Topical Delivery Systems Effectively Transport Analgesics to Areas of Localized Pain via Direct Diffusion
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Chemicals and Equipment
2.3. Tested Topical Delivery Systems (TDSs)
2.4. Open Flow Microperfusion (OFM)
2.5. Ex Vivo Experiment
2.6. In Vivo Experiment
2.7. HPLC-MS/MS
2.8. Statistics
3. Results and Discussion
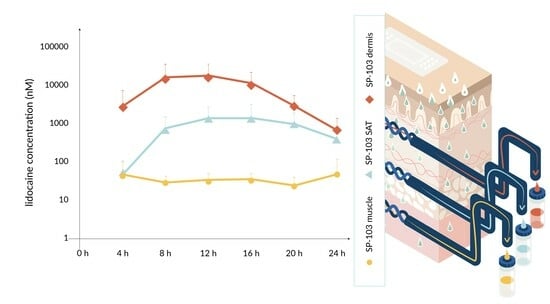
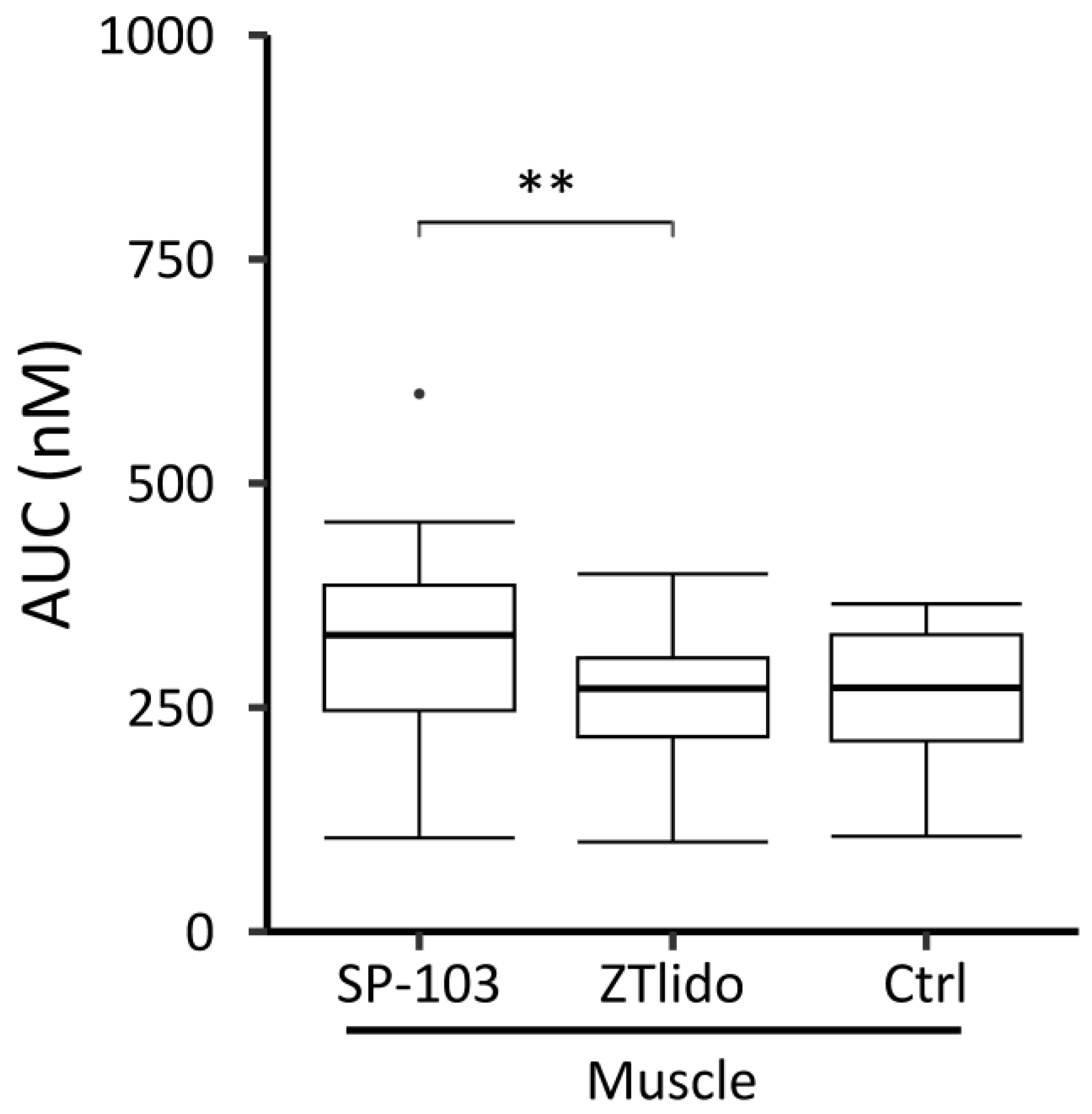
3.1. Diclofenac and Lidocaine Pharmacokinetics (Ex Vivo)
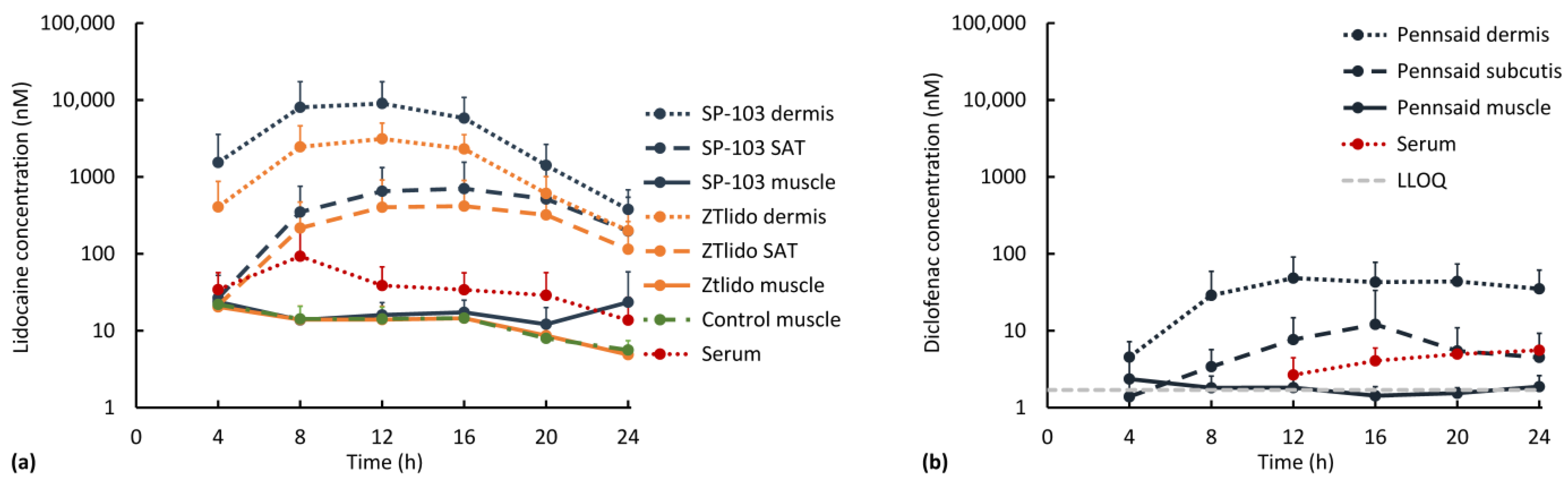
3.2. Diclofenac and Lidocaine Pharmacokinetics (In Vivo)
3.3. Limitations of Ex Vivo and In Vivo Experiments
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Leppert, W.; Malec–Milewska, M.; Zajaczkowska, R.; Wordliczek, J. Transdermal and Topical Drug Administration in the Treatment of Pain. Molecules 2018, 23, 681. [Google Scholar] [CrossRef] [PubMed]
- Stanos, S.P.; Galluzzi, K.E. Topical Therapies in the Management of Chronic Pain. Postgrad. Med. 2013, 125, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Gudin, J.; Nalamachu, S. Utility of Lidocaine as a Topical Analgesic and Improvements in Patch Delivery Systems. Postgrad. Med. 2020, 132, 28–36. [Google Scholar] [CrossRef] [PubMed]
- McCleane, G. Topical Analgesics. Anesthesiol. Clin. 2007, 25, 825–839. [Google Scholar] [CrossRef]
