Multifunctional Milk-Derived Small Extracellular Vesicles and Their Biomedical Applications
Abstract
:1. Introduction
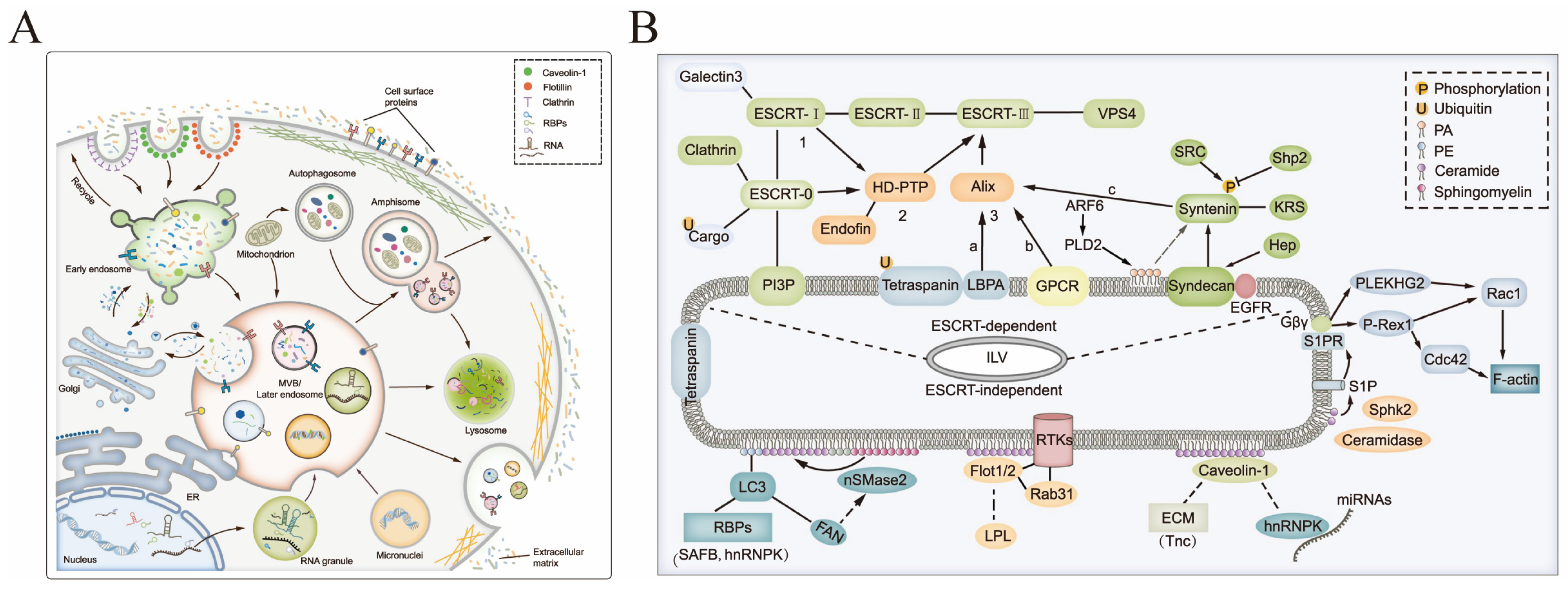
2. Biogenesis and Characteristics of sEVs
3. Isolation and Purification of msEVs
3.1. Common Methods for sEV Purification
3.2. Large-Scale Purification of High-Purity msEVs
4. Composition and Functions of msEVs
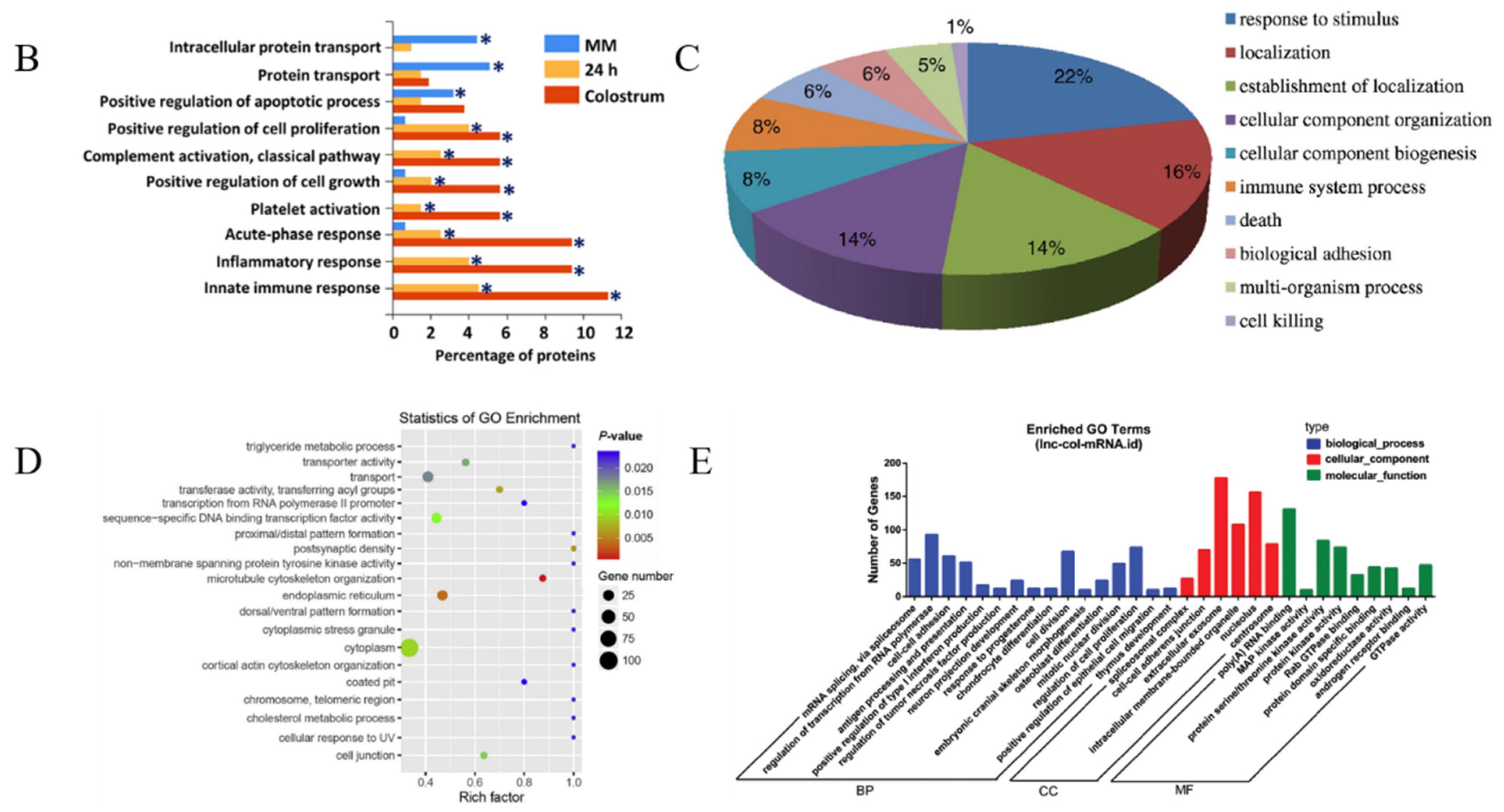
4.1. Composition of msEVs
4.1.1. Proteins
4.1.2. Nucleic Acids
4.1.3. Polysaccharides and Oligosaccharides
4.1.4. Lipids
4.2. Function of msEVs
4.2.1. Immunoregulation
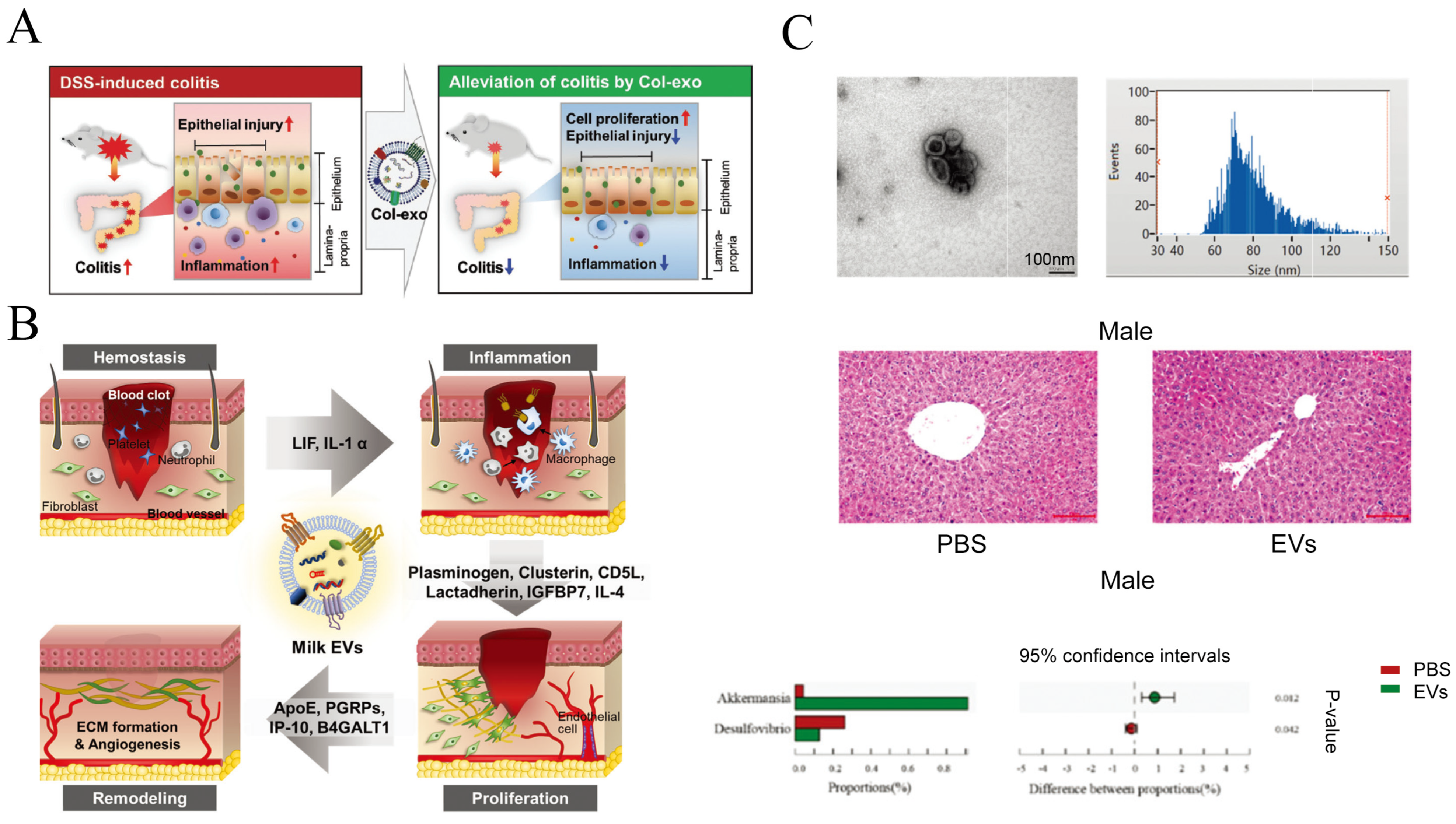
4.2.2. Regulation of Intestinal Tract Function
4.2.3. Regulation of Muscle and Bone Development
4.2.4. Promote Skin Regeneration
4.2.5. Detection of Bovine Diseases
4.2.6. Other Functions
4.3. Potential Health Risks Associated with msEVs
5. MsEVs as Drug Delivery Vehicles
5.1. Biosafety of msEVs
5.2. Engineering of msEVs
5.2.1. Cargo Loading
5.2.2. Modification of msEVs
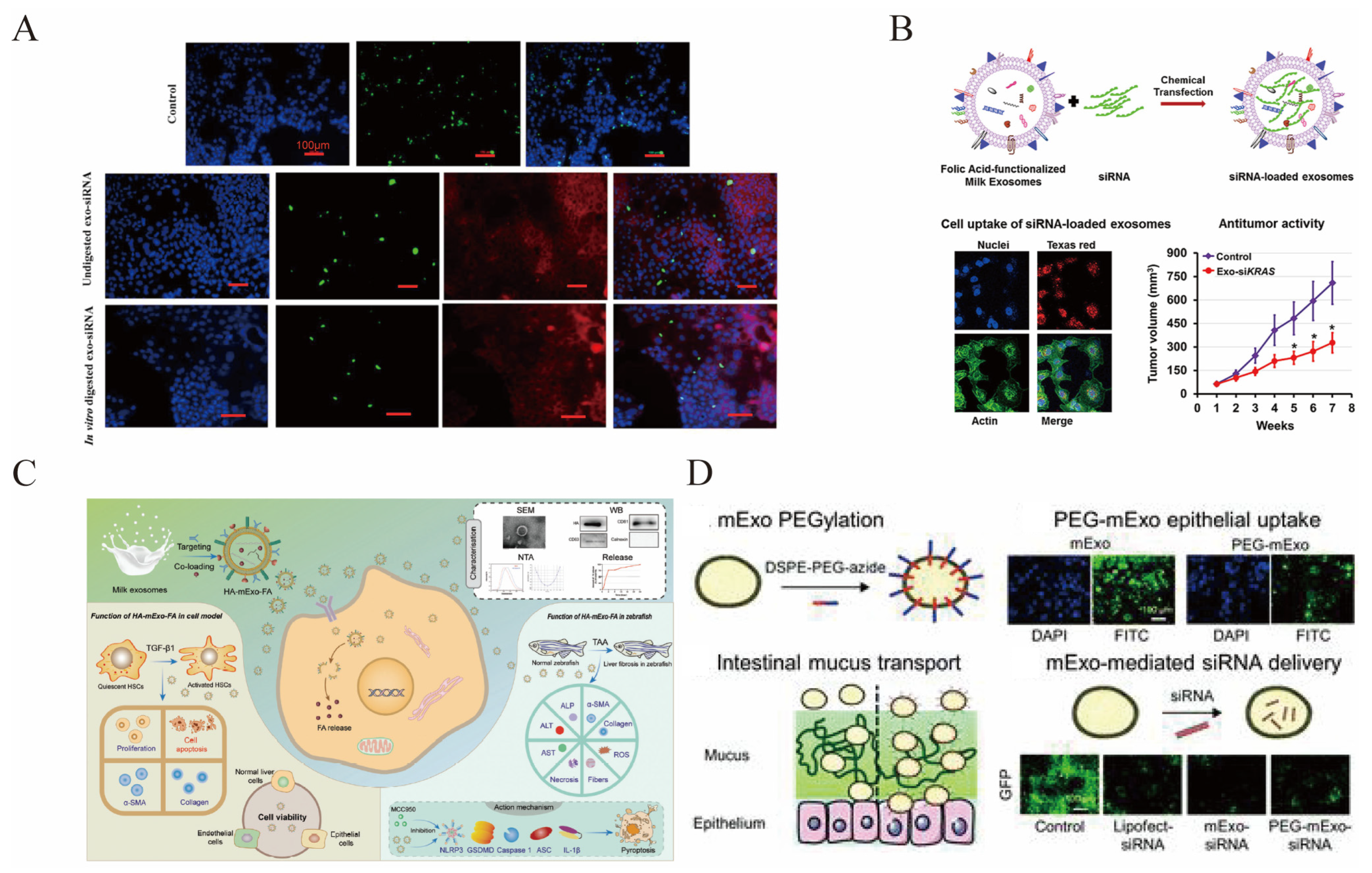
5.3. MsEVs for Drug Delivery
5.3.1. Delivery of Chemical Drugs
5.3.2. Delivery of Nucleic Acids
5.3.3. Delivery of Other Small Molecules
6. MsEVs and Nutritional Supplements
7. Conclusions and Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BLV | Bovine leukemia virus |
| BTN | Butyrophilin |
| DAI | Disease activity index |
| DNMT1 | DNA methyltransferase 1 |
| DSS | Dextran sulfate sodium salt |
| DOX | Delivering doxorubicin |
| DOX | Delivering doxorubicin |
| DSPE | 1,2-distearoyl-sn-glycero-3-phosphoethanolamine |
| DP | Dermal papillary cell |
| DHT | Dihydrotestosterone |
| ECG | Epigallocatechin gallate |
| ENP | Exosome nanopuncturer |
| ESCRT | Endosomal sorting complex required for transport |
| FA | Folic acid |
| HA | Hyaluronic acid |
| LNPs | Lipid nanoparticles |
| lncRNA | Long non-coding RNA |
| LPS | Lipopolysaccharide |
| msEVs | Milk-derived small extracellular vesicles |
| miRNA | MicroRNA |
| mRNA | Messenger RNA |
| ncRNA | Non-coding RNA |
| NEC | Necrotizing enterocolitis |
| PAC | Paclitaxel |
| PEG | Polyethylene glycol |
| sEVs | Small extracellular vesicles |
| SEC | Size-exclusion chromatography |
| siRNA | Interfering RNA |
| SAM | Severe acute malnutrition |
| TFF | Tangential flow filtration |
| TLR4 | Toll-like receptor 4 |
References
- Théry, C.; Zitvogel, L.; Amigorena, S. Exosomes: Composition, biogenesis and function reviews. Nat. Rev. Immunol. 2002, 2, 569. [Google Scholar] [CrossRef]
- Kalluri, R. The biology and function of exosomes in cancer. J. Clin. Investig. 2016, 126, 1208–1215. [Google Scholar] [CrossRef]
- Keller, S.; Sanderson, M.P.; Stoeck, A.; Altevogt, P. Exosomes: From biogenesis and secretion to biological function. Immunol. Lett. 2006, 107, 102–108. [Google Scholar] [CrossRef]
- An, Q.; van Bel, A.J.; Hückelhoven, R. Do plant cells secrete exosomes derived from multivesicular bodies? Plant Signal. Behav. 2007, 2, 4–7. [Google Scholar] [CrossRef] [PubMed]
- Xia, X.; Shi, B.; Wang, L.; Liu, Y.; Zou, Y.; Zhou, Y.; Chen, Y.; Zheng, M.; Zhu, Y.; Duan, J. From mouse to mouse-ear cress: Nanomaterials as vehicles in plant biotechnology. In Exploration; Wiley Online Library: New York, NY, USA, 2021. [Google Scholar]
- Haug, A.; Høstmark, A.T.; Harstad, O.M. Bovine milk in human nutrition—A review. Lipids Health Dis. 2007, 6, 1–16. [Google Scholar] [CrossRef]
- Zhou, Q.; Li, M.; Wang, X.; Li, Q.; Wang, T.; Zhu, Q.; Zhou, X.; Wang, X.; Gao, X.; Li, X. Immune-related microRNAs are abundant in breast milk exosomes. Int. J. Biol. Sci. 2012, 8, 118. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Xie, M.-Y.; Sun, J.-J.; Ye, R.-S.; Cheng, X.; Sun, R.-P.; Wei, L.-M.; Li, M.; Lin, D.-L.; Jiang, Q.-Y. Porcine milk-derived exosomes promote proliferation of intestinal epithelial cells. Sci. Rep. 2016, 6, 33862. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Aswath, K.; Schroeder, S.G.; Lippolis, J.D.; Reinhardt, T.A.; Sonstegard, T.S. MicroRNA expression profiles of bovine milk exosomes in response to Staphylococcus aureus infection. BMC Genom. 2015, 16, 806. [Google Scholar] [CrossRef]
- Kalluri, R.; LeBleu, V.S. The biology, function, and biomedical applications of exosomes. Science 2020, 367, eaau6977. [Google Scholar] [CrossRef]
- Yoshioka, Y.; Konishi, Y.; Kosaka, N.; Katsuda, T.; Kato, T.; Ochiya, T. Comparative marker analysis of extracellular vesicles in different human cancer types. J. Extracell. Vesicles 2013, 2, 20424. [Google Scholar] [CrossRef] [PubMed]
- Gobbo, J.; Marcion, G.; Cordonnier, M.; Dias, A.M.; Pernet, N.; Hammann, A.; Richaud, S.; Mjahed, H.; Isambert, N.; Clausse, V. Restoring anticancer immune response by targeting tumor-derived exosomes with a HSP70 peptide aptamer. J. Natl. Cancer Inst. 2016, 108, djv330. [Google Scholar] [CrossRef]
- Villarroya-Beltri, C.; Baixauli, F.; Mittelbrunn, M.; Fernández-Delgado, I.; Torralba, D.; Moreno-Gonzalo, O.; Baldanta, S.; Enrich, C.; Guerra, S.; Sánchez-Madrid, F. ISGylation controls exosome secretion by promoting lysosomal degradation of MVB proteins. Nat. Commun. 2016, 7, 13588. [Google Scholar] [CrossRef] [PubMed]
- Zitvogel, L.; Regnault, A.; Lozier, A.; Wolfers, J.; Flament, C.; Tenza, D.; Ricciardi-Castagnoli, P.; Raposo, G.; Amigorena, S. Eradication of established murine tumors using a novel cell-free vaccine: Dendritic cell derived exosomes. Nat. Med. 1998, 4, 594–600. [Google Scholar] [CrossRef]
- Konala, V.B.R.; Mamidi, M.K.; Bhonde, R.; Das, A.K.; Pochampally, R.; Pal, R. The current landscape of the mesenchymal stromal cell secretome: A new paradigm for cell-free regeneration. Cytotherapy 2016, 18, 13–24. [Google Scholar] [CrossRef]
- Raposo, G.; Stoorvogel, W. Extracellular vesicles: Exosomes, microvesicles, and friends. J. Cell Biol. 2013, 200, 373–383. [Google Scholar] [CrossRef] [PubMed]
- Hinde, E.; Thammasiraphop, K.; Duong, H.T.; Yeow, J.; Karagoz, B.; Boyer, C.; Gooding, J.J.; Gaus, K. Pair correlation microscopy reveals the role of nanoparticle shape in intracellular transport and site of drug release. Nat. Nanotechnol. 2017, 12, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.K.; Gothwal, A.; Kesharwani, P.; Alsaab, H.; Iyer, A.K.; Gupta, U. Dendrimer nanoarchitectures for cancer diagnosis and anticancer drug delivery. Drug Discov. Today 2017, 22, 314–326. [Google Scholar] [CrossRef]
- Luan, X.; Guan, Y.-Y.; Lovell, J.F.; Zhao, M.; Lu, Q.; Liu, Y.-R.; Liu, H.-J.; Gao, Y.-G.; Dong, X.; Yang, S.-C. Tumor priming using metronomic chemotherapy with neovasculature-targeted, nanoparticulate paclitaxel. Biomaterials 2016, 95, 60–73. [Google Scholar] [CrossRef]
