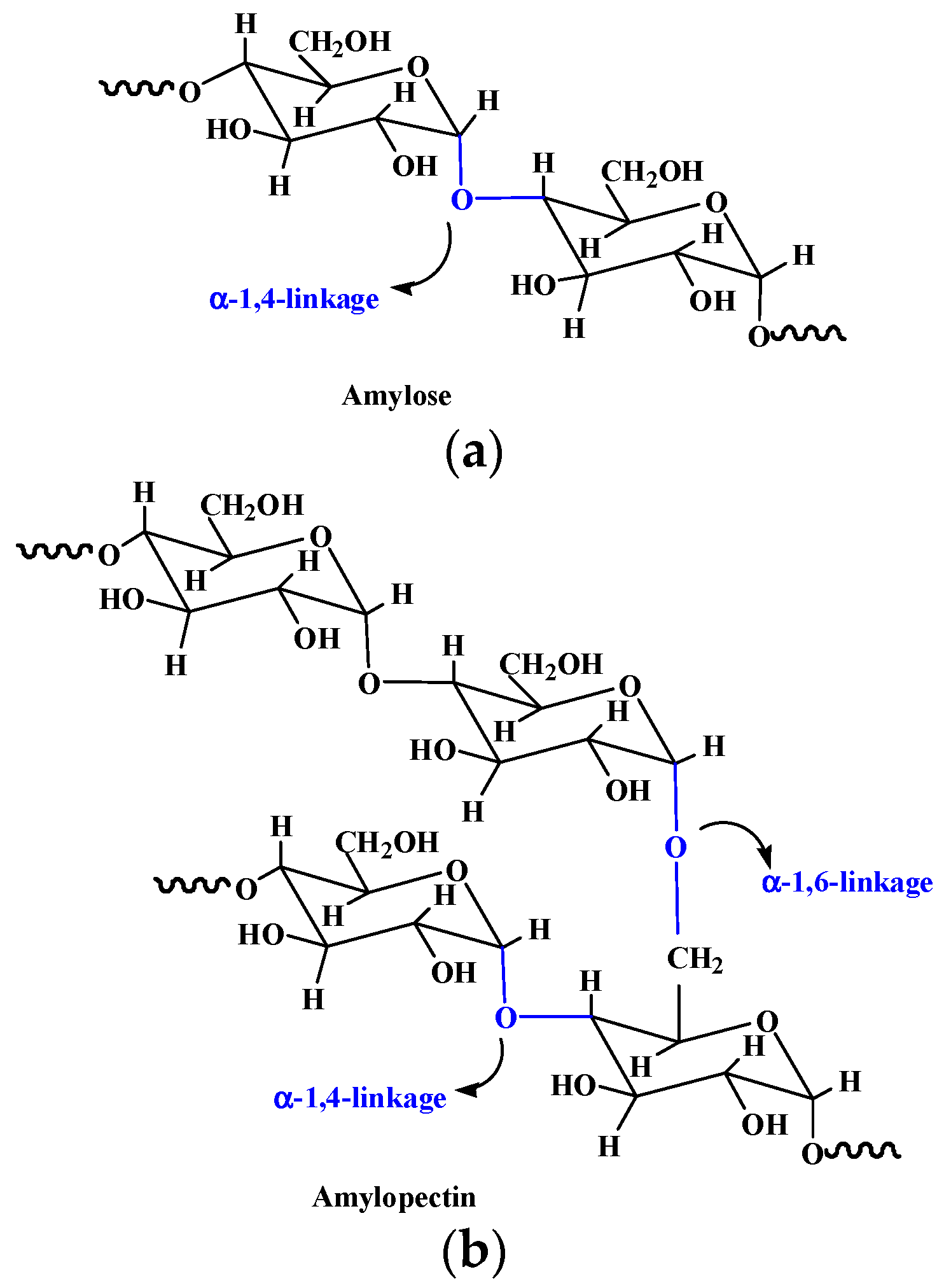
| Starch | Composed of α-D-(1-4) and α-D-(1 - 6)-glycosidic-linked glucose units | Extracted from seeds, roots, tubers, stems, fruits, and all leaves. | [15] |
| Guar gum | Composed of (1-4)-β-D-mannopyranosyl units and (1-6)-α-D-galactopyranosyl units | Isolated from the embryos of the leguminous plant Cyamopsis tetragonoloba. | [16] |
| Agarose | Consisting of alternating 1,3-linked β-D-galactose and 1,4-linked 3,6-anhydro-α-Lgalactose units | Extracted from seaweed. | [17] |
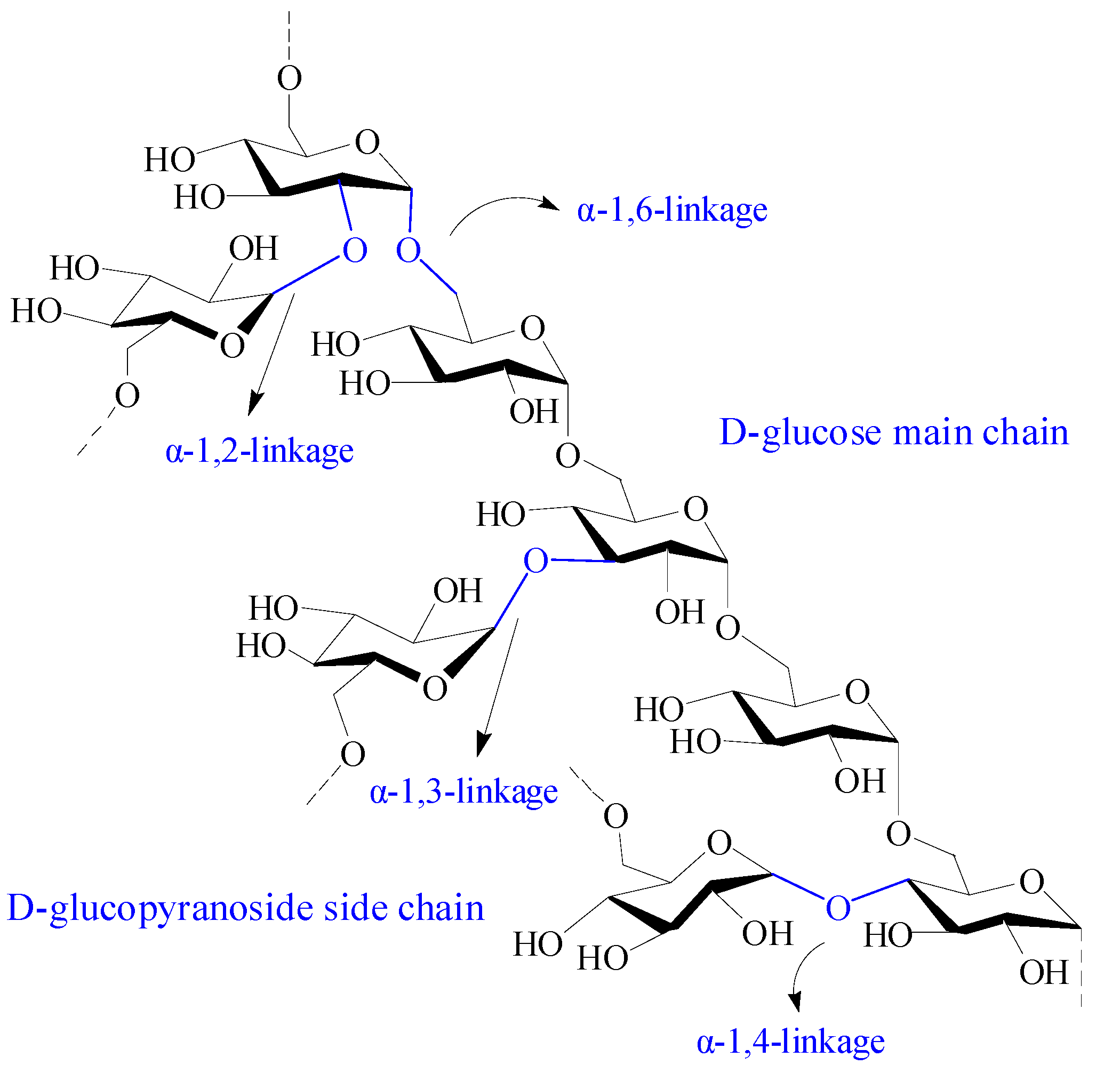
| Dextran | Composed of α-1, 6 glycosidic linkages between glucose monomers, with branches from α-1, 2, α-1, 3, and α-1, 4 linkages | 1. Produced from slime-producing bacteria called Leuconostoc Lesenteroides; 2. Produced from several Gram-positive, facultative anaerobic bacteria, such as Leuconostoc and Streptococcus strains; 3. Synthesized by cationic ring-opening polymerization of levoglucosan. | [18,19] |
| Hydrogels | Drugs | Properties and Function | Ref. |
|---|---|---|---|
| Chitosan-based micellar hydrogels | Minocycline and edaravone | Self-healing and injectable property. First-order rapid release for minocycline and zero-order sustained release for edaravone. Behavioral improvement in stroke rats. | [134] |
| Multidomain peptide hydrogels | Cyclic dinucleotide (CDN) | An eightfold slower release rate of CDN and a sixfold improvement in survival of mice compared with standard collagen hydrogel. | [135] |
| Chitosan/poly (glutamic acid)/alginate polyelectrolyte complex hydrogels | Piroxicam | Controlled colon-specific drug release and reduced gastrointestinal irritation side effect of piroxicam. | [136] |
| Host–guest interaction hydrogel system | α-CD | Temperature-responsive stepwise release of α-CD both in the solution and hydrogel states. | [137] |
| Magnetic hydrogel microrobots | Alpha-lipoic acid | Sustained drug release, targeted movement, satisfactory antioxidant properties and biosafety. | [138] |
| Hybrid gel beads based on chitosan and Fe3O4 cross-linked polyethylene glycol | Rifampicin | pH- and magnetic field-responsive asset in drug delivery. | [139] |
| CaCO3/sodium alginate/Fe3O4 hydrogel-based capsule microrobots | Indomethacin | Intravascular targeted drug delivery by following a predetermined trajectory in the blood vessel under magnetic drive. | [140] |