- Carter, G.T.; Duong, V.; Ho, S.; Ngo, K.C.; Greer, C.L.; Weeks, D.L. Side Effects of Commonly Prescribed Analgesic Medications. Phys. Med. Rehabil. Clin. N. Am. 2014, 25, 457–470. [Google Scholar] [CrossRef]
- Labianca, R.; Sarzi-Puttini, P.; Zuccaro, S.M.; Cherubino, P.; Vellucci, R.; Fornasari, D. Adverse Effects Associated with Non-Opioid and Opioid Treatment in Patients with Chronic Pain. Clin. Drug Investig. 2012, 32, 53–63. [Google Scholar] [CrossRef]
- Gunter, B.R.; Butler, K.A.; Wallace, R.L.; Smith, S.M.; Harirforoosh, S. Non-Steroidal Anti-Inflammatory Drug-Induced Cardiovascular Adverse Events: A Meta-Analysis. J. Clin. Pharm. Ther. 2017, 42, 27–38. [Google Scholar] [CrossRef]
- Baigent, C.; Bhala, N.; Emberson, J.; Merhi, A.; Abramson, S.; Arber, N.; Baron, J.A.; Bombardier, C.; Cannon, C.; Farkouh, M.E.; et al. Vascular and Upper Gastrointestinal Effects of Non-Steroidal Anti-Inflammatory Drugs: Meta-Analyses of Individual Participant Data from Randomised Trials. Lancet 2013, 382, 769–779. [Google Scholar] [CrossRef]
- Argoff, C. Topical Analgesics: A Review of Recent Clinical Trials and Their Application to Clinical Practice. Adv. Stud. Med. 2003, 3, 642–647. [Google Scholar]
- Lee, C.M.; Maibach, H.I. Deep Percutaneous Penetration into Muscles and Joints. J. Pharm. Sci. 2006, 95, 1405–1413. [Google Scholar] [CrossRef]
- Stanos, S. Topical Analgesics. Phys. Med. Rehabil. Clin. N. Am. 2020, 31, 233–244. [Google Scholar] [CrossRef] [PubMed]
