Inherently Fluorescent Peanut-Shaped Polymersomes for Active Cargo Transportation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
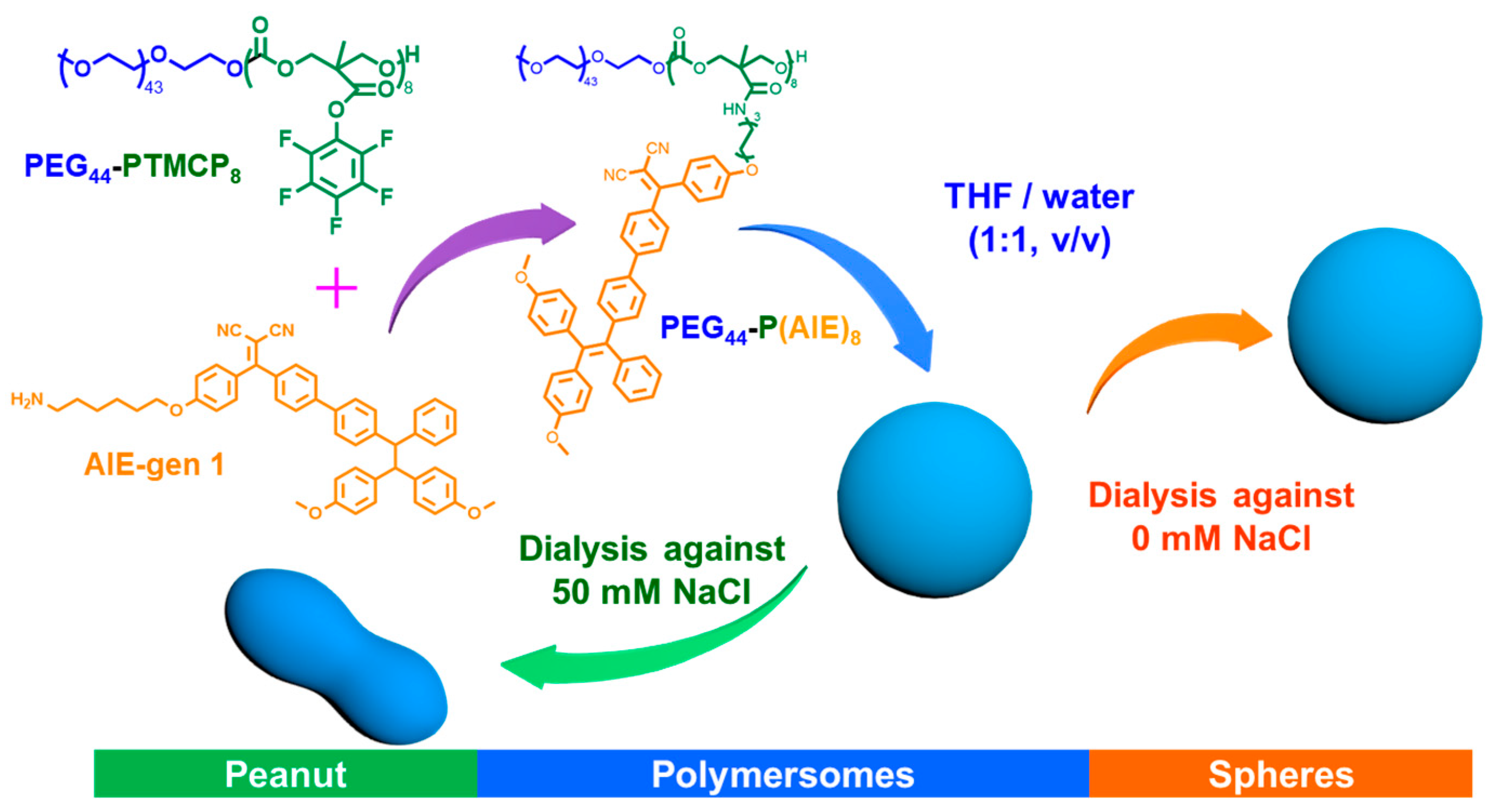
2.2. Synthesis of PEG44-P(AIE)8
2.3. Peanut Polymersome Preparation
2.4. Spherical AIE-polymersome Preparation
2.5. Singlet Oxygen (1O2) Detection
2.6. In Vitro Cell Experiments
3. Results and Discussion
3.1. Preparation and Characterization of Peanut Polymersomes
3.2. Autonomous Motion
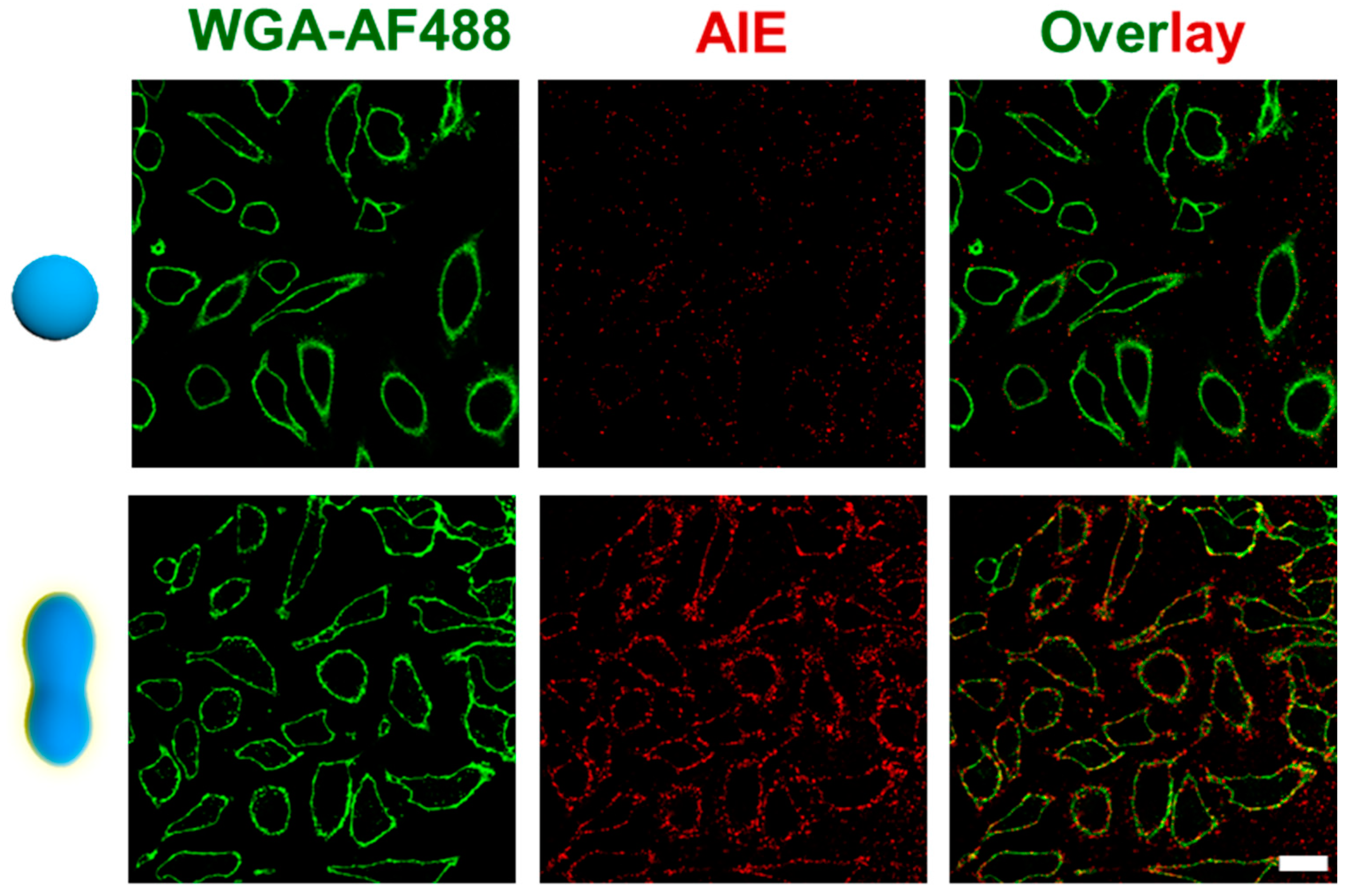
3.3. Peanut Polymersome-Meditated Cargo Transportation
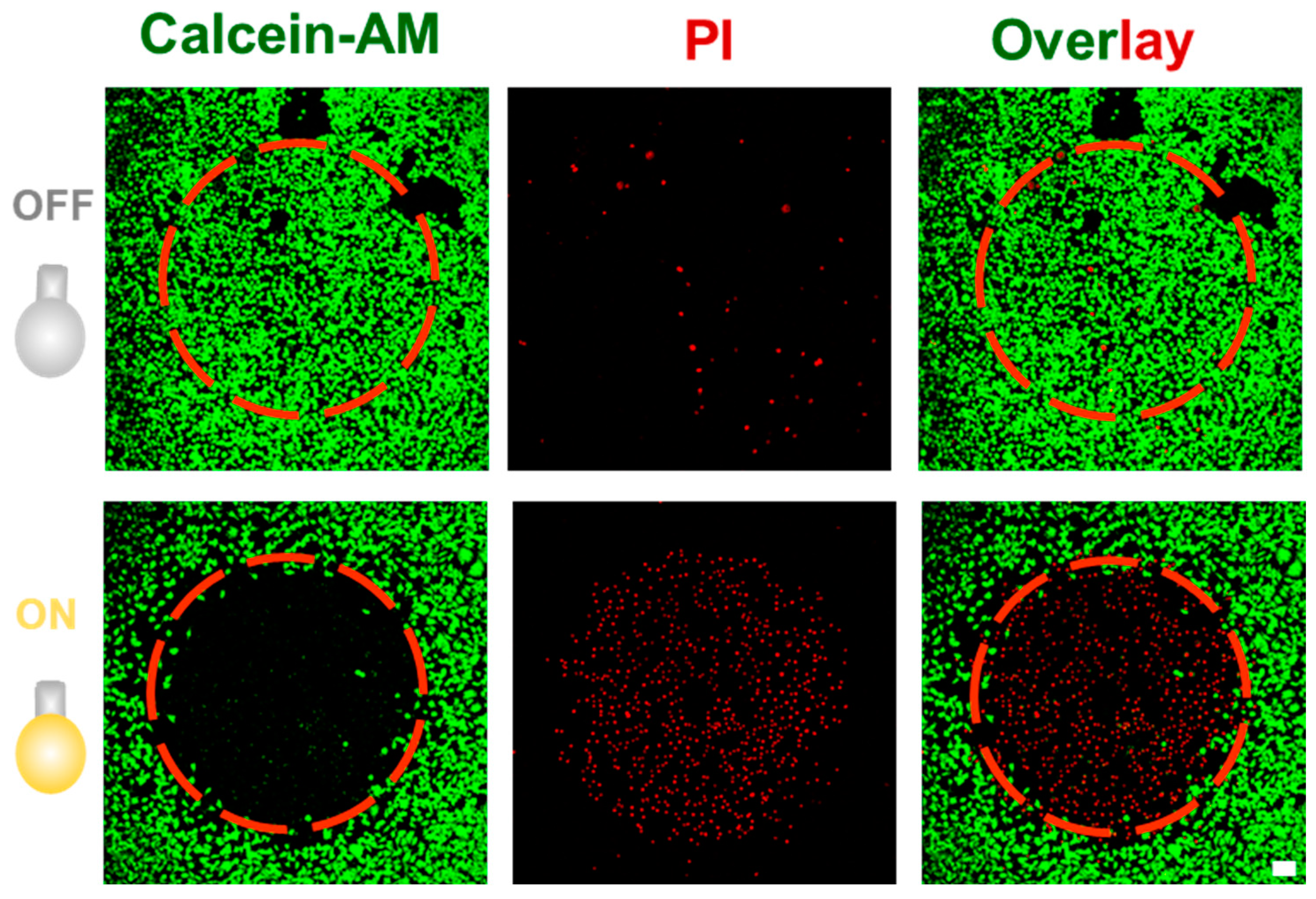
3.4. Photodynamic Therapy Using AIEgenic Peanut Polymersomes
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Somasundar, A.; Ghosh, S.; Mohajerani, F.; Massenburg, L.N.; Yang, T.; Cremer, P.S.; Velegol, D.; Sen, A. Positive and Negative Chemotaxis of Enzyme-Coated Liposome Motors. Nat. Nanotechnol. 2019, 14, 1129–1134. [Google Scholar] [CrossRef] [PubMed]
- Palagi, S.; Fischer, P. Bioinspired Microrobots. Nat. Rev. Mater. 2018, 3, 113–124. [Google Scholar] [CrossRef]
- Li, J.X.; de Ávila, B.E.; Gao, W.; Zhang, L.F.; Wang, J. Micro/Nanorobots for Biomedicine: Delivery, Surgery, Sensing, and Detoxification. Sci. Robot. 2017, 2, eaam6431. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.K.; Wu, Z.G.; Wu, Y.J.; Xuan, M.J.; He, Q. Self-Propelled Micro-/Nanomotors Based on Controlled Assembled Architectures. Adv. Mater. 2016, 28, 1060–1072. [Google Scholar] [CrossRef]
- Schattling, P.; Thingholm, B.; Städler, B. Enhanced Diffusion of Glucose-Fueled Janus Particles. Chem. Mater. 2015, 27, 7412–7418. [Google Scholar] [CrossRef]
