Assessment of Different Niosome Formulations for Optogenetic Applications: Morphological and Electrophysiological Effects
Abstract
:1. Introduction
2. Material and Methods
2.1. Elaboration of Niosomes and Nioplexes
2.2. Physicochemical Characterization of Niosomes and Nioplexes
2.3. Animal Models
2.4. Primary Neuronal Cell Extraction and Culture
2.5. In Vitro Transfection in Primary Neuronal Cell Culture
2.6. Morphological Evaluation of Transfected Cultured Cortical Neurons
2.7. Electrophysiological Recordings
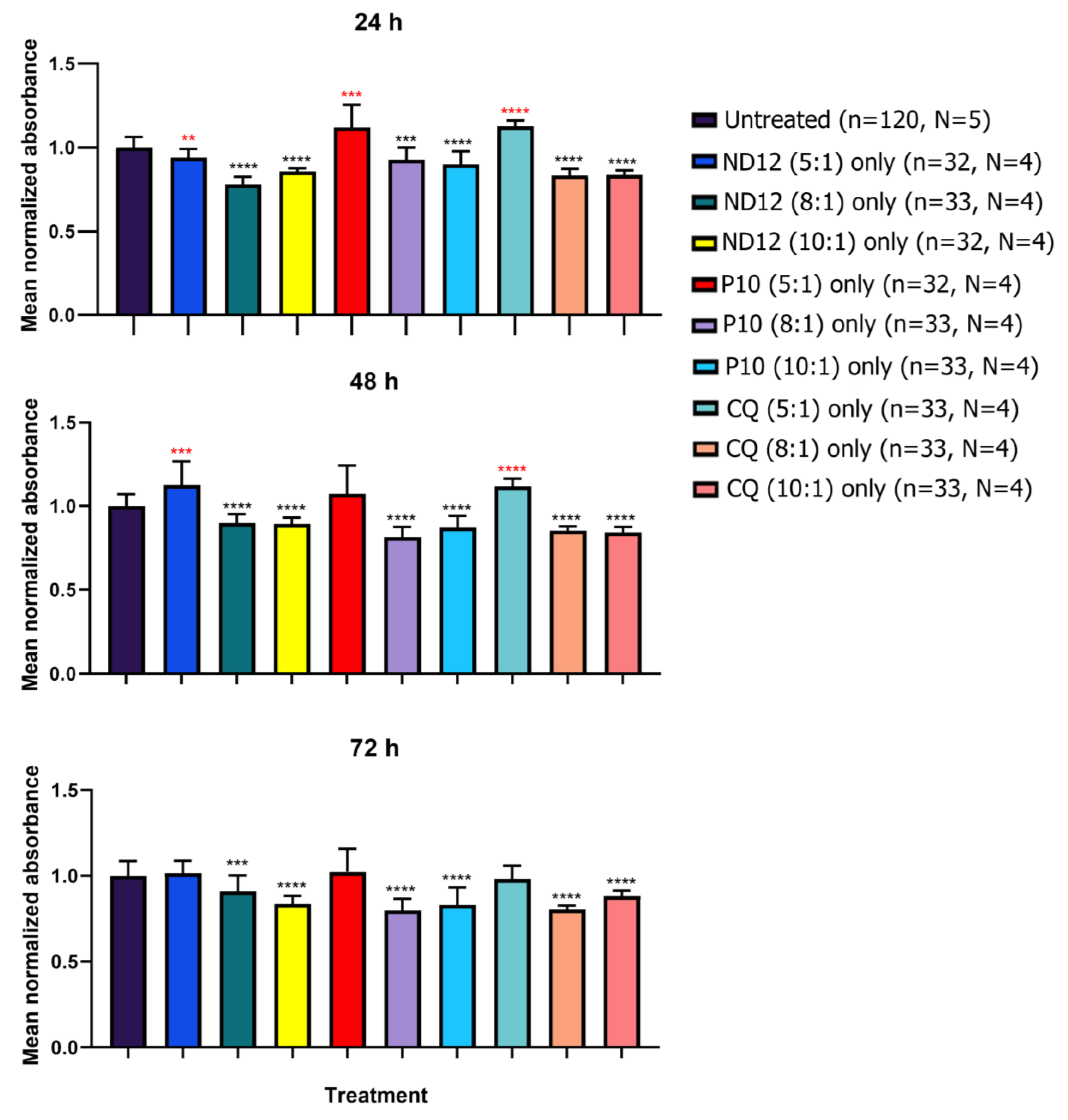
2.8. Cell Viability
2.9. Statistical Analysis
3. Results
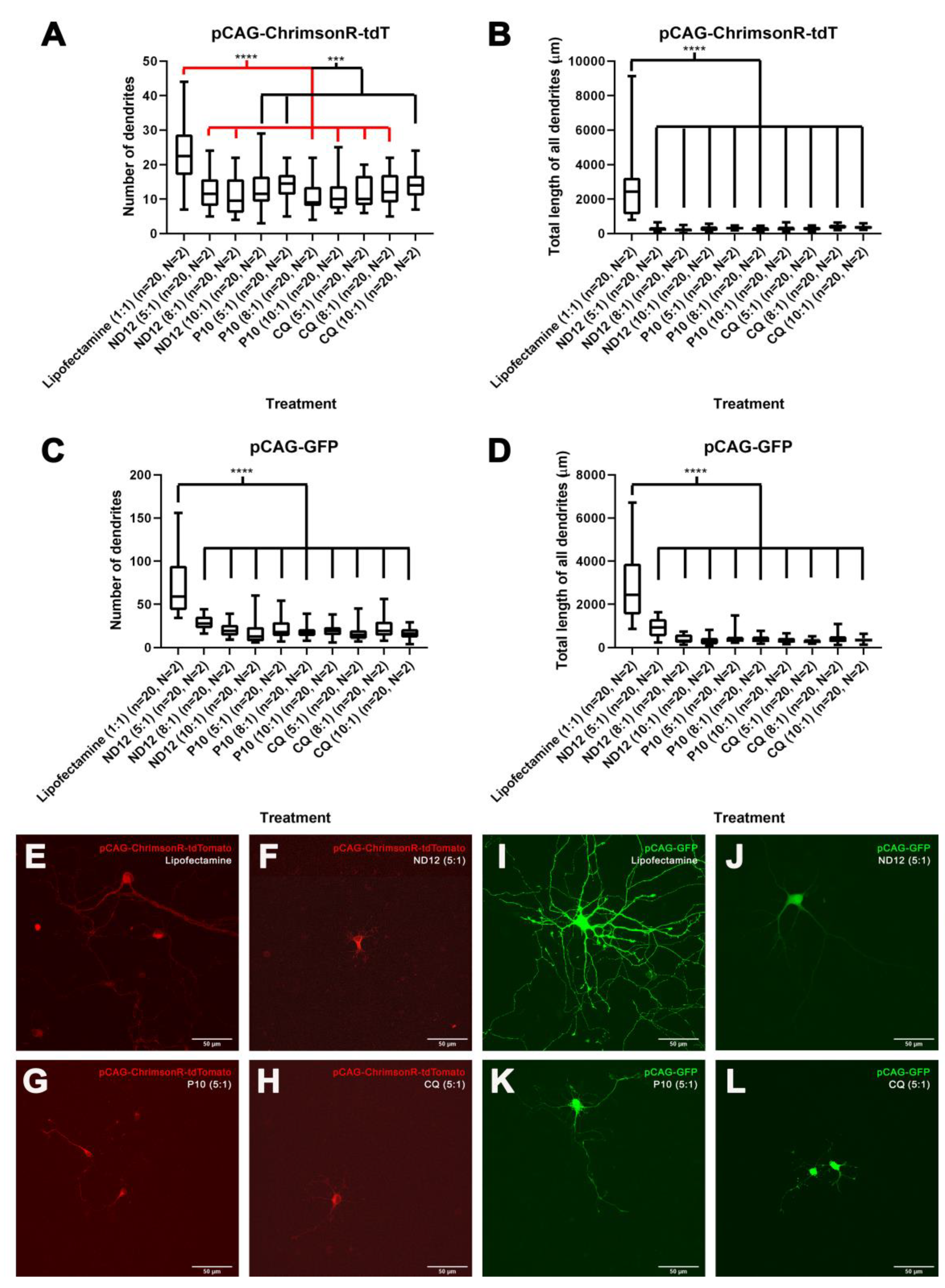
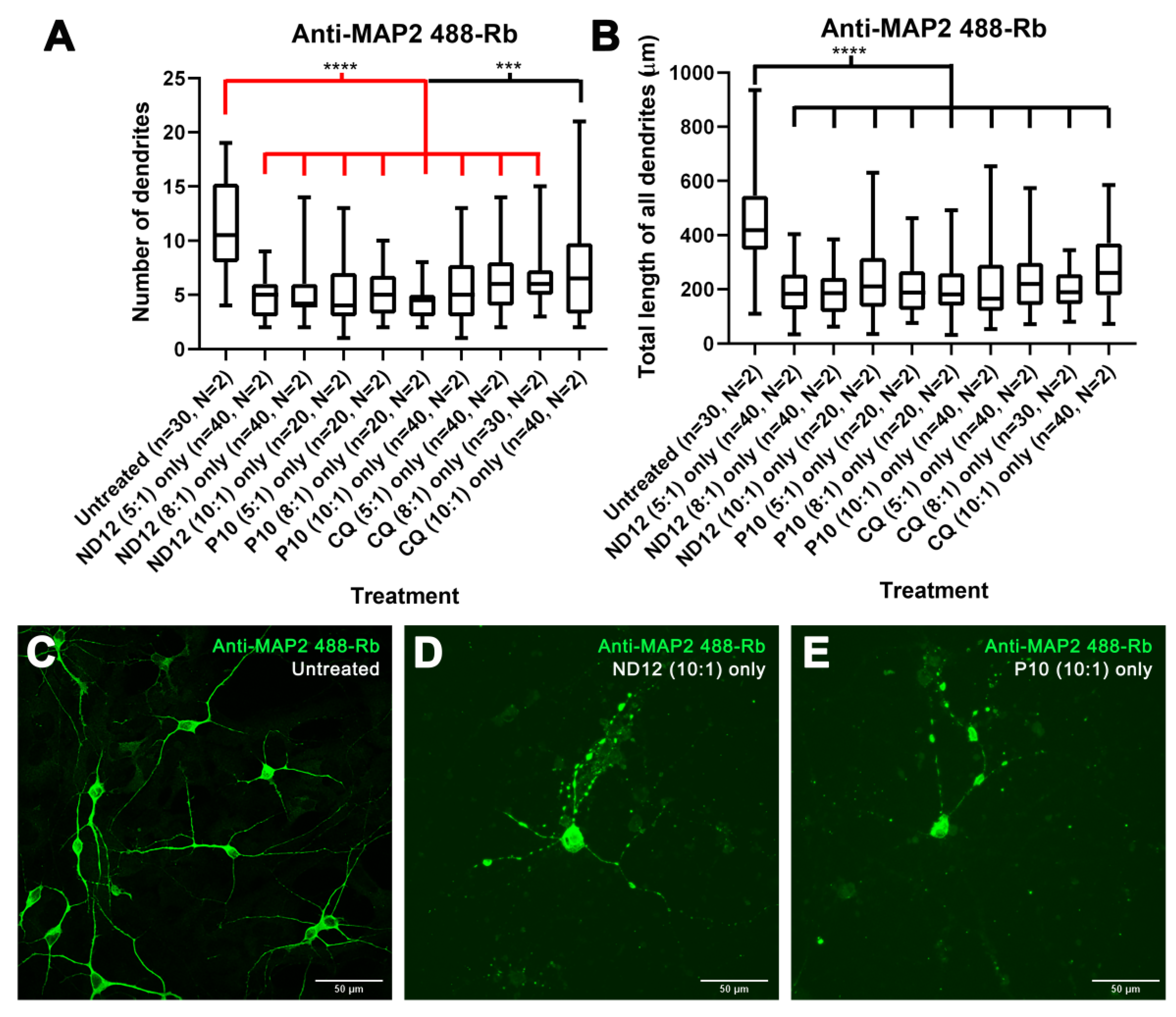
3.1. Nioplexes Transfection and Neuron Morphology
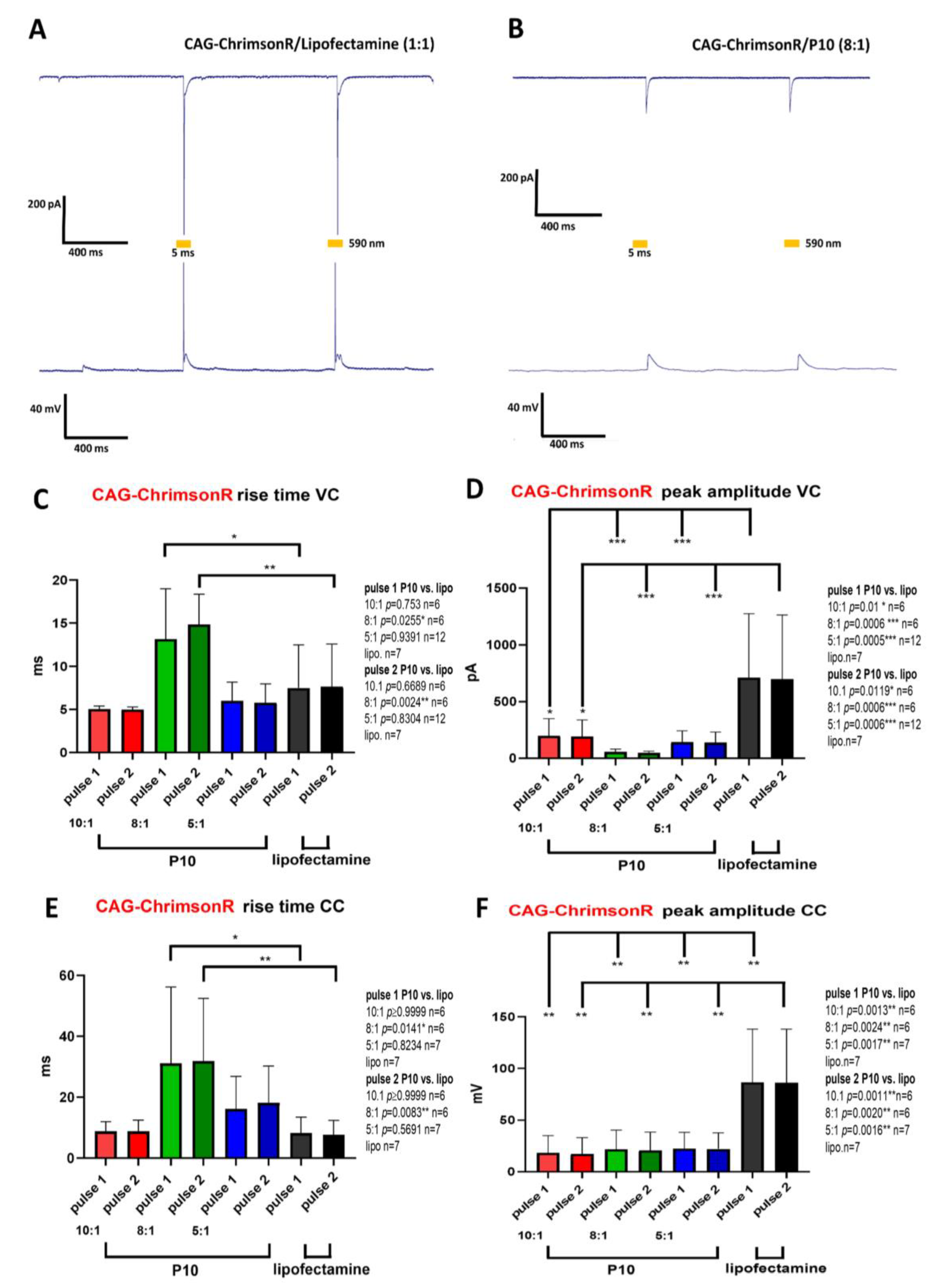
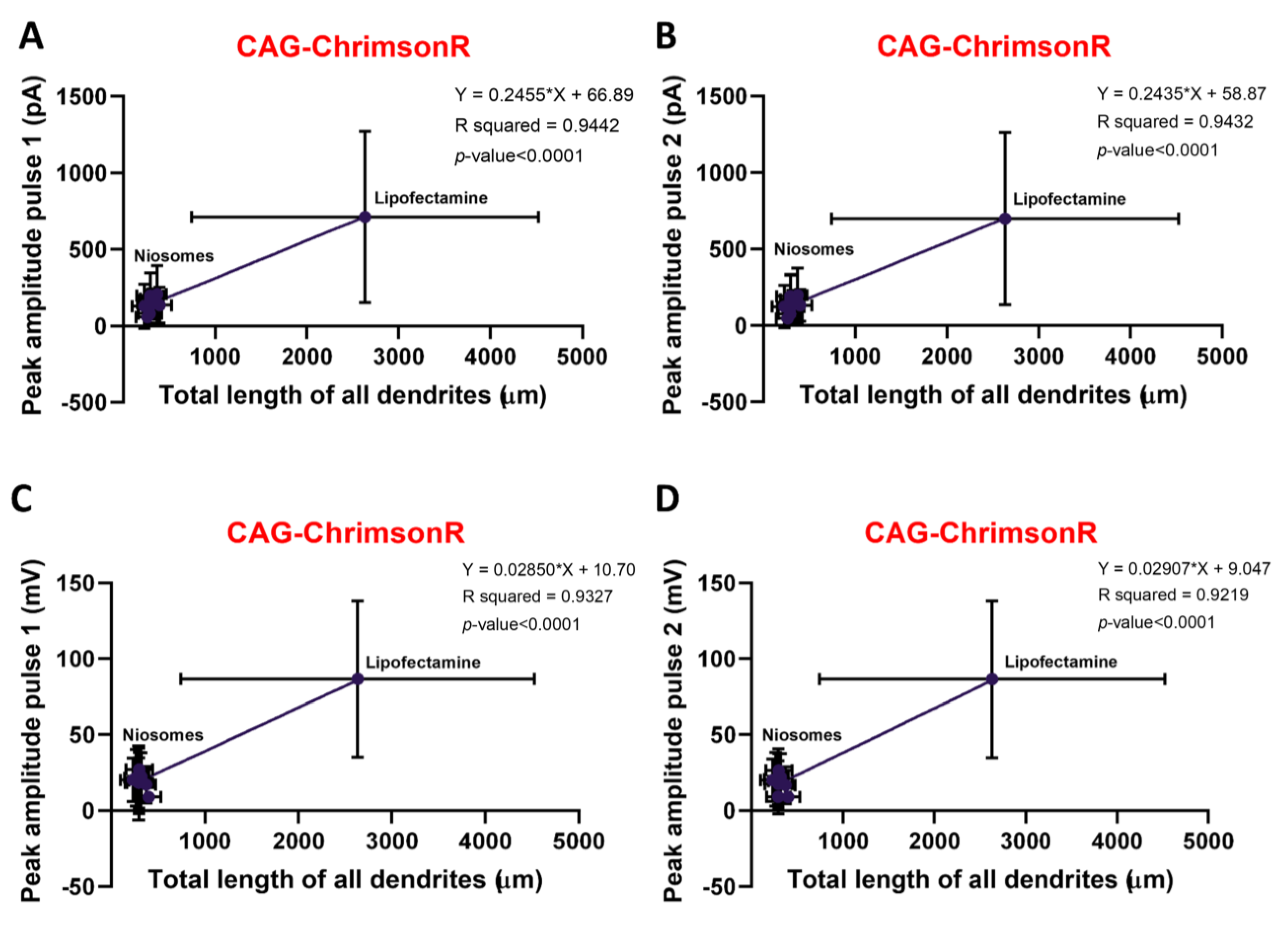
3.2. Electrophysiological Properties of Transfected Neurons
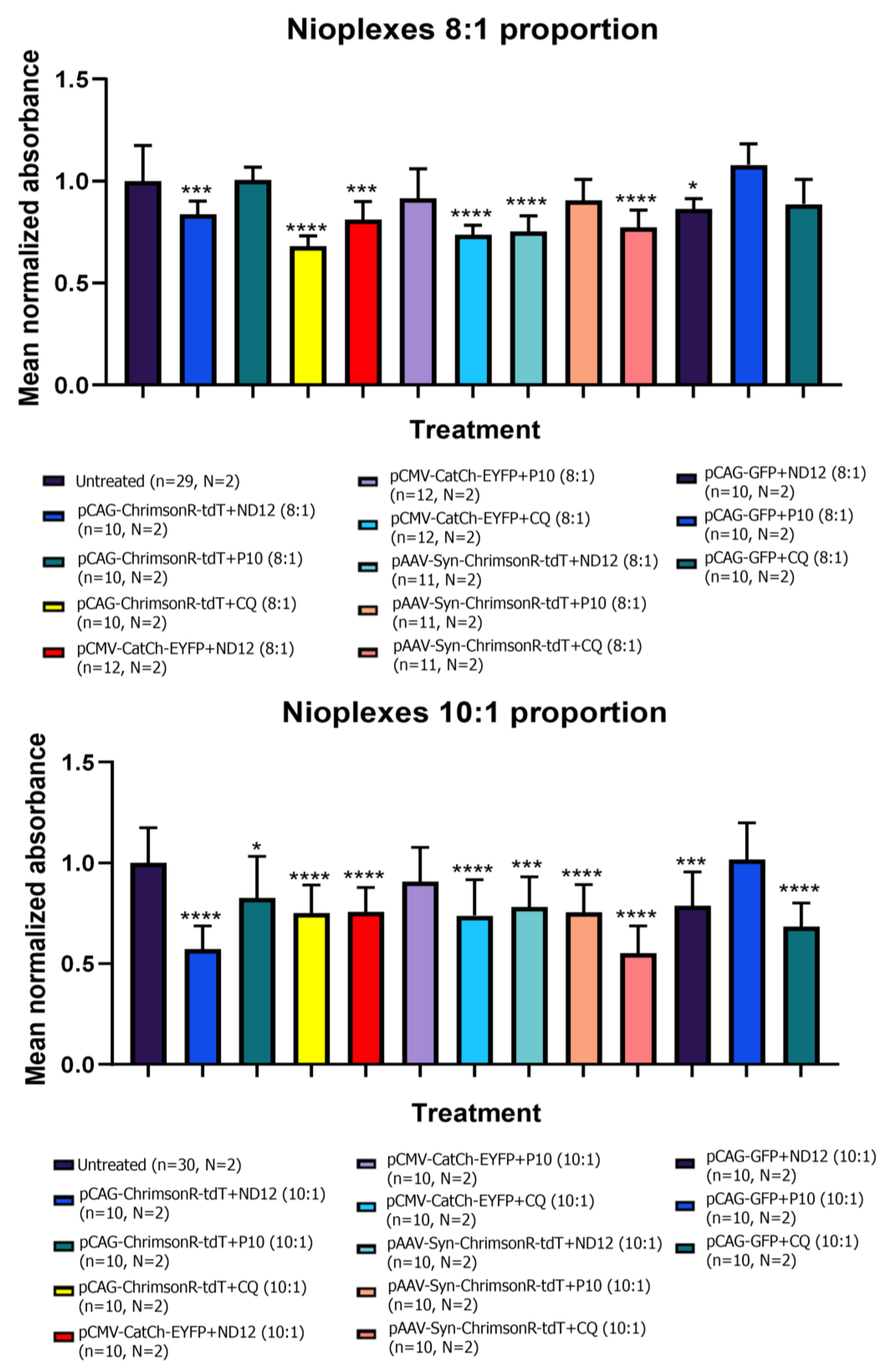
3.3. Morphological Characterization and Cell Viability Assesment of Niosomes, Nioplexes, and Naked Plasmids
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Friedmann, T.; Roblin, R. Gene therapy for human genetic disease? Science 1972, 175, 949–955. [Google Scholar] [CrossRef]
- Merz, B. Gene therapy may have future role in cancer treatment. JAMA 1987, 257, 150–151. [Google Scholar] [CrossRef]
- Shahryari, A.; Saghaeian Jazi, M.; Mohammadi, S.; Razavi Nikoo, H.; Nazari, Z.; Hosseini, E.S.; Burtscher, I.; Mowla, S.J.; Lickert, H. Development and Clinical Translation of Approved Gene Therapy Products for Genetic Disorders. Front. Genet. 2019, 10, 868. [Google Scholar] [CrossRef] [Green Version]
- Ma, C.-C.; Wang, Z.-L.; Xu, T.; He, Z.-Y.; Wei, Y.-Q. The approved gene therapy drugs worldwide: From 1998 to 2019. Biotechnol. Adv. 2020, 40, 107502. [Google Scholar] [CrossRef]
- Powell, S.K.; Rivera-Soto, R.; Gray, S.J. Viral expression cassette elements to enhance transgene target specificity and expression in gene therapy. Discov. Med. 2015, 19, 49–57. [Google Scholar]
