The Effect of Curcumin-Loaded Glucan Nanoparticles on Immune Cells: Size as a Critical Quality Attribute
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Particle Production
2.3. Particle Characterization
2.3.1. Size and Zeta Potential Measurements
2.3.2. Estimation of (1-3)-β-Glucan Content in the NPs
2.3.3. Quantification of Curcumin Loading Efficacy in Curcumin-Loaded Nanoparticles
2.3.4. Assessment of Physicochemical Stability of Curcumin-Loaded Glucan NPs at Two Different Temperatures
2.4. Hemocompatibility Studies
2.4.1. Hemolysis Assay
2.4.2. Coagulation Time Assay
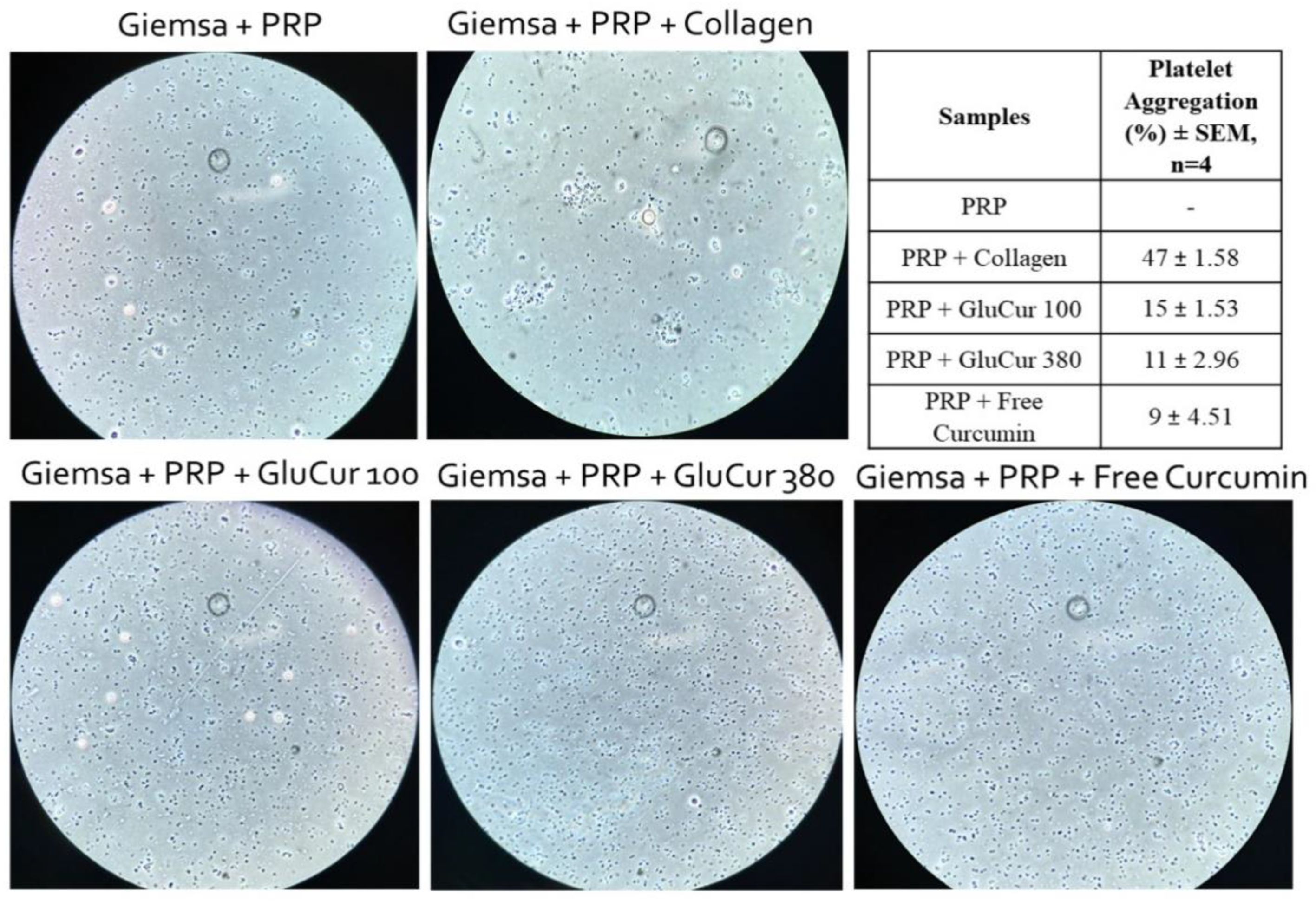
2.4.3. Platelet Aggregation Assay
2.5. In Vitro Studies with Human Peripheral Blood Mononuclear Cells
2.5.1. PBMCs Isolation by Density Gradient
2.5.2. Nanoparticle Cytotoxicity
2.5.3. Cell Proliferation Assay
2.6. Generation of Mo-DCs and Cytokines’ Assessment
2.7. In Vitro Studies with a Murine Macrophage Cell Line (RAW 264.7)
2.7.1. Nanoparticle Cytotoxicity
2.7.2. Nitric Oxide Production Assay
2.7.3. Reactive Oxygen Species Production Assay
2.7.4. Cytokine Secretion
2.8. Statistical Analysis
3. Results
3.1. Lower Concentration of Curdlan during NP Preparation Leads to Smaller-Sized and Highly Stable Curcumin-Loaded NPs
3.2. GluCur 380 NPs Present a Better Hemocompatibility Profile Than the Smaller-Sized GluCur 100 NPs
3.3. Smaller-Sized Curcumin-Loaded Glucan NPs Report a High Cytotoxic Ability Compared to GluCur 380 NPs in Human PBMCs
3.4. Both, Curcumin Nanocarriers Reveal a Tendency to Stimulate Human Dendritic Cells to Produce TNF-α
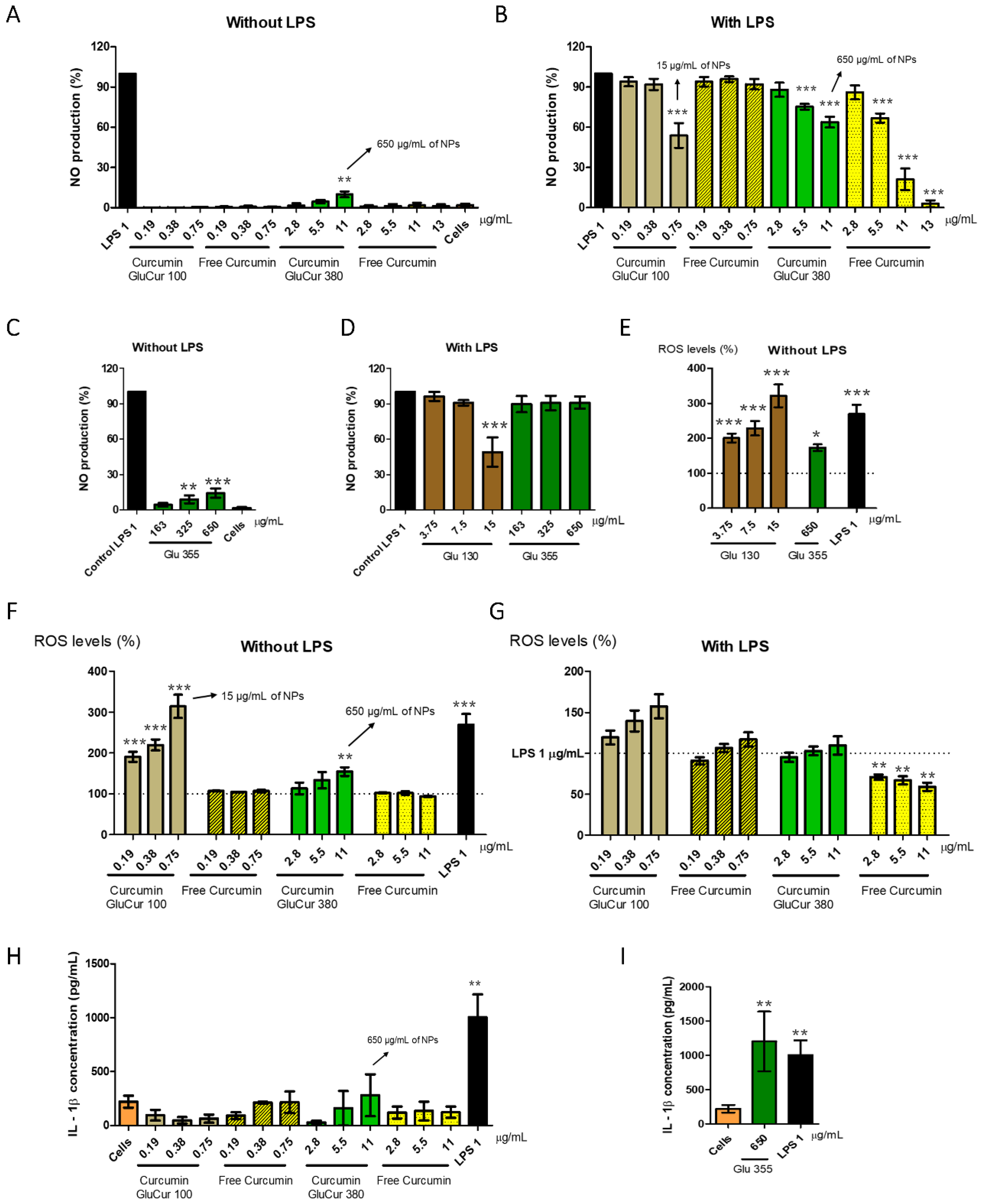
3.5. GluCur 380 NPs Decrease Curcumin-Associated Toxicity in a Murine Macrophage Cell Line
3.6. Curcumin Decreases the Inflammatory Activity of Glucan NPs in RAW 264.7 Cells
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ursini, F.; Maiorino, M.; Forman, H.J. Redox homeostasis: The Golden Mean of healthy living. Redox Biol. 2016, 8, 205–215. [Google Scholar] [CrossRef]
- Gorrini, C.; Harris, I.S.; Mak, T.W. Modulation of oxidative stress as an anticancer strategy. Nat. Rev. Drug Discov. 2013, 12, 931–947. [Google Scholar] [CrossRef]
- Joseph, A.; Wood, T.; Chen, C.-C.; Corry, K.; Snyder, J.M.; Juul, S.E.; Parikh, P.; Nance, E. Curcumin-loaded polymeric nanoparticles for neuroprotection in neonatal rats with hypoxic-ischemic encephalopathy. Nano Res. 2018, 11, 5670–5688. [Google Scholar] [CrossRef]
- Sampath, M.; Lakra, R.; Korrapati, P.; Sengottuvelan, B. Curcumin loaded poly (lactic-co-glycolic) acid nanofiber for the treatment of carcinoma. Colloids Surf. B Biointerfaces 2014, 117, 128–134. [Google Scholar] [CrossRef]
- Jurenka, J.S. Anti-inflammatory properties of curcumin, a major constituent of Curcuma longa: A review of preclinical and clinical research. Altern. Med. Rev. 2009, 14, 141–153. [Google Scholar]
- Salehi, B.; Stojanović-Radić, Z.; Matejić, J.; Sharifi-Rad, M.; Anil Kumar, N.V.; Martins, N.; Sharifi-Rad, J. The therapeutic potential of curcumin: A review of clinical trials. Eur. J. Med. Chem. 2019, 163, 527–545. [Google Scholar] [CrossRef]
