The Role of Crosslinker Content of Positively Charged NIPAM Nanogels on the In Vivo Toxicity in Zebrafish
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of NGs
2.3. Study of Monomer Conversions by 1H NMR
2.4. Nanoparticle Size by Dynamic Light Scattering
2.5. Nanoparticle Surface Charge by Electrophoretic Light Scattering
2.6. Thermoresponsive Properties of NGs by UV-Visible Spectroscopy
2.7. Zebrafish Husbandry and Maintenance
2.8. Evaluation of the Biocompatibility of NGs in Zebrafish
3. Results and Discussion
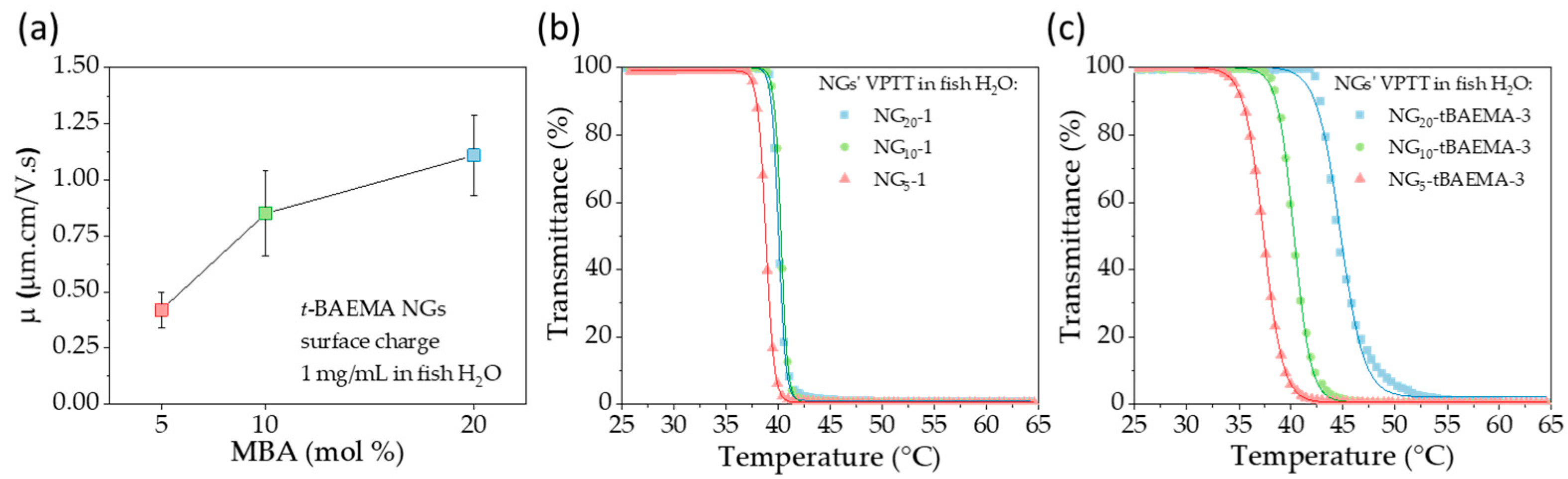
3.1. Synthesis and Characterization of Neutral and Positively Charged NGs
3.2. Impact of Surface Chemistry and Morphology of NGs on their In Vivo Toxicity on Zebrafish Model
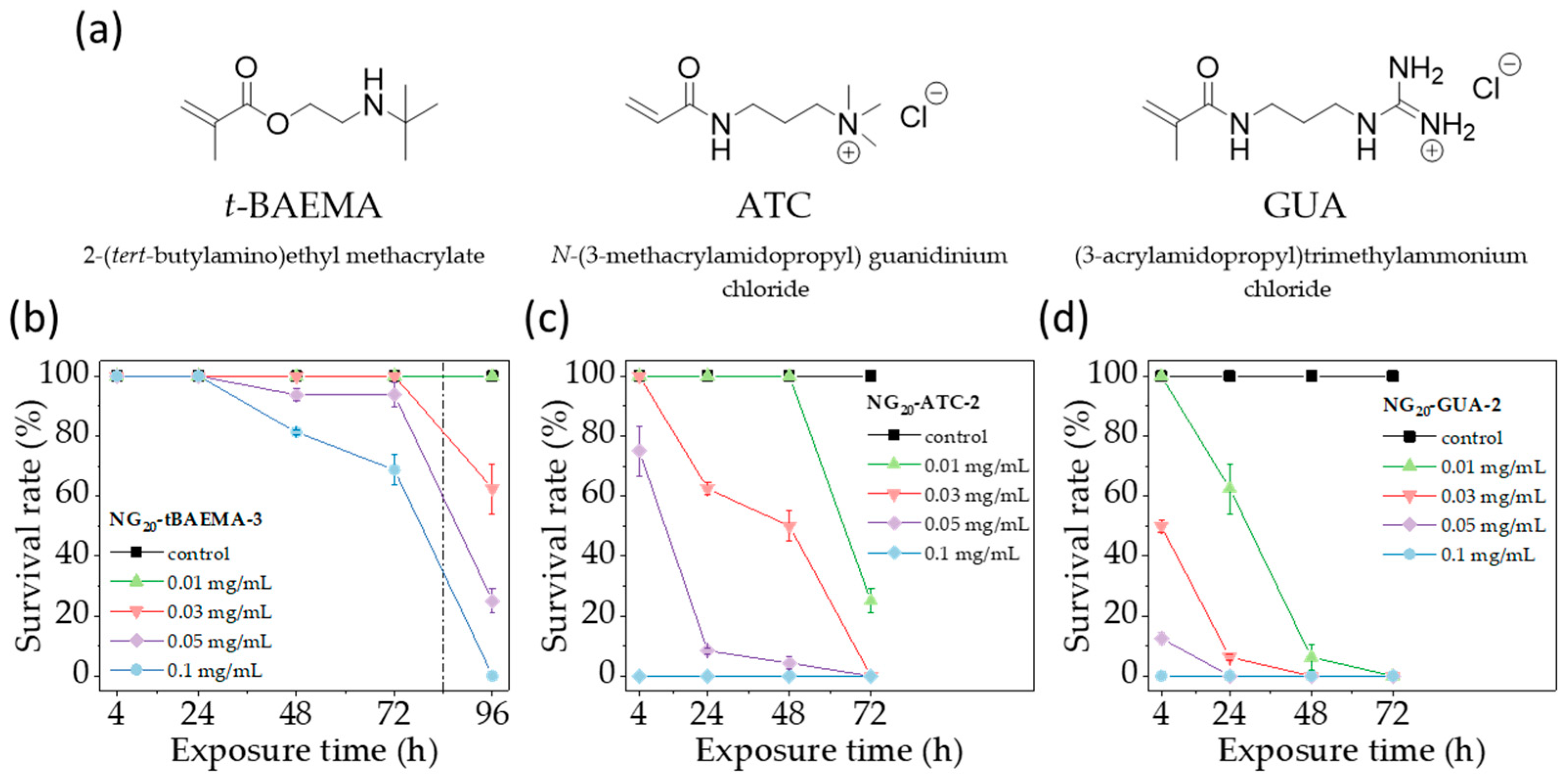
3.3. Impact of Positively Charged Group Structure on the Toxicity of NGs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Lee, B.K.; Yun, Y.H.; Park, K. Smart Nanoparticles for Drug Delivery: Boundaries and Opportunities. Chem. Eng. Sci. 2015, 125, 158–164. [Google Scholar] [CrossRef] [Green Version]
- Patra, J.K.; Das, G.; Fraceto, L.F.; Campos, E.V.R.; Rodriguez-Torres, M.D.P.; Acosta-Torres, L.S.; Diaz-Torres, L.A.; Grillo, R.; Swamy, M.K.; Sharma, S.; et al. Nano Based Drug Delivery Systems: Recent Developments and Future Prospects. J. Nanobiotechnology 2018, 16, 71. [Google Scholar] [CrossRef] [Green Version]
- Ventola, C.L. The Nanomedicine Revolution—Part 2: Current and Future Clinical Applications. Pharm. Times 2012, 37, 582–591. [Google Scholar]
- Ayub, A.; Wettig, S. An Overview of Nanotechnologies for Drug Delivery to the Brain. Pharmaceutics 2022, 14, 224. [Google Scholar] [CrossRef]
- Muhamad, N.; Plengsuriyakarn, T.; Na-Bangchang, K. Application of Active Targeting Nanoparticle Delivery System for Chemotherapeutic Drugs and Traditional/Herbal Medicines in Cancer Therapy: A Systematic Review. Int. J. Nanomed. 2018, 13, 3921–3935. [Google Scholar] [CrossRef] [Green Version]
- Alexander, A.; Agrawal, M.; Uddin, A.; Siddique, S.; Shehata, A.M.; Shaker, M.A.; Rahman, S.A.U.; Abdul, M.I.M.; Shaker, M.A. Recent Expansions of Novel Strategies towards the Drug Targeting into the Brain. Int. J. Nanomed. 2019, 14, 5895–5909. [Google Scholar] [CrossRef] [Green Version]