- Dey, K.K.; Sen, A. Chemically Propelled Molecules and Machines. J. Am. Chem. Soc. 2017, 139, 7666–7676. [Google Scholar] [CrossRef]
- Pijpers, I.A.B.; Cao, S.P.; Llopis-Lorente, A.; Zhu, J.Z.; Song, S.D.; Joosten, R.R.M.; Meng, F.H.; Friedrich, H.; Williams, D.S.; Sánchez, S.; et al. Hybrid Biodegradable Nanomotors through Compartmentalized Synthesis. Nano Lett. 2020, 20, 4472–4480. [Google Scholar]
- Wang, W.; Duan, W.T.; Ahmed, S.; Mallouk, T.E.; Sen, A. Small Power: Autonomous Nano- and Micromotors Propelled by Self-Generated Gradients. Nano Today 2013, 8, 531–554. [Google Scholar] [CrossRef]
- Fusi, A.D.; Li, Y.D.; Llopis-Lorente, A.; Patiño, T.; van Hest, J.C.M.; Abdelmohsen, L.K.E.A. Achieving Control in Micro-/Nanomotor Mobility. Angew. Chem. Int. Ed. 2023, 62, e202214754. [Google Scholar] [CrossRef]
- Wang, J. Can Man-Made Nanomachines Compete with Nature Biomotors? ACS Nano 2009, 3, 4–9. [Google Scholar] [CrossRef]
- Wu, Y.J.; Si, T.Y.; Gao, C.Y.; Yang, M.C.; He, Q. Bubble-Pair Propelled Colloidal Kayaker. J. Am. Chem. Soc. 2018, 140, 11902–11905. [Google Scholar] [CrossRef] [PubMed]
- Shao, J.S.; Cao, S.P.; Williams, D.S.; Abdelmohsen, L.K.E.A.; van Hest, J.C.M. Photoactivated Polymersome Nanomotors: Traversing Biological Barriers. Angew. Chem. Int. Ed. 2020, 59, 16918–16925. [Google Scholar] [CrossRef] [PubMed]
- Kwon, T.; Kumari, N.; Kumar, A.; Lim, J.; Son, C.Y.; Lee, I.S. Au/Pt-Egg-in-Nest Nanomotor for Glucose-Powered Catalytic Motion and Enhanced Molecular Transport to Living Cells. Angew. Chem. Int. Ed. 2021, 60, 17579–17586. [Google Scholar]
