Antitumoral and Antimicrobial Activities of Block Copolymer Micelles Containing Gold Bisdithiolate Complexes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation and Characterization of BCMs
2.2. Drug Loading Content and Efficiency
2.3. Sizes and Zeta Potentials of Micelles
2.4. Release Study
2.5. Cells and Cell-Culture Media
2.6. Determination of Cytotoxic Activity
2.7. Cellular Uptake Analysis
2.8. Antimicrobial Activities of BCMs
3. Results and Discussion
3.1. Synthesis and Characterization of Block Copolymer Micelles
3.2. Biological Studies
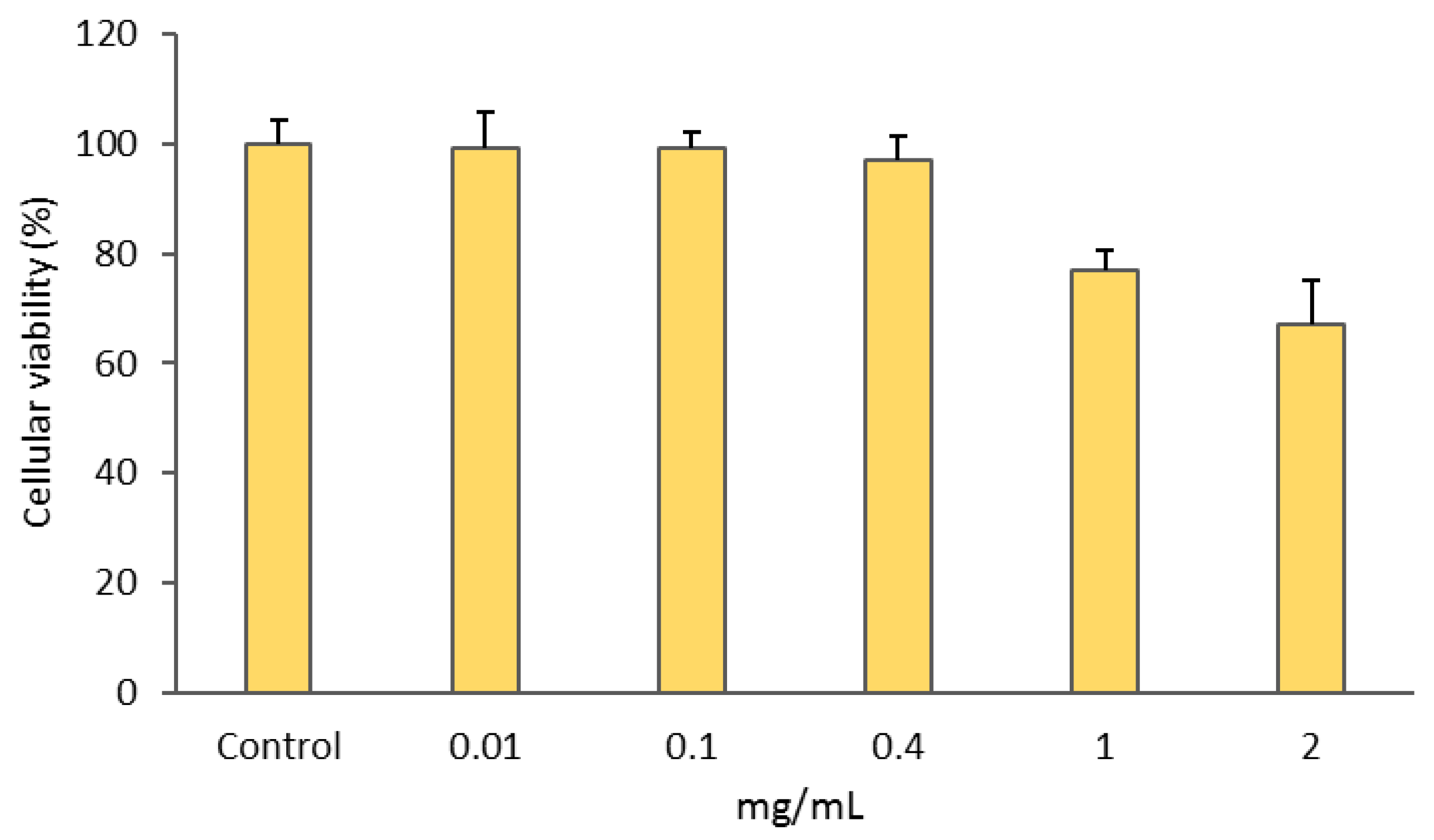
3.2.1. Cytotoxic Activity
3.2.2. Quantification of Cellular Uptake of Free and Encapsulated [Au(cdc)2]− by PIXE
3.2.3. Antimicrobial Activities
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- El-Say, K.M.; El-Sawy, H.S. Polymeric nanoparticles: Promising platform for drug delivery. Int. J. Pharm. 2017, 528, 675–691. [Google Scholar] [CrossRef] [PubMed]
- de Jong, W.H.; Borm, P.J. Drug delivery and nanoparticles: Applications and hazards. Int. J. Nanomed. 2008, 3, 133–149. [Google Scholar] [CrossRef]
- Jhaveri, A.M.; Torchilin, V.P. Multifunctional polymeric micelles for delivery of drugs and siRNA. Front. Pharmacol. 2014, 5, 77. [Google Scholar] [CrossRef] [PubMed]
- Cabral, H.; Miyata, K.; Osada, K.; Kataoka, K. Block Copolymer Micelles in Nanomedicine Applications. Chem. Rev. 2018, 118, 6844–6892. [Google Scholar] [CrossRef] [PubMed]
- Hwang, D.; Ramsey, J.D.; Kabanov, A.V. Polymeric micelles for the delivery of poorly soluble drugs: From nanoformulation to clinical approval. Adv. Drug Deliv. Rev. 2020, 156, 80–118. [Google Scholar] [CrossRef]
