Bombesins: A New Frontier in Hybrid Compound Development
Abstract
:1. Introduction
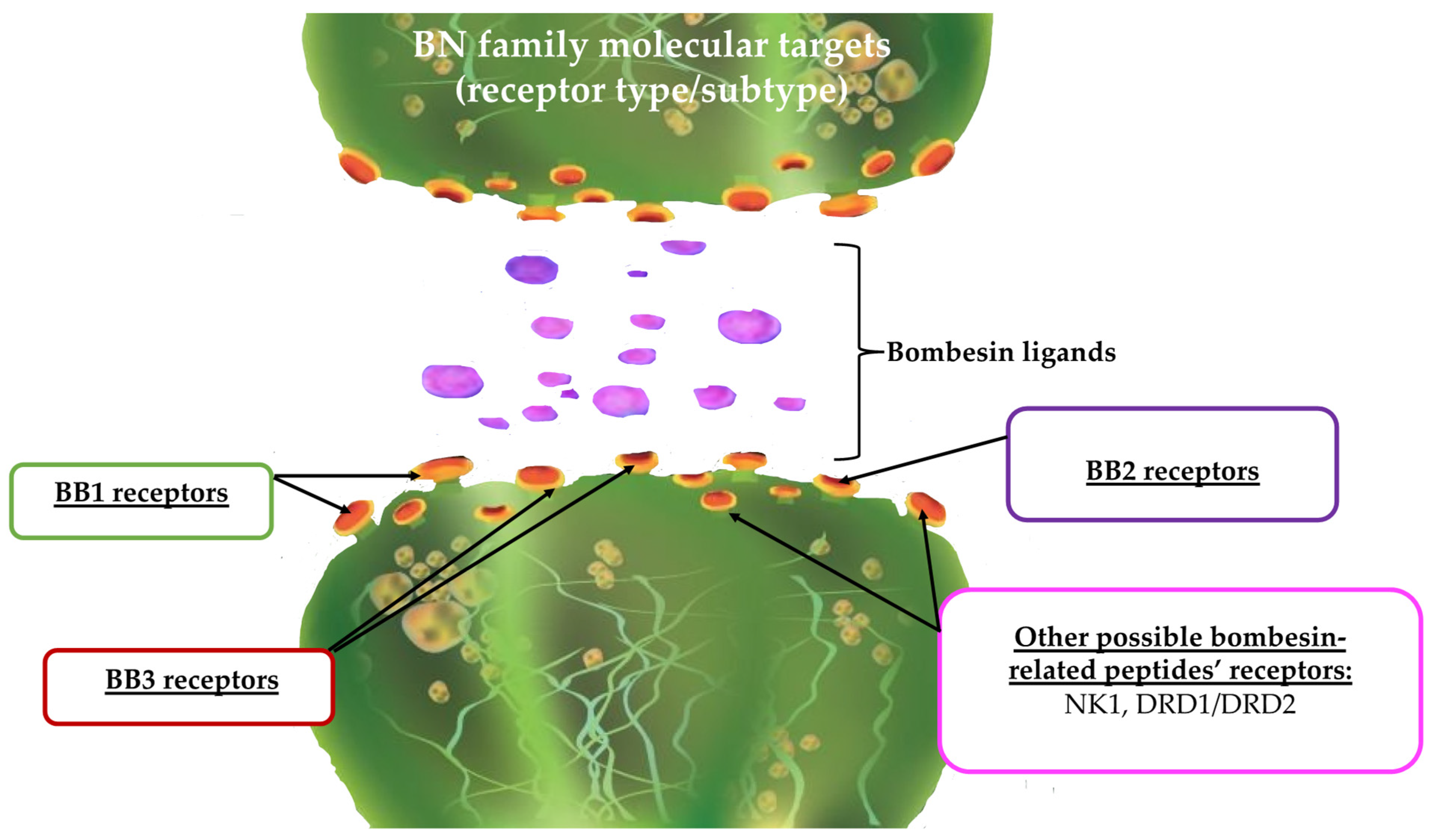
2. Bombesins and Bombesin Receptors
2.1. Mammalian Bombesins
2.2. Amphibian Bombesin-like Peptides and Their Receptors
2.3. Bombesins and Receptor Targets Other than Bombesin Receptors
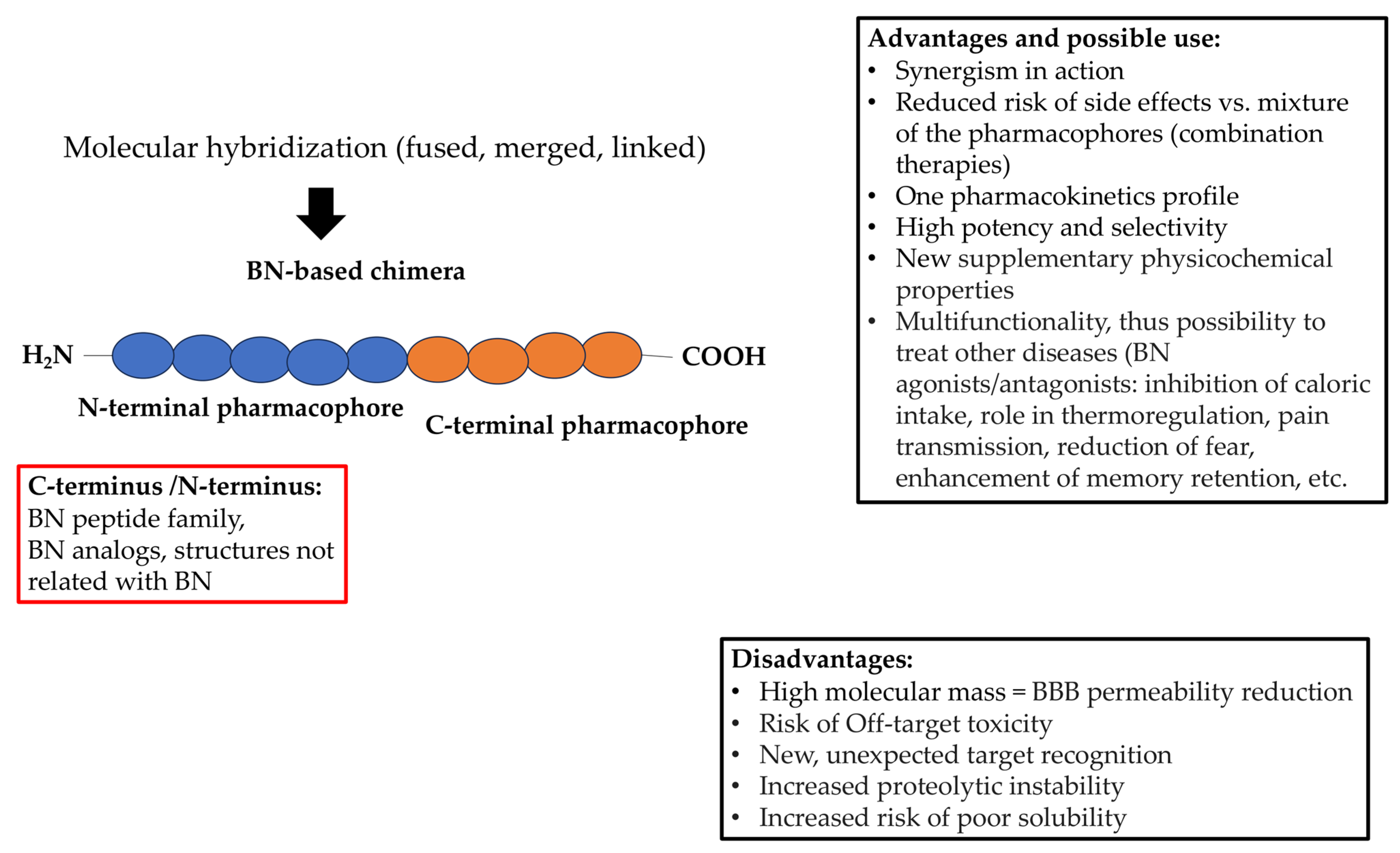
3. Hybrid Approach and Bombesins
Bombesin-Based Hybrid Compounds and Their In Vitro and/or In Vivo Efficacy
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kerru, N.; Singh, P.; Koorbanally, N.; Raj, R.; Kumar, V. Recent advances (2015–2016) in anticancer hybrids. Eur. J. Med. Chem. 2017, 142, 179–212. [Google Scholar] [CrossRef]
- Kleczkowska, P. Chimeric structures in mental illnesses—“Magic” molecules specified for complex disorders. Int. J. Mol. Sci. 2022, 23, 3739. [Google Scholar] [CrossRef] [PubMed]
- Cosledan, F.; Fraisse, L.; Pellet, A.; Guillou, F.; Mordmuller, B.; Kremsner, P.G.; Moreno, A.; Mazier, D.; Maffrand, J.-P.; Meunier, B. Selection of a trioxaquine as an antimalarial drug candidate. Proc. Natl. Acad. Sci. USA 2008, 105, 17579–17584. [Google Scholar] [CrossRef]
- Kamath, P.R.; Sunil, D.; Ajees, A.A.; Pai, K.S.R.; Das, S. Some new indole–coumarin hybrids: Synthesis, anticancer and Bcl-2 docking studies. Bioorg. Chem. 2015, 63, 101–109. [Google Scholar] [CrossRef]
- Klingenstein, R.; Lober, S.; Kujala, P.; Godsave, S.; Leliveld, S.R.; Gmeiner, P.; Peters, P.J.; Korth, C. Tricyclic antidepressants, quinacrine and a novel, synthetic chimera thereof clear prions by destabilizing detergent resistant membrane compartments. J. Neurochem. 2006, 98, 748–759. [Google Scholar] [CrossRef] [PubMed]
- Pradhan, T.K.; Katsuno, T.; Taylor, J.E.; Kim, S.H.; Ryan, R.R.; Mantey, S.A.; Donohue, P.J.; Weber, H.C.; Sainz, E.; Battey, J.F.; et al. Identification of a unique ligand which has high affinity for all four bombesin receptor subtypes. Eur. J. Pharmacol. 1998, 343, 275–287. [Google Scholar] [CrossRef] [PubMed]
- Erspamer, V.; Erspamer, G.F.; Inselvini, M. Some pharmacological actions of alytesin and bombesin. J. Pharm. Pharmacol. 1970, 22, 875–876. [Google Scholar] [CrossRef]
- Uehara, H.; Gonzales, N.; Sancho, V.; Mantey, S.; Nuche-Berenuer, B.; Pradhan, T.; Coy, D.H.; Jensen, R.T. Pharmacology and selectivity of various natural and synthetic bombesin related peptide agonists for human and rat bombesin receptors differs. Peptides 2011, 32, 1685–1699. [Google Scholar] [CrossRef]
- McDonald, T.J.; Jörnvall, H.; Nilsson, G.; Vagne, M.; Ghatei, M.; Bloom, S.R.; Mutt, V. Characterization of a gastrin releasing peptide from porcine non-antral gastric tissue. Biochem. Biophys. Res. Commun. 1979, 90, 227–233. [Google Scholar] [CrossRef]
- Taché, Y.; Vale, W.; Rivier, J.; Brown, M. Brain regulation of gastric secretion: Influence of neuropeptides. Proc. Natl. Acad. Sci. USA 1980, 77, 5515–5519. [Google Scholar] [CrossRef]
- Chen, X.J.; Sun, Y.G. Central circuit mechanisms of itch. Nat. Commun. 2020, 11, 3052. [Google Scholar] [CrossRef] [PubMed]
- Jensen, R.T.; Battey, J.F.; Spindel, E.R.; Benya, R.V. International Union of Pharmacology. LXVIII. Mammalian bombesin receptors: Nomenclature, distribution, pharmacology, signaling, and functions in normal and diseases states. Pharmacol. Rev. 2008, 60, 1–42. [Google Scholar] [CrossRef]
- Moody, T.W.; Merali, Z. Bombesin-like peptides and associated receptors within the brain: Distribution and behavioral implications. Peptides 2004, 25, 511–520. [Google Scholar] [CrossRef] [PubMed]
- Plamondon, H.; Merali, Z. Anorectic action of bombesin requires receptor for corticotropin-releasing factor but not for oxytocin. Eur. J. Pharmacol. 1997, 340, 99–109. [Google Scholar] [CrossRef] [PubMed]
- Deschodt-Lanckman, M.; Robberecht, P.; De Neef, P.; Lammens, M.; Christophe, J. In vitro action of bombesin and bombesin-like peptides on amylase secretion, calcium efflux, and adenylate cyclase activity in the rat pancreas: A comparison with other secretagogues. J. Clin. Investig. 1976, 58, 891–898. [Google Scholar] [CrossRef]