| Grapheme quantum dot/carboxymethyl cellulose-based hydrogel nanocomposite films | Doxorubicin | pH-sensitive and consecutive prolonged release of doxorubicin and nonobvious cytotoxicity on K562 cells. | [141] |
| Carbon dots/gelatin/carboxymethyl cellulose-based bionanogels | Curcumin and doxorubicin | pH-controlled release for both drugs and superior anticancer effect in comparison with free curcumin/doxorubicin. | [142] |
| Hydrogels | Properties | Effects in the Wound Healing | Ref. |
|---|---|---|---|
| Sodium alginate–polyacrylamide hydrogels | Excellent mechanical strength by zinc cross-linked hydrogel. | Antibacterial activities and promoted fibroblasts migration, vascularization, collagen deposition, and granulation tissue formation. | [150] |
| Alginate/dopamine/carboxymethyl chitosan-based hydrogels | Antibacterial, conductive, adhesive, and self-healing properties. | Photothermal antibacterial property, reduced inflammation, and increased vascular regeneration. | [151] |
| Alginate/MXene-based hydrogel | Photo- and magnetic-responsive, and precise control release of AgNPs. | Eliminated bacteria attachment and promoted M2 macrophages polarization and angiogenesis. | [152] |
| Epsilon-polylysine modified cellulose/γ-PGA double-network hydrogel | Good biocompatibility and antibacterial activity. | Improved collagen deposition, accelerated vascularization, and enhanced cell proliferation. | [153] |
| Hyaluronic acid-EN106 hydrogels | Glucose-responsive, antibacterial and anti-inflammatory abilities, and sustained release of EN106. | Ameliorated oxidative stress and improved angiogenesis. | [154] |
| Sodium alginate hydrogel containing desferrioxamine (DFO) and bioglass | Injectable and sustained release of DFO. | Promoted wound healing by increasing HIF-1α and VEGF expression and vascularization. | [155] |
| Composite hydrogels based on hyaluronic acid/collagen/deferoxamine loaded polydopamine nanoparticles | Desirable mechanical property, improved tissue adhesive and injectable performance. | Exhibited a prominent enhancement of angiogenesis, excellent anti-inflammatory and bacteriostatic effect, promoted the M2 polarization of macrophages, and enhanced diabetic wounds’ healing. | [57] |