- Pelaz, B.; Alexiou, C.; Alvarez-Puebla, R.A.; Alves, F.; Andrews, A.M.; Ashraf, S.; Balogh, L.P.; Ballerini, L.; Bestetti, A.; Brendel, C.; et al. Diverse Applications of Nanomedicine. ACS Nano 2017, 11, 2313–2381. [Google Scholar] [CrossRef]
- Khodabandehloo, H.; Zahednasab, H.; Hafez, A.A. Nanocarriers usage for drug delivery in cancer therapy. Int. J. Cancer Manag. 2016, 9, e3966. [Google Scholar] [CrossRef]
- Fricker, S.P. Medical Uses of Gold Compounds: Past, Present and Future. Gold Bull. 1996, 29, 53–60. [Google Scholar] [CrossRef]
- Berners-Price, S.J.; Filipovska, A. Gold compounds as therapeutic agents for human diseases. Metallomics 2011, 3, 863–873. [Google Scholar] [CrossRef]
- Yeo, C.I.; Ooi, K.K.; Tiekink, E.R.T. Gold-based medicine: A paradigm shift in anti-cancer therapy? Molecules 2018, 23, 1410. [Google Scholar] [CrossRef] [Green Version]
- Roder, C.; Thomson, M.J. Auranofin: Repurposing an Old Drug for a Golden New Age. Drugs R&D 2015, 15, 13–20. [Google Scholar] [CrossRef]
- Onodera, T.; Momose, I.; Kawada, M. Potential Anticancer Activity of Auranofin. Chem. Pharm. Bull. 2019, 67, 186–191. [Google Scholar] [CrossRef] [PubMed]
- Cassetta, M.I.; Marzo, T.; Fallani, S.; Novelli, A.; Messori, L. Drug repositioning: Auranofin as a prospective antimicrobial agent for the treatment of severe staphylococcal infections. BioMetals 2014, 27, 787–791. [Google Scholar] [CrossRef] [PubMed]
- Radisavljević, S.; Petrović, B. Gold(III) Complexes: An Overview on Their Kinetics, Interactions With DNA/BSA, Cytotoxic Activity, and Computational Calculations. Front. Chem. 2020, 8, 379. [Google Scholar] [CrossRef]
- Kim, J.H.; Reeder, E.; Parkin, S.; Awuah, S.G. Gold(I/III)-Phosphine Complexes as Potent Antiproliferative Agents. Sci. Rep. 2019, 9, 12335. [Google Scholar] [CrossRef]
