Tunable Drug Release Rate Using Modular Oral Dosage Forms
Abstract
:1. Introduction
2. Rationale
3. Materials and Methods
3.1. Solvent Casting of PGR-Loaded Fast-Release (F) Wafers
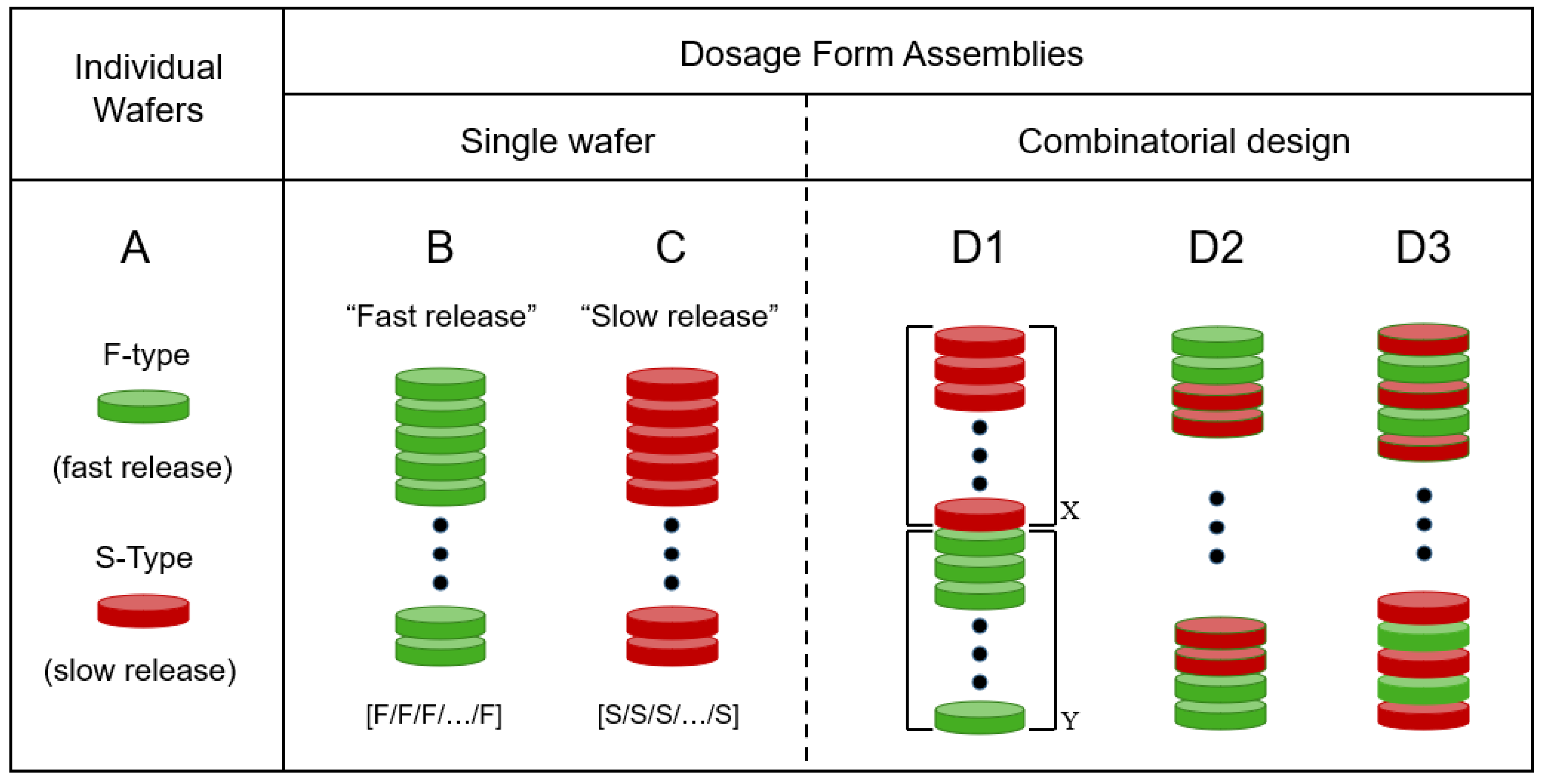
3.2. Preparation of 3D Assemblies
3.3. Drug Release from Individual Wafers and Wafer Assemblies
4. Results
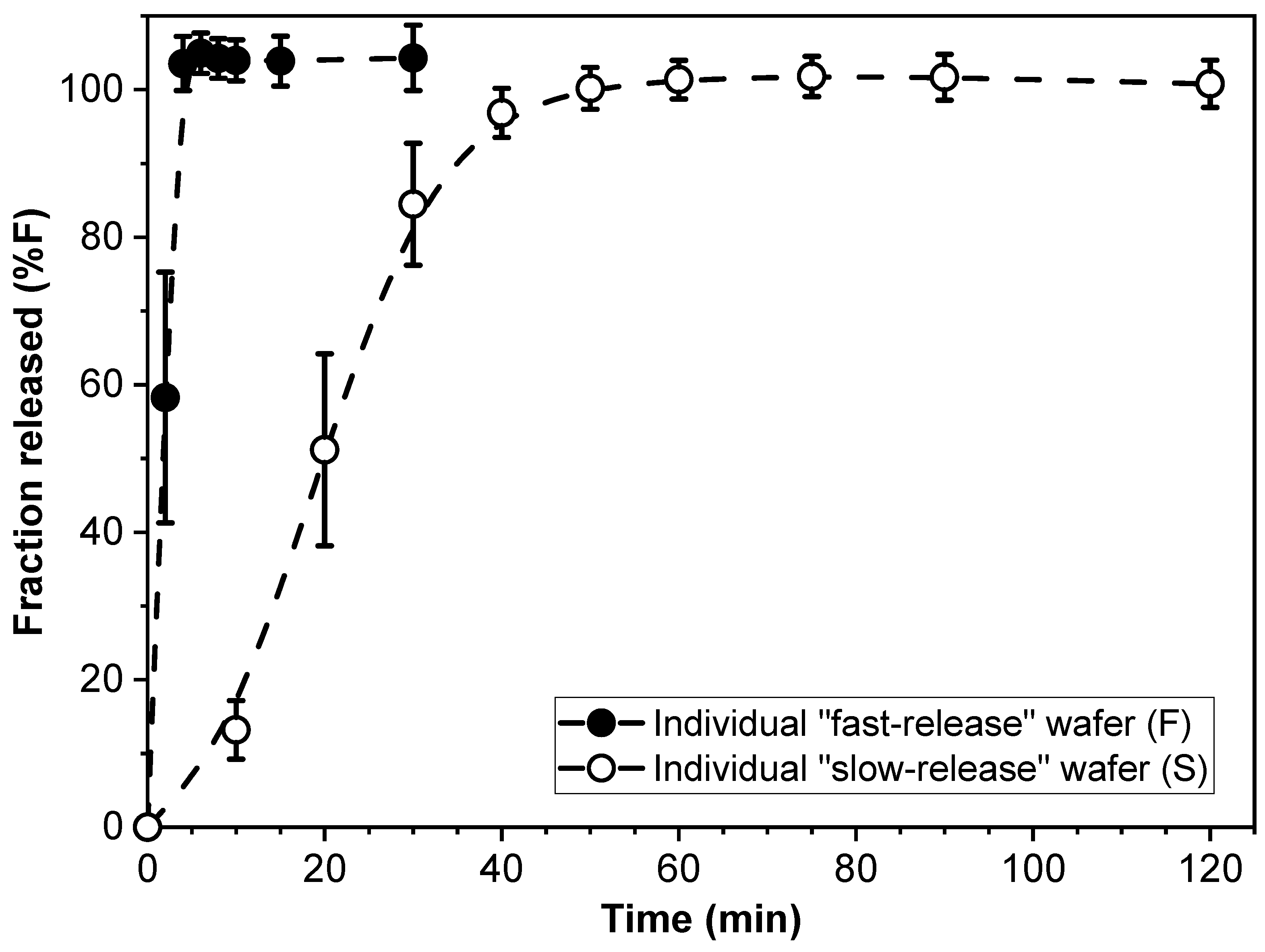
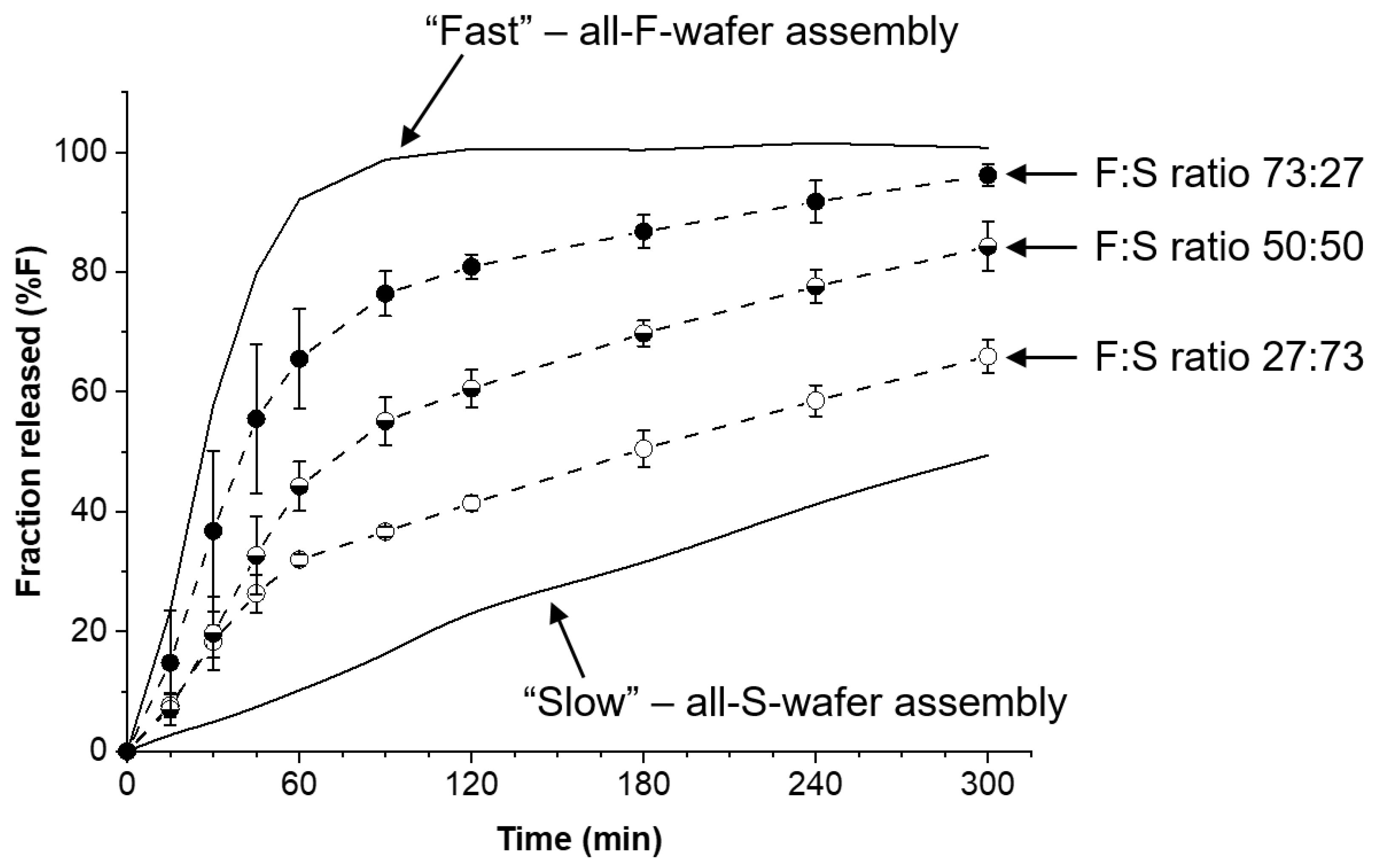
4.1. Drug Release from Individual Fast- (F) and Slow- (S) Release Modules
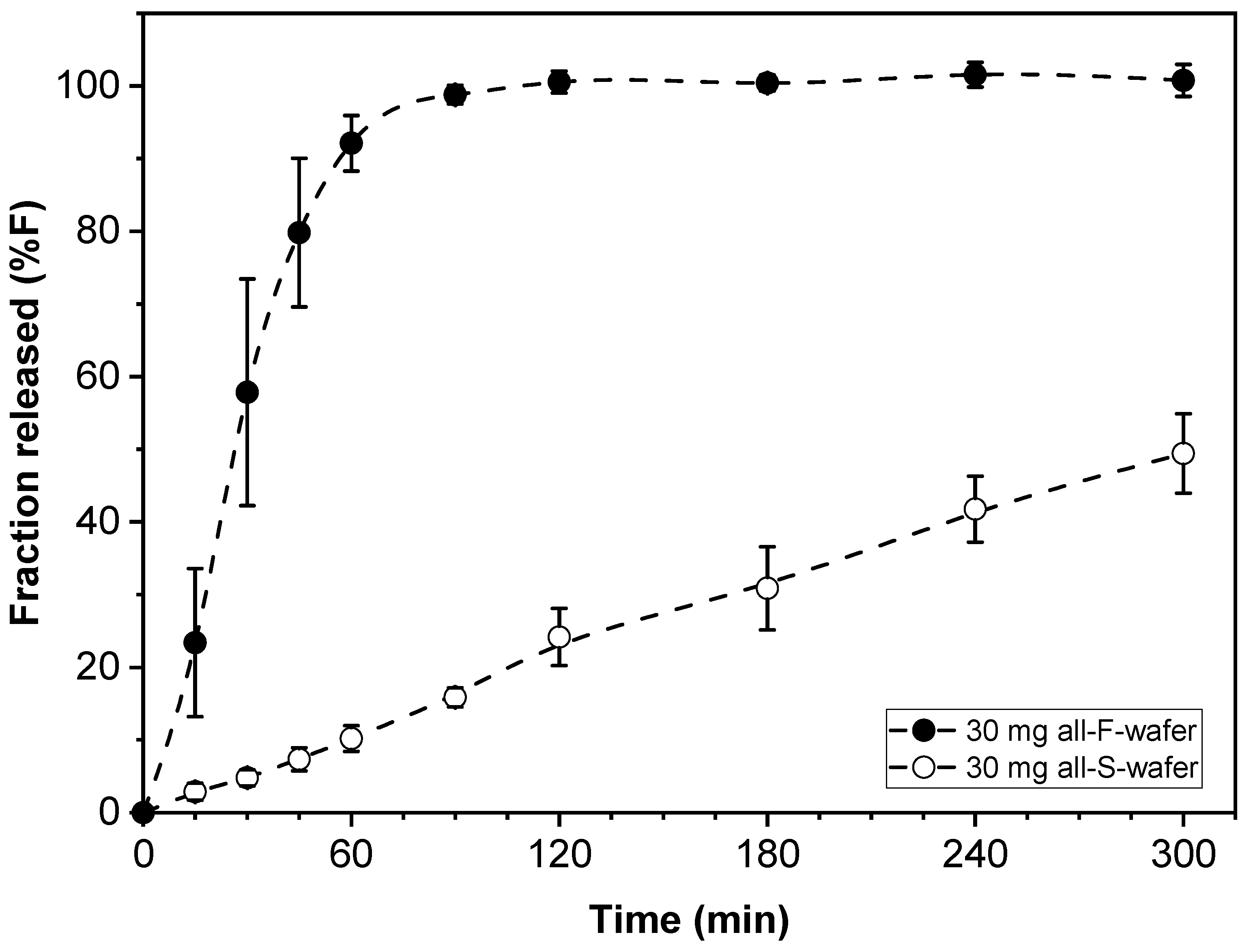
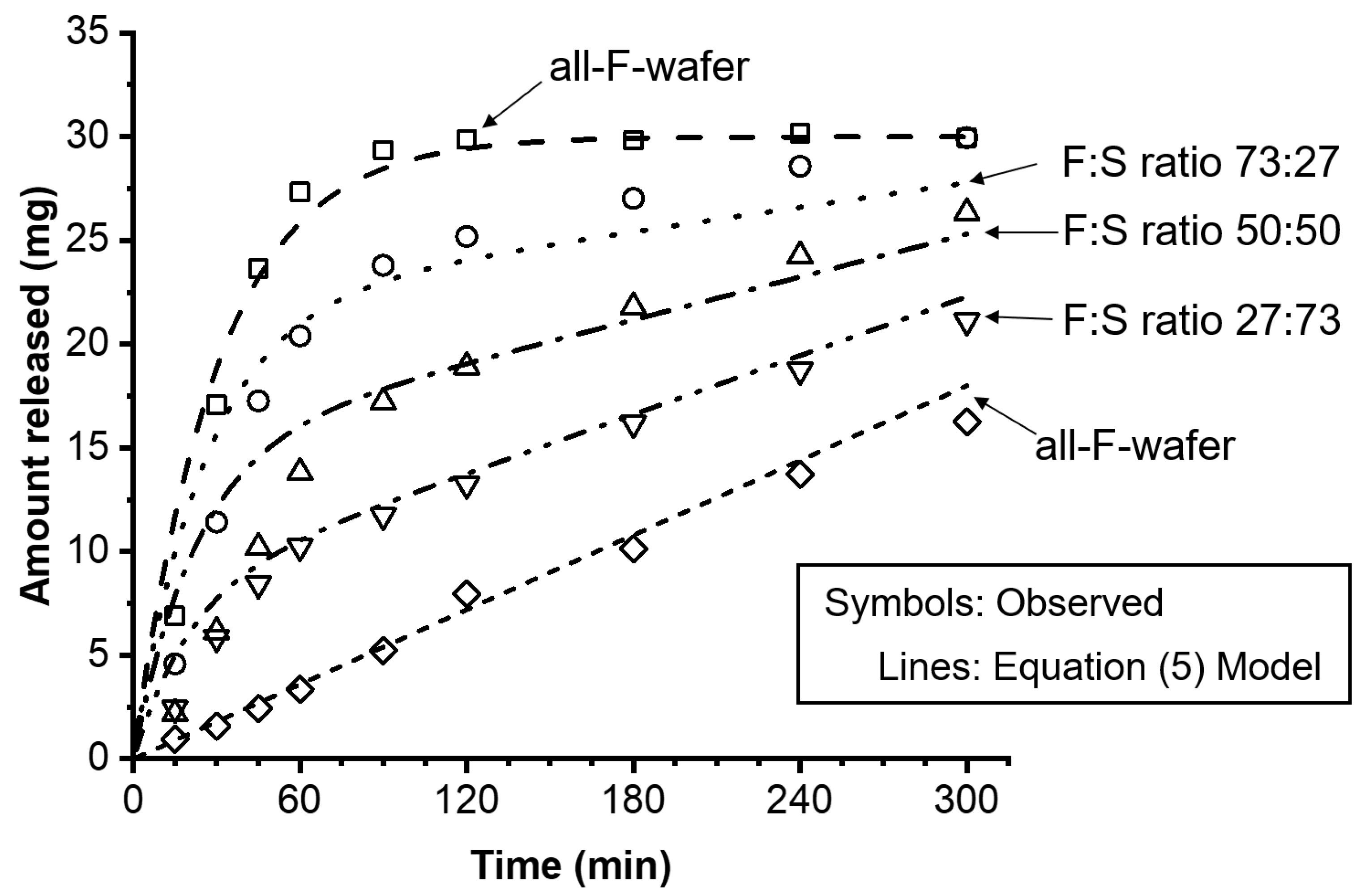
4.2. Total Drug Content in the Dosage Form
4.3. Dosage Forms with Fast and Slow Drug Release
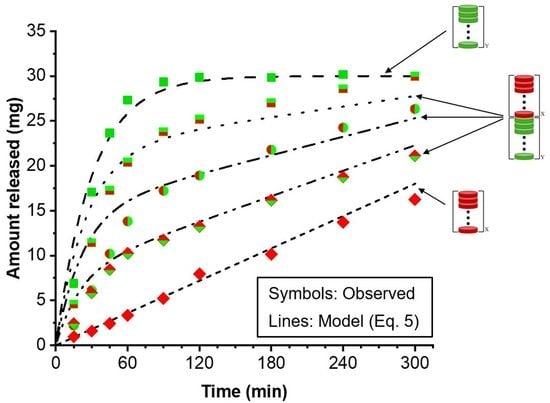
4.4. Customization of Drug Release Profile
4.5. Model for Drug Release Kinetics
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sharma, D.; Soni, M.; Kumar, S.; Gupta, G. Solubility Enhancement—Eminent Role in Poorly Soluble Drugs. Res. J. Pharm. Technol. 2009, 2, 220–224. [Google Scholar]
- A Thackaberry, E. Non-clinical toxicological considerations for pharmaceutical salt selection. Expert Opin. Drug Metab. Toxicol. 2012, 8, 1419–1433. [Google Scholar] [CrossRef] [PubMed]
- Brunaugh, A.D.; Smyth, H.D.C.; Williams, R.O. Essential Pharmaceutics; Springer: New York, NY, USA, 2019. [Google Scholar]