- Adamiak-Giera, U.; Nowak, A.; Duchnik, W.; Ossowicz-Rupniewska, P.; Czerkawska, A.; Machoy-Mokrzyńska, A.; Sulikowski, T.; Kucharski, Ł.; Białecka, M.; Klimowicz, A.; et al. Evaluation of the in Vitro Permeation Parameters of Topical Ketoprofen and Lidocaine Hydrochloride from Transdermal Pentravan® Products through Human Skin. Front. Pharmacol. 2023, 14, 1157977. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, Y.; Holmes, A.; Kwok, P.C.L.; Kumeria, T.; Namjoshi, S.; Imran, M.; Matteucci, L.; Ali, M.; Tai, W.; Benson, H.A.E.; et al. Advances and Future Perspectives in Epithelial Drug Delivery. Adv. Drug Deliv. Rev. 2022, 186, 114293. [Google Scholar] [CrossRef] [PubMed]
- Komatsu, T.; Sakurada, T. Comparison of the Efficacy and Skin Permeability of Topical NSAID Preparations Used in Europe. Eur. J. Pharm. Sci. 2012, 47, 890–895. [Google Scholar] [CrossRef] [PubMed]
- Wilhelm, I.R.; Tzabazis, A.; Likar, R.; Sittl, R.; Grießinger, N. Long-Term Treatment of Neuropathic Pain with a 5% Lidocaine Medicated Plaster. Eur. J. Anaesthesiol. 2010, 27, 169–173. [Google Scholar] [CrossRef] [PubMed]
- Nalamachu, S.; Gudin, J. Characteristics of Analgesic Patch Formulations. J. Pain Res. 2020, 13, 2343–2354. [Google Scholar] [CrossRef]
- Brown, M.B.; Martin, G.P.; Jones, S.A.; Akomeah, F.K. Dermal and Transdermal Drug Delivery Systems: Current and Future Prospects. Drug Deliv. J. Deliv. Target. Ther. Agents 2006, 13, 175–187. [Google Scholar] [CrossRef]