- Dasari, S.; Bernard Tchounwou, P. Cisplatin in cancer therapy: Molecular mechanisms of action. Eur. J. Pharmacol. 2014, 740, 364–378. [Google Scholar] [CrossRef]
- Ott, I.; Gust, R. Non platinum metal complexes as anti-cancer drugs. Arch. Pharm. 2007, 340, 117–126. [Google Scholar] [CrossRef]
- Nobili, S.; Mini, E.; Landini, I.; Gabbiani, C.; Casini, A.; Messori, L. Gold compounds as anticancer agents: Chemistry, cellular pharmacology, and preclinical studies. Med. Res. Rev. 2010, 30, 550–580. [Google Scholar] [CrossRef]
- Bindoli, A.; Rigobello, M.P.; Scutari, G.; Gabbiani, C.; Casini, A.; Messori, L. Thioredoxin reductase: A target for gold compounds acting as potential anticancer drugs. Coord. Chem. Rev. 2009, 253, 1692–1707. [Google Scholar] [CrossRef]
- Glišić, B.; Djuran, M.I. Gold complexes as antimicrobial agents: An overview of different biological activities in relation to the oxidation state of the gold ion and the ligand structure. J. Chem. Soc. Dalton Trans. 2014, 43, 5950–5969. [Google Scholar] [CrossRef]
- Jacobsen, H.; Correa, A.; Poater, A.; Costabile, C.; Cavallo, L. Understanding the M—(NHC) (NHC = N-heterocyclic carbene) bond. Coord. Chem. Rev. 2009, 253, 687–703. [Google Scholar] [CrossRef]
- Dominelli, B.; Correia, J.D.G.; Kühn, F.E. Medicinal Applications of Gold(I/III)-Based Complexes Bearing N-Heterocyclic Carbene and Phosphine Ligands. J. Organomet. Chem. 2018, 866, 153–164. [Google Scholar] [CrossRef]
- Mora, M.; Gimeno, M.C.; Visbal, R. Recent advances in gold-NHC complexes with biological properties. Chem. Soc. Rev. 2019, 48, 447–462. [Google Scholar] [CrossRef] [PubMed]