- Haque, S.; Whittaker, M.R.; McIntosh, M.P.; Pouton, C.W.; Kaminskas, L.M. Disposition and safety of inhaled biodegradable nanomedicines: Opportunities and challenges. Nanomed. Nanotechnol. Biol. Med. 2016, 12, 1703–1724. [Google Scholar] [CrossRef]
- Shan, S.; Chen, J.; Sun, Y.; Wang, Y.; Xia, B.; Tan, H.; Pan, C.; Gu, G.; Zhong, J.; Qing, G. Functionalized macrophage exosomes with panobinostat and PPM1D-siRNA for diffuse intrinsic pontine gliomas therapy. Adv. Sci. 2022, 9, 2200353. [Google Scholar] [CrossRef]
- Zhong, J.; Xia, B.; Shan, S.; Zheng, A.; Zhang, S.; Chen, J.; Liang, X.-J. High-quality milk exosomes as oral drug delivery system. Biomaterials 2021, 277, 121126. [Google Scholar] [CrossRef] [PubMed]
- Babst, M.; Katzmann, D.J.; Estepa-Sabal, E.J.; Meerloo, T.; Emr, S.D. Escrt-III: An endosome-associated heterooligomeric protein complex required for mvb sorting. Dev. Cell 2002, 3, 271–282. [Google Scholar] [CrossRef] [PubMed]
- Ostrowski, M.; Carmo, N.B.; Krumeich, S.; Fanget, I.; Raposo, G.; Savina, A.; Moita, C.F.; Schauer, K.; Hume, A.N.; Freitas, R.P. Rab27a and Rab27b control different steps of the exosome secretion pathway. Nat. Cell Biol. 2010, 12, 19–30. [Google Scholar] [CrossRef]
- Hyenne, V.; Apaydin, A.; Rodriguez, D.; Spiegelhalter, C.; Hoff-Yoessle, S.; Diem, M.; Tak, S.; Lefebvre, O.; Schwab, Y.; Goetz, J.G. RAL-1 controls multivesicular body biogenesis and exosome secretion. J. Cell Biol. 2015, 211, 27–37. [Google Scholar] [CrossRef]
- Futter, C.E.; Pearse, A.; Hewlett, L.J.; Hopkins, C.R. Multivesicular endosomes containing internalized EGF-EGF receptor complexes mature and then fuse directly with lysosomes. J. Cell Biol. 1996, 132, 1011–1023. [Google Scholar] [CrossRef] [PubMed]
- Stuffers, S.; Sem Wegner, C.; Stenmark, H.; Brech, A. Multivesicular endosome biogenesis in the absence of ESCRTs. Traffic 2009, 10, 925–937. [Google Scholar] [CrossRef]
- Conde-Vancells, J.; Rodriguez-Suarez, E.; Embade, N.; Gil, D.; Matthiesen, R.; Valle, M.; Elortza, F.; Lu, S.C.; Mato, J.M.; Falcon-Perez, J.M. Characterization and comprehensive proteome profiling of exosomes secreted by hepatocytes. J. Proteome Res. 2008, 7, 5157–5166. [Google Scholar] [CrossRef]
- Subra, C.; Grand, D.; Laulagnier, K.; Stella, A.; Lambeau, G.; Paillasse, M.; De Medina, P.; Monsarrat, B.; Perret, B.; Silvente-Poirot, S. Exosomes account for vesicle-mediated transcellular transport of activatable phospholipases and prostaglandins. J. Lipid Res. 2010, 51, 2105–2120. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Erviti, L.; Seow, Y.; Yin, H.; Betts, C.; Lakhal, S.; Wood, M.J. Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat. Biotechnol. 2011, 29, 341–345. [Google Scholar] [CrossRef] [PubMed]
- McMahon, H.T.; Boucrot, E. Membrane curvature at a glance. J. Cell Sci. 2015, 128, 1065–1070. [Google Scholar] [CrossRef]
- Han, Q.-F.; Li, W.-J.; Hu, K.-S.; Gao, J.; Zhai, W.-L.; Yang, J.-H.; Zhang, S.-J. Exosome biogenesis: Machinery, regulation, and therapeutic implications in cancer. Mol. Cancer 2022, 21, 1–26. [Google Scholar] [CrossRef]
- Munagala, R.; Aqil, F.; Jeyabalan, J.; Gupta, R.C. Bovine milk-derived exosomes for drug delivery. Cancer Lett. 2016, 371, 48–61. [Google Scholar] [CrossRef]
- Benmoussa, A.; Ly, S.; Shan, S.T.; Laugier, J.; Boilard, E.; Gilbert, C.; Provost, P. A subset of extracellular vesicles carries the bulk of microRNAs in commercial dairy cow’s milk. J. Extracell. Vesicles 2017, 6, 1401897. [Google Scholar] [CrossRef]
- Wolf, T.; Baier, S.R.; Zempleni, J. The intestinal transport of bovine milk exosomes is mediated by endocytosis in human colon carcinoma Caco-2 cells and rat small intestinal IEC-6 cells. J. Nutr. 2015, 145, 2201–2206. [Google Scholar] [CrossRef] [PubMed]
- Kusuma, R.J.; Manca, S.; Friemel, T.; Sukreet, S.; Nguyen, C.; Zempleni, J. Human vascular endothelial cells transport foreign exosomes from cow’s milk by endocytosis. Am. J. Physiol.-Cell Physiol. 2016, 310, C800–C807. [Google Scholar] [CrossRef] [PubMed]
- Blans, K.; Hansen, M.S.; Sørensen, L.V.; Hvam, M.L.; Howard, K.A.; Möller, A.; Wiking, L.; Larsen, L.B.; Rasmussen, J.T. Pellet-free isolation of human and bovine milk extracellular vesicles by size-exclusion chromatography. J. Extracell. Vesicles 2017, 6, 1294340. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Mitsialis, S.A.; Aslam, M.; Vitali, S.H.; Vergadi, E.; Konstantinou, G.; Sdrimas, K.; Fernandez-Gonzalez, A.; Kourembanas, S. Exosomes mediate the cytoprotective action of mesenchymal stromal cells on hypoxia-induced pulmonary hypertension. Circulation 2012, 126, 2601–2611. [Google Scholar] [CrossRef]
- Fernando, M.R.; Jiang, C.; Krzyzanowski, G.D.; Ryan, W.L. New evidence that a large proportion of human blood plasma cell-free DNA is localized in exosomes. PLoS ONE 2017, 12, e0183915. [Google Scholar] [CrossRef]
- Baranyai, T.; Herczeg, K.; Onódi, Z.; Voszka, I.; Módos, K.; Marton, N.; Nagy, G.; Maeger, I.; Wood, M.J.; El Andaloussi, S. Isolation of exosomes from blood plasma: Qualitative and quantitative comparison of ultracentrifugation and size exclusion chromatography methods. PLoS ONE 2015, 10, e0145686. [Google Scholar] [CrossRef]
- Cheng, X.; Zhang, G.; Zhang, L.; Hu, Y.; Zhang, K.; Sun, X.; Zhao, C.; Li, H.; Li, Y.M.; Zhao, J. Mesenchymal stem cells deliver exogenous miR-21 via exosomes to inhibit nucleus pulposus cell apoptosis and reduce intervertebral disc degeneration. J. Cell. Mol. Med. 2018, 22, 261–276. [Google Scholar] [CrossRef]
- Ferreira, A.D.F.; Cunha, P.D.S.; Carregal, V.M.; Silva, P.D.C.D.; Miranda, M.C.D.; Kunrath-Lima, M.; de Melo, M.I.A.; Faraco, C.C.F.; Barbosa, J.L.; Frezard, F.; et al. Extracellular vesicles from adipose-derived mesenchymal stem/stromal cells accelerate migration and activate AKT pathway in human keratinocytes and fibroblasts independently of miR-205 activity. Stem Cells Int. 2017, 2017, 9841035. [Google Scholar] [CrossRef] [PubMed]
- Keller, S.; Ridinger, J.; Rupp, A.-K.; Janssen, J.W.; Altevogt, P. Body fluid derived exosomes as a novel template for clinical diagnostics. J. Transl. Med. 2011, 9, 86. [Google Scholar] [CrossRef]
- Lässer, C.; Seyed Alikhani, V.; Ekström, K.; Eldh, M.; Torregrosa Paredes, P.; Bossios, A.; Sjöstrand, M.; Gabrielsson, S.; Lötvall, J.; Valadi, H. Human saliva, plasma and breast milk exosomes contain RNA: Uptake by macrophages. J. Transl. Med. 2011, 9, 1–8. [Google Scholar] [CrossRef]
- Somiya, M.; Yoshioka, Y.; Ochiya, T. Biocompatibility of highly purified bovine milk-derived extracellular vesicles. J. Extracell. Vesicles 2018, 7, 1440132. [Google Scholar] [CrossRef]