- Schaupp, L.; Ellmerer, M.; Brunner, G.A.; Wutte, A.; Sendlhofer, G.; Trajanoski, Z.; Skrabal, F.; Pieber, T.R.; Wach, P. Direct Access to Interstitial Fluid in Adipose Tissue in Humans by Use of Open-Flow Microperfusion. Am. J. Physiol. Metab. 1999, 276, E401–E408. [Google Scholar] [CrossRef]
- Hagen, M.; Baker, M. Skin Penetration and Tissue Permeation after Topical Administration of Diclofenac. Curr. Med. Res. Opin. 2017, 33, 1623–1634. [Google Scholar] [CrossRef]
- Hummer, J.; Birngruber, T.; Sinner, F.; Page, L.; Toner, F.; Roper, C.S.; Moore, D.J.; Baker, M.B.; Boncheva Bettex, M. Optimization of Topical Formulations Using a Combination of in Vitro Methods to Quantify the Transdermal Passive Diffusion of Drugs. Int. J. Pharm. 2022, 620, 121737. [Google Scholar] [CrossRef]
- Pieber, T.; Birngruber, T.; Bodenlenz, M.; Höfferer, C.; Mautner, S.; Tiffner, K. Open Flow Microperfusion: An Alternative Method to Microdialysis? In Microdialysis in Drug Development; Müller, M., Ed.; AAPS Advances in the Pharmaceutical Sciences Series; Springer New York: New York, NY, USA, 2013; Volume 4, pp. 283–302. ISBN 978-1-4614-4814-3. [Google Scholar]
- Gabrielsson, J.; Weiner, D. Non-Compartmental Analysis. In Computational Toxicology. Methods in Molecular Biology Vol 929; Reisfeld, B., Mayeno, A.N., Eds.; Humana Press: Totowa, NJ, USA, 2012; pp. 377–389. [Google Scholar]