- Pintus, A.; Aragoni, M.C.; Cinellu, M.A.; Maiore, L.; Isaia, F.; Lippolis, V.; Orrù, G.; Tuveri, E.; Zucca, A.; Arca, M. [Au(pyb-H)(mnt)]: A novel gold(III) 1,2-dithiolene cyclometalated complex with antimicrobial activity (pyb-H = C-deprotonated 2-benzylpyridine; mnt = 1,2-dicyanoethene-1,2-dithiolate). J. Inorg. Biochem. 2017, 170, 188–194. [Google Scholar] [CrossRef] [PubMed]
- Sousa, S.A.; Leitão, J.H.; Silva, R.A.; Belo, D.; Santos, I.C.; Guerreiro, J.F.; Martins, M.; Fontinha, D.; Prudêncio, M.; Almeida, M.; et al. On the path to gold: Monoanionic Au bisdithiolate complexes with antimicrobial and antitumor activities. J. Inorg. Biochem. 2020, 202, 110904. [Google Scholar] [CrossRef] [PubMed]
- Fontinha, D.; Sousa, S.A.; Morais, T.S.; Prudêncio, M.; Leitão, J.H.; Gal, Y.L.; Lorcy, D.; Silva, R.A.L.; Velho, M.F.G.; Belo, D.; et al. Gold(iii) bis(dithiolene) complexes: From molecular conductors to prospective anticancer, antimicrobial and antiplasmodial agents. Metallomics 2020, 12, 974–987. [Google Scholar] [CrossRef]
- Gheybi, H.; Niknejad, H.; Entezami, A.A. Polymer-metal complex nanoparticles-containing cisplatin and amphiphilic block copolymer for anticancer drug delivery. Des. Monomers Polym. 2014, 17, 334–344. [Google Scholar] [CrossRef]
- Adams, M.L.; Lavasanifar, A.; Kwon, G.S. Amphiphilic Block Copolymers for Drug Delivery. Pharm. Assoc. J. Pharm. Sci. 2003, 92, 1343–1355. [Google Scholar] [CrossRef]
- Wang, J.; Yao, K.; Wang, C.; Tang, C.; Jiang, X. Synthesis and drug delivery of novel amphiphilic block copolymers containing hydrophobic dehydroabietic moiety. J. Mater. Chem. B 2013, 1, 2324–2332. [Google Scholar] [CrossRef]
- Pearson, S.; Scarano, W.; Stenzel, M.H. Micelles based on gold-glycopolymer complexes as new chemotherapy drug delivery agents. Chem. Commun. 2012, 48, 4695–4697. [Google Scholar] [CrossRef]