- Domenis, R.; Zanutel, R.; Caponnetto, F.; Toffoletto, B.; Cifù, A.; Pistis, C.; Di Benedetto, P.; Causero, A.; Pozzi, M.; Bassini, F. Characterization of the proinflammatory profile of synovial fluid-derived exosomes of patients with osteoarthritis. Mediat. Inflamm. 2017, 2017, 4814987. [Google Scholar] [CrossRef]
- Coulter, M.E.; Dorobantu, C.M.; Lodewijk, G.A.; Delalande, F.; Cianferani, S.; Ganesh, V.S.; Smith, R.S.; Lim, E.T.; Xu, C.S.; Pang, S. The ESCRT-III protein CHMP1A mediates secretion of sonic hedgehog on a distinctive subtype of extracellular vesicles. Cell Rep. 2018, 24, 973–986.e978. [Google Scholar] [CrossRef]
- Samuel, M.; Chisanga, D.; Liem, M.; Keerthikumar, S.; Anand, S.; Ang, C.-S.; Adda, C.G.; Versteegen, E.; Jois, M.; Mathivanan, S. Bovine milk-derived exosomes from colostrum are enriched with proteins implicated in immune response and growth. Sci. Rep. 2017, 7, 5933. [Google Scholar] [CrossRef] [PubMed]
- Masyuk, A.I.; Huang, B.Q.; Ward, C.J.; Gradilone, S.A.; Banales, J.M.; Masyuk, T.V.; Radtke, B.; Splinter, P.L.; LaRusso, N.F. Biliary exosomes influence cholangiocyte regulatory mechanisms and proliferation through interaction with primary cilia. Am. J. Physiol.-Gastrointest. Liver Physiol. 2010, 299, G990–G999. [Google Scholar] [CrossRef] [PubMed]
- Polanco, J.C.; Scicluna, B.J.; Hill, A.F.; Götz, J. Extracellular vesicles isolated from the brains of rTg4510 mice seed tau protein aggregation in a threshold-dependent manner. J. Biol. Chem. 2016, 291, 12445–12466. [Google Scholar] [CrossRef]
- Wang, J.; Wu, Y.; Guo, J.; Fei, X.; Yu, L.; Ma, S. Adipocyte-derived exosomes promote lung cancer metastasis by increasing MMP9 activity via transferring MMP3 to lung cancer cells. Oncotarget 2017, 8, 81880. [Google Scholar] [CrossRef]
- Keller, S.; Rupp, C.; Stoeck, A.; Runz, S.; Fogel, M.; Lugert, S.; Hager, H.-D.; Abdel-Bakky, M.; Gutwein, P.; Altevogt, P. CD24 is a marker of exosomes secreted into urine and amniotic fluid. Kidney Int. 2007, 72, 1095–1102. [Google Scholar] [CrossRef]
- Théry, C.; Amigorena, S.; Raposo, G.; Clayton, A. Isolation and characterization of exosomes from cell culture supernatants and biological fluids. Curr. Protoc. Cell Biol. 2006, 30, 3–22. [Google Scholar] [CrossRef] [PubMed]
- Witwer, K.W.; Buzás, E.I.; Bemis, L.T.; Bora, A.; Lässer, C.; Lötvall, J.; Nolte-‘t Hoen, E.N.; Piper, M.G.; Sivaraman, S.; Skog, J. Standardization of sample collection, isolation and analysis methods in extracellular vesicle research. J. Extracell. Vesicles 2013, 2, 20360. [Google Scholar] [CrossRef]
- Buschmann, D.; Mussack, V.; Byrd, J.B. Separation, characterization, and standardization of extracellular vesicles for drug delivery applications. Adv. Drug Deliv. Rev. 2021, 174, 348–368. [Google Scholar] [CrossRef] [PubMed]
- Yamada, T.; Inoshima, Y.; Matsuda, T.; Ishiguro, N. Comparison of methods for isolating exosomes from bovine milk. J. Vet. Med. Sci. 2012, 74, 1523–1525. [Google Scholar] [CrossRef] [PubMed]
- Tangwattanachuleeporn, M.; Muanwien, P.; Teethaisong, Y.; Somparn, P. Optimizing Concentration of Polyethelene Glycol for Exosome Isolation from Plasma for Downstream Application. Medicina 2022, 58, 1600. [Google Scholar] [CrossRef]
- Izumi, H.; Tsuda, M.; Sato, Y.; Kosaka, N.; Ochiya, T.; Iwamoto, H.; Namba, K.; Takeda, Y. Bovine milk exosomes contain microRNA and mRNA and are taken up by human macrophages. J. Dairy Sci. 2015, 98, 2920–2933. [Google Scholar] [CrossRef]
- Lobb, R.J.; Becker, M.; Wen Wen, S.; Wong, C.S.; Wiegmans, A.P.; Leimgruber, A.; Möller, A. Optimized exosome isolation protocol for cell culture supernatant and human plasma. J. Extracell. Vesicles 2015, 4, 27031. [Google Scholar] [CrossRef]
- Szatanek, R.; Baran, J.; Siedlar, M.; Baj-Krzyworzeka, M. Isolation of extracellular vesicles: Determining the correct approach. Int. J. Mol. Med. 2015, 36, 11–17. [Google Scholar] [CrossRef]
- Li, P.; Kaslan, M.; Lee, S.H.; Yao, J.; Gao, Z. Progress in exosome isolation techniques. Theranostics 2017, 7, 789. [Google Scholar] [CrossRef]
- Kim, J.Y.; Rhim, W.-K.; Yoo, Y.-I.; Kim, D.-S.; Ko, K.-W.; Heo, Y.; Park, C.G.; Han, D.K. Defined MSC exosome with high yield and purity to improve regenerative activity. J. Tissue Eng. 2021, 12, 20417314211008626. [Google Scholar] [CrossRef] [PubMed]
- Linares, R.; Tan, S.; Gounou, C.; Arraud, N.; Brisson, A. High-speed centrifugation induces aggregation of extracellular vesicles. J. Extracell. Vesicles 2015, 4, 29509. [Google Scholar] [CrossRef] [PubMed]
- Watson, D.C.; Yung, B.C.; Bergamaschi, C.; Chowdhury, B.; Bear, J.; Stellas, D.; Morales-Kastresana, A.; Jones, J.C.; Felber, B.K.; Chen, X. Scalable, cGMP-compatible purification of extracellular vesicles carrying bioactive human heterodimeric IL-15/lactadherin complexes. J. Extracell. Vesicles 2018, 7, 1442088. [Google Scholar] [CrossRef] [PubMed]
- Foers, A.D.; Chatfield, S.; Dagley, L.F.; Scicluna, B.J.; Webb, A.I.; Cheng, L.; Hill, A.F.; Wicks, I.P.; Pang, K.C. Enrichment of extracellular vesicles from human synovial fluid using size exclusion chromatography. J. Extracell. Vesicles 2018, 7, 1490145. [Google Scholar] [CrossRef] [PubMed]
- Monguió-Tortajada, M.; Gálvez-Montón, C.; Bayes-Genis, A.; Roura, S.; Borràs, F.E. Extracellular vesicle isolation methods: Rising impact of size-exclusion chromatography. Cell. Mol. Life Sci. 2019, 76, 2369–2382. [Google Scholar] [CrossRef]
- Huang, K.; Garimella, S.; Clay-Gilmour, A.; Vojtech, L.; Armstrong, B.; Bessonny, M.; Stamatikos, A. Comparison of Human Urinary Exosomes Isolated via Ultracentrifugation Alone versus Ultracentrifugation Followed by SEC Column-Purification. J. Pers. Med. 2022, 12, 340. [Google Scholar] [CrossRef]
- Dalgleish, D.G.; Corredig, M. The structure of the casein micelle of milk and its changes during processing. Annu. Rev. Food Sci. Technol. 2012, 3, 449–467. [Google Scholar] [CrossRef]
- Obeid, S.; Guyomarc’h, F.; David-Briand, E.; Gaucheron, F.; Riaublanc, A.; Lopez, C. The phase and charge of milk polar lipid membrane bilayers govern their selective interactions with proteins as demonstrated with casein micelles. J. Colloid Interface Sci. 2019, 534, 279–290. [Google Scholar] [CrossRef]
- del Pozo-Acebo, L.; López de las Hazas, M.; Tomé-Carneiro, J.; Gil-Cabrerizo, P.; San-Cristobal, R.; Busto, R.; García-Ruiz, A.; Dávalos, A. Bovine milk-derived exosomes as a drug delivery vehicle for miRNA-based therapy. Int. J. Mol. Sci. 2021, 22, 1105. [Google Scholar] [CrossRef]
- Vaswani, K.; Koh, Y.Q.; Almughlliq, F.B.; Peiris, H.N.; Mitchell, M.D. A method for the isolation and enrichment of purified bovine milk exosomes. Reprod. Biol. 2017, 17, 341–348. [Google Scholar] [CrossRef]