- Rai, M.; Pandit, R.; Gaikwad, S.; Yadav, A.; Gade, A. Potential applications of curcumin and curcumin nanoparticles: From traditional therapeutics to modern nanomedicine. Nanotechnol. Rev. 2015, 4, 161–172. [Google Scholar] [CrossRef]
- Abu-Taweel, G.M.; Attia, M.F.; Hussein, J.; Mekawi, E.M.; Galal, H.M.; Ahmed, E.I.; Allam, A.A.; El-Naggar, M.E. Curcumin nanoparticles have potential antioxidant effect and restore tetrahydrobiopterin levels in experimental diabetes. Biomed. Pharmacother. 2020, 131, 110688. [Google Scholar] [CrossRef]
- Gómez-Estaca, J.; Balaguer, M.P.; López-Carballo, G.; Gavara, R.; Hernández-Muñoz, P. Improving antioxidant and antimicrobial properties of curcumin by means of encapsulation in gelatin through electrohydrodynamic atomization. Food Hydrocoll. 2017, 70, 313–320. [Google Scholar] [CrossRef]
- Wang, X.; Huang, H.; Chu, X.; Han, Y.; Li, M.; Li, G.; Liu, X. Encapsulation and binding properties of curcumin in zein particles stabilized by Tween 20. Colloids Surf. A Physicochem. Eng. Asp. 2019, 577, 274–280. [Google Scholar] [CrossRef]
- Wojcik, M.; Krawczyk, M.; Wojcik, P.; Cypryk, K.; Wozniak, L.A. Molecular Mechanisms Underlying Curcumin-Mediated Therapeutic Effects in Type 2 Diabetes and Cancer. Oxidative Med. Cell. Longev. 2018, 2018, 9698258. [Google Scholar] [CrossRef] [Green Version]
- Aggeli, I.-K.; Koustas, E.; Gaitanaki, C.; Beis, I. Curcumin Acts as a Pro-Oxidant Inducing Apoptosis Via JNKs in the Isolated PerfusedRana ridibundaHeart. J. Exp. Zool. Part A Ecol. Genet. Physiol. 2013, 319, 328–339. [Google Scholar] [CrossRef]
- López-Lázaro, M. Anticancer and carcinogenic properties of curcumin: Considerations for its clinical development as a cancer chemopreventive and chemotherapeutic agent. Mol. Nutr. Food Res. 2008, 52 (Suppl. S1), S103–S127. [Google Scholar] [CrossRef]
- Li, P.-M.; Li, Y.-L.; Liu, B.; Wang, W.-J.; Wang, Y.-Z.; Li, Z. Curcumin Inhibits MHCC97H Liver Cancer Cells by Activating ROS/TLR-4/Caspase Signaling Pathway. Asian Pac. J. Cancer Prev. 2014, 15, 2329–2334. [Google Scholar] [CrossRef]
- Talib, W.H.; Al-hadid, S.A.; Wild Ali, M.B.; Al-Yasari, I.H.; Abd Ali, M.R. Role of curcumin in regulating p53 in breast cancer: An overview of the mechanism of action. Breast Cancer Targets Ther. 2018, 10, 207–217. [Google Scholar] [CrossRef]
- Catanzaro, M.; Corsini, E.; Rosini, M.; Racchi, M.; Lanni, C. Immunomodulators Inspired by Nature: A Review on Curcumin and Echinacea. Molecules 2018, 23, 2778. [Google Scholar] [CrossRef] [PubMed]
- Hewlings, S.; Kalman, D. Curcumin: A Review of Its Effects on Human Health. Foods 2017, 6, 92. [Google Scholar] [CrossRef] [PubMed]
- Basnet, P.; Skalko-Basnet, N. Curcumin: An Anti-Inflammatory Molecule from a Curry Spice on the Path to Cancer Treatment. Molecules 2011, 16, 4567–4598. [Google Scholar] [CrossRef] [PubMed]
- Dei Cas, M.; Ghidoni, R. Dietary Curcumin: Correlation between Bioavailability and Health Potential. Nutrients 2019, 11, 2147. [Google Scholar] [CrossRef]