- Narvekar, M.; Xue, H.Y.; Eoh, J.Y.; Wong, H.L. Nanocarrier for poorly water-soluble anticancer drugs-Barriers of translation and solutions. AAPS PharmSciTech 2014, 15, 822–833. [Google Scholar] [CrossRef] [PubMed]
- Belo, D.; Rodrigues, C.; Santos, I.C.; Silva, S.; Eusébio, T.; Lopes, E.B.; Rodrigues, J.V.; Matos, M.J.; Almeida, M.; Duarte, M.T.; et al. Synthesis, crystal structure and magnetic properties of bis(3,4;3′,4′-ethylenedithio)2,2′,5,5′- tetrathiafulvalene-bis(cyanoimidodithiocarbonate)aurate(III), (bedt-ttf)[Au(cdc)2]. Polyhedron 2006, 25, 1209–1214. [Google Scholar] [CrossRef]
- Ribeiro, E.; Alho, I.; Marques, F.; Gano, L.; Correia, I.; Correia, J.D.; Casimiro, S.; Costa, L.; Santos, I.; Fernandes, C. Radiolabeled block copolymer micelles for image-guided drug delivery. Int. J. Pharm. 2016, 515, 692–701. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zeng, F.; Allen, C. In vivo fate of unimers and micelles of a poly(ethylene glycol)-block-poly(caprolactone) copolymer in mice following intravenous administration. Eur. J. Pharm. Biopharm. 2007, 65, 309–319. [Google Scholar] [CrossRef] [PubMed]
- Hoang, B.; Reilly, R.M.; Allen, C. Block copolymer micelles target auger electron radiotherapy to the nucleus of HER2-positive breast cancer cells. Biomacromolecules 2012, 13, 455–465. [Google Scholar] [CrossRef]
- Zhao, J.; Wu, C.; Abbruzzese, J.; Hwang, R.F.; Li, C. Cyclopamine-loaded core-cross-linked polymeric micelles enhance radiation response in pancreatic cancer and pancreatic stellate cells. Mol. Pharm. 2015, 12, 2093–2100. [Google Scholar] [CrossRef]
