Intranasal Drug Administration in Alzheimer-Type Dementia: Towards Clinical Applications
Abstract
:1. Introduction
1.1. The Pathophysiology of Alzheimer-Type Dementias
1.2. Current Treatment of Alzheimer-Type Dementias
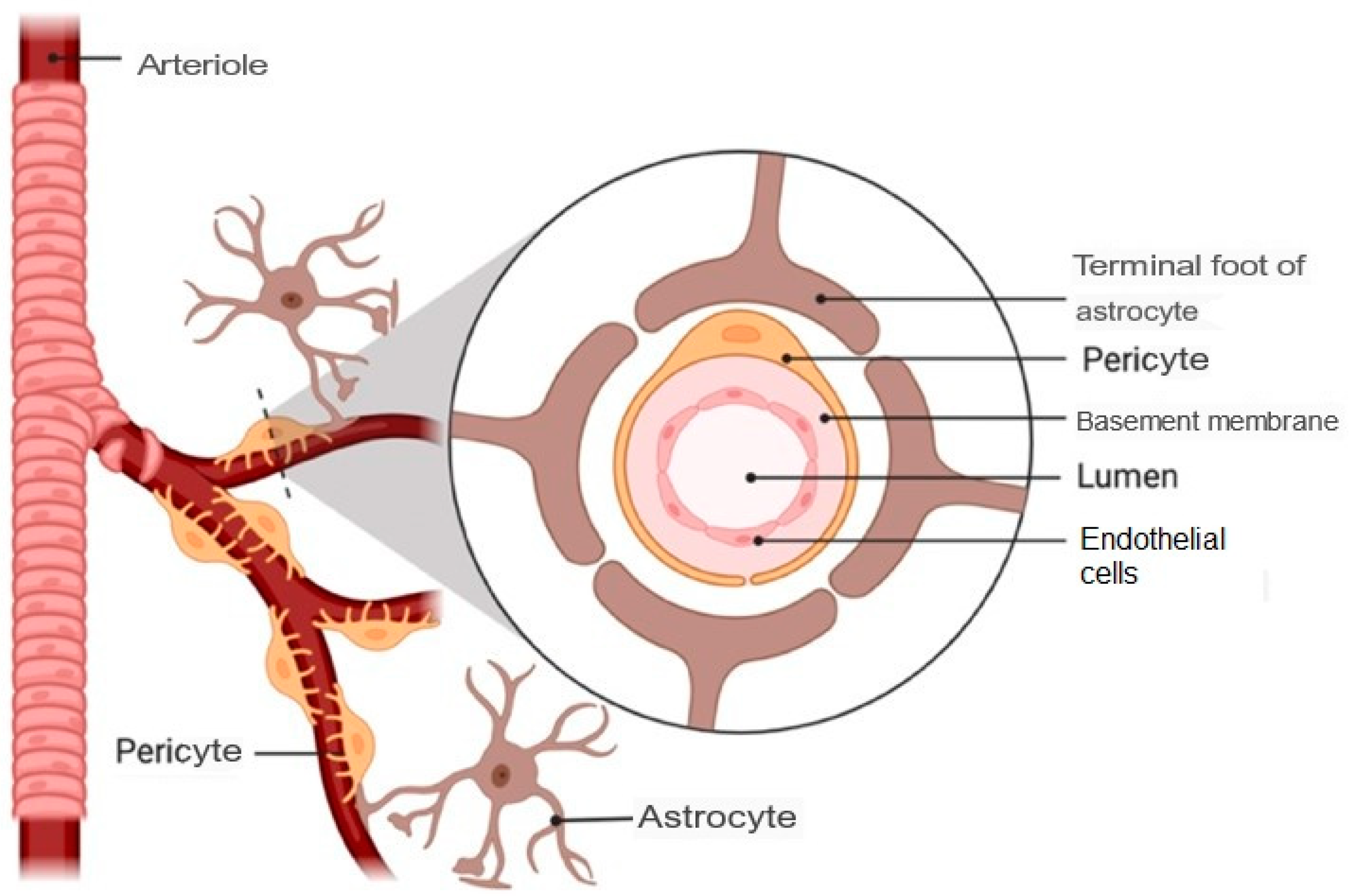
1.3. Barriers That Prevent Drug Access to the Brain
1.4. Intranasal Route for Drug Administration
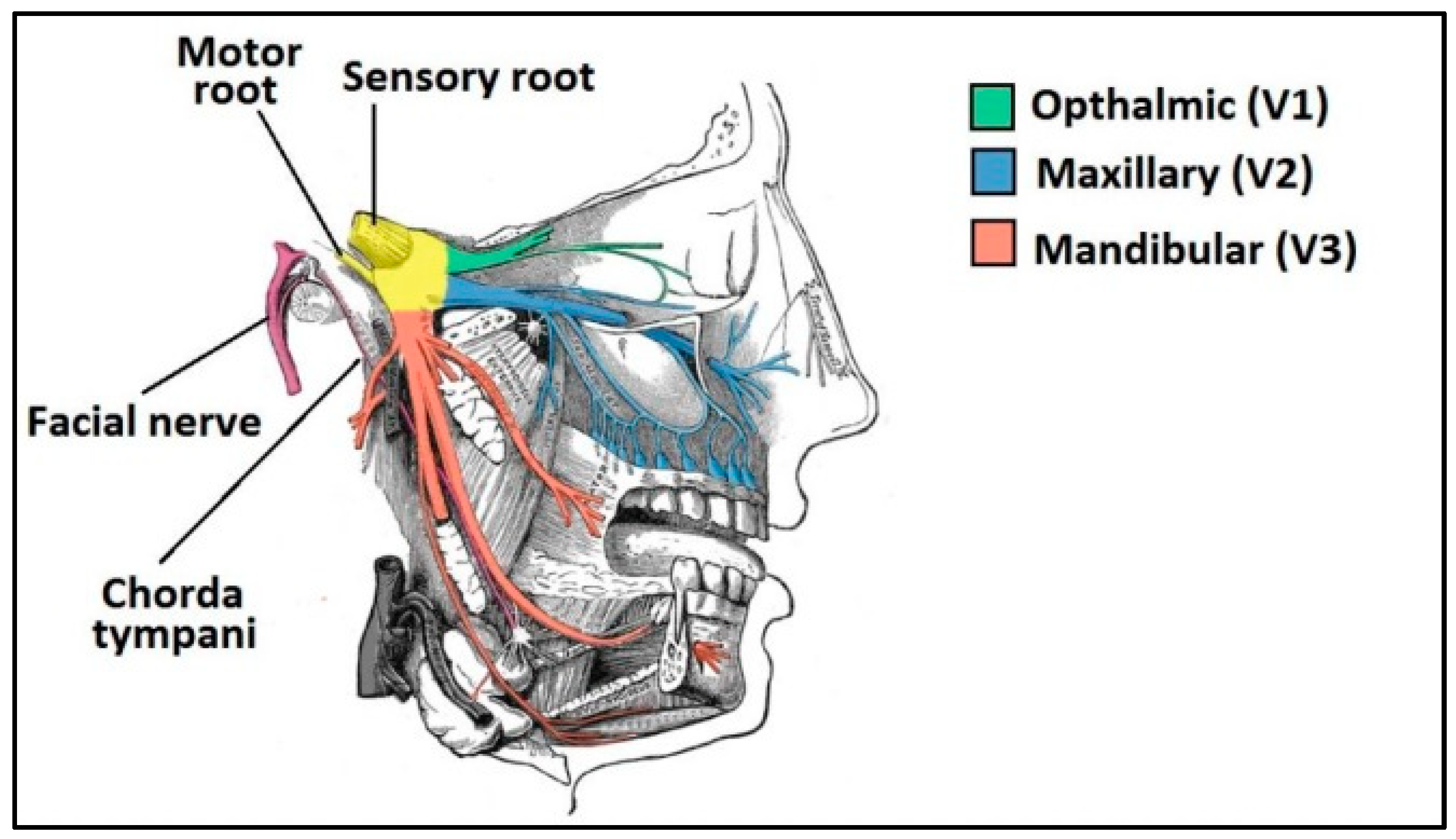
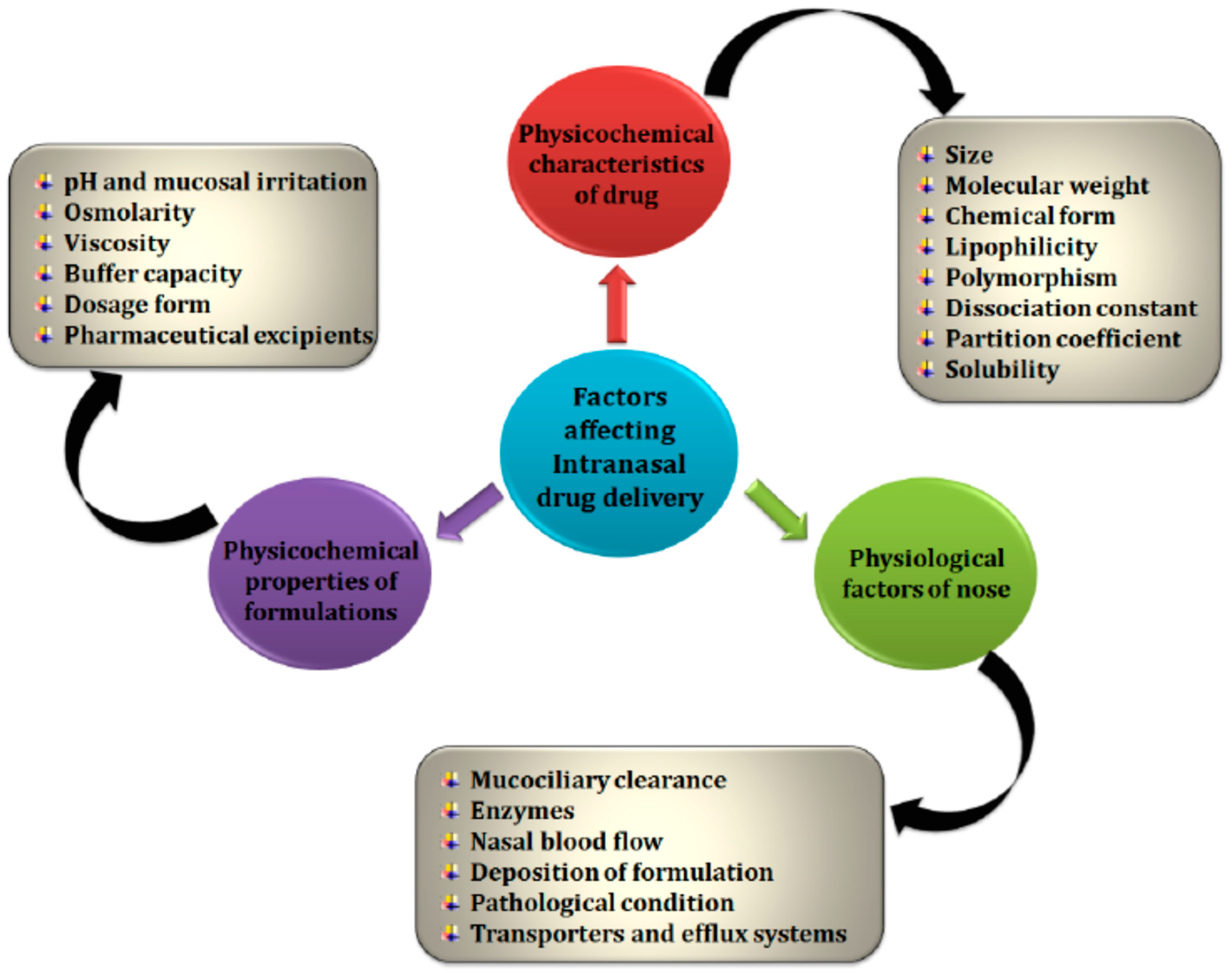
1.4.1. Nasal Cavity Anatomy
- Vestibular region: This is the most anterior area, and contains nasal hairs to filter inhaled particles and the main cell type is squamous epithelial cells. In this region, drug absorption is very limited [29].
- Respiratory region: This is the area with the largest surface (about 130 cm2) and the most vascularity, which makes it an ideal place for the systemic absorption of drugs. It encompasses the lateral walls of the nostrils, including the protruding nasal turbinates. There are four main cell types: goblet, ciliated, non-ciliated columnar and basal. In addition, the respiratory region is innervated by the maxillary and ophthalmic branches of the trigeminal nerve (V1, V2) [30].
- Olfactory region: This is located on the roof of the nasal cavity and covers only 10% of the total nasal area. There are different types of cells: basal cells, olfactory nerve cells, support cells, cilia and trigeminal neurons. In addition, the olfactory region facilitates the transport of drugs to the brain having a direct connection with the brain through the olfactory and trigeminal neurons [31].
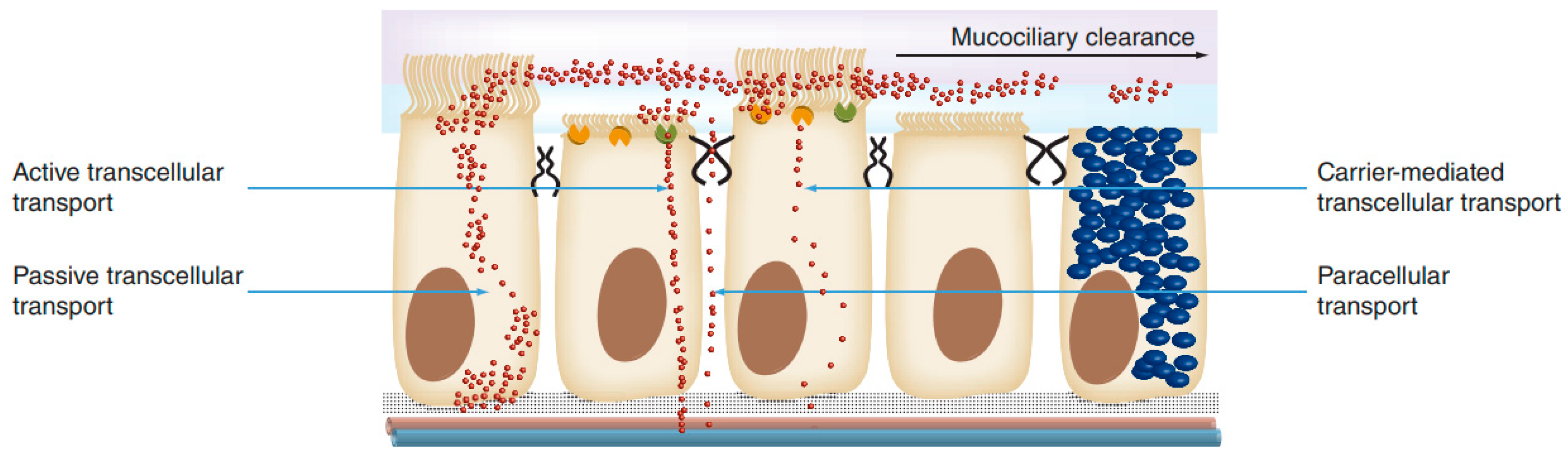
1.4.2. Intranasal Absorption
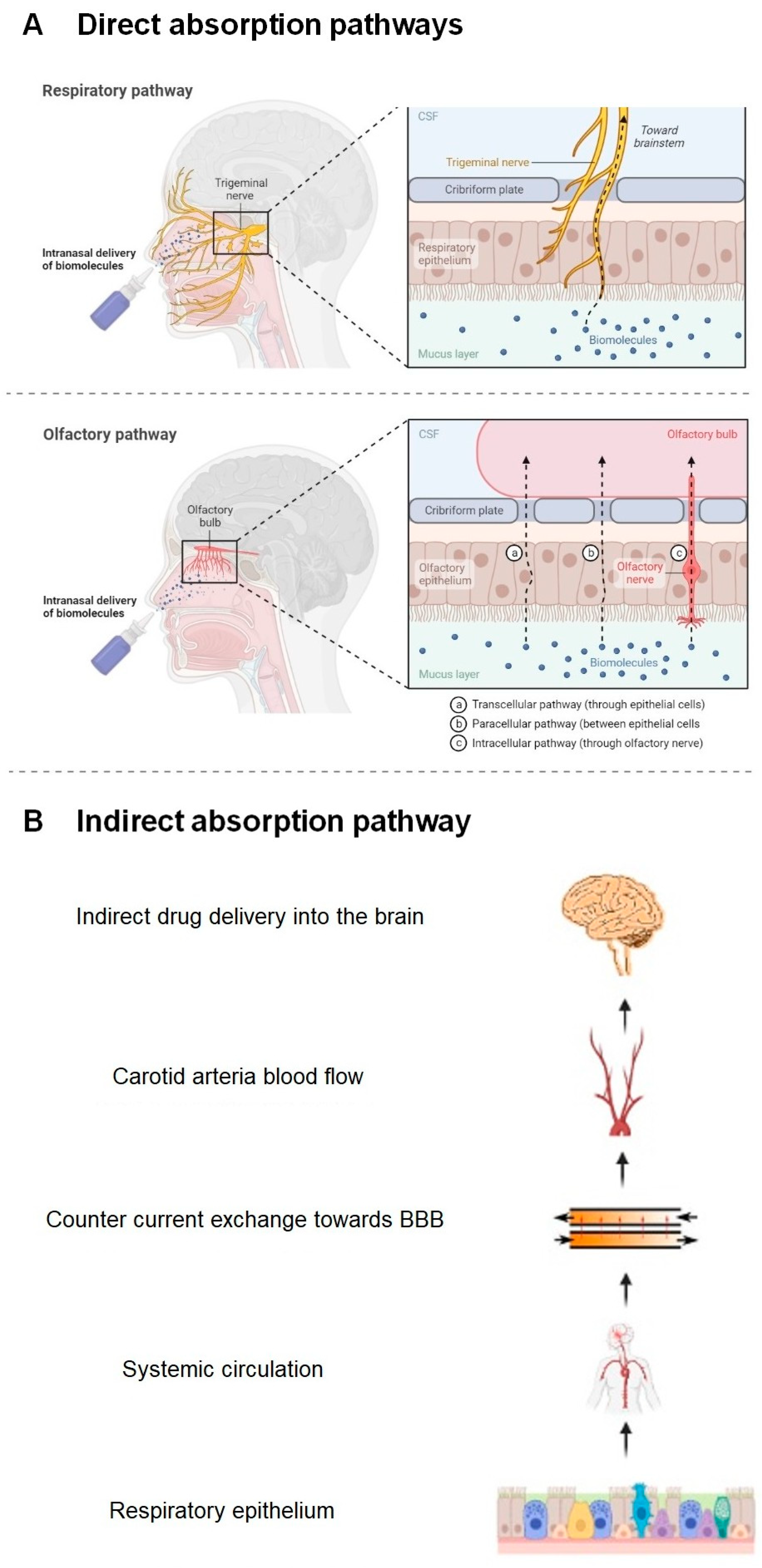
1.4.3. Nose–Brain Absorption Pathways
- (a)
- Direct Absorption Mechanism
- Olfactory Sensory Neurons
- Trigeminal Neurons
- (b)
- Indirect Absorption Mechanism: The Systemic Route
1.4.4. Advantages and Disadvantages of the Nasal Route
2. Materials and Methods
3. Results and Discussion
3.1. Possible Strategies for Overcoming the Limitations of Nasal Administration
3.1.1. Systems That Reduce Mucociliary Clearance
3.1.2. Systems That Reduce Enzymatic Degradation
3.1.3. Systems That Reduce Irritation of the Nasal Mucosa
3.1.4. Systems That Promote Nasal Permeability
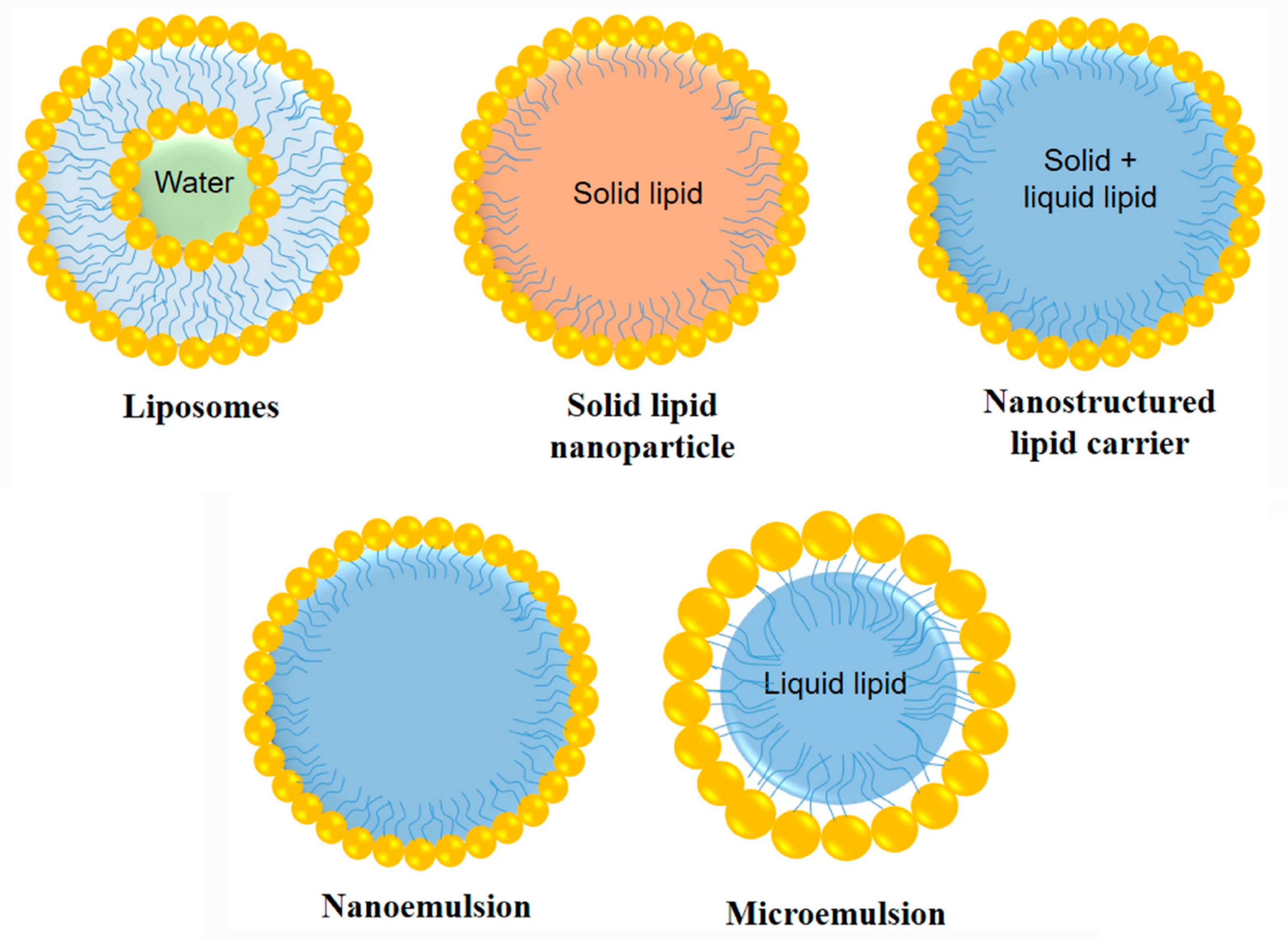
3.2. New Drug Carriers as a Potential Strategy
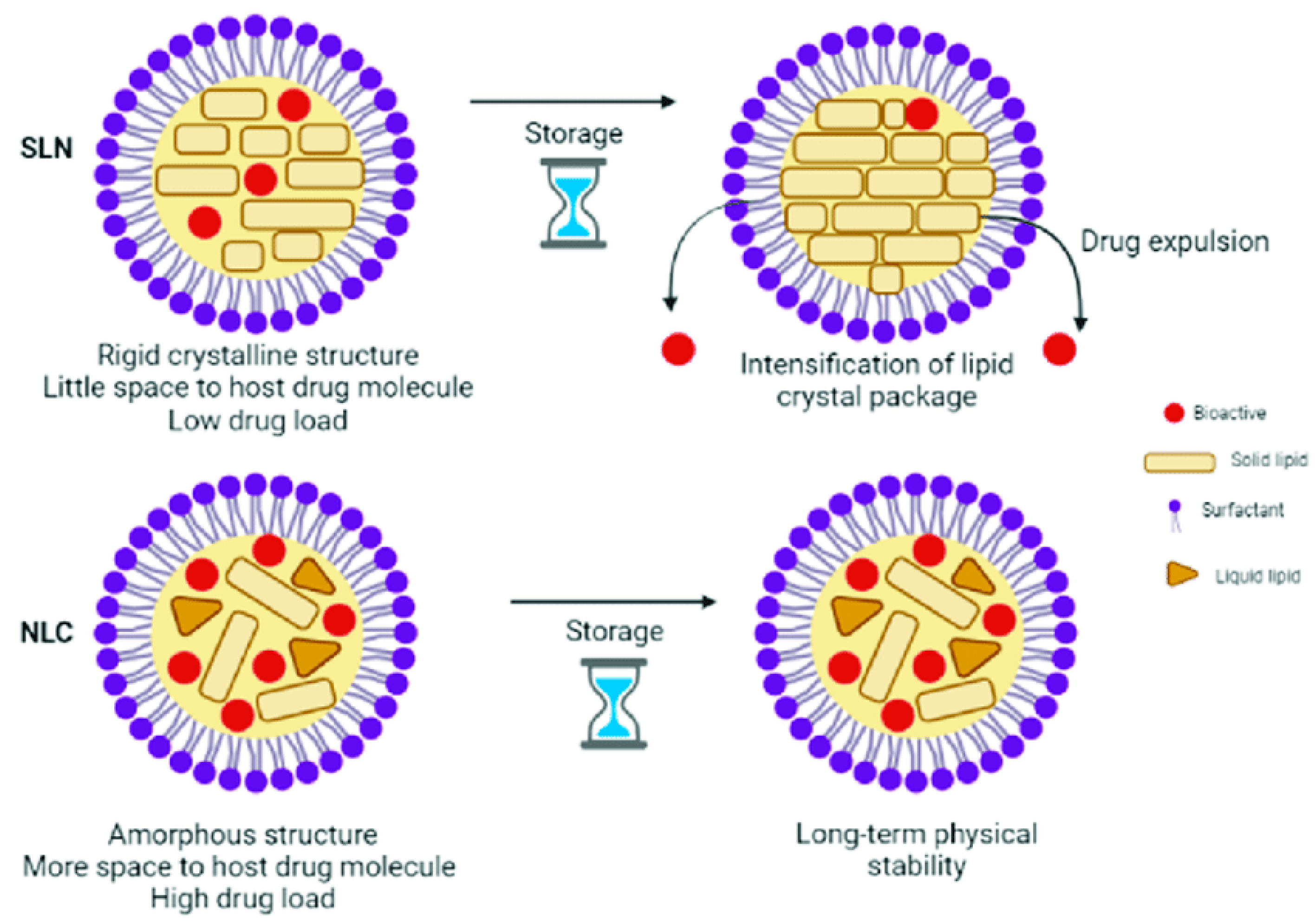
3.2.1. Nanostructured Lipid Carriers for Intranasal Delivery
Rivastigmine
Pioglitazone
Resveratrol
Berberine
Astaxanthin
Donepezil
| Preclinical Studies with Nanostructured Lipid Carriers | |||||
|---|---|---|---|---|---|
| Active Ingredient | Preparation Method | Physicochemical Properties | In Vitro Results | In Vivo Results | Reference Year |