- Mura, S.; Nicolas, J.; Couvreur, P. Stimuli-Responsive Nanocarriers for Drug Delivery. Nat. Mater. 2013, 12, 991–1003. [Google Scholar] [CrossRef]
- Cheng, R.; Meng, F.; Deng, C.; Klok, H.A.; Zhong, Z. Dual and Multi-Stimuli Responsive Polymeric Nanoparticles for Programmed Site-Specific Drug Delivery. Biomaterials 2013, 34, 3647–3657. [Google Scholar] [CrossRef]
- Soni, K.S.; Desale, S.S.; Bronich, T.K. Nanogels: An Overview of Properties, Biomedical Applications and Obstacles to Clinical Translation. J. Control. Release 2016, 240, 109–126. [Google Scholar] [CrossRef] [Green Version]
- Kabanov, A.V.; Vinogradov, S.V. Nanogels as Pharmaceutical Carriers: Finite Networks of Infinite Capabilities. Angew. Chem. Int. Ed. 2009, 48, 5418–5429. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Zhai, Y.; Wang, J.; Zhai, G. New Progress and Prospects: The Application of Nanogel in Drug Delivery. Mater. Sci. Eng. C 2016, 60, 560–568. [Google Scholar] [CrossRef]
- Zhang, X.; Malhotra, S.; Molina, M.; Haag, R. Micro- and Nanogels with Labile Crosslinks-from Synthesis to Biomedical Applications. Chem. Soc. Rev. 2015, 44, 1948–1973. [Google Scholar] [CrossRef] [Green Version]
- Vashist, A.; Kaushik, A.; Vashist, A.; Bala, J.; Nikkhah-Moshaie, R.; Sagar, V.; Nair, M. Nanogels as Potential Drug Nanocarriers for CNS Drug Delivery. Drug Discov. Today 2018, 23, 1436–1443. [Google Scholar] [CrossRef]
- Yadav, H.K.S.; Anwar, N.; Halabi, A.; Alsalloum, G.A. Nanogels as Novel Drug Delivery Systems—A Review. J. Pharm. Pharm. Res. 2017, 1, 5. [Google Scholar]
- Azadi, A.; Hamidi, M.; Rouini, M. Methotrexate-Loaded Chitosan Nanogels as ‘Trojan Horses’ for Drug Delivery to Brain: Preparation and in Vitro/in Vivo Characterization. Int. J. Biol. Macromol. 2013, 62, 523–530. [Google Scholar] [CrossRef]
- Papadimitriou, S.A.; Robin, M.P.; Ceric, D.; O’Reilly, R.K.; Marino, S.; Resmini, M. Fluorescent Polymeric Nanovehicles for Neural Stem Cell Modulation. Nanoscale 2016, 8, 17340–17349. [Google Scholar] [CrossRef] [Green Version]
- Mauri, E.; Giannitelli, S.M.; Trombetta, M.; Rainer, A. Synthesis of Nanogels: Current Trends and Future Outlook. Gels 2021, 7, 36. [Google Scholar] [CrossRef]