- Kharat, M.; Du, Z.; Zhang, G.; McClements, D.J. Physical and Chemical Stability of Curcumin in Aqueous Solutions and Emulsions: Impact of pH, Temperature, and Molecular Environment. J. Agric. Food Chem. 2017, 65, 1525–1532. [Google Scholar] [CrossRef]
- Anand, P.; Kunnumakkara, A.B.; Newman, R.A.; Aggarwal, B.B. Bioavailability of Curcumin: Problems and Promises. Mol. Pharm. 2007, 4, 807–818. [Google Scholar] [CrossRef]
- Huong, L.M.; Thu, H.P.; Thuy, N.T.B.; Ha, T.T.H.; Thi, H.T.M.; Trang, M.T.; Hang, T.T.N.; Nghi, D.H.; Phuc, N.X.; Quang, D.T. Preparation and Antitumor-promoting Activity of Curcumin Encapsulated by 1,3-β-Glucan Isolated from Vietnam Medicinal MushroomHericium erinaceum. Chem. Lett. 2011, 40, 846–848. [Google Scholar] [CrossRef]
- Wang, K.; Zhang, T.; Liu, L.; Wang, X.; Wu, P.; Chen, Z.; Ni, C.; Zhang, J.; Hu, F.; Huang, J. Novel micelle formulation of curcumin for enhancing antitumor activity and inhibiting colorectal cancer stem cells. Int. J. Nanomed. 2012, 7, 4487–4497. [Google Scholar] [CrossRef]
- Li, L.; Braiteh, F.S.; Kurzrock, R. Liposome-encapsulated curcumin. Cancer 2005, 104, 1322–1331. [Google Scholar] [CrossRef] [PubMed]
- Rafati, N.; Zarrabi, A.; Caldera, F.; Trotta, F.; Ghias, N. Pyromellitic dianhydride crosslinked cyclodextrin nanosponges for curcumin controlled release; formulation, physicochemical characterization and cytotoxicity investigations. J. Microencapsul. 2019, 36, 715–727. [Google Scholar] [CrossRef] [PubMed]
- Casula, L.; Lai, F.; Pini, E.; Valenti, D.; Sinico, C.; Cardia, M.C.; Marceddu, S.; Ailuno, G.; Fadda, A.M. Pulmonary Delivery of Curcumin and Beclomethasone Dipropionate in a Multicomponent Nanosuspension for the Treatment of Bronchial Asthma. Pharmaceutics 2021, 13, 1300. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Tang, Q.; Xu, X.; Li, N. Development and evaluation of a novel phytosome-loaded chitosan microsphere system for curcumin delivery. Int. J. Pharm. 2013, 448, 168–174. [Google Scholar] [CrossRef] [PubMed]
- Pawar, H.; Wankhade, S.R.; Yadav, D.K.; Suresh, S. Development and evaluation of co-formulated docetaxel and curcumin biodegradable nanoparticles for parenteral administration. Pharm. Dev. Technol. 2015, 21, 725–736. [Google Scholar] [CrossRef]
- Kaur, R.; Sharma, M.; Ji, D.; Xu, M.; Agyei, D. Structural Features, Modification, and Functionalities of Beta-Glucan. Fibers 2019, 8, 1. [Google Scholar] [CrossRef]
- Han, B.; Baruah, K.; Cox, E.; Vanrompay, D.; Bossier, P. Structure-Functional Activity Relationship of β-Glucans From the Perspective of Immunomodulation: A Mini-Review. Front. Immunol. 2020, 11, 658. [Google Scholar] [CrossRef]
- Zhou, J.-L.; Song, F.; Tian, J.-F.; Nie, W.-C.; Wang, X.-L.; Wang, Y.-Z. Electrostatic wrapping of doxorubicin with curdlan to construct an efficient pH-responsive drug delivery system. Nanotechnology 2017, 28, 295601. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Li, P.; Wang, F. β-glucans as potential immunoadjuvants: A review on the adjuvanticity, structure-activity relationship and receptor recognition properties. Vaccine 2018, 36, 5235–5244. [Google Scholar] [CrossRef]