| Rivastigmine | Ethanol injection method | PS: 123.2 nm ZP: 32 mV EE: 68.3% | Non-hemolytic, safe for intranasal administration | AUC: 506,731.3 ng.min/mL Cmax: 1984.23 ng/mL Tmax: 30 min t1/2: 347.65 min Increase up to 4.6–5.3 brain concentration Increased bioavailability Improves memory and cognition No or very little nasal toxicity | [72] 2017 |
| Rivastigmine hydrogen tartrate | Emulsification/ solvent evaporation | PS: 266 nm PDI: 0.23 ZP: −16.58 mV EE: 61.8% | Controlled and sustained release of 80% of the drug for 12 h Controlled diffusion, initially through surface erosion | Significant reduction in AChE1 and AchE2 activity, which enhances neuronal function Significant improvement of memory, learning and cognitive response | [73] 2019 |
| Pioglitazone | Microemulsification | PS: 211.4 nm PDI: 0.25 ZP: 14.9 mV EE: 70.18% | Sustained release for 24 h Fickian Diffusion Higuchi kinetics Increased permeability (1789 μg/cm2) No cytotoxic effect | Cmax: 1.62 μg/mL Brain/plasma ratio: 1.6 Increased bioavailability Nose–brain direct delivery | [75] 2019 |
| Resveratrol | Ultrasonic emulsification | PS: 132 nm PDI: 0.165 ZP: −23 mV EE: 74% | Drug loading of 10 ± 3% and a mucoadhesive force of the gel of 4087 ± 115 dynes/cm2 Long term stability at 2–8 °C | Cmax 2.5-fold higher in brain than NLC-based in situ gel Higher concentration in the brain with the gel in situ No nasal toxicity Faster absorption | [77] 2019 |
| Berberine | Hot homogenization and ultrasonics | PS: 180.9 nm ZP: 36.8 mV | Sustained release properties and increased ex vivo permeability through nasal mucosa | The Cbrain/Cplasma ratio at 30 min was much higher in the intranasal NLC dispersion formulation (4.56) than in the IN plain solution (2.14) or IV solution (0.26) | [79] 2022 |
| Astaxanthin | Hot high-pressure homogenization | PS: 142.8 nm PDI: 0.247 ZP: –32.2 mV EE: 94.1% | Initial burst release followed by sustained release for 24 h AST-NLCs were stable at 4–8 °C for six months | Significantly improved the cholinergic neurotransmission compared to AST solution | [81] 2023 |
| Donepezil | Melt emulsification- probe sonication method | PS: 112 nm PDI: 0.114 ZP: −35 mV EE: 79.25% | Drug loading of 7.9 ± 2.1% and a mucoadhesive force of the gel of 3189 ± 84.26 dynes/cm2 | Higher drug distribution in brain (20% increase NLC vs marketed tablets) and lower drug concentration in plasma (25% reduction NLC vs tablet) NLC administration increased residence time and penetration through olfactory lobe Improved cognitive function (IN vs. oral tablet) | [82] 2018 |