- Chiang, W.; Ho, V.T.; Huang, W.; Huang, Y.; Chern, C.; Chiu, H. Dual Stimuli-Responsive Polymeric Hollow Nanogels Designed as Carriers for Intracellular Triggered Drug Release. Langmuir 2012, 28, 15056–15064. [Google Scholar] [CrossRef]
- Escobedo, H.D.; Stansbury, J.W.; Nair, D.P. Photoreactive Nanogels as Versatile Polymer Networks with Tunable in Situ Drug Release Kinetics. J. Mech. Behav. Biomed. Mater. 2020, 108, 103755. [Google Scholar] [CrossRef]
- Vdovchenko, A.; Pearce, A.K.; Freeley, M.; O’Reilly, R.K.; Resmini, M. Effect of Heterogeneous and Homogeneous Polymerisation on the Structure of PNIPAm Nanogels. Polym. Chem. 2021, 12, 6854–6864. [Google Scholar] [CrossRef]
- Bilardo, R.; Traldi, F.; Vdovchenko, A.; Resmini, M. Influence of Surface Chemistry and Morphology of Nanoparticles on Protein Corona Formation. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnology 2022, 14, e1788. [Google Scholar] [CrossRef]
- Liu, P.; Freeley, M.; Zarbakhsh, A.; Resmini, M. Adsorption of Soft NIPAM Nanogels at Hydrophobic and Hydrophilic Interfaces: Conformation of the Interfacial Layers Determined by Neutron Reflectivity. J. Colloid Interface Sci. 2022, 623, 337–347. [Google Scholar] [CrossRef]
- Sun, H.; Zielinska, K.; Resmini, M.; Zarbakhsh, A. Interactions of NIPAM Nanogels with Model Lipid Multi-Bilayers: A Neutron Reflectivity Study. J. Colloid Interface Sci. 2019, 536, 598–608. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Pearce, C.M.; Anastasiadi, R.-M.; Resmini, M.; Castilla, A.M. Covalently Crosslinked Nanogels: An NMR Study of the Effect of Monomer Reactivity on Composition and Structure. Polymers 2019, 11, 353. [Google Scholar] [CrossRef] [Green Version]
- Traldi, F.; Liu, P.; Albino, I.; Ferreira, L.; Zarbakhsh, A.; Resmini, M. Protein-Nanoparticle Interactions Govern the Interfacial Behavior of Polymeric Nanogels: Study of Protein Corona Formation at the Air/Water Interface. Int. J. Mol. Sci. 2023, 24, 2810. [Google Scholar] [CrossRef]
- Oladipo, A.O.; Lebelo, S.L.; Msagati, T.A.M. Nanocarrier Design–Function Relationship: The Prodigious Role of Properties in Regulating Biocompatibility for Drug Delivery Applications. Chem. Biol. Interact. 2023, 377, 110466. [Google Scholar] [CrossRef]