- Zhang, Y.F.; Zhang, H.F.; Yu, H.; Ma, Y.H.; Hao, C.Y.; Lin, X.Y.; Zhang, Y.; Li, Z.Q.; Qi, X.R.; Zeng, J.; et al. Hot-melt extrusion promotes dissolution, extends “spring-parachute” process and inhibits crystallization in supersaturating microparticle systems. Particuology 2023, 78, 35–48. [Google Scholar] [CrossRef]
- Boksa, K.; Otte, A.; Pinal, R. Matrix-Assisted Cocrystallization (MAC) Simultaneous Production and Formulation of Pharmaceutical Cocrystals by Hot-Melt Extrusion. J. Pharm. Sci. 2014, 103, 2904–2910. [Google Scholar] [CrossRef] [PubMed]
- Amidon, G.L.; Lennernäs, H.; Shah, V.P.; Crison, J.R. A Theoretical Basis for a Biopharmaceutic Drug Classification: The Correlation of in Vitro Drug Product Dissolution and in Vivo Bioavailability. Pharm. Res. 1995, 12, 413–420. [Google Scholar] [CrossRef] [Green Version]
- Cano-Vega, M.A.; Deng, M.; Pinal, R. Modular solid dosage form design—Application to pH-independent release of a weak-base API. Int. J. Pharm. 2021, 601, 120518. [Google Scholar] [CrossRef]
- Nagaraju, T.; Gowthami, R.; Rajashekar, M.; Sandeep, S.; Mallesham, M.; Sathish, D.; Kumar, Y.S. Comprehensive Review On Oral Disintegrating Films. Curr. Drug Deliv. 2013, 10, 96–108. [Google Scholar] [CrossRef]
- Guo, J.-H.; Zerbe, H. Water soluble film for oral administration. Proc. Int. Symp. Control. Rel. Bioact. Mater. 1997, 24, 227–228. [Google Scholar]