- Schepetkin, I.A.; Quinn, M.T. Botanical polysaccharides: Macrophage immunomodulation and therapeutic potential. Int. Immunopharmacol. 2006, 6, 317–333. [Google Scholar] [CrossRef]
- Jesus, S.; Marques, A.P.; Duarte, A.; Soares, E.; Costa, J.P.; Colaço, M.; Schmutz, M.; Som, C.; Borchard, G.; Wick, P.; et al. Chitosan Nanoparticles: Shedding Light on Immunotoxicity and Hemocompatibility. Front. Bioeng. Biotechnol. 2020, 8, 100. [Google Scholar] [CrossRef]
- Foroozandeh, P.; Aziz, A.A. Insight into Cellular Uptake and Intracellular Trafficking of Nanoparticles. Nanoscale Res. Lett. 2018, 13, 339. [Google Scholar] [CrossRef]
- Varlamova, E.G.; Gudkov, S.V.; Plotnikov, E.Y.; Turovsky, E.A. Size-Dependent Cytoprotective Effects of Selenium Nanoparticles during Oxygen-Glucose Deprivation in Brain Cortical Cells. Int. J. Mol. Sci. 2022, 23, 7464. [Google Scholar] [CrossRef] [PubMed]
- Shang, L.; Nienhaus, K.; Nienhaus, G.U. Engineered nanoparticles interacting with cells: Size matters. J. Nanobiotechnol. 2014, 12, 5. [Google Scholar] [CrossRef]
- Colaço, M.; Marques, A.P.; Jesus, S.; Duarte, A.; Borges, O. Safe-by-Design of Glucan Nanoparticles: Size Matters When Assessing the Immunotoxicity. Chem. Res. Toxicol. 2020, 33, 915–932. [Google Scholar] [CrossRef] [PubMed]
- Nair, A.V.; Gummadi, S.N.; Doble, M. Characterization and biological activities of cyclic (1 → 3, 1 → 6)-β-glucans from Bradyrhizobium japonicum. Biotechnol. Lett. 2016, 38, 1519–1525. [Google Scholar] [CrossRef] [PubMed]
- Halamoda-Kenzaoui, B.; Bremer-Hoffmann, S. Main trends of immune effects triggered by nanomedicines in preclinical studies. Int. J. Nanomed. 2018, 13, 5419–5431. [Google Scholar] [CrossRef]
- Schneider, C.; Gordon, O.N.; Edwards, R.L.; Luis, P.B. Degradation of Curcumin: From Mechanism to Biological Implications. J. Agric. Food Chem. 2015, 63, 7606–7614. [Google Scholar] [CrossRef] [Green Version]
- Hannon, G.; Lysaght, J.; Liptrott, N.J.; Prina-Mello, A. Immunotoxicity Considerations for Next Generation Cancer Nanomedicines. Adv. Sci. 2019, 6, 1900133. [Google Scholar] [CrossRef]
- Dobrovolskaia, M.A.; McNeil, S.E. Understanding the correlation between in vitro and in vivo immunotoxicity tests for nanomedicines. J. Control. Release 2013, 172, 456–466. [Google Scholar] [CrossRef]
- ASTM E2524-08; Standard Test Method for Analysis of Hemolytic Properties of Nanoparticles. ASTM: West Conshohocken, PA, USA, 2013.
- Brenner, B.; Lisman, T. Hemostasis and Thrombosis in Extreme Physiological and Pathological Conditions. Semin. Thromb. Hemost. 2018, 44, 615–616. [Google Scholar] [CrossRef] [PubMed]
- Dobrovolskaia, M.A. Pre-clinical immunotoxicity studies of nanotechnology-formulated drugs: Challenges, considerations and strategy. J. Control. Release 2015, 220, 571–583. [Google Scholar] [CrossRef] [PubMed]