- Gao, C.Y.; Wang, Y.; Ye, Z.H.; Lin, Z.H.; Ma, X.; He, Q. Biomedical Micro-/Nanomotors: From Overcoming Biological Barriers to In Vivo Imaging. Adv. Mater. 2021, 33, 2000512. [Google Scholar] [CrossRef]
- Li, Q.Q.; Liu, L.T.; Huo, H.Q.; Su, L.C.; Wu, Y.; Lin, H.X.; Ge, X.G.; Mu, J.; Zhang, X.; Zheng, L.T.; et al. Nanosized Janus AuNR-Pt Motor for Enhancing NIR-II Photoacoustic Imaging of Deep Tumor and Pt2+ Ion-Based Chemotherapy. ACS Nano 2022, 16, 7947–7960. [Google Scholar] [CrossRef]
- Xuan, M.J.; Wu, Z.G.; Shao, J.X.; Dai, L.R.; Si, T.Y.; He, Q. Near Infrared Light-Powered Janus Mesoporous Silica Nanoparticle Motors. J. Am. Chem. Soc. 2016, 138, 6492–6497. [Google Scholar] [CrossRef]
- Yang, P.P.; Zhai, Y.G.; Qi, G.B.; Lin, Y.X.; Luo, Q.; Yang, Y.; Xu, A.P.; Yang, C.; Li, Y.S.; Wang, L.; et al. NIR Light Propulsive Janus-Like Nanohybrids for Enhanced Photothermal Tumor Therapy. Small 2016, 12, 5423–5430. [Google Scholar] [CrossRef]
- Safdar, M.; Khan, S.U.; Jänis, J. Progress toward Catalytic Micro- and Nanomotors for Biomedical and Environmental Applications. Adv. Mater. 2018, 30, 1703660. [Google Scholar] [CrossRef] [Green Version]
- Ou, J.F.; Liu, K.; Jiang, J.M.; Wilson, D.A.; Liu, L.; Wang, F.; Wang, S.H.; Tu, Y.F.; Peng, F. Micro-/Nanomotors toward Biomedical Applications: The Recent Progress in Biocompatibility. Small 2020, 16, 1906184. [Google Scholar] [CrossRef]
- Chen, H.; Shi, T.; Wang, Y.; Liu, Z.Y.; Liu, F.C.; Zhang, H.Y.; Wang, X.W.; Miao, Z.Y.; Liu, B.R.; Wan, M.M.; et al. Deep Penetration of Nanolevel Drugs and Micrometer-Level T Cells Promoted by Nanomotors for Cancer Immunochemotherapy. J. Am. Chem. Soc. 2021, 143, 12025–12037. [Google Scholar] [CrossRef]