- Chen, Y.H.; Keiser, M.S.; Davidson, B.L. Viral Vectors for Gene Transfer. Curr. Protoc. Mouse Biol. 2018, 8, e58. [Google Scholar] [CrossRef]
- Bulcha, J.T.; Wang, Y.; Ma, H.; Tai, P.W.L.; Gao, G. Viral vector platforms within the gene therapy landscape. Signal Transduct. Target. Ther. 2021, 6, 53. [Google Scholar] [CrossRef] [PubMed]
- Almarza, D.; Bussadori, G.; Navarro, M.; Mavilio, F.; Larcher, F.; Murillas, R. Risk assessment in skin gene therapy: Viral–cellular fusion transcripts generated by proviral transcriptional read-through in keratinocytes transduced with self-inactivating lentiviral vectors. Gene Ther. 2011, 18, 674–681. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ibraheem, D.; Elaissari, A.; Fessi, H. Gene therapy and DNA delivery systems. Int. J. Pharm. 2014, 459, 70–83. [Google Scholar] [CrossRef] [PubMed]
- Lundstrom, K. Viral Vectors in Gene Therapy. Diseases 2018, 6, 42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Foldvari, M.; Chen, D.W.; Nafissi, N.; Calderon, D.; Narsineni, L.; Rafiee, A. Non-viral gene therapy: Gains and challenges of non-invasive administration methods. J. Control. Release 2016, 240, 165–190. [Google Scholar] [CrossRef] [PubMed]
- Choi, W.-J.; Kim, J.-K.; Choi, S.-H.; Park, J.-S.; Ahn, W.S.; Kim, C.-K. Low toxicity of cationic lipid-based emulsion for gene transfer. Biomaterials 2004, 25, 5893–5903. [Google Scholar] [CrossRef] [PubMed]
- Rajera, R.; Nagpal, K.; Singh, S.K.; Mishra, D.N. Niosomes: A Controlled and Novel Drug Delivery System. Biol. Pharm. Bull. 2011, 34, 945–953. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ge, X.; Wei, M.; He, S.; Yuan, W.-E. Advances of Non-Ionic Surfactant Vesicles (Niosomes) and Their Application in Drug Delivery. Pharmaceutics 2019, 11, 55. [Google Scholar] [CrossRef] [Green Version]
- Karmali, P.P.; Chaudhuri, A. Cationic liposomes as non-viral carriers of gene medicines: Resolved issues, open questions, and future promises. Med. Res. Rev. 2007, 27, 696–722. [Google Scholar] [CrossRef]