- Erspamer, V.; Improta, G.; Melchiorri, P.; Sopranzi, N. Evidence of cholecystokinin release by bombesin in the dog. Br. J. Pharmacol. 1974, 52, 227–232. [Google Scholar] [CrossRef]
- Ghatei, M.A.; Jung, R.T.; Stevenson, J.C.; Hillyard, C.J.; Adrian, T.E.; Lee, Y.C.; Christofides, N.D.; Sarson, D.L.; Mashiter, K.; MacIntyre, I.; et al. Bombesin: Action on gut hormones and calcium in man. J. Clin. Endocrinol. Metab. 1982, 54, 980–985. [Google Scholar] [CrossRef]
- Rozengurt, E. Bombesin stimulation of mitogenesis. Specific receptors, signal transduction, and early events. Am. Rev. Respir. Dis. 1990, 142, S11. [Google Scholar] [CrossRef]
- Rozengurt, E.; Sinnett-Smith, J. Bombesin stimulation of DNA synthesis and cell division in cultures of Swiss 3T3 cells. Proc. Natl. Acad. Sci. USA 1983, 80, 2936–2940. [Google Scholar] [CrossRef]
- Wiedermann, C.J.; Ruff, M.R.; Pert, C.B. Bombesin-like peptides: Neuropeptides with mitogenic activity. Brain Behav. Immun. 1988, 2, 301–310. [Google Scholar] [CrossRef]
- Assimakopoulos, S.F.; Alexandris, I.H.; Scopa, C.D.; Mylonas, P.G.; Thomopoulos, K.C.; Georgiou, C.D.; Nikolopoulou, V.N.; Vagianos, C.E. Effect of bombesin and neurotensin on gut barrier function in partially hepatectomized rats. World J. Gastroenterol. 2005, 11, 6757–6764. [Google Scholar] [CrossRef]
- Assimakopoulos, S.F.; Scopa, C.D.; Zervoudakis, G.; Mylonas, P.G.; Georgiou, C.; Nikolopoulou, V.; Vagianos, C.E. Bombesin and neurotensin reduce endotoxemia, intestinal oxidative stress, and apoptosis in experimental obstructive jaundice. Ann. Surg. 2005, 241, 159–167. [Google Scholar] [CrossRef]
- Brown, D.R.; Gillespie, M.A. Actions of centrally administered neuropeptides on rat intestinal transport: Enhancement of ileal absorption by angiotensin II. Eur. J. Pharmacol. 1988, 148, 411–418. [Google Scholar] [CrossRef]
- Guarini, S.; Tagliavini, S.; Bazzani, C.; Bertolini, A. Bombesin reverses bleeding-induced hypovolemic shock, in rats. Life Sci. 1989, 45, 107–116. [Google Scholar] [CrossRef] [PubMed]
- Merali, Z.; Johnston, S.; Zalcman, S. Bombesin-induced behavioral changes: Antagonism by neuroleptics. Peptides 1983, 4, 693–697. [Google Scholar] [CrossRef]
- Baroni, A.; Perfetto, B.; Canozo, N.; Braca, A.; Farina, E.; Melito, A.; De Maria, S.; Carteni, M. Bombesin: A possible role in wound repair. Peptides 2008, 29, 1157–1166. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.G.; Chen, Z.F. A gastrin-releasing peptide receptor mediates the itch sensation in the spinal cord. Nature 2007, 448, 700–703. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Ko, M.C. Distinct functions of opioid-related peptides and gastrin-releasing peptide in regulating itch and pain in the spinal cord of primates. Sci. Rep. 2015, 5, 11676. [Google Scholar] [CrossRef]
- Green, P.G. Gastrin-releasing peptide, substance P and cytokines in rheumatoid arthritis. Arthritis Res. Ther. 2005, 7, 111–113. [Google Scholar] [CrossRef] [PubMed]
- Moody, T.W.; Carney, D.N.; Cuttitta, F.; Quattrocchi, K.; Minna, J.D. High affinity receptors for bombesin/GRP-like peptides on human small cell lung cancer. Life Sci. 1985, 37, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Rashidy-Pour, A.; Razvani, M.E. Unilateral reversible inactivations of the nucleus tractus solitarius and amygdala attenuate the effects of bombesin on memory storage. Brain Res. 1998, 1814, 127–132. [Google Scholar] [CrossRef]
- Flood, J.F.; Morley, J.E. Effects of bombesin and gastrin-releasing peptide on memory processing. Brain Res. 1988, 460, 314–322. [Google Scholar] [CrossRef]
- Ferreira, L.B.T.; Oliveira, S.L.B.; Raya, J.; Esumi, A.; Hipolide, D.C. Bombesin administration impairs memory and does not reverse memory deficit caused by sleep deprivation. Behav. Brain Res. 2017, 331, 20–24. [Google Scholar] [CrossRef]
- Mountney, C.; Sillberg, V.; Kent, P.; Anisman, H.; Merali, Z. The role of gastrin-releasing peptide on conditioned fear: Differential cortical and amygdaloid responses in the rat. Psychopharmacology 2006, 289, 287–296. [Google Scholar] [CrossRef]