- Warren, M.R.; Zhang, C.; Vedadghavami, A.; Bokvist, K.; Dhal, P.K.; Bajpayee, A.G. Milk exosomes with enhanced mucus penetrability for oral delivery of siRNA. Biomater. Sci. 2021, 9, 4260–4277. [Google Scholar] [CrossRef]
- Benmoussa, A.; Michel, S.; Gilbert, C.; Provost, P. Isolating multiple extracellular vesicles subsets, including exosomes and membrane vesicles, from bovine milk using sodium citrate and differential ultracentrifugation. Bio-Protocol 2020, 10, e3636. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Xu, Y.; Lu, Y.; Xing, W. Isolation and visible detection of tumor-derived exosomes from plasma. Anal. Chem. 2018, 90, 14207–14215. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Xu, Y.; Wang, X.; Liu, D.; Yang, F.; Zhu, X.; Lu, Y.; Xing, W. Rapid and efficient isolation and detection of extracellular vesicles from plasma for lung cancer diagnosis. Lab Chip 2019, 19, 432–443. [Google Scholar] [CrossRef]
- Zempleni, J.; Aguilar-Lozano, A.; Sadri, M.; Sukreet, S.; Manca, S.; Wu, D.; Zhou, F.; Mutai, E. Biological activities of extracellular vesicles and their cargos from bovine and human milk in humans and implications for infants. J. Nutr. 2017, 147, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Song, D.; Cao, X.; Wu, R.; Liu, B.; Ye, W.; Wu, J.; Yue, X. Comparative proteomic analysis of milk-derived exosomes in human and bovine colostrum and mature milk samples by iTRAQ-coupled LC-MS/MS. Food Res. Int. 2017, 92, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, D.; Wang, Y.; Li, M.; Fang, X.; Chen, H.; Zhang, C. The landscape of circular RNAs and mRNAs in bovine milk exosomes. J. Food Compos. Anal. 2019, 76, 33–38. [Google Scholar] [CrossRef]
- Zeng, B.; Chen, T.; Xie, M.-Y.; Luo, J.-Y.; He, J.-J.; Xi, Q.-Y.; Sun, J.-J.; Zhang, Y.-L. Exploration of long noncoding RNA in bovine milk exosomes and their stability during digestion in vitro. J. Dairy Sci. 2019, 102, 6726–6737. [Google Scholar] [CrossRef]
- Rahman, M.; Takashima, S.; Kamatari, Y.O.; Badr, Y.; Kitamura, Y.; Shimizu, K.; Okada, A.; Inoshima, Y. Proteomic profiling of milk small extracellular vesicles from bovine leukemia virus-infected cattle. Sci. Rep. 2021, 11, 2951. [Google Scholar] [CrossRef]
- Reinhardt, T.A.; Lippolis, J.D.; Nonnecke, B.J.; Sacco, R.E. Bovine milk exosome proteome. J. Proteom. 2012, 75, 1486–1492. [Google Scholar] [CrossRef]
- Vaswani, K.M.; Peiris, H.; Qin Koh, Y.; Hill, R.J.; Harb, T.; Arachchige, B.J.; Logan, J.; Reed, S.; Davies, P.S.; Mitchell, M.D. A complete proteomic profile of human and bovine milk exosomes by liquid chromatography mass spectrometry. Expert Rev. Proteom. 2021, 18, 719–735. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Ma, Z.; Li, R.; Guo, S.; Qiu, Y.; Gao, X. Proteomic analysis reveals proteins and pathways associated with lactation in bovine mammary epithelial cell-derived exosomes. J. Proteome Res. 2020, 19, 3211–3219. [Google Scholar] [CrossRef]
- Maity, S.; Bhat, A.H.; Giri, K.; Ambatipudi, K. BoMiProt: A database of bovine milk proteins. J. Proteom. 2020, 215, 103648. [Google Scholar] [CrossRef] [PubMed]
- Hata, T.; Murakami, K.; Nakatani, H.; Yamamoto, Y.; Matsuda, T.; Aoki, N. Isolation of bovine milk-derived microvesicles carrying mRNAs and microRNAs. Biochem. Biophys. Res. Commun. 2010, 396, 528–533. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Guo, H.; Zhang, H.; Xie, X.; Wen, P.; Ren, F. Yak-milk-derived exosomes promote proliferation of intestinal epithelial cells in an hypoxic environment. J. Dairy Sci. 2019, 102, 985–996. [Google Scholar] [CrossRef]
- Martin, C.; Patel, M.; Williams, S.; Arora, H.; Sims, B. Human breast milk-derived exosomes attenuate cell death in intestinal epithelial cells. Innate Immun. 2018, 24, 278–284. [Google Scholar] [CrossRef]
- Chen, X.; Gao, C.; Li, H.; Huang, L.; Sun, Q.; Dong, Y.; Tian, C.; Gao, S.; Dong, H.; Guan, D. Identification and characterization of microRNAs in raw milk during different periods of lactation, commercial fluid, and powdered milk products. Cell Res. 2010, 20, 1128–1137. [Google Scholar] [CrossRef]
- Shome, S.; Jernigan, R.; Beitz, D.C.; Clark, S.; Testroet, E. Non-coding RNA in raw and commercially processed milk and putative targets related to growth and immune-response. BMC Genom. 2021, 22, 749. [Google Scholar] [CrossRef]
- Sukreet, S.; Braga, C.P.; An, T.T.; Adamec, J.; Cui, J.; Zempleni, J. Ultrasonication of Milk Decreases the Content of Exosomes and MicroRNAs in an Exosome-defined Rodent Diet. J. Nutr. 2022, 152, 961–970. [Google Scholar] [CrossRef]
- He, Y.; He, Z.; Leone, S.; Liu, S. Milk Exosomes Transfer Oligosaccharides into Macrophages to Modulate Immunity and Attenuate Adherent-Invasive E. coli (AIEC) Infection. Nutrients 2021, 13, 3198. [Google Scholar] [CrossRef]
- Chen, W.; Wang, R.; Li, D.; Zuo, C.; Wen, P.; Liu, H.; Chen, Y.; Fujita, M.; Wu, Z.; Yang, G. Comprehensive analysis of the glycome and glycoproteome of bovine milk-derived exosomes. J. Agric. Food Chem. 2020, 68, 12692–12701. [Google Scholar] [CrossRef] [PubMed]
- Ortega-Anaya, J.; Jiménez-Flores, R. Symposium review: The relevance of bovine milk phospholipids in human nutrition—Evidence of the effect on infant gut and brain development. J. Dairy Sci. 2019, 102, 2738–2748. [Google Scholar] [CrossRef] [PubMed]
- Hullin-Matsuda, F.; Colosetti, P.; Rabia, M.; Luquain-Costaz, C.; Delton, I. Exosomal lipids from membrane organization to biomarkers: Focus on an endolysosomal-specific lipid. Biochimie 2022, 203, 77–92. [Google Scholar] [CrossRef]
- Skotland, T.; Hessvik, N.P.; Sandvig, K.; Llorente, A. Exosomal lipid composition and the role of ether lipids and phosphoinositides in exosome biology. J. Lipid Res. 2019, 60, 9–18. [Google Scholar] [CrossRef]
- Suga, K.; Matsui, D.; Watanabe, N.; Okamoto, Y.; Umakoshi, H. Insight into the Exosomal Membrane: From Viewpoints of Membrane Fluidity and Polarity. Langmuir 2021, 37, 11195–11202. [Google Scholar] [CrossRef] [PubMed]
- Isaac, R.; Reis, F.C.G.; Ying, W.; Olefsky, J.M. Exosomes as mediators of intercellular crosstalk in metabolism. Cell Metab. 2021, 33, 1744–1762. [Google Scholar] [CrossRef]
- Betker, J.L.; Angle, B.M.; Graner, M.W.; Anchordoquy, T.J. The potential of exosomes from cow milk for oral delivery. J. Pharm. Sci. 2019, 108, 1496–1505. [Google Scholar] [CrossRef]
- Vella, L.J.; Hill, A.F.; Cheng, L. Focus on extracellular vesicles: Exosomes and their role in protein trafficking and biomarker potential in Alzheimer’s and Parkinson’s disease. Int. J. Mol. Sci. 2016, 17, 173. [Google Scholar] [CrossRef]
- Camussi, G.; Deregibus, M.C.; Bruno, S.; Cantaluppi, V.; Biancone, L. Exosomes/microvesicles as a mechanism of cell-to-cell communication. Kidney Int. 2010, 78, 838–848. [Google Scholar] [CrossRef]