- Dabkowska, A.P.; Barlow, D.J.; Campbell, R.A.; Hughes, A.V.; Quinn, P.J.; Lawrence, M.J. Effect of Helper Lipids on the Interaction of DNA with Cationic Lipid Monolayers Studied by Specular Neutron Reflection. Biomacromolecules 2012, 13, 2391–2401. [Google Scholar] [CrossRef]
- Ojeda, E.; Puras, G.; Agirre, M.; Zarate, J.; Grijalvo, S.; Eritja, R.; DiGiacomo, L.; Caracciolo, G.; Pedraz, J.-L. The role of helper lipids in the intracellular disposition and transfection efficiency of niosome formulations for gene delivery to retinal pigment epithelial cells. Int. J. Pharm. 2016, 503, 115–126. [Google Scholar] [CrossRef] [Green Version]
- Taymouri, S.; Varshosaz, J. Effect of different types of surfactants on the physical properties and stability of carvedilol nano-niosomes. Adv. Biomed. Res. 2016, 5, 48. [Google Scholar] [CrossRef]
- Villate-Beitia, I.; Gallego, I.; Martínez-Navarrete, G.; Zárate, J.; López-Méndez, T.; Soto-Sánchez, C.; Santos-Vizcaíno, E.; Puras, G.; Fernández, E.; Pedraz, J.L. Polysorbate 20 non-ionic surfactant enhances retinal gene delivery efficiency of cationic niosomes after intravitreal and subretinal administration. Int. J. Pharm. 2018, 550, 388–397. [Google Scholar] [CrossRef]
- Mashal, M.; Attia, N.; Puras, G.; Martínez-Navarrete, G.; Fernández, E.; Pedraz, J.L. Retinal gene delivery enhancement by lycopene incorporation into cationic niosomes based on DOTMA and polysorbate 60. J. Control. Release 2017, 254, 55–64. [Google Scholar] [CrossRef]
- Ojeda, E.; Puras, G.; Agirre, M.; Zarate, J.; Grijalvo, S.; Eritja, R.; Martinez-Navarrete, G.; Soto-Sánchez, C.; Diaz-Tahoces, A.; Aviles-Trigueros, M.; et al. The influence of the polar head-group of synthetic cationic lipids on the transfection efficiency mediated by niosomes in rat retina and brain. Biomaterials 2016, 77, 267–279. [Google Scholar] [CrossRef] [Green Version]