- Mountney, C.; Anisman, H.; Merali, Z. Effects of gastrin-releasing peptide agonist and antagonist administered to the basolateral nucleus of the amygdala on conditioned fear in the rat. Psychopharmacology 2008, 200, 51–58. [Google Scholar] [CrossRef]
- Sakamoto, H.; Matsuda, K.; Zuloaga, D.G.; Hongu, H.; Wada, E.; Wada, K.; Jordan, C.L.; Breedlove, S.M.; Kawata, M. Sexually dimorphic gastrin releasing peptide system in the spinal cord controls male reproductive functions. Nat. Neurosci. 2008, 11, 634–636. [Google Scholar] [CrossRef] [PubMed]
- DeWitt, R.C.; Wu, Y.; Renegar, K.B.; King, B.K.; Li, J.; Kudsk, K.A. Bombesin recovers gut-associated lymphoid tissue and preserves immunity to bacterial pneumonia in mice receiving total parenteral nutrition. Ann. Surg. 2000, 231, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Dal-Pizzol, F.; Pons Di Leone, L.; Ritter, C.; Martins, M.R.; Reinke, A.; Gelain, D.P.; Zanotto-Filho, A.; de Souza, L.F.; Andrades, M.; Frediani Barberio, D.; et al. Gastrin-releasing peptide receptor antagonists effects on an animal model of sepsis. Am. J. Respir. Crit. Care Med. 2006, 173, 84–90. [Google Scholar] [CrossRef]
- Ohki-Hamazaki, H.; Iwabuchi, M.; Maekawa, F. Development and function of bombesin-like peptides and their receptors. Int. J. Dev. Biol. 2005, 49, 293–300. [Google Scholar] [CrossRef]
- Bedard, T.; Mountney, C.; Kent, P.; Anisman, H.; Merali, Z. Role of gastrin-releasing peptide and Neuromedin B in anxiety and fear-related behavior. Behav. Brain Res. 2007, 179, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Matusiak, D.; Glover, S.; Nathaniel, R.; Matkowskyj, K.; Yang, J.; Benya, R.V. Neuromedin B and its receptor are mitogens in both normal and malignant epithelial cells lining the colon. Am. J. Physiol. Gastrointest. Liver Physiol. 2005, 288, G718–G728. [Google Scholar] [CrossRef]
- Moody, T.W.; Jensen, R.T.; Garcia, L.; Leyton, J. Nonpeptide Neuromedin B receptor antagonists inhibit the proliferation of C6 cells. Eur. J. Pharmacol. 2000, 409, 133–142. [Google Scholar] [CrossRef]
- Von Schrenck, T.; Heinz-Erian, P.; Moran, T.; Mantey, S.A.; Gardner, J.D.; Jensen, R.T. Neuromedin B receptor in esophagus: Evidence for subtypes of bombesin receptors. Am. J. Physiol. 1989, 256, G747–G758. [Google Scholar] [CrossRef] [PubMed]
- Fleming, M.S.; Ramos, D.; Han, S.B.; Zhao, J.; Son, Y.-J.; Luo, W. The majority of dorsal spinal cord gastrin releasing peptide is synthesized locally whereas neuromedin B is highly expressed in pain- and itch-sensing somatosensory neurons. Mol. Pain. 2012, 8, 52. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.K.; Holzman, S.; Hoon, M.A. A nociceptive signaling role for neuromedin B. J. Neurosci. 2012, 32, 8686–8695. [Google Scholar] [CrossRef] [PubMed]
- Wan, L.; Jin, H.; Liu, X.Y.; Jeffry, J.; Barry, D.M.; Shen, K.F.; Peng, J.H.; Liu, X.T.; Jin, J.H.; Sun, Y.; et al. Distinct roles of NMB and GRP in itch transmission. Sci. Rep. 2017, 7, 15466. [Google Scholar] [CrossRef] [PubMed]
- Boughton, C.K.; Patel, S.A.; Thompson, E.L.; Patterson, M.; Curtis, A.E.; Amin, A.; Chen, K.; Ghatei, M.A.; Bloom, S.R.; Murphy, K.G. Neuromedin B stimulates the hypothalamic–pituitary–gonadal axis in male rats. Regul. Pept. 2013, 187, 6–11. [Google Scholar] [CrossRef] [PubMed]
- Todman, M.G.; Han, S.K.; Herbison, A.E. Profiling neurotransmitter receptor expression in mouse gonadotropin-releasing hormone neurons using green fluorescent protein-promoter transgenics and microarrays. Neuroscience 2005, 132, 703–712. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Guo, Z.; Xiang, W.; Wang, P. The neuroendocrine pathways and mechanisms for the control of the reproduction in female pigs. Anim. Reprod. 2021, 18, e20210063. [Google Scholar] [CrossRef]
- Park, H.J.; Kim, M.K.; Kim, Y.; Kim, H.J.; Bae, S.K.; Bae, M.K. Neuromedin B modulates phosphate-induced vascular calcification. BMB Rep. 2021, 54, 569–574. [Google Scholar] [CrossRef]