- Pieters, B.C.; Arntz, O.J.; Bennink, M.B.; Broeren, M.G.; van Caam, A.P.; Koenders, M.I.; van Lent, P.L.; van den Berg, W.B.; de Vries, M.; van der Kraan, P.M. Commercial cow milk contains physically stable extracellular vesicles expressing immunoregulatory TGF-β. PLoS ONE 2015, 10, e0121123. [Google Scholar] [CrossRef]
- Yu, S.; Zhao, Z.; Sun, L.; Li, P. Fermentation results in quantitative changes in milk-derived exosomes and different effects on cell growth and survival. J. Agric. Food Chem. 2017, 65, 1220–1228. [Google Scholar] [CrossRef]
- Baier, S.R.; Nguyen, C.; Xie, F.; Wood, J.R.; Zempleni, J. MicroRNAs are absorbed in biologically meaningful amounts from nutritionally relevant doses of cow milk and affect gene expression in peripheral blood mononuclear cells, HEK-293 kidney cell cultures, and mouse livers. J. Nutr. 2014, 144, 1495–1500. [Google Scholar] [CrossRef] [PubMed]
- Naqvi, A.R.; Fordham, J.B.; Nares, S. miR-24, miR-30b, and miR-142-3p regulate phagocytosis in myeloid inflammatory cells. J. Immunol. 2015, 194, 1916–1927. [Google Scholar] [CrossRef] [PubMed]
- Benmoussa, A.; Gotti, C.; Bourassa, S.; Gilbert, C.; Provost, P. Identification of protein markers for extracellular vesicle (EV) subsets in cow’s milk. J. Proteom. 2019, 192, 78–88. [Google Scholar] [CrossRef] [PubMed]
- Melnik, B.C.; Schmitz, G. MicroRNAs: Milk’s epigenetic regulators. Best Pract. Res. Clin. Endocrinol. Metab. 2017, 31, 427–442. [Google Scholar] [CrossRef]
- Lin, D.; Chen, T.; Xie, M.; Li, M.; Zeng, B.; Sun, R.; Zhu, Y.; Ye, D.; Wu, J.; Sun, J. Oral administration of bovine and porcine milk exosome alter miRNAs profiles in piglet serum. Sci. Rep. 2020, 10, 6983. [Google Scholar] [CrossRef]
- Ross, M.; Atalla, H.; Karrow, N.; Mallard, B.A. The bioactivity of colostrum and milk exosomes of high, average, and low immune responder cows on human intestinal epithelial cells. J. Dairy Sci. 2021, 104, 2499–2510. [Google Scholar] [CrossRef]
- Wang, L.; Shi, Z.; Wang, X.; Mu, S.; Xu, X.; Shen, L.; Li, P. Protective effects of bovine milk exosomes against oxidative stress in IEC-6 cells. Eur. J. Nutr. 2021, 60, 317–327. [Google Scholar] [CrossRef]
- Wang, L.; Wang, X.; Shi, Z.; Shen, L.; Zhang, J.; Zhang, J. Bovine milk exosomes attenuate the alteration of purine metabolism and energy status in IEC-6 cells induced by hydrogen peroxide. Food Chem. 2021, 350, 129142. [Google Scholar] [CrossRef]
- Gao, H.; Ren, F.; Wen, P.; Xie, L.; Wang, R.; Yang, Z.; Li, Y. Yak milk–derived exosomal microRNAs regulate intestinal epithelial cells on proliferation in hypoxic environment. J. Dairy Sci. 2021, 104, 1291–1303. [Google Scholar] [CrossRef]
- Good, M.; Sodhi, C.P.; Egan, C.E.; Afrazi, A.; Jia, H.; Yamaguchi, Y.; Lu, P.; Branca, M.F.; Ma, C.; Prindle, T. Breast milk protects against the development of necrotizing enterocolitis through inhibition of Toll-like receptor 4 in the intestinal epithelium via activation of the epidermal growth factor receptor. Mucosal Immunol. 2015, 8, 1166–1179. [Google Scholar] [CrossRef] [PubMed]
- Stremmel, W.; Weiskirchen, R.; Melnik, B.C. Milk exosomes prevent intestinal inflammation in a genetic mouse model of ulcerative colitis: A pilot experiment. Inflamm. Intest. Dis. 2020, 5, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Kittana, H.; Shu, J.; Kachman, S.D.; Cui, J.; Ramer-Tait, A.E.; Zempleni, J. Dietary depletion of milk exosomes and their microRNA cargos elicits a depletion of miR-200a-3p and elevated intestinal inflammation and chemokine (CXC motif) ligand 9 expression in Mdr1a−/− mice. Curr. Dev. Nutr. 2019, 3, nzz122. [Google Scholar] [CrossRef]
- Li, B.; Hock, A.; Wu, R.Y.; Minich, A.; Botts, S.R.; Lee, C.; Antounians, L.; Miyake, H.; Koike, Y.; Chen, Y. Bovine milk-derived exosomes enhance goblet cell activity and prevent the development of experimental necrotizing enterocolitis. PLoS ONE 2019, 14, e0211431. [Google Scholar] [CrossRef]
- Han, G.; Cho, H.; Kim, H.; Jang, Y.; Jang, H.; Kim, E.S.; Kim, E.H.; Hwang, K.Y.; Kim, K.; Yang, Y. Bovine colostrum derived-exosomes prevent dextran sulfate sodium-induced intestinal colitis via suppression of inflammation and oxidative stress. Biomater. Sci. 2022, 10, 2076–2087. [Google Scholar] [CrossRef] [PubMed]
- Reif, S.; Elbaum Shiff, Y.; Golan-Gerstl, R. Milk-derived exosomes (MDEs) have a different biological effect on normal fetal colon epithelial cells compared to colon tumor cells in a miRNA-dependent manner. J. Transl. Med. 2019, 17, 1–10. [Google Scholar] [CrossRef]
- Gao, H.; Hu, H.; Wen, P.; Lian, S.; Xie, X.; Song, H.; Yang, Z.; Ren, F. Yak milk–derived exosomes alleviate lipopolysaccharide-induced intestinal inflammation by inhibiting PI3K/AKT/C3 pathway activation. J. Dairy Sci. 2021, 104, 8411–8424. [Google Scholar] [CrossRef]
- Maghraby, M.K.; Li, B.; Chi, L.; Ling, C.; Benmoussa, A.; Provost, P.; Postmus, A.C.; Abdi, A.; Pierro, A.; Bourdon, C. Extracellular vesicles isolated from milk can improve gut barrier dysfunction induced by malnutrition. Sci. Rep. 2021, 11, 7635. [Google Scholar] [CrossRef]
- Tong, L.; Hao, H.; Zhang, X.; Zhang, Z.; Lv, Y.; Zhang, L.; Yi, H. Oral Administration of Bovine Milk-Derived Extracellular Vesicles Alters the Gut Microbiota and Enhances Intestinal Immunity in Mice. Mol. Nutr. Food Res. 2020, 64, 1901251. [Google Scholar] [CrossRef]
- Zhou, F.; Paz, H.A.; Sadri, M.; Cui, J.; Kachman, S.D.; Fernando, S.C.; Zempleni, J. Dietary bovine milk exosomes elicit changes in bacterial communities in C57BL/6 mice. Am. J. Physiol.-Gastrointest. Liver Physiol. 2019, 317, G618–G624. [Google Scholar] [CrossRef]
- Aarts, J.; Boleij, A.; Pieters, B.C.; Feitsma, A.L.; van Neerven, R.; Ten Klooster, J.P.; M’Rabet, L.; Arntz, O.J.; Koenders, M.I.; van de Loo, F.A. Flood Control: How Milk-Derived Extracellular Vesicles Can Help to Improve the Intestinal Barrier Function and Break the Gut–Joint Axis in Rheumatoid Arthritis. Front. Immunol. 2021, 12, 2931. [Google Scholar] [CrossRef] [PubMed]
- Tong, L.; Hao, H.; Zhang, Z.; Lv, Y.; Liang, X.; Liu, Q.; Liu, T.; Gong, P.; Zhang, L.; Cao, F. Milk-derived extracellular vesicles alleviate ulcerative colitis by regulating the gut immunity and reshaping the gut microbiota. Theranostics 2021, 11, 8570. [Google Scholar] [CrossRef] [PubMed]
- Melnik, B.C.; John, S.M.; Schmitz, G. Milk is not just food but most likely a genetic transfection system activating mTORC1 signaling for postnatal growth. Nutr. J. 2013, 12, 103. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, M.C.; Arntz, O.J.; Davidson, E.N.B.; van Lent, P.L.; Koenders, M.I.; van der Kraan, P.M.; van den Berg, W.B.; Ferreira, A.V.; van de Loo, F.A. Milk extracellular vesicles accelerate osteoblastogenesis but impair bone matrix formation. J. Nutr. Biochem. 2016, 30, 74–84. [Google Scholar] [CrossRef] [PubMed]