- Lincha, V.R.; Zhao, J.; Wen, X.; Xiong, C.; Chow, D.S.-L.; Li, C. A polymeric micellar drug delivery system developed through a design of Experiment approach improves pancreatic tumor accumulation of calcipotriol and paclitaxel. Int. J. Pharm. 2021, 601, 120523. [Google Scholar] [CrossRef]
- Arendrup, M.C.; Cuenca-Estrella, M.; Lass-Flörl, C.; Hope, W.; EUCAST-AFST. EUCAST technical note on the EUCAST definitive document EDef 7.2: Method for the determination of broth dilution minimum inhibitory concentrations of antifungal agents for yeasts EDef 7.2 (EUCAST-AFST). Clin. Microbiol. Infect. 2012, 18, E246–E247. [Google Scholar] [CrossRef]
- Costa, J.P.; Pinheiro, T.; Martins, M.S.; Carvalho, M.F.N.N.; Feliciano, J.R.; Leitão, J.H.; Silva, R.A.L.; Guerreiro, J.F.; Alves, L.M.C.; Custódio, I.; et al. Tuning the Biological Activity of Camphorimine Complexes through Metal Selection. Antibiotics 2022, 11, 1010. [Google Scholar] [CrossRef]
- Borst, A.; Raimer, M.T.; Warnock, D.W.; Morrison, C.J.; Arthington-Skaggs, B.A. Rapid acquisition of stable azole resistance by Candida glabrata isolates obtained before the clinical introduction of fluconazole. Antimicrob. Agents Chemother. 2005, 49, 783–787. [Google Scholar] [CrossRef] [Green Version]
- Duthie, E.S.; Lorenz, L.L. Staphylococcal Coagulase: Mode of Action and Antigenicity. J. Gen. Microbiol. 1952, 6, 95–107. [Google Scholar] [CrossRef] [PubMed]
- Díez-Martínez, R.; García-Fernández, E.; Manzano, M.; Martínez, Á.; Domenech, M.; Vallet-Regí, M.; García, P. Auranofin-loaded nanoparticles as a new therapeutic tool to fight streptococcal infections. Sci. Rep. 2016, 6, 19525. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, E.; Marques, F.; Gano, L.; Correia, J.D.G.; Santos, I.; Fernandes, C. Docetaxel-loaded block copolymer micelles labeled with 188Re for combined radiochemotherapy. J. Drug. Deliv. Sci. Technol. 2020, 60, 101898. [Google Scholar] [CrossRef]