- Cross, S.E.; Wu, Z.; Roberts, M.S. The Effect of Protein Binding on the Deep Tissue Penetration and Efflux of Dermally Applied Salicylic Acid, Lidocaine and Diazepam in the Perfused Rat Hindlimb. J. Pharmacol. Exp. Ther. 1996, 277, 366–374. [Google Scholar] [PubMed]
- Matzneller, P.; Oesterreicher, Z.; Wulkersdorfer, B.; al Jalali, V.; Mascher, D.; Mascher, H.; Zeitlinger, M. Microdialysis as a Potential Tool for Comparative Assessment of Tissue Pharmacokinetics of Two Different Patches Containing Lidocaine: A Crossover Pilot Study. Int. J. Clin. Pharmacol. Ther. 2021, 59, 603–609. [Google Scholar] [CrossRef] [PubMed]
- Oni, G.; Brown, S.; Kenkel, J. Comparison of Five Commonly-Available, Lidocaine-Containing Topical Anesthetics and Their Effect on Serum Levels of Lidocaine and Its Metabolite Monoethylglycinexylidide (MEGX). Aesthetic Surg. J. 2012, 32, 495–503. [Google Scholar] [CrossRef] [PubMed]
- Cordero, J.A.; Alarcon, L.; Escribano, E.; Obach, R.; Domenech, J. A Comparative Study of the Transdermal Penetration of a Series of Nonsteroidal Antiinflammatory Drugs. J. Pharm. Sci. 1997, 86, 503–508. [Google Scholar] [CrossRef] [PubMed]
- Altman, R.; Bosch, B.; Brune, K.; Patrignani, P.; Young, C. Advances in NSAID Development: Evolution of Diclofenac Products Using Pharmaceutical Technology. Drugs 2015, 75, 859. [Google Scholar] [CrossRef]