- Trayhurn, P.; Wood, I.S. Adipokines: Inflammation and the pleiotropic role of white adipose tissue. Br. J. Nutr. 2004, 92, 347–355. [Google Scholar] [CrossRef]
- Kroog, G.S.; Jensen, R.T.; Battey, J.F. Mammalian bombesin receptors. Med. Res. Rev. 1995, 15, 389–417. [Google Scholar] [CrossRef] [PubMed]
- Jensen, R.T.; Moody, T.W. Bombesin-related peptides. In Handbook of Biologically Active Peptides, 2nd ed.; Kastin, A.J., Ed.; Academic Press: Cambridge, MA, USA, 2013; pp. 1188–1196. [Google Scholar]
- Hildebrand, P.; Lehmann, F.S.; Ketterer, S.; Christ, A.D.; Stingelin, T.; Beltinger, J.; Gibbons, A.H.; Coy, D.H.; Calam, J.; Larsen, F.; et al. Regulation of gastric function by endogenous gastrin releasing peptide in humans: Studies with a specific gastrin releasing peptide receptor antagonist. Gut 2001, 49, 23–28. [Google Scholar] [CrossRef]
- Hirschowitz, B.J.; Gibson, R.G. Stimulation of gastrin release and gastric secretion: Effect of bombesin and a nonapeptide in fistula dogs with and without fundic vagotomy. Digestion 1978, 18, 227–239. [Google Scholar] [CrossRef] [PubMed]
- Lenz, J.H.; Forquignon, I.; Druge, G.; Greten, H. Effects of neuropeptides on gastric acid and duodenal bicarbonate secretions in freely moving rats. Regul. Pept. 1989, 24, 293–300. [Google Scholar] [CrossRef] [PubMed]
- Varner, A.A.; Modlin, I.M.; Walsh, J.H. High potency of bombesin for stimulation of human gastrin release and gastric acid secretion. Regul. Pept. 1981, 1, 289–296. [Google Scholar] [CrossRef]
- Beltran, B.; Barrachina, M.D.; Mendez, A.; Quintero, E.; Esplugues, J.V. Synthesis of nitric oxide in the dorsal motor nucleus of the vagus mediates the inhibition of gastric acid secretion by central bombesin. Br. J. Pharmacol. 1999, 127, 1603–1610. [Google Scholar] [CrossRef]
- Bertaccini, G.; Erspamer, V.; Impicciatore, M. The actions of bombesin on gastric secretion of the dog and the rat. Br. J. Pharmacol. 1973, 49, 437–444. [Google Scholar] [CrossRef]
- Martinez, V.; Tache, Y. Bombesin and the brain-gut axis. Peptides 2000, 21, 1617–1625. [Google Scholar] [CrossRef]
- Gonzalez, N.; Moody, T.W.; Igarashi, H.; Ito, T.; Jensen, R.T. Bombesin-related peptides and their receptors: Recent advances in their role in physiology and disease states. Curr. Opin. Endocrinol. Diabetes Obes. 2008, 15, 58–64. [Google Scholar] [CrossRef]
- Hirooka, A.; Hamada, M.; Fujiyama, D.; Takanami, K.; Kobayashi, Y.; Oti, T.; Katayama, Y.; Sakamoto, T.; Sakamoto, H. The gastrin-releasing peptide/bombesin system revisited by a reverse-evolutionary study considering Xenopus. Sci. Rep. 2021, 11, 13315. [Google Scholar] [CrossRef]
- Fathi, Z.; Corjay, M.H.; Shapira, H.; Wada, E.; Benya, R.; Jensen, R.; Viallet, J.; Sausville, E.A.; Battey, J.F. BRS-3: A novel bombesin receptor subtype selectively expressed in testis and lung carcinoma cells. J. Biol. Chem. 1993, 268, 5979–5984. [Google Scholar] [CrossRef]
- Wada, E.; Way, J.; Lebacq-Verheyden, A.M.; Battey, J.F. Neuromedin B and gastrin-releasing peptide mRNA are differentially distributed in the rat nervous system. J. Neurosci. 1990, 10, 2917–2930. [Google Scholar] [CrossRef]
- Wang, H.; Bian, J.; Chen, Z.; Miao, Y.; Li, W. A novel bombesin-like peptide from skin of Rana shuchinae. Mol. Biol. Rep. 2011, 38, 3599–3603. [Google Scholar] [CrossRef]
- De Sousa, N.A.; Marani, M.M.; Lopes, A.L.F.; Silva, E.M.; Barbosa, E.A.; Vasconcelos, A.G.; Kuzniewski, F.T.B.; Lustosa, S.S.; Gomes, K.P.; Colugnati, D.B.; et al. BR-bombesin: A novel bombesin-related peptide from the skin secretion of the Chaco tree frog (Boana raniceps) with physiological gastric effects. Amino Acids 2022, 54, 733–747. [Google Scholar] [CrossRef] [PubMed]
- Miao, Y.; Li, W.; Duan, L.; Xiao, Y. A bombesin-like peptide from skin of Sanguirana varians. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2010, 155, 106–109. [Google Scholar] [CrossRef] [PubMed]
- Endean, R.; Erspamer, V.; Erspamer, G.F.; Improta, G.; Melchiorri, P.; Negri, L.; Sopranzi, N. Parallel bioassay of bombesin and litorin, a bombesin-like peptide from the skin of Litoria aurea. Br. J. Pharmacol. 1975, 55, 213–219. [Google Scholar] [CrossRef]