- Zhang, L.; Wu, L.; Si, Y.; Shu, K. Size-Dependent Cytotoxicity of Silver Nanoparticles to Azotobacter Vinelandii: Growth Inhibition, Cell Injury, Oxidative Stress and Internalization. PLoS ONE 2018, 13, e0209020. [Google Scholar] [CrossRef] [Green Version]
- Yuan, Y.G.; Zhang, S.; Hwang, J.Y.; Kong, I.K. Silver Nanoparticles Potentiates Cytotoxicity and Apoptotic Potential of Camptothecin in Human Cervical Cancer Cells. Oxid. Med. Cell. Longev. 2018, 2018, 6121328. [Google Scholar] [CrossRef] [Green Version]
- Huo, S.; Jin, S.; Ma, X.; Xue, X.; Yang, K.; Kumar, A.; Wang, P.C.; Zhang, J.; Hu, Z.; Liang, X.J. Ultrasmall Gold Nanoparticles as Carriers for Nucleus-Based Gene Therapy Due to Size-Dependent Nuclear Entry. ACS Nano 2014, 8, 5852–5862. [Google Scholar] [CrossRef]
- Soenen, S.J.; Parak, W.J.; Rejman, J.; Manshian, B. (Intra)Cellular Stability of Inorganic Nanoparticles: Effects on Cytotoxicity, Particle Functionality, and Biomedical Applications. Chem. Rev. 2015, 115, 2109–2135. [Google Scholar] [CrossRef]
- Moyano, D.F.; Goldsmith, M.; Solfiell, D.J.; Landesman-Milo, D.; Miranda, O.R.; Peer, D.; Rotello, V.M. Nanoparticle Hydrophobicity Dictates Immune Response. J. Am. Chem. Soc. 2012, 134, 3965–3967. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Waku, T.; Nishigaki, S.; Kitagawa, Y.; Koeda, S.; Kawabata, K.; Kunugi, S.; Kobori, A.; Tanaka, N. Effect of the Hydrophilic-Hydrophobic Balance of Antigen-Loaded Peptide Nanofibers on Their Cellular Uptake, Cellular Toxicity, and Immune Stimulatory Properties. Int. J. Mol. Sci. 2019, 20, 3781. [Google Scholar] [CrossRef] [Green Version]
- Bewersdorff, T.; Gruber, A.; Eravci, M.; Dumbani, M.; Klinger, D.; Haase, A. Amphiphilic Nanogels: Influence of Surface Hydrophobicity on Protein Corona, Biocompatibility and Cellular Uptake. Int. J. Nanomed. 2019, 14, 7861–7878. [Google Scholar] [CrossRef] [Green Version]
- Sukhanova, A.; Bozrova, S.; Sokolov, P.; Berestovoy, M.; Karaulov, A.; Nabiev, I. Dependence of Nanoparticle Toxicity on Their Physical and Chemical Properties. Nanoscale Res. Lett. 2018, 13, 44. [Google Scholar] [CrossRef] [Green Version]
- Huang, Y.W.; Cambre, M.; Lee, H.J. The Toxicity of Nanoparticles Depends on Multiple Molecular and Physicochemical Mechanisms. Int. J. Mol. Sci. 2017, 18, 2702. [Google Scholar] [CrossRef] [Green Version]