- Nosrati, H.; Barzegari, P.; Danafar, H.; Kheiri Manjili, H. Biotin-functionalized copolymeric PEG-PCL micelles for in vivo tumour-targeted delivery of artemisinin. Artif. Cells Nanomed. Biotechnol. 2019, 47, 104–114. [Google Scholar] [CrossRef] [PubMed]
- Rashidi, M.; Seghatoleslam, A.; Namavari, M.; Amiri, A.; Fahmidehkar, M.A.; Ramezani, A.; Eftekhar, E.; Hosseini, A.; Erfani, N.; Fakher, S. Selective cytotoxicity and apoptosis-induction of Cyrtopodion scabrum extract against digestive cancer cell lines. Int. J. Cancer Manag. 2017, 10, e8633. [Google Scholar] [CrossRef]
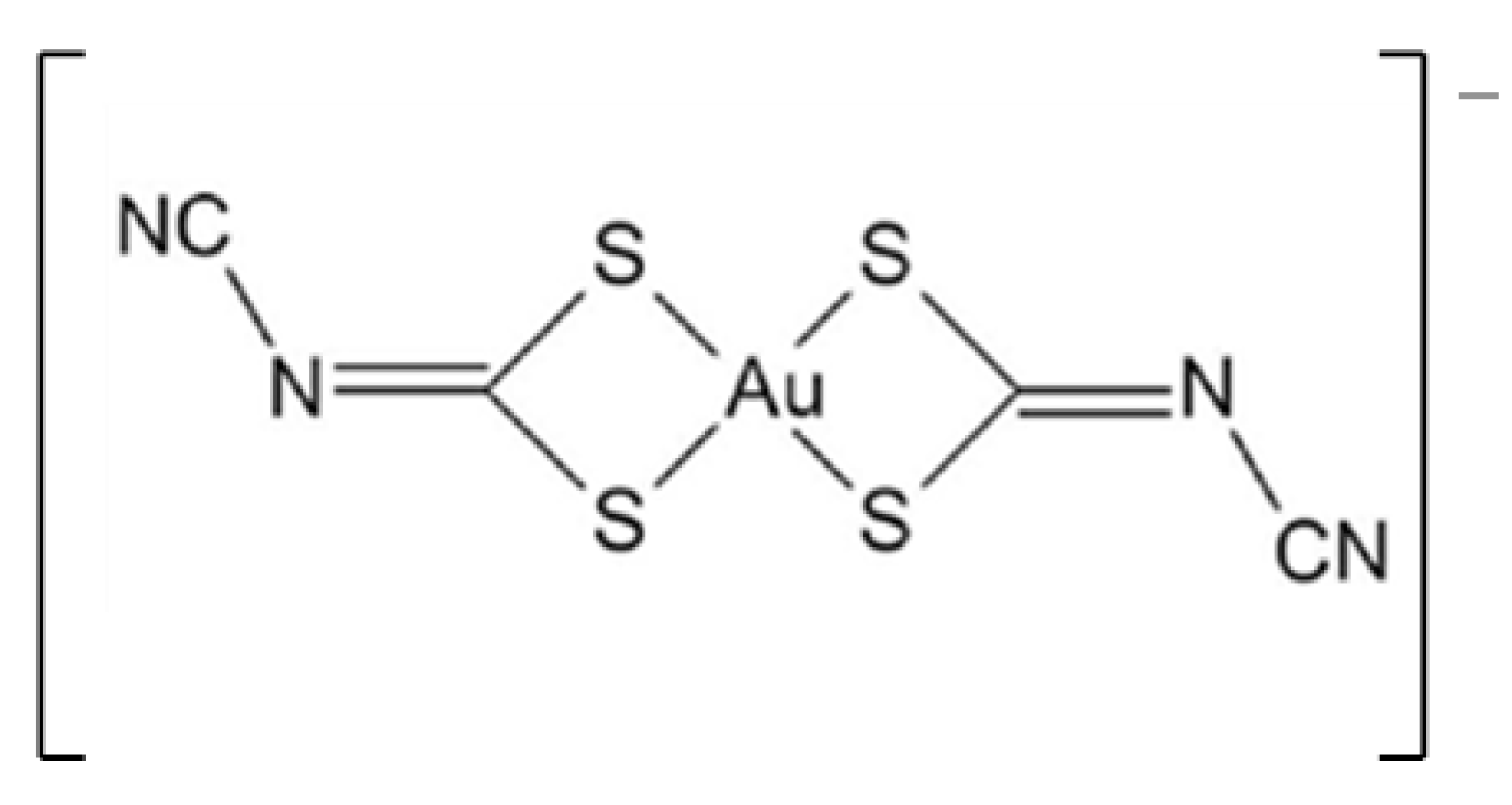
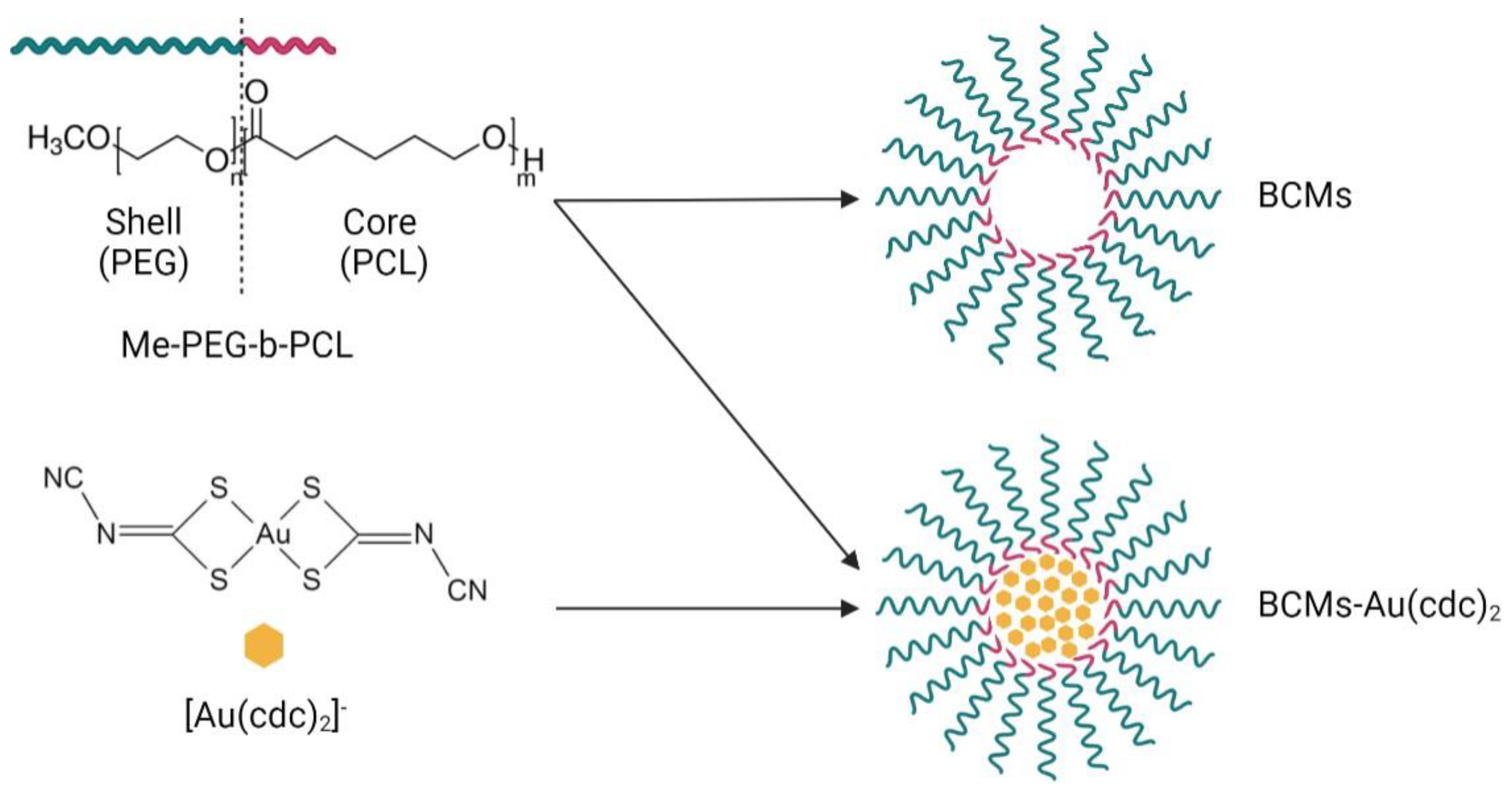
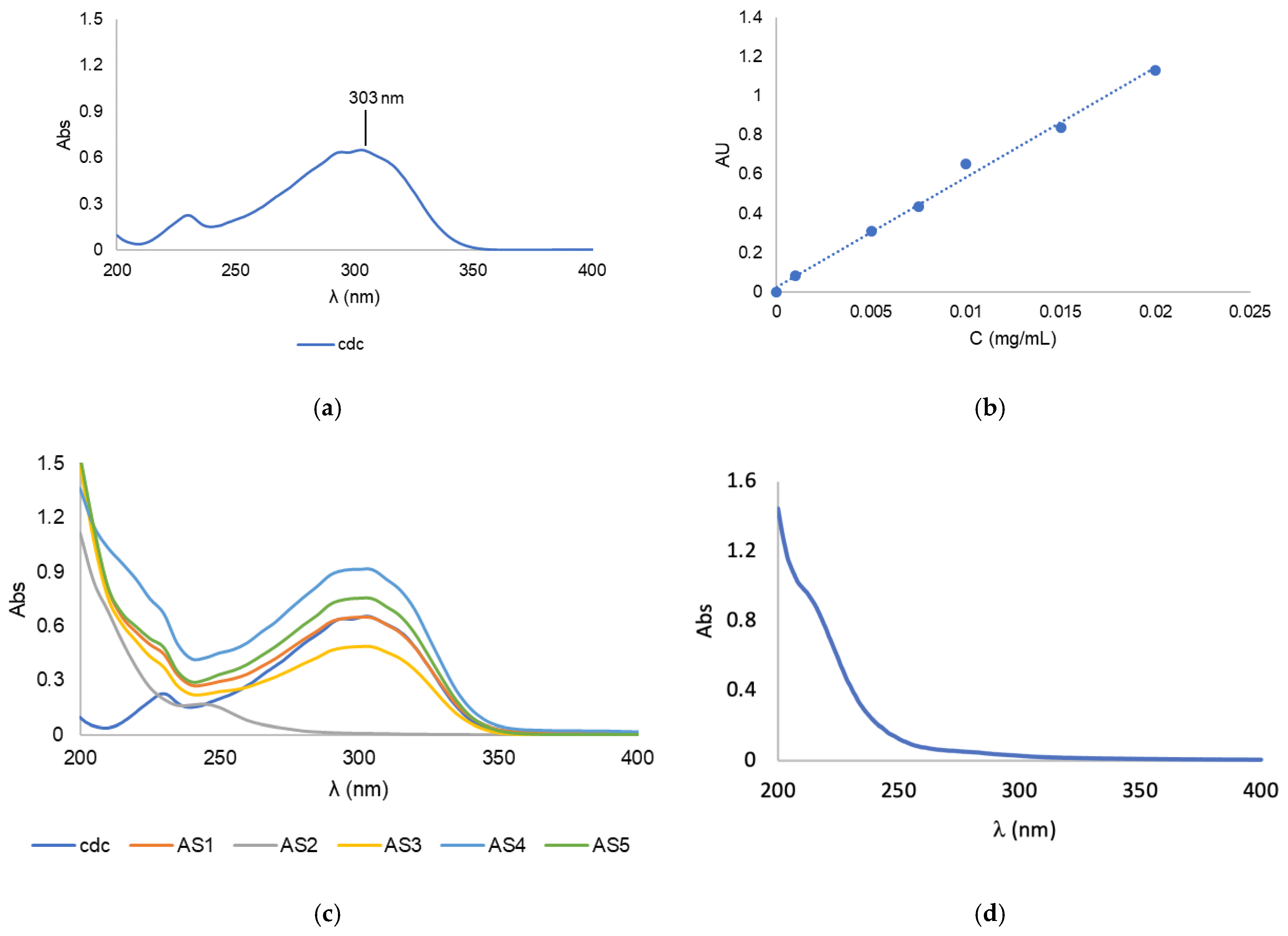

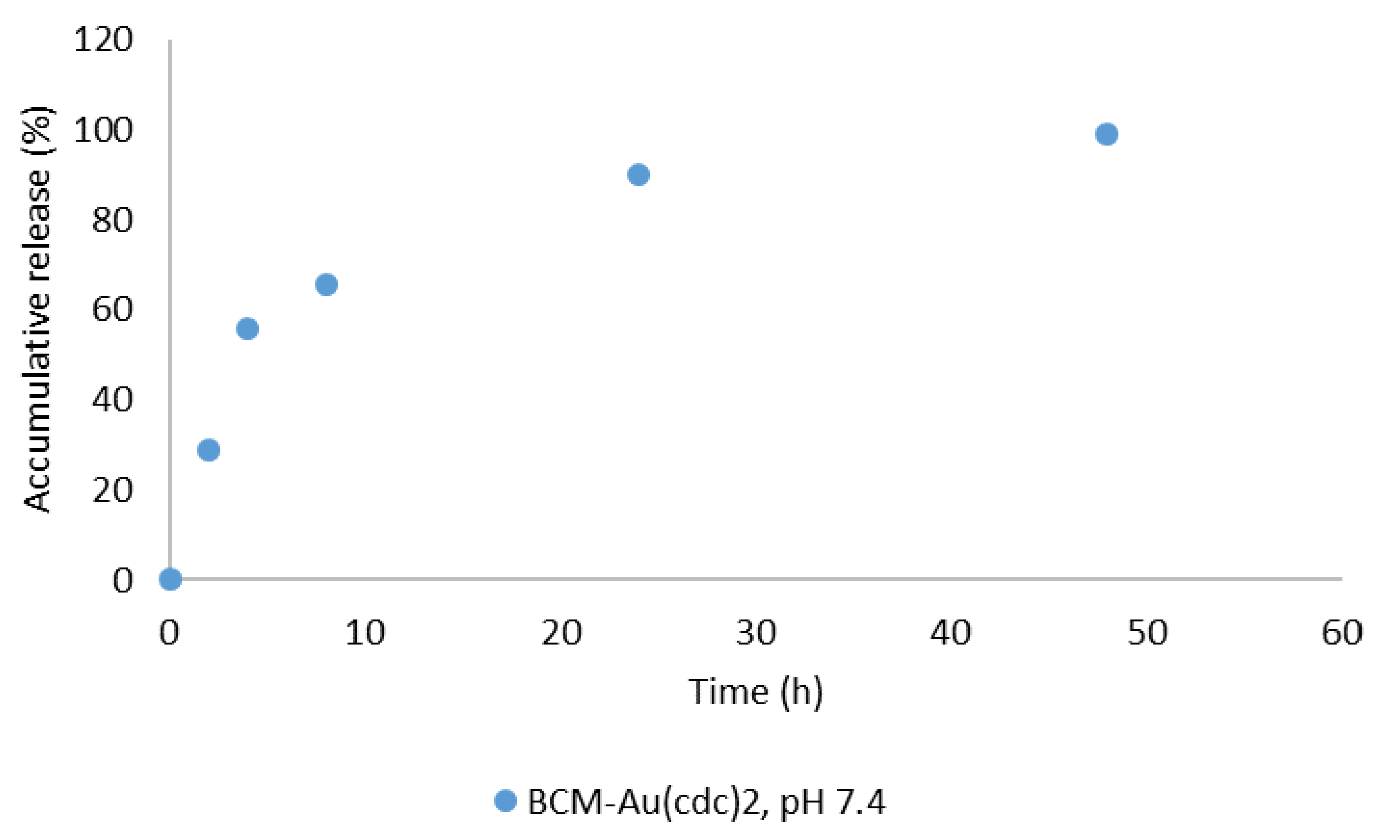
| Complex (mg) | Polymer (mg) | Solvents for Complex Dissolution | Solvents for Thin-Film Hydration | |
|---|---|---|---|---|
| AS1 | 2 | 25 | DMF + DCM | PBS |
| AS2 | ACN | H2O | ||
| AS3 | ACN | PBS | ||
| AS4 | CHCl3 | H2O | ||
| AS5 | CHCl3 | PBS |
| mtotal Au(cdc) (mg) | mBCM (mg) | LE (%) | LC (mgcdc/gBCM) | |
|---|---|---|---|---|
| AS1 | 0.145 | 34.5 | 7.3 | 4.21 |
| AS2 | 0.000 | 5.9 | 0.0 | 0.00 |
| AS3 | 0.070 | 22.8 | 3.5 | 3.05 |
| AS4 | 0.096 | 16.9 | 4.8 | 5.68 |
| AS5 | 0.118 | 22.4 | 5.9 | 5.27 |
| mAu(cdc) (mg) | LE (%) | LC (mg[Au(cdc)2]−/gBCM) | |
|---|---|---|---|
| AS6 | 2 | 64.59 | 35.29 |
| AS7 | 4 | 7.81 | 8.47 |
| AS8 | 8 | 3.34 | 7.11 |
| AS9 | 1 | 65.68 | 24.25 |
| dh (nm) | PdI | Zp (mV) | |
|---|---|---|---|
| AS1 | 50.96 ± 20.06 | 0.42 | −63.03 ± 9.75 |
| AS4 | 70.56 ± 35.49 | 0.30 | −51.10 ± 9.92 |