- Liang, A.C.; Chen, L.-L.H. Fast-dissolving intraoral drug delivery systems. Expert Opin. Ther. Patents 2001, 11, 981–986. [Google Scholar] [CrossRef]
- Borsadia, S.B.; O’Halloran, D.; Osborne, J.L. Oral film technology. Drug Deliv. 2003, 3, 63–66. [Google Scholar]
- Pharmaceuticals, S. FDA Approves Strativa Pharmaceuticals’ Zuplenz® (ondansetron) Oral Soluble Film. 2010. Available online: https://www.prnewswire.com/news-releases/fda-approves-strativa-pharmaceuticals-zuplenz-ondansetron-oral-soluble-film-97696804.html (accessed on 7 July 2023).
- Dixit, R.P.; Puthli, S.P. Oral strip technology: Overview and future potential. J. Control. Release 2009, 139, 94–107. [Google Scholar] [CrossRef] [PubMed]
- Borges, A.F.; Silva, C.; Coelho, J.F.J.; Simoes, S. Oral films: Current status and future perspectives I—Galenical development and quality attributes. J. Control. Rel. 2015, 206, 1–19. [Google Scholar] [CrossRef] [Green Version]
- Borges, A.F.; Silva, C.; Coelho, J.F.J.; Simoes, S. Oral films: Current status and future perspectives II—Intellectual property, technologies and market needs. J. Control. Rel. 2015, 206, 108–121. [Google Scholar] [CrossRef] [Green Version]
- Vinarov, Z.; Dobreva, P.; Tcholakova, S. Effect of surfactant molecular structure on Progesterone solubilization. J. Drug Deliv. Sci. Technol. 2018, 43, 44–49. [Google Scholar] [CrossRef]