- Puras, G.; Mashal, M.; Zárate, J.; Agirre, M.; Ojeda, E.; Grijalvo, S.; Eritja, R.; Diaz-Tahoces, A.; Navarrete, G.M.; Avilés-Trigueros, M.; et al. A novel cationic niosome formulation for gene delivery to the retina. J. Control. Release 2014, 174, 27–36. [Google Scholar] [CrossRef]
- Gallego, I.; Villate-Beitia, I.; Navarrete, G.M.; Menéndez, M.; López-Méndez, T.; Soto-Sánchez, C.; Zarate, J.; Puras, G.; Fernández, E.; Pedraz, J.L. Non-viral vectors based on cationic niosomes and minicircle DNA technology enhance gene delivery efficiency for biomedical applications in retinal disorders. Nanomed. Nanotechnol. Biol. Med. 2019, 17, 308–318. [Google Scholar] [CrossRef] [PubMed]
- Attia, N.; Mashal, M.; Grijalvo, S.; Eritja, R.; Zárate, J.; Puras, G.; Pedraz, J.L. Stem cell-based gene delivery mediated by cationic niosomes for bone regeneration. Nanomed. Nanotechnol. Biol. Med. 2018, 14, 521–531. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Attia, N.; Mashal, M.; Soto-Sánchez, C.; Martínez-Navarrete, G.; Fernández, E.; Grijalvo, S.; Eritja, R.; Puras, G.; Pedraz, J.L. Gene transfer to rat cerebral cortex mediated by polysorbate 80 and poloxamer 188 nonionic surfactant vesicles. Drug Des. Dev. Ther. 2018, 12, 3937–3949. [Google Scholar] [CrossRef] [Green Version]
- Mashal, M.; Attia, N.; Soto-Sánchez, C.; Navarrete, G.M.; Fernández, E.; Puras, G.; Pedraz, J.L. Non-viral vectors based on cationic niosomes as efficient gene delivery vehicles to central nervous system cells into the brain. Int. J. Pharm. 2018, 552, 48–55. [Google Scholar] [CrossRef]
- Carballo-Pedrares, N.; Kattar, A.; Concheiro, A.; Alvarez-Lorenzo, C.; Rey-Rico, A. Niosomes-based gene delivery systems for effective transfection of human mesenchymal stem cells. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 128, 112307. [Google Scholar] [CrossRef]
- Qin, Y.; Tian, Y.; Liu, Y.; Li, D.; Zhang, H.; Yang, Y.; Qi, J.; Wang, H.; Gan, L. Hyaluronic acid-modified cationic niosomes for ocular gene delivery: Improving transfection efficiency in retinal pigment epithelium. J. Pharm. Pharmacol. 2018, 70, 1139–1151. [Google Scholar] [CrossRef] [PubMed]