- They promote greater cerebral uptake than simple drug solutions, both intravenously and intranasally, leading to increased drug concentrations in the brain.
- They are more efficiently absorbed after intranasal administration and their small diameter facilitates their passage through the neuronal pathway.
- They provide a controlled and sustained release profile of the drug, unlike conventional drug delivery systems.
- They can be coated with polymers, such as chitosan, or dispersed in hydrogels, giving the formulation excellent mucoadhesive properties, prolonging the residence time in the nasal cavity and improving permeability through the nasal epithelium.
- They offer better drug residence and formulation stability during storage and greater physiological compatibility than other formulations.
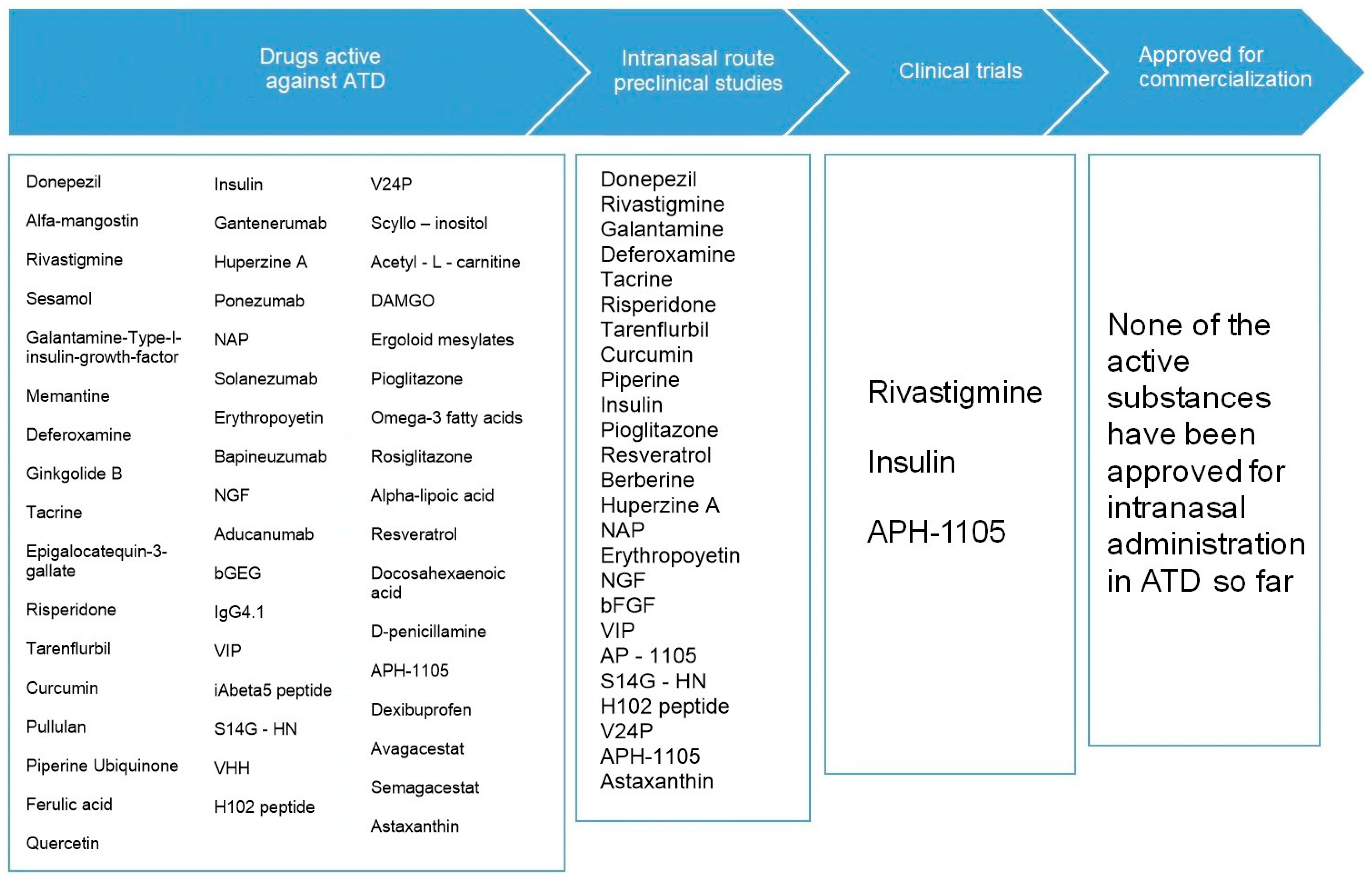
3.3. Current Situation of Intranasal Administration in the Treatment of ATD
| Clinical Trials for Nasal Administration in Alzheimer-Type Dementias | ||||
|---|---|---|---|---|
| Active Ingredient | Date | Phase | Results | Reference |
| Insulin | 2007–2012 | Phase 2 | An improvement in memory retention and attention was observed after administration of 20 IU daily of intranasal insulin compared to placebo. | NCT00438568 [91] |
| Insulin | 2012 | Phase 2 | No results have been published. | NCT01547169 [92] |
| Insulin | 2012–2018 | Phase 2 | Treatment with 20 IU of insulin improved delayed memory, and both insulin doses (20 and 40 IU) preserved general cognition as assessed via the ADAS-cog score and functional abilities as assessed via the ADCS-ADL scale. There were no treatment-related serious adverse events. | NCT01595646 [47,93] |
| Insulin | 2013–2020 | Phase 2 | A total of 289 participants were randomized. Among the first 49 participants, the ViaNase device was used to deliver intranasal insulin, while the rest of the participants used a second device (device 2). After one year of treatment, participants using device 2 did not show cognitive or functional benefits, while patients using the ViaNase device did show a slowdown in cognitive decline according to the ADAS-cog-12 scale and in everyday activities. No clinically important adverse events were associated. | NCT01767909 [94] |
| Insulin | 2015–2020 | Phase 2 | No results have been published. | NCT02462161 [95] |
| Insulin | 2015–2020 | Phase 2 | Intranasal insulin glulisine had no significant effect on improving cognition or mood; however, the number of subjects successfully enrolled and the duration of the study were limited. Intranasal insulin glulisine was relatively safe and well tolerated. | NCT02503501 [96] |
| Insulin | 2021 | Phase 2 | In recruiting status. | NCT05081219 [89] |