- Ogihara, T.; Matsumoto, S.; Ohnishi, S. Functional characterization of active transport of progesterone to adrenal cells. J. Pharm. Pharmacol. 2004, 56, 79–84. [Google Scholar] [CrossRef] [PubMed]
- Talevi, A.; Bellera, C.L. Active and facilitated transport in drug absorption. In The ADME Encyclopedia: A Comprehensive Guide on Biopharmacy and Pharmacokinetics; Talevi, A., Ed.; Springer International Publishing: Cham, Switzerland, 2022; pp. 62–68. [Google Scholar]
- Campos-Aldrete, M.E.; Villafuerte-Robles, L. Influence of the viscosity grade and the particle size of HPMC on metronidazole release from matrix tablets. Eur. J. Pharm. Biopharm. 1997, 43, 173–178. [Google Scholar] [CrossRef]
- Zema, L.; Maroni, A.; Foppoli, A.; Palugan, L.; Sangalli, M.; Gazzaniga, A. Different HPMC Viscosity Grades as Coating Agents for an Oral Time and/or Site-Controlled Delivery System: An Investigation into the Mechanisms Governing Drug Release. J. Pharm. Sci. 2007, 96, 1527–1536. [Google Scholar] [CrossRef]
- Kumar, S.P.; Kumar, S.P.; Darwhekar, G.N.; Birendra, S. An overview about novel fast dissolving oral films. Int. J. Drug Reg. Aff. 2018, 6, 1–7. [Google Scholar]
- He, M.; Zhu, L.; Yang, N.; Li, H.; Yang, Q. Recent advances of oral film as platform for drug delivery. Int. J. Pharm. 2021, 604, 120759. [Google Scholar] [CrossRef]