- Leiferman, A.; Shu, J.; Grove, R.; Cui, J.; Adamec, J.; Zempleni, J. A diet defined by its content of bovine milk exosomes and their RNA cargos has moderate effects on gene expression, amino acid profiles and grip strength in skeletal muscle in C57BL/6 mice. J. Nutr. Biochem. 2018, 59, 123–128. [Google Scholar] [CrossRef]
- Yun, B.; Maburutse, B.; Kang, M.; Park, M.; Park, D.; Kim, Y.; Oh, S. Dietary bovine milk–derived exosomes improve bone health in an osteoporosis-induced mouse model. J. Dairy Sci. 2020, 103, 7752–7760. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Kim, D.E.; Han, G.; Lim, N.R.; Kim, E.H.; Jang, Y.; Cho, H.; Jang, H.; Kim, K.H.; Kim, S.H. Harnessing the Natural Healing Power of Colostrum: Bovine Milk-Derived Extracellular Vesicles from Colostrum Facilitating the Transition from Inflammation to Tissue Regeneration for Accelerating Cutaneous Wound Healing. Adv. Healthc. Mater. 2022, 11, 2102027. [Google Scholar] [CrossRef]
- Yamamoto, T.; Kosaka, N.; Ochiya, T. Latest advances in extracellular vesicles: From bench to bedside. Sci. Technol. Adv. Mater. 2019, 20, 746–757. [Google Scholar] [CrossRef]
- Colitti, M.; Sgorlon, S.; Stefanon, B. Exosome cargo in milk as a potential marker of cow health. J. Dairy Res. 2020, 87, 79–83. [Google Scholar] [CrossRef]
- Abeysinghe, P.; Turner, N.; Morean Garcia, I.; Mosaad, E.; Peiris, H.N.; Mitchell, M.D. The role of exosomal epigenetic modifiers in cell communication and fertility of dairy cows. Int. J. Mol. Sci. 2020, 21, 9106. [Google Scholar] [CrossRef]
- Ma, S.; Tong, C.; Ibeagha-Awemu, E.M.; Zhao, X. Identification and characterization of differentially expressed exosomal microRNAs in bovine milk infected with Staphylococcus aureus. BMC Genom. 2019, 20, 934. [Google Scholar] [CrossRef]
- Reinhardt, T.A.; Sacco, R.E.; Nonnecke, B.J.; Lippolis, J.D. Bovine milk proteome: Quantitative changes in normal milk exosomes, milk fat globule membranes and whey proteomes resulting from Staphylococcus aureus mastitis. J. Proteom. 2013, 82, 141–154. [Google Scholar] [CrossRef] [PubMed]
- Yamada, T.; Shigemura, H.; Ishiguro, N.; Inoshima, Y. Cell Infectivity in relation to bovine leukemia virus gp51 and p24 in bovine milk exosomes. PLoS ONE 2013, 8, e77359. [Google Scholar] [CrossRef] [PubMed]
- Arntz, O.J.; Pieters, B.C.; Oliveira, M.C.; Broeren, M.G.; Bennink, M.B.; de Vries, M.; van Lent, P.L.; Koenders, M.I.; van den Berg, W.B.; van der Kraan, P.M. Oral administration of bovine milk derived extracellular vesicles attenuates arthritis in two mouse models. Mol. Nutr. Food Res. 2015, 59, 1701–1712. [Google Scholar] [CrossRef] [PubMed]
- Matic, S.; D’Souza, D.H.; Wu, T.; Pangloli, P.; Dia, V.P. Bovine milk exosomes affect proliferation and protect macrophages against cisplatin-induced cytotoxicity. Immunol. Investig. 2020, 49, 711–725. [Google Scholar] [CrossRef] [PubMed]
- Mokarizadeh, A.; Hassanzadeh, K.; Abdi, M.; Soraya, H.; Faryabi, M.R.; Mohammadi, E.; Ahmadi, A. Transdermal delivery of bovine milk vesicles in patients with multiple sclerosis: A novel strategy to induce MOG-specific tolerance. Med. Hypotheses 2015, 85, 141–144. [Google Scholar] [CrossRef]
- Bae, I.-S.; Kim, S.H. Milk Exosome-Derived MicroRNA-2478 Suppresses Melanogenesis through the Akt-GSK3β Pathway. Cells 2021, 10, 2848. [Google Scholar] [CrossRef]
- Zhang, C.; Lu, X.; Hu, J.; Li, P.; Yan, J.; Ling, X.; Xiao, J. Bovine Milk Exosomes Alleviate Cardiac Fibrosis via Enhancing Angiogenesis in Vivo and in Vitro. J. Cardiovasc. Transl. Res. 2021, 15, 560–570. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Jang, Y.; Kim, E.; Jang, H.; Cho, H.; Han, G.; Song, H.; Kim, S.; Yang, Y. Potential of Colostrum-Derived Exosomes for Promoting Hair Regeneration through the Transition from Telogen to Anagen Phase. Front. Cell Dev. Biol. 2022, 10, 815205. [Google Scholar] [CrossRef]
- Shi, C.; Zhang, M.; Tong, M.; Yang, L.; Pang, L.; Chen, L.; Xu, G.; Chi, X.; Hong, Q.; Ni, Y. miR-148a is associated with obesity and modulates adipocyte differentiation of mesenchymal stem cells through Wnt signaling. Sci. Rep. 2015, 5, 9930. [Google Scholar] [CrossRef]
- Du, C.; Quan, S.; Nan, X.; Zhao, Y.; Shi, F.; Luo, Q.; Xiong, B. Effects of oral milk extracellular vesicles on the gut microbiome and serum metabolome in mice. Food Funct. 2021, 12, 10938–10949. [Google Scholar] [CrossRef] [PubMed]
- Melnik, B.C. Synergistic effects of milk-derived exosomes and galactose on α-synuclein pathology in Parkinson’s disease and type 2 diabetes mellitus. Int. J. Mol. Sci. 2021, 22, 1059. [Google Scholar] [CrossRef]
- Samuel, M.; Fonseka, P.; Sanwlani, R.; Gangoda, L.; Chee, S.H.; Keerthikumar, S.; Spurling, A.; Chitti, S.V.; Zanker, D.; Ang, C.-S. Oral administration of bovine milk-derived extracellular vesicles induces senescence in the primary tumor but accelerates cancer metastasis. Nat. Commun. 2021, 12, 3950. [Google Scholar] [CrossRef]
- Melnik, B.C. Lifetime impact of cow’s milk on overactivation of mTORC1: From fetal to childhood overgrowth, acne, diabetes, cancers, and neurodegeneration. Biomolecules 2021, 11, 404. [Google Scholar] [CrossRef]
- Vader, P.; Mol, E.A.; Pasterkamp, G.; Schiffelers, R.M. Extracellular vesicles for drug delivery. Adv. Drug Deliv. Rev. 2016, 106, 148–156. [Google Scholar] [CrossRef]
- Malhotra, H.; Sheokand, N.; Kumar, S.; Chauhan, A.S.; Kumar, M.; Jakhar, P.; Boradia, V.M.; Raje, C.I.; Raje, M. Exosomes: Tunable nano vehicles for macromolecular delivery of transferrin and lactoferrin to specific intracellular compartment. J. Biomed. Nanotechnol. 2016, 12, 1101–1114. [Google Scholar] [CrossRef] [PubMed]
- Rupert, D.L.; Claudio, V.; Lässer, C.; Bally, M. Methods for the physical characterization and quantification of extracellular vesicles in biological samples. Biochim. Biophys. Acta (BBA)-Gen. Subj. 2017, 1861, 3164–3179. [Google Scholar] [CrossRef]
- Hood, J.L. Post isolation modification of exosomes for nanomedicine applications. Nanomedicine 2016, 11, 1745–1756. [Google Scholar] [CrossRef] [PubMed]
- Tian, M.-Y.; Hao, D.-X.; Liu, Y.; He, J.; Zhao, Z.-H.; Guo, T.-Y.; Li, X.; Zhang, Y. Milk exosomes: An oral drug delivery system with great application potential. Food Funct. 2023, 14, 1320–1337. [Google Scholar] [CrossRef]
- Benmoussa, A.; Diallo, I.; Salem, M.; Michel, S.; Gilbert, C.; Sévigny, J.; Provost, P. Concentrates of two subsets of extracellular vesicles from cow’s milk modulate symptoms and inflammation in experimental colitis. Sci. Rep. 2019, 9, 14661. [Google Scholar] [CrossRef]