| Insulin | 2022 | Phase 2 | Not recruiting yet. | NCT05006599 [90] |
| Rivastigmine | 2016 | Phase 1 | Rivastigmine nasal spray had superior absolute bioavailability compared to historical values for the oral capsule and transdermal patch determined by other investigators. It had a rapid onset of action, and a favorable safety and tolerability profile. No clinically important adverse events were associated. | ACTRN12614001313628 [87] |
| APH-1105 | 2023–2024 | Phase 2 | Not recruiting yet. | NCT03806478 [86] |
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Scheltens, P.; De Strooper, B.; Kivipelto, M.; Holstege, H.; Chételat, G.; Teunissen, C.E.; Cummings, J.; van der Flier, W.M. Alzheimer’s disease. Lancet 2021, 397, 1577–1590. [Google Scholar] [CrossRef] [PubMed]
- Villarejo-Galende, A.; Eimil-Ortiz, M.; Llamas-Velasco, S.; Llanero-Luque, M.; López de Silanes de Miguel, C.; Prieto Jurczynska, C. Informe de la Fundación del Cerebro. Impacto social de la enfermedad de Alzheimer y otras demencias. Neurología 2021, 36, 39–49. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.alz.org/alzheimers-dementia/what-is-alzheimers (accessed on 8 January 2023).
- Breijyeh, Z.; Karaman, R. Comprehensive Review on Alzheimer’s Disease: Causes and Treatment. Molecules 2020, 25, 5789. [Google Scholar] [CrossRef]
- Prince, M.J.; Wimo, A.; Guerchet, M.M.; Ali, G.C.; Wu, Y.-T.; Prina, M. World Alzheimer Report 2015. The Global Impact of Dementia: An Analysis of Prevalence, Incidence, Cost and Trends; Alzehimer Disease International: London, UK, 2015; Available online: http://www.alz.co.uk/research/world-report-2015 (accessed on 3 October 2022).
- Li, X.; Feng, X.; Sun, X.; Hou, N.; Han, F.; Liu, Y. Global, regional, and national burden of Alzheimer’s disease and other dementias, 1990–2019. Front. Aging Neurosci. Lausanne 2022, 14, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Alzheimer’s Disease Fact Sheet. National Institute on Aging. 2022. Available online: https://www.nia.nih.gov/health/alzheimers-disease-fact-sheet (accessed on 3 October 2022).
- Mendez, M.F. Early-onset Alzheimer’s disease: Nonamnestic subtypes and type 2 AD. Arch. Med. Res. 2012, 43, 677–685. [Google Scholar] [CrossRef] [PubMed]
- Burns, A.; Iliffe, S. Alzheimer’s disease. Brit. Med. J. 2009, 338, b158. [Google Scholar] [CrossRef]
- Hippius, H.; Neundörfer, G. The Discovery of Alzheimer disease. Dialogues. Clin. Neurosci. 2003, 5, 101–108. [Google Scholar] [CrossRef]
- Agrawal, M.; Dethe, M.R.; Ahmed, H.; Yadav, A.; Gupta, U.; Alexander, A. Nose-to-brain drug delivery for the treatment of Alzheimer’s disease: Current advancements and challenges. Expert Opin. Drug Deliv. 2022, 19, 87–102. [Google Scholar]