- Rrivier, C.; Rivier, J.; Vale, W. The effect of bombesin and related peptides on prolactin and growth hormone secretion in the rat. Endocrinology 1978, 102, 519–522. [Google Scholar] [CrossRef]
- Modlin, I.M.; Lamers, C.B.H.; Walsh, J.H. Stimulation of canine pancreatic polypeptides gastrin and gastric acid secretion by rantensin, litorin, bombesin on a peptide and substance P. Regul. Pept. 1981, 1, 279–288. [Google Scholar] [CrossRef]
- Mitsuma, T.; Nogimori, T.; Sun, D.H.; Chaya, M. Litorin (bombesin family) inhibits thyrotropin secretion in rats. Exp. Clin. Endocrinol. 1986, 87, 162–168. [Google Scholar] [CrossRef]
- de Caro, G.; Massi, M.; Micossi, L.G.; Perfumi, M. Drinking and feeding inhibition by ICV pulse injection or infusion of bombesin, ranatensin and litorin to rats. Peptides 1984, 5, 607–613. [Google Scholar] [CrossRef] [PubMed]
- Gibbs, J.; Kulkosky, P.J.; Smith, G.P. Effects of peripheral and central bombesin on feeding behavior of rats. Peptides 1981, 2, 179–183. [Google Scholar] [CrossRef] [PubMed]
- Kulkosky, P.J.; Sanchez, M.R.; Marrinan, D.A. Bombesin reduces alcohol choice in nutritive expectancy and limited-access procedures. Alcohol 1992, 9, 123–127. [Google Scholar] [CrossRef]
- Geller, R.G.; Govier, W.C.; Pisano, J.J.; Tanimura, T.; Van Clineschmidt, B. The action of ranatensin, a new polypeptide from amphibian skin, on the blood pressure of experimental animals. Br. J. Pharmacol. 1970, 40, 605–616. [Google Scholar] [CrossRef] [PubMed]
- Howard, J.M.; Jensen, R.T.; Gardner, J.D. Bombesin-induced residual stimulation of amylase release from mouse pancreatic acini. Am. J. Physiol. 1985, 248, G196–G199. [Google Scholar] [CrossRef]
- Jensen, R.T.; Gardner, J.D. Identification and characterization of receptors for secretagogues on pancreatic acinar cells. Federation Proc. 1981, 40, 2486–2496. [Google Scholar]
- Cline, M.A.; Fouse, D.N.; Prall, B.C. Central and peripheral alytesin cause short-term anorexigenic effects in neonatal chicks. Neuropeptides 2008, 42, 283–291. [Google Scholar] [CrossRef]
- Negri, L.; Improta, G.; Briccardo, M.; Melchiorri, P. Phyllolitorins: A new family of bombesin-like peptides. Ann. N. Y. Acad. Sci. 1988, 547, 415–428. [Google Scholar] [CrossRef]
- King, K.A.; Torday, J.S.; Sunday, M.E. Bombesin and [Leu8]phyllolitorin promote fetal mouse lung branching morphogenesis via a receptor-mediated mechanism. Proc. Natl. Acad. Sci. USA 1995, 92, 4357–4361. [Google Scholar] [CrossRef]
- Broccardo, M.; Cadamone, A. The effects of a new amphibian peptide, Leu8phyllolitorin, on thermoregulation in the rat. Peptides 1985, 6, 99–102. [Google Scholar] [CrossRef]
- Merali, Z.; Johnston, S.; Sistek, J. Role of dopaminergic system(s) in mediation of the behavioral effects of bombesin. Pharmacol. Biochem. Behav. 1985, 23, 243–248. [Google Scholar] [CrossRef]
- Van Wimersma Greidanus, T.B.; Maigret, C.; Torn, M.; Ronner, E.; Van der Kracht, S.; Van der Wee, N.J.; Versteeg, D.H. Dopamine D-1 and D-2 receptor agonists and antagonists and neuropeptide-induced excessive grooming. Eur. J. Pharmacol. 1989, 173, 227–231. [Google Scholar] [CrossRef] [PubMed]
- Van Wimersma Greidanus, T.B.; van de Brug, F.; de Bruijckere, L.M.; Pabst, P.H.; Ruesink, R.W.; Hulshof, R.L.; van Berckel, B.N.; Arissen, S.M.; de Koning, E.J.; Donker, D.K. Comparison of bombesin-, ACTH-, and beta-endorphin-induced grooming. Antagonism by haloperidol, naloxone, and neurotensin. Ann. N. Y. Acad. Sci. 1988, 525, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.Z.; Ji, X.Q.; Wu, S.X.; Zou, G. Sulpiride attenuates ranatensin-M-induced antinociception. Zhongguo Yao Li Xue Bao 1991, 12, 291–293. [Google Scholar]
- Laskowska, A.K.; Szudzik, M.; Ścieżyńska, A.; Komorowski, M.; Szűcs, E.; Gombos, D.; Bączek, B.; Lipka- Miciuk, J.; Benyhe, S.; Kleczkowska, P. The role of a natural amphibian skin-based peptide, ranatensin, in pancreatic cancers expressing dopamine D2 receptors. Cancers 2022, 14, 5535. [Google Scholar] [CrossRef]