- Liang, Y.; Duan, L.; Lu, J.; Xia, J. Engineering exosomes for targeted drug delivery. Theranostics 2021, 11, 3183. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.S.; Haney, M.J.; Zhao, Y.; Mahajan, V.; Deygen, I.; Klyachko, N.L.; Inskoe, E.; Piroyan, A.; Sokolsky, M.; Okolie, O. Development of exosome-encapsulated paclitaxel to overcome MDR in cancer cells. Nanomed. Nanotechnol. Biol. Med. 2016, 12, 655–664. [Google Scholar] [CrossRef] [PubMed]
- Luo, S.; Sun, X.; Huang, M.; Ma, Q.; Du, L.; Cui, Y. Enhanced neuroprotective effects of epicatechin gallate encapsulated by bovine milk-derived exosomes against parkinson’s disease through antiapoptosis and antimitophagy. J. Agric. Food Chem. 2021, 69, 5134–5143. [Google Scholar] [CrossRef] [PubMed]
- Hao, R.; Yu, Z.; Du, J.; Hu, S.; Yuan, C.; Guo, H.; Zhang, Y.; Yang, H. A High-Throughput Nanofluidic Device for Exosome Nanoporation to Develop Cargo Delivery Vehicles. Small 2021, 17, 2102150. [Google Scholar] [CrossRef]
- Zhang, D.; Lee, H.; Zhu, Z.; Minhas, J.K.; Jin, Y. Enrichment of selective miRNAs in exosomes and delivery of exosomal miRNAs in vitro and in vivo. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2017, 312, L110–L121. [Google Scholar] [CrossRef]
- Tsai, S.J.; Atai, N.A.; Cacciottolo, M.; Nice, J.; Salehi, A.; Guo, C.; Sedgwick, A.; Kanagavelu, S.; Gould, S.J. Exosome-mediated mRNA delivery in vivo is safe and can be used to induce SARS-CoV-2 immunity. J. Biol. Chem. 2021, 297, 101266. [Google Scholar] [CrossRef]
- Lin, Y.; Wu, J.; Gu, W.; Huang, Y.; Tong, Z.; Huang, L.; Tan, J. Exosome–liposome hybrid nanoparticles deliver CRISPR/Cas9 system in MSCs. Adv. Sci. 2018, 5, 1700611. [Google Scholar] [CrossRef]
- Li, D.; Yao, S.; Zhou, Z.; Shi, J.; Huang, Z.; Wu, Z. Hyaluronan decoration of milk exosomes directs tumor-specific delivery of doxorubicin. Carbohydr. Res. 2020, 493, 108032. [Google Scholar] [CrossRef]
- Kandimalla, R.; Aqil, F.; Alhakeem, S.S.; Jeyabalan, J.; Tyagi, N.; Agrawal, A.; Yan, J.; Spencer, W.; Bondada, S.; Gupta, R.C. Targeted oral delivery of paclitaxel using colostrum-derived exosomes. Cancers 2021, 13, 3700. [Google Scholar] [CrossRef]
- Gong, L.; Zhou, H.; Zhang, S.; Wang, C.; Fu, K.; Ma, C.; Zhang, Y.; Peng, C.; Li, Y. CD44-targeting Drug Delivery System of Exosomes Loading Forsythiaside A Combats Liver Fibrosis via Regulating NLRP3-mediated Pyroptosis. Adv. Healthc. Mater. 2023, 12, 2202228. [Google Scholar] [CrossRef]
- Pullan, J.; Dailey, K.; Bhallamudi, S.; Feng, L.; Alhalhooly, L.; Froberg, J.; Osborn, J.; Sarkar, K.; Molden, T.; Sathish, V. Modified Bovine Milk Exosomes for Doxorubicin Delivery to Triple-Negative Breast Cancer Cells. ACS Appl. Bio Mater. 2022, 5, 2163–2175. [Google Scholar] [CrossRef] [PubMed]
- Koh, E.; Lee, E.J.; Nam, G.-H.; Hong, Y.; Cho, E.; Yang, Y.; Kim, I.-S. Exosome-SIRPα, a CD47 blockade increases cancer cell phagocytosis. Biomaterials 2017, 121, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Benmoussa, A.; Lee, C.H.C.; Laffont, B.; Savard, P.; Laugier, J.; Boilard, E.; Gilbert, C.; Fliss, I.; Provost, P. Commercial dairy cow milk microRNAs resist digestion under simulated gastrointestinal tract conditions. J. Nutr. 2016, 146, 2206–2215. [Google Scholar] [CrossRef]
- Agrawal, A.K.; Aqil, F.; Jeyabalan, J.; Spencer, W.A.; Beck, J.; Gachuki, B.W.; Alhakeem, S.S.; Oben, K.; Munagala, R.; Bondada, S. Milk-derived exosomes for oral delivery of paclitaxel. Nanomed. Nanotechnol. Biol. Med. 2017, 13, 1627–1636. [Google Scholar] [CrossRef] [PubMed]
- Vashisht, M.; Rani, P.; Onteru, S.K.; Singh, D. Curcumin encapsulated in milk exosomes resists human digestion and possesses enhanced intestinal permeability in vitro. Appl. Biochem. Biotechnol. 2017, 183, 993–1007. [Google Scholar] [CrossRef]
- Chen, J.; Cao, F.; Cao, Y.; Wei, S.; Zhu, X.; Xing, W. Targeted Therapy of Lung Adenocarcinoma by the Nanoplatform Based on Milk Exosomes Loaded with Paclitaxel. J. Biomed. Nanotechnol. 2022, 18, 1075–1083. [Google Scholar] [CrossRef]
- Munagala, R.; Aqil, F.; Jeyabalan, J.; Agrawal, A.K.; Mudd, A.M.; Kyakulaga, A.H.; Singh, I.P.; Vadhanam, M.V.; Gupta, R.C. Exosomal formulation of anthocyanidins against multiple cancer types. Cancer Lett. 2017, 393, 94–102. [Google Scholar] [CrossRef]
- Mudd, A.M.; Gu, T.; Munagala, R.; Jeyabalan, J.; Egilmez, N.K.; Gupta, R.C. Chemoprevention of colorectal cancer by Anthocyanidins and mitigation of metabolic shifts induced by dysbiosis of the gut microbiome. Cancer Prev. Res. 2020, 13, 41–52. [Google Scholar] [CrossRef]
- Shandilya, S.; Rani, P.; Onteru, S.K.; Singh, D. Small interfering RNA in milk exosomes is resistant to digestion and crosses the intestinal barrier in vitro. J. Agric. Food Chem. 2017, 65, 9506–9513. [Google Scholar] [CrossRef]
- Aqil, F.; Munagala, R.; Jeyabalan, J.; Agrawal, A.K.; Kyakulaga, A.-H.; Wilcher, S.A.; Gupta, R.C. Milk exosomes-Natural nanoparticles for siRNA delivery. Cancer Lett. 2019, 449, 186–195. [Google Scholar] [CrossRef]
- Matsuda, A.; Patel, T. Milk-derived extracellular vesicles for therapeutic delivery of small interfering RNAs. In Extracellular RNA; Springer: Berlin/Heidelberg, Germany, 2018; pp. 187–197. [Google Scholar]
- Tao, H.; Xu, H.; Zuo, L.; Li, C.; Qiao, G.; Guo, M.; Zheng, L.; Leitgeb, M.; Lin, X. Exosomes-coated bcl-2 siRNA inhibits the growth of digestive system tumors both in vitro and in vivo. Int. J. Biol. Macromol. 2020, 161, 470–480. [Google Scholar] [CrossRef]
- Xu, M.; Chen, G.; Dong, Y.; Yang, J.; Liu, Y.; Song, H.; Song, H.; Wang, Y. Liraglutide-Loaded Milk Exosomes Lower Blood Glucose When Given by Sublingual Route. ChemMedChem 2022, 17, e202100758. [Google Scholar] [CrossRef]
- Sánchez, C.; Franco, L.; Regal, P.; Lamas, A.; Cepeda, A.; Fente, C. Breast milk: A source of functional compounds with potential application in nutrition and therapy. Nutrients 2021, 13, 1026. [Google Scholar] [CrossRef] [PubMed]
- Kleinjan, M.; Van Herwijnen, M.J.; Libregts, S.F.; van Neerven, R.J.; Feitsma, A.L.; Wauben, M.H. Regular industrial processing of bovine milk impacts the integrity and molecular composition of extracellular vesicles. J. Nutr. 2021, 151, 1416–1425. [Google Scholar] [CrossRef] [PubMed]
- Reis, M.G.; Harris, P.; Berry, C.; Nguyen, H.; Maclean, P.; Weeks, M. Tracking changes in volatile components and lipids after homogenisation and thermal processing of milk. Int. Dairy J. 2020, 103, 104624. [Google Scholar] [CrossRef]
- Mutai, E.; Ngu, A.K.H.; Zempleni, J. Preliminary evidence that lectins in infant soy formula apparently bind bovine milk exosomes and prevent their absorption in healthy adults. BMC Nutr. 2022, 8, 7. [Google Scholar] [CrossRef] [PubMed]