- Todd, P.; Sorkin, E. Diclofenac Sodium. A Reappraisal of Its Pharmacodynamic and Pharmacokinetic Properties, and Therapeutic Efficacy. Drugs 1988, 35, 545–558. [Google Scholar] [CrossRef]
- Sheets, M.F.; Hanck, D.A. Molecular Action of Lidocaine on the Voltage Sensors of Sodium Channels. J. Gen. Physiol. 2003, 121, 163. [Google Scholar] [CrossRef]
- Weinberg, L.; Peake, B.; Tan, C.; Nikfarjam, M. Pharmacokinetics and Pharmacodynamics of Lignocaine: A Review. World J. Anesthesiol. 2015, 4, 17–29. [Google Scholar] [CrossRef]
- Binder, A.; Bruxelle, J.; Rogers, P.; Hans, G.; Bösl, I.; Baron, R. Topical 5% Lidocaine (Lignocaine) Medicated Plaster Treatment for Post-Herpetic Neuralgia: Results of a Double-Blind, Placebo-Controlled, Multinational Efficacy and Safety Trial. Clin. Drug Investig. 2009, 29, 393–408. [Google Scholar] [CrossRef]
- Galer, B.S.; Rowbotham, M.C.; Perander, J.; Friedman, E. Topical Lidocaine Patch Relieves Postherpetic Neuralgia More Effectively than a Vehicle Topical Patch: Results of an Enriched Enrollment Study. Pain 1999, 80, 533–538. [Google Scholar] [CrossRef]
- Rowbotham, M.C.; Davies, P.S.; Verkempinck, C.; Galer, B.S. Lidocaine Patch: Double-Blind Controlled Study of a New Treatment Method for Post-Herpetic Neuralgia. Pain 1996, 65, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Scilex Pharmaceuticals. ZTLidoTM Prescribing Information. Available online: www.fda.gov/medwatch (accessed on 23 June 2023).