- Wan, J.; Wang, J.H.; Liu, T.; Xie, Z.; Yu, X.F.; Li, W. Surface Chemistry but Not Aspect Ratio Mediates the Biological Toxicity of Gold Nanorods in Vitro and in Vivo. Sci. Rep. 2015, 5, 11398. [Google Scholar] [CrossRef] [Green Version]
- Yang, W.; Liu, S.; Bai, T.; Keefe, A.J.; Zhang, L.; Ella-Menye, J.R.; Li, Y.; Jiang, S. Poly(Carboxybetaine) Nanomaterials Enable Long Circulation and Prevent Polymer-Specific Antibody Production. Nano Today 2014, 9, 10–16. [Google Scholar] [CrossRef]
- Huang, Y.W.; Lee, H.J.; Tolliver, L.M.; Aronstam, R.S. Delivery of Nucleic Acids and Nanomaterials by Cell-Penetrating Peptides: Opportunities and Challenges. BioMed Res. Int. 2015, 2015, 834079. [Google Scholar] [CrossRef] [Green Version]
- Bannunah, A.M.; Vllasaliu, D.; Lord, J.; Stolnik, S. Mechanisms of Nanoparticle Internalization and Transport across an Intestinal Epithelial Cell Model: Effect of Size and Surface Charge. Mol. Pharm. 2014, 11, 4363–4373. [Google Scholar] [CrossRef]
- Sun, H.; Jiang, C.; Wu, L.; Bai, X.; Zhai, S. Cytotoxicity-Related Bioeffects Induced by Nanoparticles: The Role of Surface Chemistry. Front. Bioeng. Biotechnol. 2019, 7, 414. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.; Mccrate, J.M.; Lee, J.C.M.; Li, H. The Role of Surface Charge on the Uptake and Biocompatibility of Hydroxyapatite Nanoparticles with Osteoblast Cells. Nanotechnology 2011, 22, 5708. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hühn, D.; Kantner, K.; Geidel, C.; Brandholt, S.; De Cock, I.; Soenen, S.J.H.; Riveragil, P.; Montenegro, J.M.; Braeckmans, K.; Müllen, K.; et al. Polymer-Coated Nanoparticles Interacting with Proteins and Cells: Focusing on the Sign of the Net Charge. ACS Nano 2013, 7, 3253–3263. [Google Scholar] [CrossRef]
- Kai, W.; Xiaojun, X.; Ximing, P.; Zhenqing, H.; Qiqing, Z. Cytotoxic Effects and the Mechanism of Three Types of Magnetic Nanoparticles on Human Hepatoma BEL-7402 Cells. Nanoscale Res. Lett. 2011, 6, 480. [Google Scholar] [CrossRef] [Green Version]
- Baek, M.; Kim, M.K.; Cho, H.J.; Lee, J.A.; Yu, J.; Chung, H.E.; Choi, S.J. Factors Influencing the Cytotoxicity of Zinc Oxide Nanoparticles: Particle Size and Surface Charge. J. Phys. Conf. Ser. 2011, 304, 2044. [Google Scholar] [CrossRef]