- Wang, S.H.; Xu, J.; Zhou, Q.; Geng, P.W.; Wang, B.; Zhou, Y.F.; Liu, K.; Peng, F.; Tu, Y.F. Biodegradability of Micro/Nanomotors: Challenges and Opportunities. Adv. Healthc. Mater. 2021, 10, 2100335. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.K.; Laos, A.J.; Soeriyadi, A.H.; Wiedenmann, J.; Curmi, P.M.G.; Gooding, J.J.; Marquis, C.P.; Stenzel, M.H.; Thordarson, P. Polymersomes Prepared from Thermoresponsive Fluorescent Protein- Polymer Bioconjugates: Capture of and Report on Drug and Protein Payloads. Angew. Chem. Int. Ed. 2015, 54, 5317–5322. [Google Scholar] [CrossRef] [PubMed]
- Wauters, A.C.; Pijpers, I.A.B.; Mason, A.F.; Williams, D.S.; Tel, J.; Abdelmohsen, L.K.E.A.; van Hest, J.C.M. Development of Morphologically Discrete PEG-PDLLA Nanotubes for Precision Nanomedicine. Biomacromolecules 2019, 20, 177–183. [Google Scholar] [CrossRef]
- Chen, M.J.; Yin, M.Z. Design and Development of Fluorescent Nanostructures for Bioimaging. Prog. Polym. Sci. 2014, 39, 365–395. [Google Scholar] [CrossRef]
- Hu, R.R.; Leung, N.L.C.; Tang, B.Z. AIE Macromolecules: Syntheses, Structures and Functionalities. Chem. Soc. Rev. 2014, 43, 4494–4562. [Google Scholar] [CrossRef] [PubMed]
- Mei, J.; Hong, Y.N.; Lam, J.W.Y.; Qin, A.J.; Tang, Y.H.; Tang, B.Z. Aggregation-Induced Emission: The Whole Is More Brilliant than the Parts. Adv. Mater. 2014, 26, 5429–5479. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Chen, H.; Fan, Y.J.; Zhou, L.; Trépout, S.; Guo, J.; Li, M.H. Fluorescent Polymersomes with Aggregation-Induced Emission. ACS Nano 2018, 12, 4025–4035. [Google Scholar] [CrossRef]