- Speer, I.; Preis, M.; Breitkreutz, J. Prolonged drug release properties for orodispersible films by combining hot-melt extrusion and solvent casting methods. Eur. J. Pharm. Biopharm. 2018, 129, 66–73. [Google Scholar] [CrossRef]
- Speer, I.; Lenhart, V.; Preis, M.; Breitkreutz, J. Prolonged release from orodispersible films by incorporation of diclofenac-loaded micropellets. Int. J. Pharm. 2019, 554, 149–160. [Google Scholar] [CrossRef]
- Speer, I.; Preis, M.; Breitkreutz, J. Dissolution testing of oral film preparations: Experimental comparison of compendial and non-compendial methods. Int. J. Pharm. 2019, 561, 124–134. [Google Scholar] [CrossRef] [PubMed]
- Pepin, X.; Goetschy, M.; Abrahmsén-Alami, S. Mechanistic Models for USP2 Dissolution Apparatus, Including Fluid Hydrodynamics and Sedimentation. J. Pharm. Sci. 2022, 111, 185–196. [Google Scholar] [CrossRef]
- Sullivan, J.G.; Webster, L. Novel Buccal Film Formulation of Buprenorphine-Naloxone for the Maintenance Treatment of Opioid Dependence: A 12-Week Conversion Study. Clin. Ther. 2015, 37, 1064–1075. [Google Scholar] [CrossRef] [Green Version]
- Swetha, M.; Sirisolla, J.D. A Comprehensive Review on Orally Dissolving Film Drug Delivery System. J. Pharm. Neg. Resul. 2022, 13, 507–515. [Google Scholar]