- Food and Drug Administration. ARICEPT (Donepezil Hydrochloride) Prescribing Information [Internet]. 2021. Available online: http://www.aricept.com (accessed on 3 October 2022).
- Food and Drug Administration. ADLARITY (Donepezil Transdermal System) Prescribing Information [Internet]. 2022. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/212304s000lbl.pdf (accessed on 24 April 2023).
- Food and Drug Administration. RAZADYNE® (Galantamine Hydrobromide) Prescribing Information. 2021. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/021169s035,021615s026lbl.pdf (accessed on 3 October 2022).
- Food and Drug Administration. Exelon® Patch (Rivastigmine Transdermal System) Prescribing Information. 2020. Available online: https://www.novartis.com/us-en/sites/novartis_us/files/exelonpatch.pdf (accessed on 15 July 2022).
- Food and Drug Administration. RIVASTIGMINE TARTRATE Capsules Prescribing Information. 2019. Available online: https://www.apotex.com/products/us/downloads/pre/riva_imcp_ins.pdf (accessed on 15 July 2022).
- Food and Drug Administration. NAMENDA (Memantine HCl) Prescribing Information [Internet]. 2019. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/022525s015lbl.pdf (accessed on 15 July 2022).
- Binda, A.; Murano, C.; Rivolta, I. Innovative Therapies and Nanomedicine Applications for the Treatment of Alzheimer’s Disease: A State-of-the-Art (2017–2020). Int. J. Nanomed. 2020, 15, 6113–6135. [Google Scholar] [CrossRef]
- Food and Drug Administration. ADUHELM® (Aducanumab-Avwa) Injection Prescribing Information. 2022. Available online: https://www.biogencdn.com/us/aduhelm-pi.pdf (accessed on 15 July 2022).
- European Medicines Agency. Refusal of the Marketing Authorization for Aduhelm (Aducanumab). 2022. Available online: https://www.ema.europa.eu/en/medicines/human/summaries-opinion/aduhelm#opinion-section (accessed on 3 October 2022).
- Agrawal, M.; Saraf, S.; Saraf, S.; Antimisiaris, S.G.; Chougule, M.B.; Shoyele, S.A.; Alexander, A. Nose-to-brain drug delivery: An update on clinical challenges and progress toward approval of anti-Alzheimer drugs. J. Control. Release 2018, 281, 139–177. [Google Scholar] [CrossRef]
- Tarriot, P.N.; Braeckman, R.; Oh, C. Comparison of steady-state pharmacokinetics of Donepezil transdermal delivery system with oral Donepezil. J. Alzheimer’s Dis. 2022, 90, 161–172. [Google Scholar] [CrossRef] [PubMed]
- Food and Drug Administration. NAMZARIC (Memantine and Donepezil Hydrochlorides) Prescribing Information. 2019. Available online: https://www.rxabbvie.com/pdf/namzaric_pi.pdf (accessed on 15 July 2022).