- Jenne, C.N.; Kubes, P. Platelets in inflammation and infection. Platelets 2015, 26, 286–292. [Google Scholar] [CrossRef] [PubMed]
- Tsoupras, A.; Zabetakis, I.; Lordan, R. Platelet aggregometry assay for evaluating the effects of platelet agonists and antiplatelet compounds on platelet function in vitro. MethodsX 2019, 6, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.K.; Thareja, S. In vitro and in vivo characterization of pharmaceutical nanocarriers used for drug delivery. Artif. Cells Nanomed. Biotechnol. 2019, 47, 524–539. [Google Scholar] [CrossRef] [PubMed]
- Deters, M.; Knochenwefel, H.; Lindhorst, D.; Koal, T.; Meyer, H.H.; Hänsel, W.; Resch, K.; Kaever, V. Different Curcuminoids Inhibit T-Lymphocyte Proliferation Independently of Their Radical Scavenging Activities. Pharm. Res. 2008, 25, 1822–1827. [Google Scholar] [CrossRef]
- Bol, K.F.; Schreibelt, G.; Rabold, K.; Wculek, S.K.; Schwarze, J.K.; Dzionek, A.; Teijeira, A.; Kandalaft, L.E.; Romero, P.; Coukos, G.; et al. The clinical application of cancer immunotherapy based on naturally circulating dendritic cells. J. ImmunoTherapy Cancer 2019, 7, 109. [Google Scholar] [CrossRef]
- Rothen-Rutishauser, B.; Bourquin, J.; Petri-Fink, A. Nanoparticle-Cell Interactions: Overview of Uptake, Intracellular Fate and Induction of Cell Responses. In Biological Responses to Nanoscale Particles; Springer: Cham, Switzerland, 2019; pp. 153–170. [Google Scholar] [CrossRef]
- Martín-Sánchez, F.; Diamond, C.; Zeitler, M.; Gomez, A.I.; Baroja-Mazo, A.; Bagnall, J.; Spiller, D.; White, M.; Daniels, M.J.D.; Mortellaro, A.; et al. Inflammasome-dependent IL-1β release depends upon membrane permeabilisation. Cell Death Differ. 2016, 23, 1219–1231. [Google Scholar] [CrossRef] [PubMed]
- Tsuda, T. Curcumin as a functional food-derived factor: Degradation products, metabolites, bioactivity, and future perspectives. Food Funct. 2018, 9, 705–714. [Google Scholar] [CrossRef]
- Mirzaei, H.; Shakeri, A.; Rashidi, B.; Jalili, A.; Banikazemi, Z.; Sahebkar, A. Phytosomal curcumin: A review of pharmacokinetic, experimental and clinical studies. Biomed. Pharmacother. 2017, 85, 102–112. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Duan, B.; Chen, H.; Xu, X. A Novel Strategy for Treating Inflammatory Bowel Disease by Targeting Delivery of Methotrexate through Glucan Particles. Adv. Healthc. Mater. 2020, 9, 1901805. [Google Scholar] [CrossRef] [PubMed]
- Lopalco, A.; Denora, N. Nanoformulations for Drug Delivery: Safety, Toxicity, and Efficacy. In Computational Toxicology Methods in Molecular Biology; Nicolotti, O., Ed.; Humana Press: New York, NY, USA, 2018; Volume 1800, pp. 347–365. [Google Scholar]
- Kraegeloh, A.; Suarez-Merino, B.; Sluijters, T.; Micheletti, C. Implementation of Safe-by-Design for Nanomaterial Development and Safe Innovation: Why We Need a Comprehensive Approach. Nanomaterials 2018, 8, 239. [Google Scholar] [CrossRef]
- Deka, C.; Aidew, L.; Devi, N.; Buragohain, A.K.; Kakati, D.K. Synthesis of curcumin-loaded chitosan phosphate nanoparticle and study of its cytotoxicity and antimicrobial activity. J. Biomater. Sci. Polym. Ed. 2016, 27, 1659–1673. [Google Scholar] [CrossRef]