- Merali, Z.; Graitson, S.; MacKay, J.C.; Kent, P. Stress and eating: A dual role for bombesin-like peptides. Front. Neurosci. 2013, 7, 193. [Google Scholar] [CrossRef]
- Roesler, R.; Luft, T.; Oliveira, S.H.S.; Farias, C.B.; Almeida, V.R.; Quevedo, J.; Dal Pizzol, F.; Schroder, N.; Izquierdo, I.; Schwartsmann, G. Molecular mechanisms mediating gastrin-releasing peptide receptor modulation of memory consolidation in the hippocampus. Neuropharmacology 2006, 51, 350–357. [Google Scholar] [CrossRef]
- Buhot, M.C. Serotonin receptors in cognitive behaviors. Curr. Opin. Neurobiol. 1997, 7, 243–254. [Google Scholar] [CrossRef]
- Chaouloff, F.; Berton, O.; Mormède, P. Serotonin and stress. Neuropsychopharmacology 1999, 21, 28–32. [Google Scholar] [CrossRef]
- Merali, Z.; Bedard, T.; Andrews, N.; Davis, B.; McKnight, A.T.; Gonzalez, M.I.; Pritchard, M.; Kent, P.; Anisman, H. Bombesin receptors as a novel anti-anxiety therapeutic target: BB1 receptor actions on anxiety through alterations of serotonin activity. J. Neurosci. 2006, 26, 10387–10396. [Google Scholar] [CrossRef]
- Pinnock, R.D.; Reynolds, T.; Woodruff, G.N. Different types of bombesin receptors on neurons in the dorsal raphe nucleus and the rostral hypothalamus in rat brain slices in vitro. Brain Res. 1994, 653, 119–124. [Google Scholar] [CrossRef] [PubMed]
- Piqueras, L.; Taché, Y.; Martínez, V. Somatostatin receptor type 2 mediates bombesin-induced inhibition of gastric acid secretion in mice. J. Physiol. 2003, 549, 889–901. [Google Scholar] [CrossRef]
- Liu, X.Y.; Ginosar, Y.; Yazdi, J.; Hincker, A.; Chen, Z.F. Cross-talk between human spinal cord μ-opioid receptor 1Y isoform and gastrin-releasing peptide receptor mediates opioid-induced scratching behavior. Anesthesiology 2019, 131, 381–391. [Google Scholar] [CrossRef]
- Gmerek, D.E.; Cowan, A. Role of opioid receptors in bombesin-induced grooming. Ann. N. Y. Acad. Sci. 1988, 525, 291–300. [Google Scholar] [CrossRef] [PubMed]
- Bungo, T.; Ando, R.; Kawakami, S.-I.; Ohgushi, A.; Shimojo, M.; Masuda, Y.; Furuse, M. Central bombesin inhibits food intake and the orexigenic effect of neuropeptide Y in the neonatal chick. Physiol. Behav. 2000, 70, 573–576. [Google Scholar] [CrossRef]
- Chang, M.M.; Leeman, S.E.; Niall, H.D. Amino-acid sequence of substance P. Nat. New Biol. 1971, 232, 86–87. [Google Scholar] [CrossRef] [PubMed]
- Grimsholm, O.; Rantapää-Dahlqvist, S.; Forsgren, S. Levels of gastrin-releasing peptide and substance P in synovial fluid and serum correlate with levels of cytokines in rheumatoid arthritis. Arthritis Res. Ther. 2005, 7, R416. [Google Scholar] [CrossRef]
- Regoli, D.; Dion, S.; Rhaleb, N.E.; Drapeau, G.; Rouissi, N.; Orléans-Juste, P. Receptors for neurokinins, tachykinins, and bombesin: A pharmacological study. Ann. N. Y. Acad. Sci. 1988, 547, 158–173. [Google Scholar] [CrossRef]
- Sakurada, T.; Manome, Y.; Katsumata, K.; Uchiumi, H.; Tan-No, K.; Sakurada, S.; Kisara, K. Naloxone-reversible effect of spantide on the spinally mediated behavioural response induced by neurokinin-2 and -3 receptor agonists. Naunyn Schmiedebergs Arch. Pharmacol. 1992, 346, 69–75. [Google Scholar] [CrossRef]
- Moura, E.G.; Santos, C.V.; Santos, R.M.; Pazos-Moura, C.C. Interaction between substance P and gastrin-releasing peptide on thyrotropin secretion by rat pituitary in vitro. Braz. J. Med. Biol. Res. 1999, 32, 1155–1160. [Google Scholar] [CrossRef]
- Laskowska, A.K.; Kleczkowska, P. Anticancer efficacy of endo- and exogenous potent ligands acting at dopaminergic receptor-expressing cancer cells. Eur. J. Pharmacol. 2022, 932, 175230. [Google Scholar] [CrossRef]
- Bruna-Larenas, T.; Gomez-Jeria, J.S. A DFT and semiempirical model-based study of opioid receptor affinity and selectivity in a group of molecules with a morphine structural core. Int. J. Med. Chem. 2012, 2012, 682495. [Google Scholar] [CrossRef]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 2001, 46, 3–26. [Google Scholar] [CrossRef]