- Terstappen, G.C.; Meyer, A.H.; Bell, R.D.; Zhang, W. Strategies for delivering therapeutics across the blood–brain barrier. Nat. Rev. Drug Discov. 2021, 20, 362–383. [Google Scholar] [CrossRef]
- Tripathi, S.; Gupta, U.; Ujjwal, R.R.; Yadav, A.K. Nano-lipidic formulation and therapeutic strategies for Alzheimer’s disease via intranasal route. J. Microencapsul. 2021, 38, 572–593. [Google Scholar] [CrossRef]
- Riccardi, C.; Napolitano, F.; Montesarchio, D.; Sampaolo, S.; Melone, M.A.B. Nanoparticle-Guided Brain Drug Delivery: Expanding the Therapeutic Approach to Neurodegenerative Diseases. Pharmaceutics 2021, 13, 1897. [Google Scholar] [CrossRef] [PubMed]
- Bell, R.D. Considerations When Developing Blood-Brain Barrier Crossing Drug Delivery Technology. Handb. Exp. Pharmacol. 2022, 273, 83–95. [Google Scholar]
- Sobiesk, J.L.; Munakomi, S. Anatomy, Head and Neck, Nasal Cavity; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Akel, H.; Ismail, R.; Csóka, I. Progress and perspectives of brain-targeting lipid-based nanosystems via the nasal route in Alzheimer’s disease. Eur. J. Pharm. Biopharm. 2020, 148, 38–53. [Google Scholar] [CrossRef] [PubMed]
- Lobaina Mato, Y. Nasal route for vaccine and drug delivery: Features and current opportunities. Int. J. Pharm. 2019, 572, 118813. [Google Scholar] [CrossRef]
- Lee, D.; Minko, T. Nanotherapeutics for Nose-to-Brain Drug Delivery: An Approach to Bypass the Blood Brain Barrier. Pharmaceutics 2021, 13, 2049. [Google Scholar] [CrossRef] [PubMed]
- Agu, R.U. Challenges in nasal drug absorption: How far have we come? Ther. Deliv. 2016, 7, 495–510. [Google Scholar] [CrossRef] [PubMed]
- Dando, S.J.; Mackay-Sim, A.; Norton, R.; Currie, B.J.; St John, J.A.; Ekberg, J.A.; Batzloff, M.; Ulett, G.C.; Beacham, I.R. Pathogens penetrating the central nervous system: Infection pathways and the cellular and molecular mechanisms of invasion. Clin. Microbiol. Rev. 2014, 27, 691–726. [Google Scholar] [CrossRef]
- Dhas, N.L.; Kudarha, R.R.; Mehta, T.A. Intranasal Delivery of Nanotherapeutics/Nanobiotherapeutics for the Treatment of Alzheimer’s Disease: A Proficient Approach. Crit. Rev. Ther. Drug Carrier Syst. 2019, 36, 373–447. [Google Scholar] [CrossRef] [PubMed]
- Poovaiah, N.; Davoudi, Z.; Peng, H.; Schlichtmann, B.; Mallapragada, S.; Narasimhan, B.; Wang, Q. Treatment of neurodegenerative disorders through the blood-brain barrier using nanocarriers. Nanoscale 2018, 10, 16962–16983. [Google Scholar] [CrossRef] [PubMed]
- Erdő, F.; Bors, L.A.; Farkas, D.; Bajza, Á.; Gizurarson, S. Evaluation of intranasal delivery route of drug administration for brain targeting. Brain Res. Bull. 2018, 143, 155–170. [Google Scholar] [CrossRef] [PubMed]
- Selvaraj, K.; Gowthamarajan, K.; Reddy-Karri, V.V.S. Nose to brain transport pathways an overview: Potential of nanostructured lipid carriers in nose to brain targeting. Artif. CellsNanomed. Biotechnol. 2018, 46, 2088–2095. [Google Scholar] [CrossRef]
- Bahadur, S.; Pardhi, D.M.; Rautio, J.; Rosenholm, J.M.; Pathak, K. Intranasal Nanoemulsions for Direct Nose-to-Brain Delivery of Actives for CNS Disorders. Pharmaceutics 2020, 12, 1230. [Google Scholar] [CrossRef]
- The Mandibular Division of the Trigeminal Nerve (CNV3)—TeachMeAnatomy. Available online: https://teachmeanatomy.info/head/nerves/mandibular-nerve/ (accessed on 28 March 2023).
- Bourganis, V.; Kammona, O.; Alexopoulos, A.; Kiparissides, C. Recent advances in carrier mediated nose-to-brain delivery of pharmaceuticals. Eur. J. Pharm. Biopharm. 2018, 128, 337–362. [Google Scholar] [CrossRef]
- Bicker, J.; Fortuna, A.; Alves, G.; Falcão, A. Nose-to-brain Delivery of Natural Compounds for the Treatment of Central Nervous System Disorders. Curr. Pharm. Des. 2020, 26, 594–619. [Google Scholar] [CrossRef]
- Cunha, S.; Forbes, B.; Sousa Lobo, J.M.; Silva, A.C. Improving Drug Delivery for Alzheimer’s Disease Through Nose-to-Brain Delivery Using Nanoemulsions, Nanostructured Lipid Carriers (NLC) and in situ Hydrogels. Int. J. Nanomed. 2021, 16, 4373–4390. [Google Scholar] [CrossRef]
- Fonseca, L.C.; Lopes, J.A.; Vieira, J.; Viegas, C.; Oliveira, C.S.; Hartmann, R.P.; Fonte, P. Intranasal drug delivery for treatment of Alzheimer’s disease. Drug Deliv. Transl. Res. 2021, 11, 411–425. [Google Scholar] [CrossRef]
- Bustamante-Marin, X.M.; Ostrowski, L.E. Cilia and Mucociliary Clearance. Cold Spring Harb. Perspect Biol. 2017, 9, a028241. [Google Scholar] [CrossRef]
- Brannigan, R.P.; Khutoryanskiy, V.V. Progress and current trends in the synthesis of novel polymers with enhanced mucoadhesive properties. Macromol. Biosci. 2019, 19, 1900194. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Xiong, G.; Tsang, W.C.; Schätzlein, A.G.; Uchegbu, I.F. Nose-to-Brain Delivery. J. Pharmacol. Exp. Ther. 2019, 370, 593–601. [Google Scholar] [CrossRef] [PubMed]
- Craft, S.; Claxton, A.; Baker, L.D.; Hanson, A.J.; Cholerton, B.; Trittschuh, E.H.; Dahl, D.; Caulder, E.; Neth, B.; Montine, T.J.; et al. Effects of regular and long-acting insulin on cognition and Alzheimer’s disease biomarkers: A pilot clinical trial. J. Alzheimers Dis. 2017, 57, 1325–1334. [Google Scholar] [CrossRef] [PubMed]
- Food and Drug Administration. ONZETRATM XsailTM (Sumatriptan Nasal Powder) Prescribing Information. 2016. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/206099s000lbl.pdf (accessed on 31 March 2022).
- Ghadiri, M.; Young, P.; Traini, D. Strategies to Enhance Drug Absorption via Nasal and Pulmonary Routes. Pharmaceutics 2019, 11, 113. [Google Scholar] [CrossRef]
- Bakker, C.; van der Aart, J.; Hart, E.P.; Klaassen, E.S.; Bergmann, K.R.; van Esdonk, M.J.; Kay, D.G.; Groeneveld, G.J. Safety, pharmacokinetics, and pharmacodynamics of Gln-1062, a prodrug of galantamine. A&D Transl. Res. Clin. Interv. 2020, 6, e12093. Available online: https://onlinelibrary.wiley.com/doi/10.1002/trc2.12093 (accessed on 28 March 2023).