- Ruenraroengsak, P.; Tetley, T.D. Differential Bioreactivity of Neutral, Cationic and Anionic Polystyrene Nanoparticles with Cells from the Human Alveolar Compartment: Robust Response of Alveolar Type 1 Epithelial Cells. Part. Fibre Toxicol. 2015, 12, 19. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Li, W.; Lao, F.; Liu, Y.; Wang, L.; Bai, R.; Zhao, Y.; Chen, C. Intracellular Dynamics of Cationic and Anionic Polystyrene Nanoparticles without Direct Interaction with Mitotic Spindle and Chromosomes. Biomaterials 2011, 32, 8291–8303. [Google Scholar] [CrossRef]
- Ali, S.; Champagne, D.L.; Spaink, H.P.; Richardson, M.K. Zebrafish Embryos and Larvae: A New Generation of Disease Models and Drug Screens. Birth Defects Res. Part C Embryo Today Rev. 2011, 93, 115–133. [Google Scholar] [CrossRef] [PubMed]
- Lopes, S.S. The Importance of Zebrafish in Biomedical Research. Acta Med. Port. 2013, 26, 583–592. [Google Scholar]
- Streisinger, G.; Walker, C.; Dower, N.; Knauber, D.; Singer, F. Production of Clones of Homozygous Diploid Zebra Fish (Brachydanio rerio). Nature 1981, 291, 293–296. [Google Scholar] [CrossRef]
- Saleem, S.; Kannan, R.R. Zebrafish: An Emerging Real-Time Model System to Study Alzheimer’s Disease and Neurospecific Drug Discovery. Nanoscale Res. Lett. 2018, 4, 45. [Google Scholar] [CrossRef] [Green Version]
- McGrath, P.; Li, C.Q. Zebrafish: A Predictive Model for Assessing Drug-Induced Toxicity. Drug Discov. Today 2008, 13, 394–401. [Google Scholar] [CrossRef] [PubMed]
- Westerfield, M. The Zebrafish Book. A Guide for the Laboratory Use of Zebrafish (Danio rerio), 4th ed.; University of Oregon Press: Eugene, OR, USA, 2000. [Google Scholar]
- Graham, N.B.; Cameron, A. Nayonogels and Microgels: The New Polymeric Materials Playground. Pure Appl. Chem. 1998, 70, 1271–1275. [Google Scholar] [CrossRef] [Green Version]
- Morse, A.J.; Dupin, D.; Thompson, K.L.; Armes, S.P.; Ouzineb, K.; Mills, P.; Swart, R. Novel Pickering Emulsifiers Based on PH-Responsive Poly(tert-Butylaminoethyl Methacrylate) Latexes. Langmuir 2012, 28, 11733–11744. [Google Scholar] [CrossRef]
- Salinas, Y.; Castilla, A.M.; Resmini, M. An L-Proline Based Thermoresponsive and PH-Switchable Nanogel as a Drug Delivery Vehicle. Polym. Chem. 2018, 9, 2271–2280. [Google Scholar] [CrossRef] [Green Version]