- Ghaffari, M.; Kalantar, S.M.; Hemati, M.; Firoozabadi, A.D.; Asri, A.; Shams, A.; Ghalekohneh, S.J.; Haghiralsadat, F. Co-delivery of miRNA-15a and miRNA-16–1 using cationic PEGylated niosomes downregulates Bcl-2 and induces apoptosis in prostate cancer cells. Biotechnol. Lett. 2021, 43, 981–994. [Google Scholar] [CrossRef]
- Maurer, V.; Altin, S.; Seleci, D.A.; Zarinwall, A.; Temel, B.; Vogt, P.; Strauß, S.; Stahl, F.; Scheper, T.; Bucan, V.; et al. In-Vitro Application of Magnetic Hybrid Niosomes: Targeted siRNA-Delivery for Enhanced Breast Cancer Therapy. Pharmaceutics 2021, 13, 394. [Google Scholar] [CrossRef]
- Pengnam, S.; Plianwong, S.; Patrojanasophon, P.; Radchatawedchakoon, W.; Yingyongnarongkul, B.-E.; Opanasopit, P.; Charoensuksai, P. Synergistic Effect of Doxorubicin and siRNA-Mediated Silencing of Mcl-1 Using Cationic Niosomes against 3D MCF-7 Spheroids. Pharmaceutics 2021, 13, 550. [Google Scholar] [CrossRef]
- Gharbavi, M.; Johari, B.; Rismani, E.; Mousazadeh, N.; Taromchi, A.H.; Sharafi, A. NANOG Decoy Oligodeoxynucleotide–Encapsulated Niosomes Nanocarriers: A Promising Approach to Suppress the Metastatic Properties of U87 Human Glioblastoma Multiforme Cells. ACS Chem. Neurosci. 2020, 11, 4499–4515. [Google Scholar] [CrossRef]
- Boyden, E.S.; Zhang, F.; Bamberg, E.; Nagel, G.; Deisseroth, K. Millisecond-timescale, genetically targeted optical control of neural activity. Nat. Neurosci. 2005, 8, 1263–1268. [Google Scholar] [CrossRef] [PubMed]
- Aravanis, A.M.; Wang, L.-P.; Zhang, F.; Meltzer, L.A.; Mogri, M.Z.; Schneider, M.B.; Deisseroth, K. An optical neural interface: In vivo control of rodent motor cortex with integrated fiberoptic and optogenetic technology. J. Neural Eng. 2007, 4, S143–S156. [Google Scholar] [CrossRef] [PubMed]
- Yamanaka, A.; Tsunematsu, T. New approaches for the study of orexin function. J. Neuroendocr. 2010, 22, 818–824. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, H.; Zhao, H.; Zeng, C.; Van Dort, C.; Faingold, C.L.; Taylor, N.; Solt, K.; Feng, H.-J. Optogenetic activation of 5-HT neurons in the dorsal raphe suppresses seizure-induced respiratory arrest and produces anticonvulsant effect in the DBA/1 mouse SUDEP model. Neurobiol. Dis. 2018, 110, 47–58. [Google Scholar] [CrossRef]