- Movia, D.; Bruni-Favier, S.; Prina-Mello, A. In vitro Alternatives to Acute Inhalation Toxicity Studies in Animal Models—A Perspective. Front. Bioeng. Biotechnol. 2020, 8, 549. [Google Scholar] [CrossRef]
- Pires, P.C.; Santos, A.O. Nanosystems in nose-to-brain drug delivery: A review of non-clinical brain targeting studies. J. Control. Release 2018, 270, 89–100. [Google Scholar] [CrossRef]
- Vitorino, C.; Silva, S.; Bicker, J.; Falcão, A.; Fortuna, A. Antidepressants and nose-to-brain delivery: Drivers, restraints, opportunities and challenges. Drug Discov. Today 2019, 24, 1911–1923. [Google Scholar] [CrossRef]
- Hong, S.S.; Oh, K.T.; Choi, H.G.; Lim, S.J. Liposomal Formulations for Nose-to-Brain Delivery: Recent Advances and Future Perspectives. Pharmaceutics 2019, 11, 540. [Google Scholar] [CrossRef]
- Pokharkar, V.; Patil-Gadhe, A.; Palla, P. Efavirenz loaded nanostructured lipid carrier engineered for brain targeting through intranasal route: In-vivo pharmacokinetic and toxicity study. Biomed. Pharmacother. 2017, 94, 150–164. [Google Scholar] [CrossRef]
- Ross, C.; Taylor, M.; Fullwood, N.; Allsop, D. Liposome delivery systems for the treatment of Alzheimer’s disease. Int. J. Nanomed. 2018, 13, 8507–8522. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, P.; Deepak Rawtani, D.; Barot, T. Design, development and in-vitro/in-vivo evaluation of intranasally delivered Rivastigmine and N-Acetyl Cysteine loaded bifunctional niosomes for applications in combinative treatment of Alzheimer’s disease. Eur. J. Pharm. Biopharm. 2021, 163, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Gharbavi, M.; Amani, J.; Kheiri-Manjili, H.; Danafar, H.; Sharafi, A.J. Niosome: A Promising Nanocarrier for Natural Drug Delivery through Blood-Brain Barrier. Adv. Pharmacol. Pharm. Sci. 2018, 2018, 6847971. [Google Scholar] [CrossRef] [PubMed]
- Ferraz de Souza, I.F.; Queiroz dos Santos, T.; Rodrigo Vicentino, P.; Aparecida Mangerona, B.; Chiva Carvalho, F.; Bergamin Boralli, V.; Morais Ruela, A.L.; Ribeiro Pereira, G. The liquid crystalline phase behaviour of a nasal formulation modifies the brain disposition of donepezil in rats in the treatment of Alzheimer’s disease. Colloids Surf. B Biointerfaces 2021, 203, 111721. [Google Scholar] [CrossRef] [PubMed]
- Kaur, A.; Nigam, K.; Bhatnagar, I.; Sukhpal, H.; Awasthy, S.; Shankar, S.; Tyagi, A.; Dang, S. Treatment of Alzheimer’s diseases using donepezil nanoemulsion: An intranasal approach. Drug Deliv. Transl. Res. 2020, 10, 1862–1875. [Google Scholar] [CrossRef] [PubMed]
- Haider, M.; Abdin, S.M.; Kamal, L.; Orive, G. Nanostructured Lipid Carriers for Delivery of Chemotherapeutics: A Review. Pharmaceutics 2020, 12, 288. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Nguyen, T.T.D.; Tran, N.M.; Van Vo, G. Lipid-Based Nanocarriers via Nose-to-Brain Pathway for Central Nervous System Disorders. Neurochem. Res. 2022, 47, 552–573. [Google Scholar] [CrossRef]
- Cunha, S.; Amaral, M.H.; Lobo, J.M.S.; Silva, A.C. Lipid Nanoparticles for Nasal/Intranasal Drug Delivery. Crit. Rev. Ther. Drug Carr. Syst. 2017, 34, 257–282. [Google Scholar] [CrossRef]
- Agrawal, M.; Saraf, S.; Saraf, S.; Dubey, S.K.; Puri, A.; Patel, R.J.; Ravichandiran, V.; Murty, U.S.; Alexander, A. Recent strategies and advances in the fabrication of nano lipid carriers and their application towards brain targeting. J. Control. Release 2020, 321, 372–415. [Google Scholar] [CrossRef]
- Agrawal, M.; Saraf, S.; Saraf, S.; Antimisiaris, S.G.; Hamano, N.; Li, S.-D.; Chougule, M.; Shoyele, S.A.; Gupta, U.; Ajazuddin; et al. Recent advancements in the field of nanotechnology for the delivery of anti-Alzheimer drug in the brain region. Expert Opin. Drug Deliv. 2018, 15, 598–617. [Google Scholar] [CrossRef]
- de Souza Guedes, L.; Martinez, R.M.; Bou-Chacra, N.A.; Velasco, M.V.R.; Rosado, C.; Baby, A.R. An Overview on Topical Administration of Carotenoids and Coenzyme Q10 Loaded in Lipid Nanoparticles. Antioxidants 2021, 10, 1034. [Google Scholar] [CrossRef] [PubMed]
- Costa, C.; Moreira, J.N.; Amaral, M.H.; Sousa Lobo, J.M.; Silva, A.C. Nose-to-brain delivery of lipid-based nanosystems for epileptic seizures and anxiety crises. J. Control. Release 2019, 295, 187–200. [Google Scholar] [CrossRef] [PubMed]
- Gadhave, D.G.; Kokare, C.R. Nanostructured lipid carriers engineered for intranasal delivery of teriflunomide in multiple sclerosis: Optimization and in vivo studies. Drug Dev. Ind. Pharm. 2019, 45, 839–851. [Google Scholar] [CrossRef] [PubMed]
- Desfrançois, C.; Auzély, R.; Texier, I. Lipid Nanoparticles and Their Hydrogel Composites for Drug Delivery: A Review. Pharmaceuticals 2018, 11, 118. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.-T.-L.; Maeng, H.-J. Pharmacokinetics and Pharmacodynamics of Intranasal Solid Lipid Nanoparticles and Nanostructured Lipid Carriers for Nose-to-Brain Delivery. Pharmaceutics 2022, 14, 572. [Google Scholar] [CrossRef]
- Nguyen, K.; Hoffman, H.; Chakkamparambil, B.; Grossberg, G.T. Evaluation of rivastigmine in Alzheimer’s disease. Neurodegener. Dis. Manag. 2021, 11, 35–48. [Google Scholar] [CrossRef]
- Wavikar, P.; Pai, R.; Vavia, P. Nose to Brain Delivery of Rivastigmine by In Situ Gelling Cationic Nanostructured Lipid Carriers: Enhanced Brain Distribution and Pharmacodynamics. J. Pharm. Sci. 2017, 106, 3613–3622. [Google Scholar] [CrossRef]
- Anand, A.; Arya, M.; Kaithwas, G.; Singh, G.; Saraf, S.A. Sucrose stearate as a biosurfactant for development of rivastigmine containing nanostructured lipid carriers and assessment of its activity against dementia in C. elegans model. J. Drug Deliv. Sci. Technol. 2019, 49, 219–226. [Google Scholar] [CrossRef]
- Saunders, A.M.; Burns, D.K.; Gottschalk, W.K. Reassessment of Pioglitazone for Alzheimer’s Disease. Front. Neurosci. 2021, 15, 666958. [Google Scholar] [CrossRef]
- Jojo, G.M.; Kuppusamy, G.; De, A.; Reddy-Karri, V.V.S.N. Formulation and optimization of intranasal nanolipid carriers of pioglitazone for the repurposing in Alzheimer’s disease using Box-Behnken design. Drug Dev. Ind. Pharm. 2019, 45, 1061–1072. [Google Scholar] [CrossRef]
- Rao, Y.L.; Ganaraja, B.; Joy, T.; Pai, M.M.; Ullal, S.D.; Murlimanju, B.V. Neuroprotective effects of resveratrol in Alzheimer’s disease. Front. Biosci. (Elite Ed.) 2020, 12, 139–149. [Google Scholar] [CrossRef] [PubMed]
- Rajput, A.P.; Butani, S.B. Resveratrol anchored nanostructured lipid carrier loaded in situ gel via nasal route: Formulation, optimization and in vivo characterization. J. Drug Deliv. Sci. Technol. 2019, 51, 214–223. [Google Scholar] [CrossRef]
- Akbar, M.; Shabbir, A.; Rehman, K.; Akash, M.S.H.; Shah, M.A. Neuroprotective potential of berberine in modulating Alzheimer’s disease via multiple signaling pathways. J. Food Biochem. 2021, 45, e13936. [Google Scholar] [CrossRef] [PubMed]
- Abo-El-Enin, H.A.; Elkomy, M.H.; Naguib, I.A.; Ahmed, M.F.; Alsaidan, O.A.; Alsalahat, I.; Ghoneim, M.M.; Eid, H.M. Lipid Nanocarriers Overlaid with Chitosan for Brain Delivery of Berberine via the Nasal Route. Pharmaceuticals 2022, 15, 281. [Google Scholar] [CrossRef] [PubMed]
- Kales, H.C.; Lyketsos, C.G.; Miller, E.M.; Ballard, C. Management of behavioral and psychological symptoms in people with Alzheimer’s disease: An international Delphi consensus. Int. Psychogeriatr. 2019, 31, 83–90. [Google Scholar] [CrossRef]
- Shehata, M.K.; Ismail, A.A.; Kamel, M.A. Nose to Brain Delivery of Astaxanthin–Loaded Nanostructured Lipid Carriers in Rat Model of Alzheimer’s Disease: Preparation, in vitro and in vivo Evaluation. Int. J. Nanomed. 2023, 18, 1631–1658. [Google Scholar] [CrossRef]
- Rajput, A.P.; Butani, S.B. Fabrication of an ion-sensitive in situ gel loaded with nanostructured lipid carrier for nose to brain delivery of donepezil. Asian J. Pharm. 2018, 12, 293. [Google Scholar]
- Younis, M.A.; Tawfeek, H.M.; Abdellatif, A.A.H.; Abdel-Aleem, J.A.; Harashima, H. Clinical translation of nanomedicines: Challenges, opportunities, and keys. Adv. Drug Deliv. Rev. 2022, 181, 114083. [Google Scholar] [CrossRef]
- Dubey, S.K.; Lakshmi, K.K.; Krishna, K.V.; Agrawal, M.; Singhvi, G.; Saha, R.N.; Saraf, S.; Saraf, S.; Shukla, R.; Alexander, A. Insulin mediated novel therapies for the treatment of Alzheimer’s disease. Life Sci. 2020, 249, 117540. [Google Scholar] [CrossRef]
- Morgan, T.M.; Soh, B. Absolute bioavailability and safety of a novel rivastigmine nasal spray in healthy elderly individuals. Br. J. Clin. Pharmacol. 2017, 83, 510–516. [Google Scholar] [CrossRef]
- Aphios. Safety, Tolerability and Efficacy Assessment of Intranasal Nanoparticles of APH-1105, A Novel Alpha Secretase Modulator For Mild to Moderate Cognitive Impairment Due to Alzheimer’s Disease (AD). clinicaltrials.gov; Report No.: NCT03806478. 2021. Available online: https://clinicaltrials.gov/ct2/show/NCT03806478 (accessed on 28 March 2023).