- Huang, M.-H.; Yang, M.-C. Evaluation of glucan/poly(vinyl alcohol) blend wound dressing using rat models. Int. J. Pharm. 2008, 346, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.-C.; Ku, S.-K.; Bae, J.-S. Anticoagulant activities of curcumin and its derivative. BMB Rep. 2012, 45, 221–226. [Google Scholar] [CrossRef]
- Duse, L.; Agel, M.R.; Pinnapireddy, S.R.; Schäfer, J.; Selo, M.A.; Ehrhardt, C.; Bakowsky, U. Photodynamic Therapy of Ovarian Carcinoma Cells with Curcumin-Loaded Biodegradable Polymeric Nanoparticles. Pharmaceutics 2019, 11, 282. [Google Scholar] [CrossRef]
- Keihanian, F.; Saeidinia, A.; Bagheri, R.K.; Johnston, T.P.; Sahebkar, A. Curcumin, hemostasis, thrombosis, and coagulation. J. Cell. Physiol. 2018, 233, 4497–4511. [Google Scholar] [CrossRef]
- Perrone, D.; Ardito, F.; Giannatempo, G.; Dioguardi, M.; Troiano, G.; Lo Russo, L.; De Lillo, A.; Laino, L.; Lo Muzio, L. Biological and therapeutic activities, and anticancer properties of curcumin. Exp. Ther. Med. 2015, 10, 1615–1623. [Google Scholar] [CrossRef] [PubMed]
- Ngo, T.; Kim, K.; Bian, Y.; An, G.-J.; Bae, O.-N.; Lim, K.-M.; Chung, J.-H. Cyclocurcumin from Curcuma longa selectively inhibits shear stress-induced platelet aggregation. J. Funct. Foods 2019, 61, 103462. [Google Scholar] [CrossRef]
- Tabeshpour, J.; Hashemzaei, M.; Sahebkar, A. The regulatory role of curcumin on platelet functions. J. Cell. Biochem. 2018, 119, 8713–8722. [Google Scholar] [CrossRef] [PubMed]
- Le, M.H.; Do, H.D.; Tran Thi, H.H.; Dung, L.V.; Nguyen, H.N.; Tran Thi, H.N.; Nguyen, L.D.; Hoang, C.K.; Le, H.C.; Le Thi, T.H.; et al. The dual effect of curcumin nanoparticles encapsulated by 1-3/1-6 β-glucan from medicinal mushrooms Hericium erinaceus and Ganoderma lucidum. Adv. Nat. Sci. Nanosci. Nanotechnol. 2016, 7, 045019. [Google Scholar] [CrossRef]
- Busari, Z.A.; Dauda, K.A.; Morenikeji, O.A.; Afolayan, F.; Oyeyemi, O.T.; Meena, J.; Sahu, D.; Panda, A.K. Antiplasmodial Activity and Toxicological Assessment of Curcumin PLGA-Encapsulated Nanoparticles. Front. Pharmacol. 2017, 8, 622. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhu, R.; Sun, D.; Sun, X.; Geng, Z.; Liu, H.; Wang, S.-L. Intracellular Uptake of Curcumin-Loaded Solid Lipid Nanoparticles Exhibit Anti-Inflammatory Activities Superior to Those of Curcumin Through the NF-κB Signaling Pathway. J. Biomed. Nanotechnol. 2015, 11, 403–415. [Google Scholar] [CrossRef]
- Hirayama, D.; Lida, T.; Nakase, H. The Phagocytic Function of Macrophage-Enforcing Innate Immunity and Tissue Homeostasis. Int. J. Mol. Sci. 2017, 19, 92. [Google Scholar] [CrossRef]
- Khatun, B.; Banik, N.; Hussain, A.; Ramteke, A.; Maji, T. Genipin crosslinked curcumin loaded chitosan/montmorillonite K-10 (MMT) nanoparticles for controlled drug delivery applications. J. Microencapsul. 2018, 35, 439–453. [Google Scholar] [CrossRef]