- Valverde, S.; Vandecasteele, M.; Piette, C.; Derousseaux, W.; Gangarossa, G.; Arbelaiz, A.A.; Touboul, J.; Degos, B.; Venance, L. Deep brain stimulation-guided optogenetic rescue of parkinsonian symptoms. Nat. Commun. 2020, 11, 2388. [Google Scholar] [CrossRef]
- Sahel, J.-A.; Boulanger-Scemama, E.; Pagot, C.; Arleo, A.; Galluppi, F.; Martel, J.N.; Degli Esposti, S.; Delaux, A.; de Saint Aubert, J.-B.; de Montleau, C.; et al. Partial recovery of visual function in a blind patient after optogenetic therapy. Nat. Med. 2021, 27, 1223–1229. [Google Scholar] [CrossRef]
- Klapoetke, N.C.; Murata, Y.; Kim, S.S.; Pulver, S.R.; Birdsey-Benson, A.; Cho, Y.K.; Morimoto, T.K.; Chuong, A.S.; Carpenter, E.J.; Tian, Z.; et al. Independent optical excitation of distinct neural populations. Nat. Methods 2014, 11, 338–346. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kleinlogel, S.; Feldbauer, K.; Dempski, R.E.; Fotis, H.; Wood, P.G.; Bamann, C.; Bamberg, E. Ultra light-sensitive and fast neuronal activation with the Ca2+-permeable channelrhodopsin CatCh. Nat. Neurosci. 2011, 14, 513–518. [Google Scholar] [CrossRef] [Green Version]
- Matsuda, T.; Cepko, C.L. Electroporation and RNA interference in the rodent retina in vivo and in vitro. Proc. Natl. Acad. Sci. USA 2003, 101, 16–22. [Google Scholar] [CrossRef]
- AL Qtaish, N.; Gallego, I.; Paredes, A.J.; Villate-Beitia, I.; Soto-Sánchez, C.; Martínez-Navarrete, G.; Sainz-Ramos, M.; Lopez-Mendez, T.B.; Fernández, E.; Puras, G.; et al. Nanodiamond Integration into Niosomes as an Emerging and Efficient Gene Therapy Nanoplatform for Central Nervous System Diseases. ACS Appl. Mater. Interfaces 2022, 14, 13665–13677. [Google Scholar] [CrossRef]