- ANZCTR. Evaluation of the Pharmacokinetics and Safety of Rivastigmine Intranasal Spray. Report No.: ACTRN12614001313628. 2016. Available online: https://www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=367562 (accessed on 28 March 2023).
- Hallschmid, M. Intranasal insulin. J. Neuroendocrinol. 2021, 33, e12934. [Google Scholar] [CrossRef] [PubMed]
- Wake Forest University Health Sciences. Study of Nasal Insulin to Fight Forgetfulness—Combination Intranasal Insulin and Empagliflozin Trial. clinicaltrials.gov;. Report No.: NCT05081219. 2021. Available online: https://clinicaltrials.gov/ct2/show/NCT05081219 (accessed on 28 March 2023).
- Wake Forest University Health Sciences. Study of Nasal Insulin to Fight Forgetfulness (SNIFF)—3-Week Aptar CPS Device. clinicaltrials.gov; Report No.: NCT05006599. 2021. Available online: https://clinicaltrials.gov/ct2/show/NCT05006599 (accessed on 28 March 2023).
- University of Washington. Therapeutic Effects of Intranasal Insulin Administration in AD. clinicaltrials.gov; Report No.: NCT00438568. 2012. Available online: https://clinicaltrials.gov/ct2/show/NCT00438568 (accessed on 28 March 2023).
- Craft, S. Study of Nasal Insulin to Fight Forgetfulness—Long-Acting Insulin Detemir—21 Days. clinicaltrials.gov;. Report No.: NCT01547169. 2012. Available online: https://clinicaltrials.gov/ct2/show/NCT01547169 (accessed on 28 March 2023).
- Wake Forest University Health Sciences. Study of Nasal Insulin to Fight Forgetfulness—Long-Acting Insulin Detemir—120 Days (SL120). clinicaltrials.gov; Report No.: NCT01595646. 2020. Available online: https://clinicaltrials.gov/ct2/show/NCT01595646 (accessed on 29 March 2023).
- Aisen, P. Therapeutic Effects of Intranasally-Administered Insulin in Adults with Amnestic Mild Cognitive Impairment (aMCI) or Mild Alzheimer’s Disease (AD). clinicaltrials.gov; Report No.: NCT01767909. Available online: https://clinicaltrials.gov/ct2/show/NCT01767909 (accessed on 28 March 2023).
- Wake Forest University Health Sciences. Study of Nasal Insulin to Fight Forgetfulness—Short-Acting Insulin Aspart. clinicaltrials.gov; Report No.: NCT02462161. 2020. Available online: https://clinicaltrials.gov/ct2/show/NCT02462161 (accessed on 28 March 2023).
- Health Partners Institute. A Phase II, Single Center, Randomized, Double-Blind, Placebo-Controlled Study of the Safety and the Therapeutic Effectiveness of Intranasal Glulisine in Amnestic Mild Cognitive Impairment and Probable Mild Alzheimer’s Disease. clinicaltrials.gov; Report No.: NCT02503501. 2020. Available online: https://clinicaltrials.gov/ct2/show/NCT02503501 (accessed on 29 March 2023).



| Active Ingredient | Route of Administration | Pharmaceutical Form | FDA Reference |
|---|---|---|---|
| Rivastigmine | Oral | Capsules Oral solution | [15,16] |
| Transdermal | Transdermal patches | ||
| Galantamine | Oral | Tablets Extended release capsules | [14] |
| Memantine | Oral | Tablets Oral solution Extended release capsules | [17] |
| Donepezil | Oral | Tablets Orodispersible tablets | [12,13,22] |
| Transdermal | Transdermal patches | ||
| Combination of memantine and donepezil | Oral | Controlled release capsules | [23] |
| Aducanumab | Parenteral | IV bolus | [19] |
| Advantages | Disadvantages |
|---|---|
|
|
| Strategies to Improve Nasal Absorption | |||||
|---|---|---|---|---|---|
| Promoters of Absorption | Promoters of Absorption | Promoters of Absorption | Promoters of Absorption | Promoters of Absorption | Promoters of Absorption |
| Sodium lauryl sulfate Poloxamer Tween® Span® Sodium glucodeoxycholate Sodium taurodeoxycholate Taurodihydrofusidate Oleic acids Lauric acid Caprylic acid Phosphatidylcholine EDTA Citric acid Sodium salicytate Peppermint oil Cyclodextrins Chitosan Carbopol Starch Aminated gelatin | Comostat amylase Bestatin Aprotinin Amastatin Boroleucine Bacitracin Puromycin EDTA | Carboxymethylcellu-lose Microcrystalline cellulose Hydroxypropylcellu-lose Carbopol 971P Carbopol 934P Carbopol 981P Eudragrit Chitosan Cyclodextrins Stearylamine | MEMOGAIN® (Gln-106 | Optinase nasal device ViaNase-electronic atomizer Precision-Olfactory device Naltos device | Polymeric nanoparticles |
| Polymeric nanogels Core/shell nanoparticles Polymeric micelles Dendrimers | |||||
| Inorganic nanoparticles | |||||
| Silica nanoparticles Carbon nanotubes Magnetic nanoparticles Gold nanoparticles | |||||
| Lipid nanoparticles | |||||
| Nanoemulsions Microemulsions Liposomes Solid lipid nanoparticles Nanostructured lipid carriers | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Taléns-Visconti, R.; de Julián-Ortiz, J.V.; Vila-Busó, O.; Diez-Sales, O.; Nácher, A. Intranasal Drug Administration in Alzheimer-Type Dementia: Towards Clinical Applications. Pharmaceutics 2023, 15, 1399. https://doi.org/10.3390/pharmaceutics15051399
Taléns-Visconti R, de Julián-Ortiz JV, Vila-Busó O, Diez-Sales O, Nácher A. Intranasal Drug Administration in Alzheimer-Type Dementia: Towards Clinical Applications. Pharmaceutics. 2023; 15(5):1399. https://doi.org/10.3390/pharmaceutics15051399
Chicago/Turabian StyleTaléns-Visconti, Raquel, Jesus Vicente de Julián-Ortiz, Ofelia Vila-Busó, Octavio Diez-Sales, and Amparo Nácher. 2023. "Intranasal Drug Administration in Alzheimer-Type Dementia: Towards Clinical Applications" Pharmaceutics 15, no. 5: 1399. https://doi.org/10.3390/pharmaceutics15051399