- Rideau, E.; Dimova, R.; Schwille, P.; Wurm, F.R.; Landfester, K. Liposomes and Polymersomes: A Comparative Review Towards Cell Mimicking. Chem. Soc. Rev. 2018, 47, 8572–8610. [Google Scholar] [CrossRef] [Green Version]
- Cao, S.P.; Shao, J.S.; Wu, H.L.; Song, S.D.; De Martino, M.T.; Pijpers, I.A.B.; Friedrich, H.; Abdelmohsen, L.K.E.A.; Williams, D.S.; van Hest, J.C.M. Photoactivated Nanomotors via Aggregation Induced Emission for Enhanced Phototherapy. Nat. Commun. 2021, 12, 2077. [Google Scholar] [CrossRef]
- Shao, J.S.; Cao, S.P.; Wu, H.L.; Abdelmohsen, L.K.E.A.; van Hest, J.C.M. Therapeutic Stomatocytes with Aggregation Induced Emission for Intracellular Delivery. Pharmaceutics 2021, 13, 1833. [Google Scholar] [CrossRef]
- Cao, S.P.; Wu, H.L.; Pijpers, I.A.B.; Shao, J.S.; Abdelmohsen, L.K.E.A.; Williams, D.S.; van Hest, J.C.M. Cucurbit-Like Polymersomes with Aggregation-Induced Emission Properties Show enzyme-Mediated Motility. ACS Nano 2021, 15, 18270–18278. [Google Scholar] [CrossRef] [PubMed]
- Mei, J.; Leung, N.L.C.; Kwok, R.T.K.; Lam, J.W.Y.; Tang, B.Z. Aggregation-Induced Emission: Together We Shine, United We Soar! Chem. Rev. 2015, 115, 11718–11940. [Google Scholar] [CrossRef] [PubMed]
- Hu, F.; Xu, S.D.; Liu, B. Photosensitizers with Aggregation-Induced Emission: Materials and Biomedical Applications. Adv. Mater. 2018, 30, 1801350. [Google Scholar] [CrossRef]
- Wang, J.H.; Li, D.; Fan, Y.; Shi, M.H.; Yang, Y.X.; Wang, L.; Peng, Y.T.; Shen, M.W.; Shi, X.Y. Core-Shell Tecto Dendrimers Formed via Host-Guest Supramolecular Assembly as pH-Responsive Intelligent Carriers for Enhanced Anticancer Drug Delivery. Nanoscale 2019, 11, 22343–22350. [Google Scholar] [CrossRef] [PubMed]