- AL Qtaish, N.; Gallego, I.; Beitia, I.V.; Sainz-Ramos, M.; Martínez-Navarrete, G.; Soto-Sánchez, C.; Fernández, E.; Gálvez-Martín, P.; Lopez-Mendez, T.B.; Puras, G.; et al. Sphingolipid extracts enhance gene delivery of cationic lipid vesicles into retina and brain. Eur. J. Pharm. Biopharm. 2021, 169, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Sainz-Ramos, M.; Villate-Beitia, I.; Gallego, I.; Qtaish, N.A.; Lopez-Mendez, T.B.; Eritja, R.; Grijalvo, S.; Puras, G.; Pedraz, J.L. Non-viral mediated gene therapy in human cystic fibrosis airway epithelial cells recovers chloride channel functionality. Int. J. Pharm. 2020, 588, 119757. [Google Scholar] [CrossRef] [PubMed]
- Cui, S.; Wang, Y.; Gong, Y.; Lin, X.; Zhao, Y.; Zhi, D.; Zhou, Q.; Zhang, S. Correlation of the cytotoxic effects of cationic lipids with their headgroups. Toxicol. Res. 2018, 7, 473–479. [Google Scholar] [CrossRef]
- Torkzadeh-Mahani, M.; Hajizadeh, M.R.; Maleki, H.; Barani, M.; Fahmidehkar, M.A.; Mahmoodi, M. In vitro cytotoxicity assay of D-limonene niosomes: An efficient nano-carrier for enhancing solubility of plant-extracted agents. Res. Pharm. Sci. 2019, 14, 448–458. [Google Scholar] [CrossRef]
- Guevara, M.L.; Persano, S.; Persano, F. Lipid-Based Vectors for Therapeutic mRNA-Based Anti-Cancer Vaccines. Curr. Pharm. Des. 2019, 25, 1443–1454. [Google Scholar] [CrossRef] [PubMed]
- Davarpanah, F.; Yazdi, A.K.; Barani, M.; Mirzaei, M.; Torkzadeh-Mahani, M. Magnetic delivery of antitumor carboplatin by using PEGylated-Niosomes. DARU J. Pharm. Sci. 2018, 26, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Meng, N.; Grimm, D. Membrane-destabilizing ionizable phospholipids: Novel components for organ-selective mRNA delivery and CRISPR–Cas gene editing. Signal Transduct. Target. Ther. 2021, 6, 206. [Google Scholar] [CrossRef]
- Zhao, N.; Fogg, J.M.; Zechiedrich, L.; Zu, Y. Transfection of shRNA-encoding Minivector DNA of a few hundred base pairs to regulate gene expression in lymphoma cells. Gene Ther. 2010, 18, 220–224. [Google Scholar] [CrossRef] [Green Version]