| Exosome-loaded hydrogels based on α-Lipoic acid modified chitosan | Strong adhesion, photoinduced self-healing and pH/H2O2/glucose responsiveness | Accelerate diabetic wound healing by regulating the wound environment, such as reducing oxidative stress, lowering blood glucose levels, and promoting angiogenesis. | [156] |
| Hydrogels | Properties | Functions | Ref. |
|---|---|---|---|
| Collagen/alginate/fibrin-based hydrogels | Thermosensitivity and mechanical stiffness similar to native soft tissues | Enhanced osteogenic potential of human mesenchymal stem cells and improved aggregation MIN6 β-cells with the indication of pseudoislets formation. | [49] |
| Gelatin/FAPi-loaded microspheres composite hydrogels | Antioxidant properties | Promoted repair of osteoporotic bone defects by rescuing ROS microenvironment and guiding the immune response in bilateral OVX-induced osteoporotic rats. | [114] |
| RGD-modified alginate-based osteoconductive hydrogels | Tunable mechanical properties and biodegradability | Complete bone regeneration around ailing dental implants with peri-implant bone loss in a rat model. | [115] |
| GelMA-based hydrogel scaffolds containing anisotropic microchannels | Improved robustness and versatility | Encapsulated live cells at high viability levels in desired cellular alignments to fabricate muscle–tendon unit and muscle–microvascular unit | [117] |
| Magneto-patterned cellular hydrogels based on methacrylated hyaluronic acid | Prepositioned diamagnetic objects in 3D hydrogels. | Fabricated cartilage constructs similar to natural tissue with gradient cellularity and maintained these cell gradients in the extracellular matrix content. | [118] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, L.; Zhou, Y.; Zhang, J.; Liang, H.; Chen, X.; Tan, H. Natural Polymer-Based Hydrogels: From Polymer to Biomedical Applications. Pharmaceutics 2023, 15, 2514. https://doi.org/10.3390/pharmaceutics15102514
Zhao L, Zhou Y, Zhang J, Liang H, Chen X, Tan H. Natural Polymer-Based Hydrogels: From Polymer to Biomedical Applications. Pharmaceutics. 2023; 15(10):2514. https://doi.org/10.3390/pharmaceutics15102514
Chicago/Turabian StyleZhao, Lingling, Yifan Zhou, Jiaying Zhang, Hongze Liang, Xianwu Chen, and Hui Tan. 2023. "Natural Polymer-Based Hydrogels: From Polymer to Biomedical Applications" Pharmaceutics 15, no. 10: 2514. https://doi.org/10.3390/pharmaceutics15102514