- Judah, H.L.; Liu, P.; Zarbakhsh, A.; Resmini, M. Influence of Buffers, Ionic Strength, and PH on the Volume Phase Transition Behavior of Acrylamide-Based Nanogels. Polymers 2020, 12, 2590. [Google Scholar] [CrossRef] [PubMed]
- Holder, E.L.; Conmy, R.N.; Venosa, A.D. Comparative Laboratory-Scale Testing of Dispersant Effectiveness of 23 Crude Oils Using Four Different Testing Protocols. J. Environ. Prot. 2015, 6, 628–639. [Google Scholar] [CrossRef] [Green Version]
- Yonamine, Y.; Yoshimatsu, K.; Lee, S.H.; Hoshino, Y.; Okahata, Y.; Shea, K.J. Polymer Nanoparticle-Protein Interface. Evaluation of the Contribution of Positively Charged Functional Groups to Protein Affinity. ACS Appl. Mater. Interfaces 2013, 5, 374–379. [Google Scholar] [CrossRef]
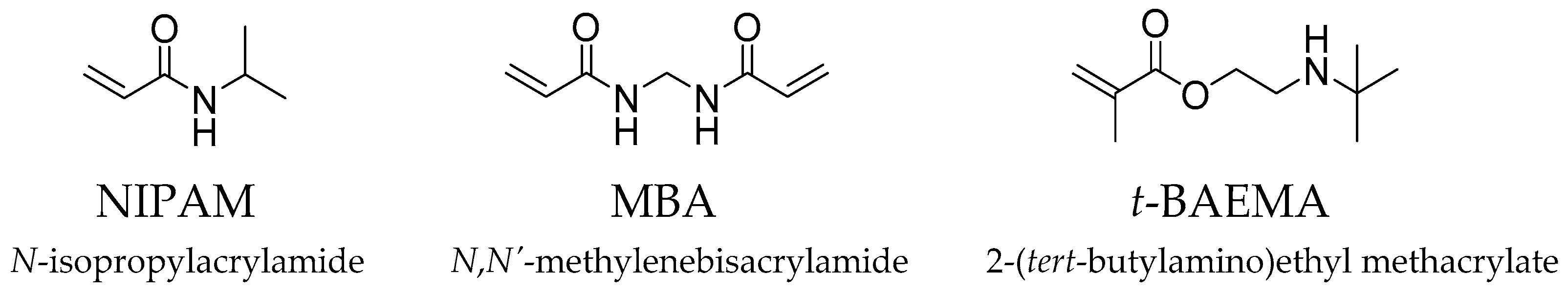

| Nanogel | Composition (mol%) | CM (%) | Monomer Conversion (%) | Yield (%) | Dh (nm) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| NIPAM | MBA | t-BAEMA | NIPAM | MBA | t-BAEMA | Overall | D.I Water | Fish Water | |||
| NG20-1 | 80 | 20 | - | 1 | 86 | 97 | - | 88 | 81 | 8.0 ± 0.4 | 8.4 ± 0.6 |
| NG10-1 | 90 | 10 | - | 1 | 82 | 93 | - | 83 | 74 | 4.7 ± 0.4 | 4.2 ± 0.5 |
| NG5-1 | 95 | 5 | - | 1 | 70 | 86 | - | 71 | 71 | 3.6 ± 0.4 | 3.3 ± 0.5 |
| NG20-tBAEMA-3 | 70 | 20 | 10 | 3 | 90 | 98 | 99 | 92 | 77 | 5.3 ± 0.3 | 8.5 ± 0.9 |
| NG10-tBAEMA-3 | 80 | 10 | 10 | 3 | 87 | 95 | 99 | 89 | 66 | 3.9 ± 0.2 | 4.8 ± 0.8 |
| NG5-tBAEMA-3 | 85 | 5 | 10 | 3 | 82 | 88 | 99 | 84 | 62 | 2.7 ± 0.3 | 4.3 ± 0.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bilardo, R.; Traldi, F.; Brennan, C.H.; Resmini, M. The Role of Crosslinker Content of Positively Charged NIPAM Nanogels on the In Vivo Toxicity in Zebrafish. Pharmaceutics 2023, 15, 1900. https://doi.org/10.3390/pharmaceutics15071900
Bilardo R, Traldi F, Brennan CH, Resmini M. The Role of Crosslinker Content of Positively Charged NIPAM Nanogels on the In Vivo Toxicity in Zebrafish. Pharmaceutics. 2023; 15(7):1900. https://doi.org/10.3390/pharmaceutics15071900
Chicago/Turabian StyleBilardo, Roberta, Federico Traldi, Caroline H. Brennan, and Marina Resmini. 2023. "The Role of Crosslinker Content of Positively Charged NIPAM Nanogels on the In Vivo Toxicity in Zebrafish" Pharmaceutics 15, no. 7: 1900. https://doi.org/10.3390/pharmaceutics15071900