- Akiyama, T.; Tominaga, M.; Takamori, K.; Carstens, M.I.; Carstens, E. Role of spinal bombesin-responsive neurons in nonhistaminergic itch. J. Neurophysiol. 2014, 112, 2283–2289. [Google Scholar] [CrossRef]
- Sun, Y.-G.; Zhao, Z.-Q.; Meng, X.-L.; Yin, J.; Liu, X.-Y.; Chen, Z.-F. Cellular basis of itch sensation. Science 2009, 325, 1531–1534. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Yang, H.; Wan, L.; Cai, H.; Li, S.; Li, Y.; Cheng, J.; Lu, X. Enhancement of cytotoxicity of antimicrobial peptide magainin II in tumor cells by bombesin-targeted delivery. Acta Pharmacol. Sin. 2011, 32, 79–88. [Google Scholar] [CrossRef]
- Lehmann, J.; Retz, M.; Sidhu, S.S.; Suttmann, H.; Sell, M.; Paulsen, F.; Harder, J.; Untergger, G.; Stockle, M. Antitumor activity of the antimicrobial peptide magainin II against bladder cancer cell lines. Eur. Urol. 2006, 50, 141–147. [Google Scholar] [CrossRef]
- Cruciani, R.A.; Barker, J.L.; Zasloff, M.; Chen, H.C.; Colamonici, O. Antibiotic magainins exert cytolytic activity against transformed cell lines through channel formation. Proc. Natl. Acad. Sci. USA 1991, 88, 3792–3796. [Google Scholar] [CrossRef] [PubMed]
- Serafin, P.; Kowalczyk, P.; Stefanucci, A.; Laskowska, A.K.; Zawadzka, M.; Kramkowski, K.; Kleczkowska, P. Evaluation of antimicrobial activities against various E. coli strains of a novel hybrid peptide—LENART01. Molecules 2023, 28, 4955. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Lai, R. The chemistry and biological activities of peptides from amphibian skin secretions. Chem. Rev. 2015, 115, 1760–1846. [Google Scholar] [CrossRef] [PubMed]
- Stein, C. Targeting pain and inflammation by peripherally acting opioids. Front. Pharmacol. 2013, 4, 123. [Google Scholar] [CrossRef]
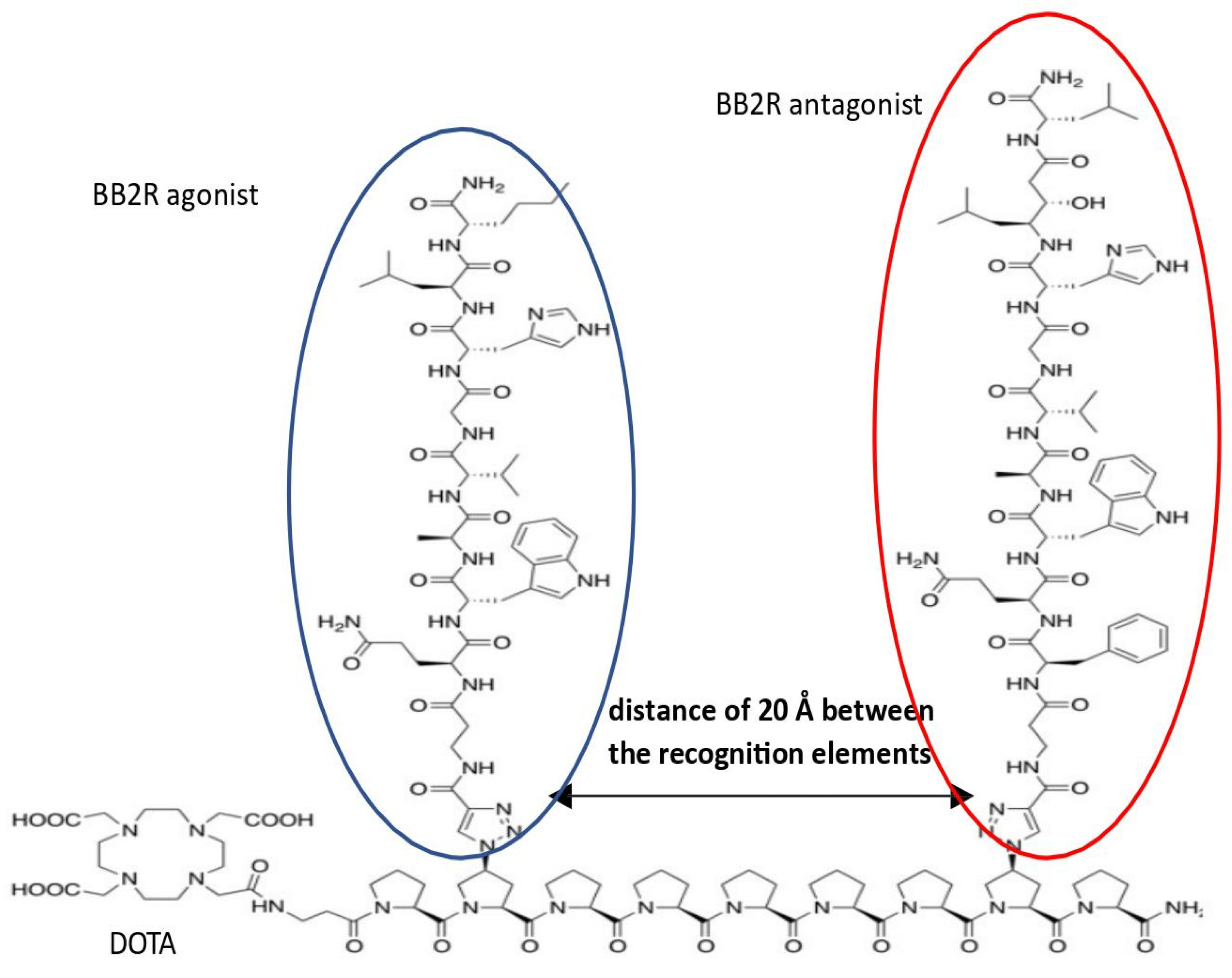
- Kroll, C.; Mansi, R.; Braun, F.; Dobitz, S.; Maecke, H.R.; Wennemers, H. Hybrid bombesin analogues: Combining an agonist and an antagonist in defined distances for optimized tumor targeting. J. Am. Chem. Soc. 2013, 135, 16793–16796. [Google Scholar] [CrossRef]
- Santos-Cuevas, C.L.; Ferro-Flores, G.; Arteaga de Murphy, C.; Ramirez, F.M.; Luna-Gutierez, M.A.; Pedraza-Lopez, M.; Garcia-Becerra, R.; Ordaz-Rosado, D. Design, preparation, in vitro and in vivo evaluation of (99m)Tc-N2S2-Tat(49-57)-bombesin: A target-specific hybrid radiopharmaceutical. Int. J. Pharm. 2009, 375, 75–83. [Google Scholar] [CrossRef]
- Begum, A.A.; Toth, I.; Moyle, P.M. Gastrin-releasing peptide receptor-targeted hybrid peptide/phospholipid pDNA/siRNA delivery systems. Nanomedicine 2019, 14, 1153–1171. [Google Scholar] [CrossRef] [PubMed]
- Nagy, A.; Armatis, P.; Cai, R.Z.; Szepeshazi, K.; Halmos, G.; Schally, A.V. Design, synthesis, and in vitro evaluation of cytotoxic analogs of bombesin-like peptides containing doxorubicin or its intensely potent derivative, 2-pyrrolinodoxorubicin. Proc. Natl. Acad. Sci. USA 1997, 94, 652–656. [Google Scholar] [CrossRef] [PubMed]
- Kiaris, H.; Schally, A.; Nagy, A.; Sun, B.; Armatis, P.; Szepeshazi, K. Targeted cytotoxic analogue of bombesin/gastrin-releasing peptide inhibits the growth of H-69 human small-cell lung carcinoma in nude mice. Br. J. Cancer 1999, 81, 966–971. [Google Scholar] [CrossRef]
- Gibbens-Bandala, B.; Morales-Avila, E.; Ferro-Flores, G.; Santos-Cuevas, C.; Melendez-Alafort, L.; Trujillo-Nolasco, M.; Ocampo-Garcia, B. 177Lu-Bombesin-PLGA (paclitaxel): A targeted controlled-release nanomedicine for bimodal therapy of breast cancer. Mater. Sci. Eng. C 2019, 105, 110043. [Google Scholar] [CrossRef]
- Yang, S.; Zhu, J.; Lu, Y.; Liang, B.; Yang, C. Body distribution of camptothecin solid lipid nanoparticles after oral administration. Pharm. Res. 1999, 1, 751–757. [Google Scholar] [CrossRef]
- Mi, Z.; Burke, T.G. Differential interactions of camptothecin lactone and carboxylate forms with human blood components. Biochemistry 1994, 33, 10325–10336. [Google Scholar] [CrossRef]
- Gottlieb, J.A.; Luce, J.K. Treatment of malignant melanoma with camptothecin (NSC-100880). Cancer Chemother. Rep. 1972, 56, 103–105. [Google Scholar] [PubMed]