- Golan-Gerstl, R.; Elbaum Shiff, Y.; Moshayoff, V.; Schecter, D.; Leshkowitz, D.; Reif, S. Characterization and biological function of milk-derived miRNAs. Mol. Nutr. Food Res. 2017, 61, 1700009. [Google Scholar] [CrossRef]
- López de las Hazas, M.-C.; del Pozo-Acebo, L.; Hansen, M.S.; Gil-Zamorano, J.; Mantilla-Escalante, D.C.; Gómez-Coronado, D.; Marín, F.; Garcia-Ruiz, A.; Rasmussen, J.T.; Dávalos, A. Dietary bovine milk miRNAs transported in extracellular vesicles are partially stable during GI digestion, are bioavailable and reach target tissues but need a minimum dose to impact on gene expression. Eur. J. Nutr. 2022, 61, 1043–1056. [Google Scholar] [CrossRef] [PubMed]



| sEVs Proteins | MW (kDa) | Classification | Function | Specimen Source |
|---|---|---|---|---|
| CD63 | 63 | Four transmembrane proteins | It is a lysosomal membrane protein with the activity of activating platelet surface antigens; | Bovine milk [34,36,37], mesenchymal stromal cells [38], and plasma [39,40]; |
| CD9 | 24–27 | Four transmembrane proteins | Participate in the interaction between cells and the outside world; | Bovine milk [36], mesenchymal stem cells [41,42], and saliva [43,44]; |
| CD81 | 81 | Four transmembrane proteins | Key structural sites for perceiving external signals in cells; | Bovine milk [37,45], synovial fluid [46], and cerebrospinal fluid [47]; |
| TSG101 | 44 | Internal signature proteins | A component of the functional ESCRT-I complex that regulates vesicular transport; | Bovine milk [34,48], rat serum [40], and bile [49]; |
| ALIX | 95 | Internal signature proteins | A phylogenetically conserved cytosolic scaffold protein; | Bovine milk [34,36,48], brain [50], and fibroblast [51]; |
| HSP70 | 70 | Internal signature proteins | It is an important member of the heat shock protein family. | Bovine milk [34,45], plasma [39,44], and urine [52,53]. |
| Separation Method | Principle | Purity | Production | Time Consuming | References |
|---|---|---|---|---|---|
| PEG precipitation purification | Macromolecule aggregation precipitation; | + | +++ | + | [57] |
| Ultracentrifugation | Difference of sedimentation coefficient; | ++ | ++ | ++ | [53,58,59] |
| Density gradient centrifugation | Density gradient difference; | +++ | + | +++ | [60] |
| Ultrafiltration centrifugation | Specific molecular weight cutoff; | ++ | +++ | + | [61] |
| Tangential Flow Filtration | Tangential filtering; | ++ | +++ | + | [62] |
| Size exclusion chromatography | Particle size difference; | +++ | ++ | ++ | [63,64,65] |
| Affinity purification | Intermolecular specific binding. | ++++ | + | ++ | [66] |
| Function | Therapeutic Agent | Effect | Mechanism | References |
|---|---|---|---|---|
| Immunoregulation | TGF-β and miRNA-30b | Play a crucial role in the biogenesis and improvement of infant immune function. | Regulation of immune-related factors (such as miRNAs and antibodies). | [100,101,102,103,104,105,106] |
| Regulation of intestinal tract function | bta-miR-34a, miR-155, and miR-146a | Antioxidant stress; Resistance to hypoxic injury; Reduce the inflammatory response induced by LPS, DSS, and other factors in the mouse intestinal model. | Enhance cell activity, inhibit inflammation, regulate intestinal flora, etc. | [107,108,109,110,111,112,113,114,115,116,117,118,119,120,121,122,123] |
| Development of muscle and bone | miR-21 and miR-29a | The growth and development of muscle and bone can be altered by miRNAs from msEVs. | Increase the number of osteoblasts, promote bone formation and osteoblast differentiation, and encourage myofiber formation in myotube cells. | [124,125,126,127] |
| Promote skin regeneration | TGF-β and miRNA-21 | Promote the transformation of inflammation into tissue and further promote the healing of skin wounds. | Induce ECM deposition and regulate tissue regeneration by regulating the phosphorylation of the Smad pathway. | [128] |
| Detection of bovine diseases | Bovines can be monitored for infection with pathogenic bacteria such as Staphylococcus aureus or their health status. | Examining the nucleic acids, proteins, and lipids in msEVs to identify illness signs. | [129,130,131,132,133,134] | |
| Alleviates arthritis symptoms | immunoregulatory microRNAs (miR-30a, miR-223, miR-92a), beta-lactoglobulin mRNA, and milk-specific beta-casein | Delayed the onset of arthritis, and histology showed diminished cartilage pathology and bone marrow inflammation in both models. | Decreased MCP-1 and IL-6 production by splenic cells in serum; Decreased splenic Th1 (Tbet) and Th17 (RORT) mRNA levels, which were also associated with decreased anticollagen IgG2a levels. | [135] |
| Reduce the side effects of chemotherapy | Protect cells from chemotherapeutic drug-induced cytotoxicity. | Affect the cell cycle of RAW 264.7 with and without cisplatin. | [136] | |
| Use in MOG-specific immunotherapy | butyrophilin (BTN) | Could be thought of as an attractive method to help patients with multiple sclerosis develop MOG-specific tolerance. | The BTN content of these vesicles can pass past the skin’s epidermis and other biological barriers, serving as Trojan horses for the body. | [137] |
| Can be a useful medicinal ingredient for improving skin lightening | miR-2478 | Inhibit melanin production. | Through the Akt-GSK3 signaling pathway, Rap1a expression inhibition reduced melanogenesis. | [138] |
| Might become a potential way to treat cardiac fibrosis | Improved heart performance in cardiac fibrosis rats. | Significant improvements were made to the proangiogenic growth factors. | [139] | |
| Have the potential to promote hair regeneration. | Promote hair regeneration. | Promote dermal papillary cell (DP) proliferation and rescue dihydrotestosterone (DHT)-induced follicular development arrest. | [140] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhong, Y.; Wang, X.; Zhao, X.; Shen, J.; Wu, X.; Gao, P.; Yang, P.; Chen, J.; An, W. Multifunctional Milk-Derived Small Extracellular Vesicles and Their Biomedical Applications. Pharmaceutics 2023, 15, 1418. https://doi.org/10.3390/pharmaceutics15051418
Zhong Y, Wang X, Zhao X, Shen J, Wu X, Gao P, Yang P, Chen J, An W. Multifunctional Milk-Derived Small Extracellular Vesicles and Their Biomedical Applications. Pharmaceutics. 2023; 15(5):1418. https://doi.org/10.3390/pharmaceutics15051418
Chicago/Turabian StyleZhong, Youxiu, Xudong Wang, Xian Zhao, Jiuheng Shen, Xue Wu, Peifen Gao, Peng Yang, Junge Chen, and Wenlin An. 2023. "Multifunctional Milk-Derived Small Extracellular Vesicles and Their Biomedical Applications" Pharmaceutics 15, no. 5: 1418. https://doi.org/10.3390/pharmaceutics15051418