- Singh, A.; Lavkush; Kureel, A.K.; Dutta, P.K.; Kumar, S.; Rai, A.K. Curcumin loaded chitin-glucan quercetin conjugate: Synthesis, characterization, antioxidant, in vitro release study, and anticancer activity. Int. J. Biol. Macromol. 2018, 110, 234–244. [Google Scholar] [CrossRef]
- Nishanth, R.P.; Jyotsna, R.G.; Schlager, J.J.; Hussain, S.M.; Reddanna, P. Inflammatory responses of RAW 264.7 macrophages upon exposure to nanoparticles: Role of ROS-NFκB signaling pathway. Nanotoxicology 2011, 5, 502–516. [Google Scholar] [CrossRef]
- Ben, P.; Liu, J.; Lu, C.; Xu, Y.; Xin, Y.; Fu, J.; Huang, H.; Zhang, Z.; Gao, Y.; Luo, L.; et al. Curcumin promotes degradation of inducible nitric oxide synthase and suppresses its enzyme activity in RAW 264.7 cells. Int. Immunopharmacol. 2011, 11, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Yang, X.; Wu, W.; Long, Z.; Xiao, H.; Luo, F.; Shen, Y.; Lin, Q. Elaboration of curcumin-loaded rice bran albumin nanoparticles formulation with increased in vitro bioactivity and in vivo bioavailability. Food Hydrocoll. 2018, 77, 834–842. [Google Scholar] [CrossRef]
- Li, P.; Zhang, X.; Cheng, Y.; Li, J.; Xiao, Y.; Zhang, Q.; Zong, A.; Zhong, C.; Wang, F. Preparation and in vitro immunomodulatory effect of curdlan sulfate. Carbohydr. Polym. 2014, 102, 852–861. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Castejon, G.; Brough, D. Understanding the mechanism of IL-1β secretion. Cytokine Growth Factor Rev. 2011, 22, 189–195. [Google Scholar] [CrossRef]
- Elder, M.J.; Webster, S.J.; Chee, R.; Williams, D.L.; Hill Gaston, J.S.; Goodall, J.C. β-Glucan Size Controls Dectin-1-Mediated Immune Responses in Human Dendritic Cells by Regulating IL-1β Production. Front. Immunol. 2017, 8, 791. [Google Scholar] [CrossRef]
- Yin, H.; Guo, Q.; Li, X.; Tang, T.; Li, C.; Wang, H.; Sun, Y.; Feng, Q.; Ma, C.; Gao, C.; et al. Curcumin Suppresses IL-1β Secretion and Prevents Inflammation through Inhibition of the NLRP3 Inflammasome. J. Immunol. 2018, 200, 2835–2846. [Google Scholar] [CrossRef] [PubMed]
- Guglielmo, A.; Sabra, A.; Elbery, M.; Cerveira, M.M.; Ghenov, F.; Sunasee, R.; Ckless, K. A mechanistic insight into curcumin modulation of the IL-1β secretion and NLRP3 S-glutathionylation induced by needle-like cationic cellulose nanocrystals in myeloid cells. Chem.-Biol. Interact. 2017, 274, 1–12. [Google Scholar] [CrossRef]
- Filin, I.Y.; Kitaeva, K.V.; Rutland, C.S.; Rizvanov, A.A.; Solovyeva, V.V. Recent Advances in Experimental Dendritic Cell Vaccines for Cancer. Front. Oncol. 2021, 11, 824. [Google Scholar] [CrossRef]
- Zimara, N.; Chanyalew, M.; Aseffa, A.; van Zandbergen, G.; Lepenies, B.; Schmid, M.; Weiss, R.; Rascle, A.; Wege, A.K.; Jantsch, J.; et al. Dectin-1 Positive Dendritic Cells Expand after Infection with Leishmania major Parasites and Represent Promising Targets for Vaccine Development. Front. Immunol. 2018, 9, 263. [Google Scholar] [CrossRef]
- Bao, M.; Ehexige, E.; Xu, J.; Ganbold, T.; Han, S.; Baigude, H. Oxidized curdlan activates dendritic cells and enhances antitumor immunity. Carbohydr. Polym. 2021, 264, 117988. [Google Scholar] [CrossRef]