- Muggia, F.M.; Creaven, P.J.; Hansen, H.H.; Cohen, M.H.; Selawry, O.S. Phase I trial of weekly and daily treatment with camptothecin (NSC-100880): Correlation with preclinical studies. Cancer Chemother. Rep. 1972, 56, 515–521. [Google Scholar]
- Moody, T.W.; Mantey, S.A.; Pradhan, T.K.; Schumann, R.; Nakagawa, T.; Martinez, A.; Fusilier, J.; Coy, D.H.; Jensen, R.T. Development of high affinity camptothecin-bombesin conjugates that have targeted cytotoxicity for bombesin receptor-containing tumor cells. J. Biol. Chem. 2004, 279, 23580–23589. [Google Scholar] [CrossRef] [PubMed]
- Moody, T.W.; Sun, L.C.; Mantey, S.A.; Pradhan, T.; Mackey, L.V.; Gonzales, N.; Fuselier, J.A.; Coy, D.H.; Jensen, R.T. In vitro and in vivo antitumor effects of cytotoxic camptothecin-bombesin conjugates are mediated by specific interaction with cellular bombesin receptors. J. Pharmacol. Exp. Ther. 2006, 318, 1265–1272. [Google Scholar] [CrossRef] [PubMed]
- Aranda-Lara, L.; Ferro-Flores, G.; Ramirez, F.M.; Ocampo-Garcia, B.; Santos-Cueva, C.; Diaz-Nieto, L.; Isaac-Olive, K. Improved radiopharmaceutical based on 99mTc-Bombesin-folate for breast tumour imaging. Nucl. Med. Commun. 2016, 37, 377–386. [Google Scholar] [CrossRef] [PubMed]
- Pieroth, R.; Paver, S.; Day, S.; Lammersfeld, C. Folate and its impact on cancer risk. Curr. Nutr. Rep. 2018, 7, 70–84. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, P.; Chen, H.; Wei, B.; Xiao, X.; Da, J.; Skinner, K.; Hicks, D.G.; Bu, H.; Tang, P.; et al. Folate receptor [alpha] associated with triple-negative breast cancer and poor prognosis. Arch. Pathol. Lab. Med. 2014, 138, 890–895. [Google Scholar] [CrossRef]
- Accardo, A.; Salsano, G.; Morisco, A.; Aurilio, M.; Parisi, A.; Maione, F.; Cicala, C.; Tesauro, D.; Aloj, L.; De Rosa, G.; et al. Peptide-modified liposomes for selective targeting of bombesin receptors overexpressed by cancer cells: A potential theranostic agent. Int. J. Nanomed. 2012, 7, 2007–2017. [Google Scholar]
- Wang, C.; Sun, X.; Wang, K.; Wang, Y.; Yang, F.; Wang, H. Breast cancer targeted chemotherapy based on doxorubicin-loaded bombesin peptide modified nanocarriers. Drug Deliv. 2016, 23, 2697–2702. [Google Scholar] [CrossRef]


| Peptide | Amino Acid Sequence | Affinities at Bombesin Receptor Subtypes * IC50 [nM] | ||
|---|---|---|---|---|
| BB1 | BB2 | BB3 | ||
| Bombesin (BN) | pGlu-Gln-Arg-Leu-Gly-Asn-Gln-Trp-Ala-Val-Gly-His-Leu-Met-NH2 | 1.77 ± 0.04 | 0.07 ± 0.01 | >3000 |
| Gastrin-releasing peptide (GRP) | Ala-Pro-Val-Ser-Val-Gly-Gly-Gly-Thr-Val-Leu-Ala-Lys-Met-Tyr-Pro-Arg-Gly-Asn-His-Trp-Ala-Val-Gly-His-Leu-Met-NH2 | 148.0 ± 8.00 | 0.17 ± 0.01 | >3000 |
| Neuromedin B | Gly-Asn-Leu-Trp-Ala-Thr-Gly-His-Phe-Met-NH2 | 0.052 ± 0.003 | 50.1 ± 2.50 | >3000 |
| Peptide | Amino Acid Sequence | Affinities at Bombesin Receptor Subtypes * IC50 [nM] | ||
|---|---|---|---|---|
| BB1 | BB2 | BB3 | ||
| Bombesin (BN) | pGlu-Gln-Arg-Leu-Gly-Asn-Gln-Trp-Ala-Val-Gly-His-Leu-Met-NH2 | 34 | 4 | >10,000 |
| Alytensin | pGlu-Gly-Arg-Leu-Gly-Thr-Gln-Trp-Ala-Val-Gly-His-Leu-Met-NH2 | 460 | 62 | >10,000 |
| Litorin | pGlu-Gln-Trp-Ala-Val-Gly-His-Phe-Met-NH2 | 7 | 6 | >10,000 |
| Phyllolitorin | pGlu-Leu-Trp-Ala-Val-Gly-Ser-Phe-Met-NH2 | 47 | 240 | >10,000 |
| [Leu8]-phyllolitorin | pGlu-Leu-Trp-Ala-Val-Gly-Ser-Leu-Met-NH2 | 372 | 295 | >3000 |
| [Thr5,Leu8]-phyllolitorin (R-phyllolitorin) | pGlu-Leu-Trp-Ala-Thr-Gly-Ser-Leu-Met-NH2 | unknown | unknown | unknown |
| Ranatensin | pGlu-Val-Pro-Gln-Trp-Ala-Val-Gly-His-Phe-Met-NH2 | 13 | 2 | >10,000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Serafin, P.; Kleczkowska, P. Bombesins: A New Frontier in Hybrid Compound Development. Pharmaceutics 2023, 15, 2597. https://doi.org/10.3390/pharmaceutics15112597
Serafin P, Kleczkowska P. Bombesins: A New Frontier in Hybrid Compound Development. Pharmaceutics. 2023; 15(11):2597. https://doi.org/10.3390/pharmaceutics15112597
Chicago/Turabian StyleSerafin, Pawel, and Patrycja Kleczkowska. 2023. "Bombesins: A New Frontier in Hybrid Compound Development" Pharmaceutics 15, no. 11: 2597. https://doi.org/10.3390/pharmaceutics15112597