- Palmer, M. On the Use of the Pfizer and the Moderna COVID-19 mRNA Vaccines in Children and Adolescents. 2022. Available online: https://doctors4covidethics.org/wp-content/uploads/2022/05/pfizer-moderna-public.pdf (accessed on 15 January 2023).
- Kole, A.J.; Annis, R.P.; Deshmukh, M. Mature neurons: Equipped for survival. Cell Death Dis. 2013, 4, e689. [Google Scholar] [CrossRef] [PubMed] [Green Version]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Celdrán, J.D.; Humphreys, L.; González, D.; Soto-Sánchez, C.; Martínez-Navarrete, G.; Maldonado, I.; Gallego, I.; Villate-Beitia, I.; Sainz-Ramos, M.; Puras, G.; et al. Assessment of Different Niosome Formulations for Optogenetic Applications: Morphological and Electrophysiological Effects. Pharmaceutics 2023, 15, 1860. https://doi.org/10.3390/pharmaceutics15071860
Celdrán JD, Humphreys L, González D, Soto-Sánchez C, Martínez-Navarrete G, Maldonado I, Gallego I, Villate-Beitia I, Sainz-Ramos M, Puras G, et al. Assessment of Different Niosome Formulations for Optogenetic Applications: Morphological and Electrophysiological Effects. Pharmaceutics. 2023; 15(7):1860. https://doi.org/10.3390/pharmaceutics15071860
Chicago/Turabian StyleCeldrán, José David, Lawrence Humphreys, Desirée González, Cristina Soto-Sánchez, Gema Martínez-Navarrete, Iván Maldonado, Idoia Gallego, Ilia Villate-Beitia, Myriam Sainz-Ramos, Gustavo Puras, and et al. 2023. "Assessment of Different Niosome Formulations for Optogenetic Applications: Morphological and Electrophysiological Effects" Pharmaceutics 15, no. 7: 1860. https://doi.org/10.3390/pharmaceutics15071860
APA StyleCeldrán, J. D., Humphreys, L., González, D., Soto-Sánchez, C., Martínez-Navarrete, G., Maldonado, I., Gallego, I., Villate-Beitia, I., Sainz-Ramos, M., Puras, G., Pedraz, J. L., & Fernández, E. (2023). Assessment of Different Niosome Formulations for Optogenetic Applications: Morphological and Electrophysiological Effects. Pharmaceutics, 15(7), 1860. https://doi.org/10.3390/pharmaceutics15071860