- Mahboob, M.B.H.; Riaz, T.; Jamshaid, M.; Bashir, I.; Zulfiqar, S. Oral Films: A Comprehensive Review. Int. Curr. Pharm. J. 2016, 5, 111–117. [Google Scholar] [CrossRef] [Green Version]
- Rozo, J.I.J.; Zarow, A.; Zhou, B.; Pinal, R.; Iqbal, Z.; Romañach, R.J. Complementary Near-Infrared and Raman Chemical Imaging of Pharmaceutical Thin Films. J. Pharm. Sci. 2011, 100, 4888–4895. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Slipchenko, M.N.; Zhou, B.; Pinal, R.; Cheng, J.-X. Mechanisms of Epi-Detected Stimulated Raman Scattering Microscopy. IEEE J. Sel. Top. Quantum Electron. 2011, 18, 384–388. [Google Scholar] [CrossRef]
- Porwal, A.; Dwivedi, H.; Pathak, K. Gastroretentive bilayer film for sustained release of atorvastatin calcium and immediate release of amlodipine besylate: Pharmaceutical, pharmacokinetic evaluation, and IVIVC. Pharm. Dev. Technol. 2019, 25, 416–431. [Google Scholar] [CrossRef] [PubMed]
- Eleftheriadis, G.K.; Monou, P.K.; Bouropoulos, N.; Boetker, J.; Rantanen, J.; Jacobsen, J.; Vizirianakis, I.S.; Fatouros, D.G. Fabrication of Mucoadhesive Buccal Films for Local Administration of Ketoprofen and Lidocaine Hydrochloride by Combining Fused Deposition Modeling and Inkjet Printing. J. Pharm. Sci. 2020, 109, 2757–2766. [Google Scholar] [CrossRef]
- Preis, M.; Woertz, C.; Schneider, K.; Kukawka, J.; Broscheit, J.; Roewer, N.; Breitkreutz, J. Design and evaluation of bilayered buccal film preparations for local administration of lidocaine hydrochloride. Eur. J. Pharm. Biopharm. 2014, 86, 552–561. [Google Scholar] [CrossRef] [PubMed]