- Chen, J.; Zhao, Y.; Jiang, Y.; Gao, S.; Wang, Y.; Wang, D.; Wang, A.; Yi, H.; Gu, R.; Yi, Q.; et al. Interleukin-33 Contributes to the Induction of Th9 Cells and Antitumor Efficacy by Dectin-1-Activated Dendritic Cells. Front. Immunol. 2018, 9, 787. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.-S.; Agrawal, S.; Gupta, S.; Agrawal, A. Human Dendritic Cells Activated via Dectin-1 Are Efficient at Priming Th17, Cytotoxic CD8 T and B Cell Responses. PLoS ONE 2010, 5, e13418. [Google Scholar] [CrossRef]
- Kim, H.S.; Park, K.H.; Lee, H.K.; Kim, J.S.; Kim, Y.G.; Lee, J.H.; Kim, K.H.; Yun, J.; Hwang, B.Y.; Hong, J.T.; et al. Curdlan activates dendritic cells through dectin-1 and toll-like receptor 4 signaling. Int. Immunopharmacol. 2016, 39, 71–78. [Google Scholar] [CrossRef]
- Kim, S.J.; Kim, H.S.; Seo, Y.R. Understanding of ROS-Inducing Strategy in Anticancer Therapy. Oxidative Med. Cell. Longev. 2019, 2019, 5381692. [Google Scholar] [CrossRef]
- Yu, Y.; Shen, M.; Song, Q.; Xie, J. Biological activities and pharmaceutical applications of polysaccharide from natural resources: A review. Carbohydr. Polym. 2018, 183, 91–101. [Google Scholar] [CrossRef]
- Fatima, N.; Upadhyay, T.; Sharma, D.; Sharma, R. Particulate beta-glucan induces early and late phagosomal maturation in murine macrophages. Front. Biosci. 2017, 9, 129–140. [Google Scholar] [CrossRef]
- Jia, X.; Liu, Q.; Zou, S.; Xu, X.; Zhang, L. Construction of selenium nanoparticles/β-glucan composites for enhancement of the antitumor activity. Carbohydr. Polym. 2015, 117, 434–442. [Google Scholar] [CrossRef]
- Fernando, W.; Rupasinghe, H.P.V.; Hoskin, D.W. Dietary phytochemicals with anti-oxidant and pro-oxidant activities: A double-edged sword in relation to adjuvant chemotherapy and radiotherapy? Cancer Lett. 2019, 452, 168–177. [Google Scholar] [CrossRef]
- Guo, M.; Li, Y.; Lin, Z.; Zhao, M.; Xiao, M.; Wang, C.; Xu, T.; Xia, Y.; Zhu, B. Surface decoration of selenium nanoparticles with curcumin induced HepG2 cell apoptosis through ROS mediated p53 and AKT signaling pathways. RSC Adv. 2017, 7, 52456–52464. [Google Scholar] [CrossRef] [Green Version]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Colaço, M.; Roquito, T.; Costa, J.P.; Cruz, M.T.; Borges, O. The Effect of Curcumin-Loaded Glucan Nanoparticles on Immune Cells: Size as a Critical Quality Attribute. Pharmaceutics 2023, 15, 623. https://doi.org/10.3390/pharmaceutics15020623
Colaço M, Roquito T, Costa JP, Cruz MT, Borges O. The Effect of Curcumin-Loaded Glucan Nanoparticles on Immune Cells: Size as a Critical Quality Attribute. Pharmaceutics. 2023; 15(2):623. https://doi.org/10.3390/pharmaceutics15020623
Chicago/Turabian StyleColaço, Mariana, Tiago Roquito, João Panão Costa, Maria Teresa Cruz, and Olga Borges. 2023. "The Effect of Curcumin-Loaded Glucan Nanoparticles on Immune Cells: Size as a Critical Quality Attribute" Pharmaceutics 15, no. 2: 623. https://doi.org/10.3390/pharmaceutics15020623