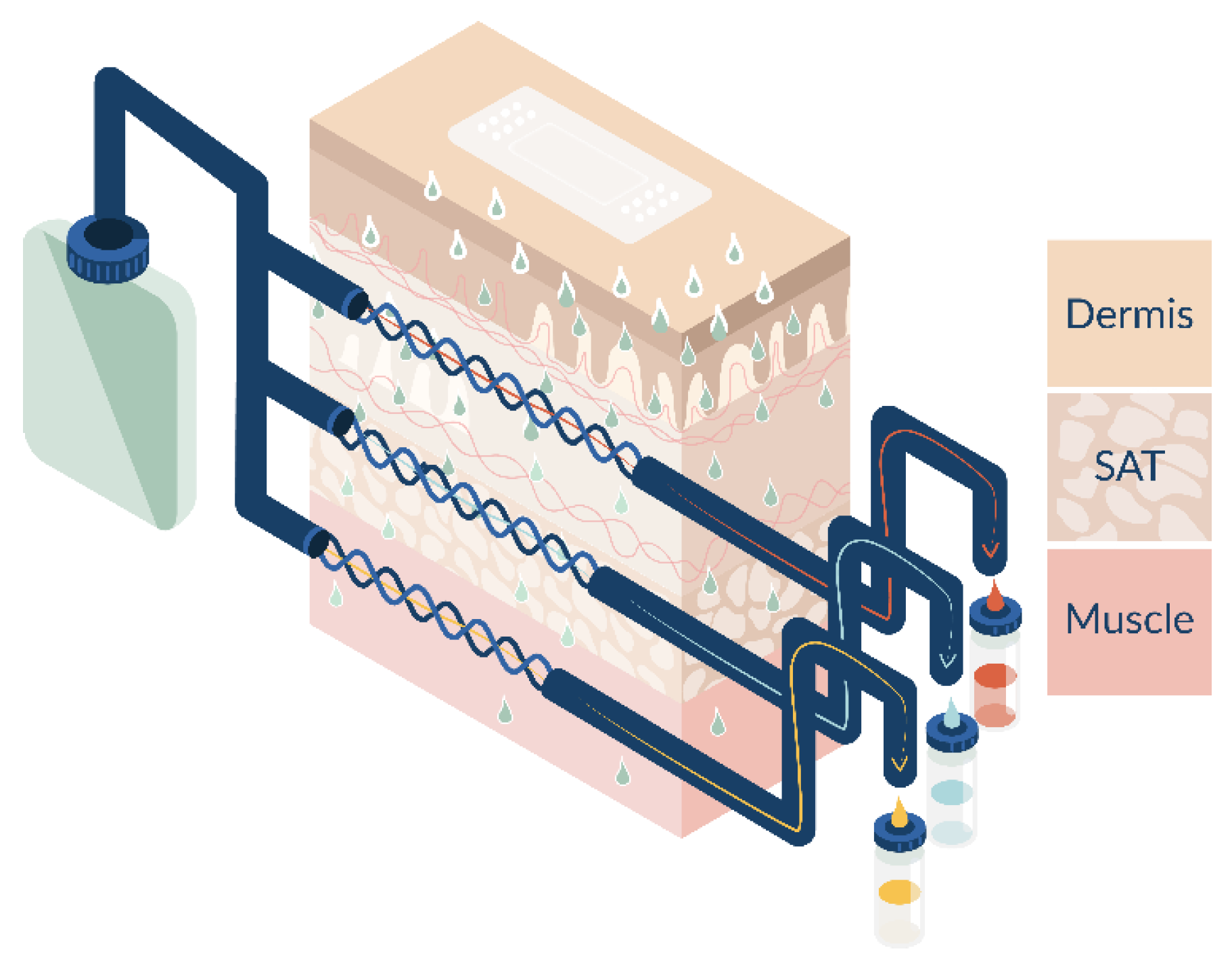
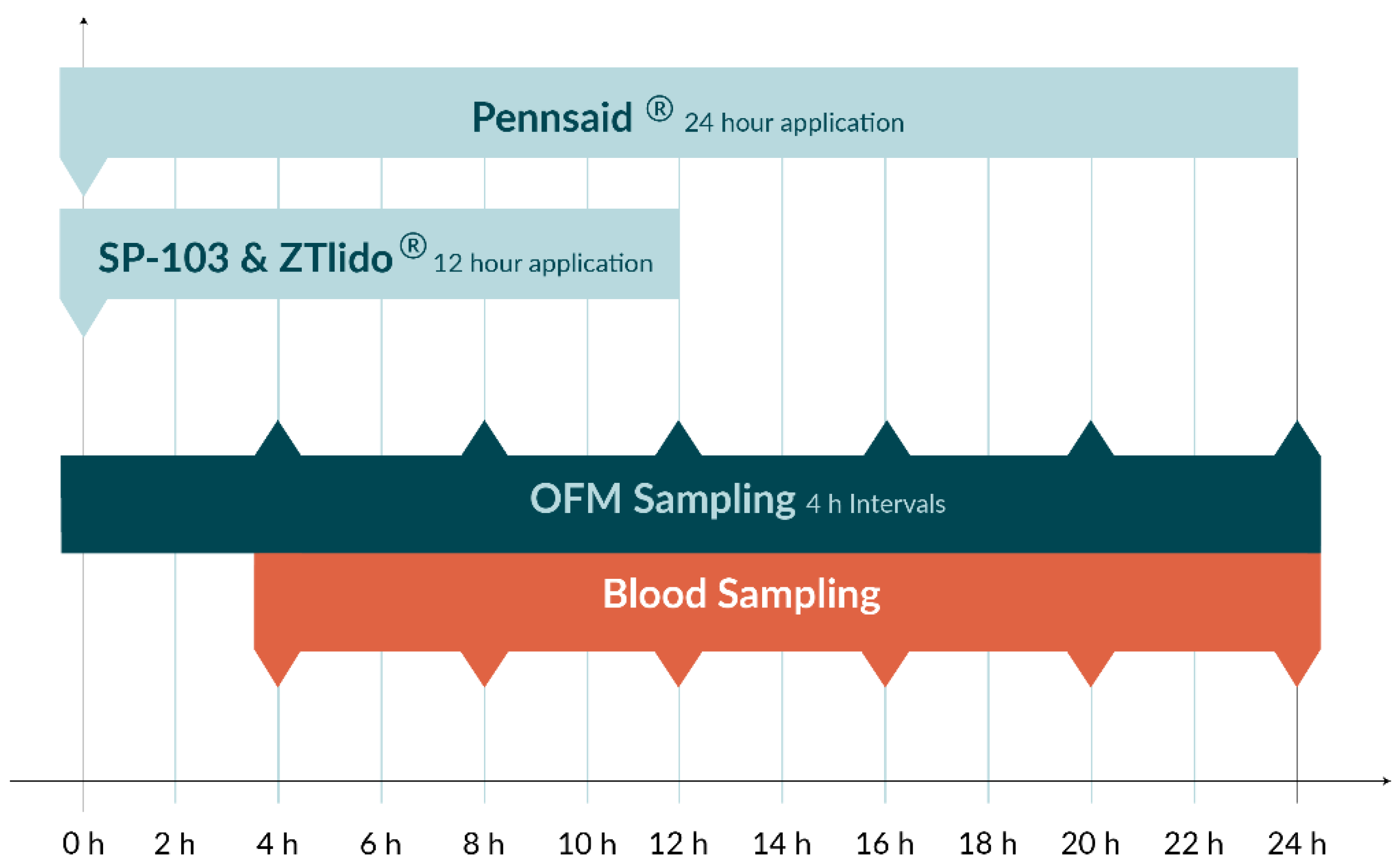

| Product Strength/Concentration | Test Setup | Drug | Drug Load per TDS (mg) | Dimensions of Application Site | Drug Load per Application Site (mg) |
|---|---|---|---|---|---|
| SP-103 5.4% | ev vivo/in vivo | lidocaine | 108 | 8 × 2.5 cm | 15.4 |
| ZTlido® 1.8% | in vivo | lidocaine | 36 | 8 × 2.5 cm | 5.1 |
| Pennsaid® 2% | ev vivo/in vivo | diclofenac | n.a. | 8 × 2.5 cm | 6.0 (15 mg/cm2) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Birngruber, T.; Vought, K.; Schwingenschuh, S.; Reisenegger, P.; Maibach, H.; Lissin, D. Topical Delivery Systems Effectively Transport Analgesics to Areas of Localized Pain via Direct Diffusion. Pharmaceutics 2023, 15, 2563. https://doi.org/10.3390/pharmaceutics15112563
Birngruber T, Vought K, Schwingenschuh S, Reisenegger P, Maibach H, Lissin D. Topical Delivery Systems Effectively Transport Analgesics to Areas of Localized Pain via Direct Diffusion. Pharmaceutics. 2023; 15(11):2563. https://doi.org/10.3390/pharmaceutics15112563
Chicago/Turabian StyleBirngruber, Thomas, Kip Vought, Simon Schwingenschuh, Peter Reisenegger, Howard Maibach, and Dmitri Lissin. 2023. "Topical Delivery Systems Effectively Transport Analgesics to Areas of Localized Pain via Direct Diffusion" Pharmaceutics 15, no. 11: 2563. https://doi.org/10.3390/pharmaceutics15112563