- Li, A.; Khan, I.N.; Khan, I.U.; Yousaf, A.M.; Shahzad, Y. Gellan Gum-Based Bilayer Mucoadhesive Films Loaded with Moxifloxacin Hydrochloride and Clove Oil for Possible Treatment of Periodontitis. Drug Des. Dev. Ther. 2021, 15, 3937–3952. [Google Scholar] [CrossRef]
- Preis, M.; Breitkreutz, J.; Sandler, N. Perspective: Concepts of printing technologies for oral film formulations. Int. J. Pharm. 2015, 494, 578–584. [Google Scholar] [CrossRef] [PubMed]
- Govender, R.; Abrahmsén-Alami, S.; Larsson, A.; Borde, A.; Liljeblad, A.; Folestad, S. Independent Tailoring of Dose and Drug Release via a Modularized Product Design Concept for Mass Customization. Pharmaceutics 2020, 12, 771. [Google Scholar] [CrossRef] [PubMed]
- Eleftheriadis, G.K.; Genina, N.; Boetker, J.; Rantanen, J. Modular design principle based on compartmental drug delivery systems q. Adv. Drug Deliv. Rev. 2021, 178, 113921. [Google Scholar] [CrossRef] [PubMed]
- Govender, R.; Abrahmsén-Alami, S.; Folestad, S.; Olsson, M.; Larsson, A. Enabling modular dosage form concepts for individualized multidrug therapy: Expanding the design window for poorly water-soluble drugs. Int. J. Pharm. 2021, 602, 120625. [Google Scholar] [CrossRef]