| AS6 | 77.31 ± 27.00 | 0.18 | −57.20 ± 12.10 |
| Compound | IC50 (µM) | ||
|---|---|---|---|
| A2780 | A2780cisR | V79 | |
| [Au(cdc)2]− | 1.11 ± 0.24 | 1.63 ± 0.41 | 3.36 ± 0.92 |
| BCM-[Au(cdc)2] | 1.83 ± 0.62 | 1.69 ± 0.43 | 3.65 ± 0.95 |
| Compound | Celular Uptake ng Au/106 A2780 Cells |
|---|---|
| [Au(cdc)2]− | 321 ± 16 |
| BCM-[Au(cdc)2] | 387 ± 13 |
| MIC (µg/mL) | ||||
|---|---|---|---|---|
| BCMs-[Au(cdc)2]− | BCMs | Free [Au(cdc)2]− | ||
| Total | [Au(cdc)2]− Amount | |||
| S. aureus Newman | 498.9 ± 142.9 | 18.7 ± 5.4 | >1250 | 15.3 ± 1.3 * |
| C. glabrata CBS138 | 490.5 ± 241.8 | 18.9 ± 8.4 | >1250 | 7.0 ± 0.8 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sousa, A.; Santos, J.F.; Silva, F.; Sousa, S.A.; Leitão, J.H.; Matos, A.P.; Pinheiro, T.; Silva, R.A.L.; Belo, D.; Almeida, M.; et al. Antitumoral and Antimicrobial Activities of Block Copolymer Micelles Containing Gold Bisdithiolate Complexes. Pharmaceutics 2023, 15, 564. https://doi.org/10.3390/pharmaceutics15020564
Sousa A, Santos JF, Silva F, Sousa SA, Leitão JH, Matos AP, Pinheiro T, Silva RAL, Belo D, Almeida M, et al. Antitumoral and Antimicrobial Activities of Block Copolymer Micelles Containing Gold Bisdithiolate Complexes. Pharmaceutics. 2023; 15(2):564. https://doi.org/10.3390/pharmaceutics15020564
Chicago/Turabian StyleSousa, Andreia, Joana F. Santos, Francisco Silva, Sílvia A. Sousa, Jorge H. Leitão, António P. Matos, Teresa Pinheiro, Rafaela A. L. Silva, Dulce Belo, Manuel Almeida, and et al. 2023. "Antitumoral and Antimicrobial Activities of Block Copolymer Micelles Containing Gold Bisdithiolate Complexes" Pharmaceutics 15, no. 2: 564. https://doi.org/10.3390/pharmaceutics15020564