| Drug Loading | All-F-Wafer | All-S-Wafer | ||
|---|---|---|---|---|
| Parameter | Value | Parameter | Value | |
| 15 mg | k1 | 0.046 ± 0.008 | k0 | 0.229 ± 0.59 |
| R2 | 0.9246 | R2 | 0.9480 | |
| 30 mg | k1 | 0.032 ± 0.008 | k0 | 0.198 ± 0.014 |
| R2 | 0.9329 | R2 | 0.9755 | |
| 50 mg | k1 | 0.023 ± 0.002 | k0 | 0.171 ± 0.018 |
| R2 | 0.9346 | R2 | 0.9920 | |
| D0 (mg) | q (S-Section) | p (F-Section) | k0 (%·min−1) | k1 (min−1) | AFE | AAFE |
|---|---|---|---|---|---|---|
| 30 | 0.00 | 1.00 | N/A | 0.033 | 1.06 | 1.09 |
| 30 | 0.27 | 0.73 | 0.242 | 0.039 | 1.11 | 1.18 |
| 30 | 0.50 | 0.50 | 0.229 | 0.045 | 1.31 | 1.34 |
| 30 | 0.73 | 0.27 | 0.216 | 0.050 | 1.16 | 1.16 |
| 30 | 1.00 | 0.00 | 0.200 | N/A | 1.04 | 1.08 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cano-Vega, M.A.; Arango-Salazar, L.M.; Pinal, R. Tunable Drug Release Rate Using Modular Oral Dosage Forms. Pharmaceutics 2023, 15, 1905. https://doi.org/10.3390/pharmaceutics15071905
Cano-Vega MA, Arango-Salazar LM, Pinal R. Tunable Drug Release Rate Using Modular Oral Dosage Forms. Pharmaceutics. 2023; 15(7):1905. https://doi.org/10.3390/pharmaceutics15071905
Chicago/Turabian StyleCano-Vega, Mario A., Laura M. Arango-Salazar, and Rodolfo Pinal. 2023. "Tunable Drug Release Rate Using Modular Oral Dosage Forms" Pharmaceutics 15, no. 7: 1905. https://doi.org/10.3390/pharmaceutics15071905