- Ji, X.; Yang, H.Y.; Liu, W.; Ma, Y.D.; Wu, J.P.; Zong, X.Q.; Yuan, P.F.; Chen, X.J.; Yang, C.Q.; Li, X.D.; et al. Multifunctional Parachute-like Nanomotors for Enhanced Skin Penetration and Synergistic Antifungal Therapy. ACS Nano 2021, 15, 14218–14228. [Google Scholar] [CrossRef]
- Liu, T.Y.; Xie, L.; Price, C.H.; Liu, J.; He, Q.; Kong, B. Controlled Propulsion of Micro/Nanomotors: Operational Mechanisms, Motion Manipulation and Potential Biomedical Applications. Chem. Soc. Rev. 2022, 51, 10083–10119. [Google Scholar] [CrossRef]
- Che, H.L.; Zhu, J.Z.; Song, S.D.; Mason, A.F.; Cao, S.P.; Pijpers, I.A.B.; Abdelmohsen, L.K.E.A.; van Hest, J.C.M. ATP-Mediated Transient Behavior of Stomatocyte Nanosystems. Angew. Chem. Int. Ed. 2019, 58, 13113–13118. [Google Scholar] [CrossRef]
- Wilson, D.A.; Nolte, R.J.M.; van Hest, J.C.M. Autonomous Movement of Platinum-Loaded Stomatocytes. Nat. Chem. 2012, 4, 268–274. [Google Scholar] [CrossRef]
- Abdelmohsen, L.K.E.A.; Nijemeisland, M.; Pawar, G.M.; Janssen, G.A.; Nolte, R.J.M.; van Hest, J.C.M.; Wilson, D.A. Dynamic Loading and Unloading of Proteins in Polymeric Stomatocytes: Formation of an Enzyme-Loaded Supramolecular Nanomotor. ACS Nano 2016, 10, 2652–2660. [Google Scholar] [CrossRef] [Green Version]
- Camelio, A.M.; Wright, R.J.; Knight, N.T.; Jazdzewski, B.A. Ir-Catalyzed C-H Amidation and Borylation of Anthraquinones. J. Org. Chem. 2019, 84, 4940–4947. [Google Scholar] [CrossRef]
- Du, X.B.; Qi, J.; Zhang, Z.Q.; Ma, D.G.; Wang, Z.Y. Efficient Non-doped Near Infrared Organic Light-Emitting Devices Based on Fluorophores with Aggregation-Induced Emission Enhancement. Chem. Mater. 2012, 24, 2178–2185. [Google Scholar] [CrossRef]
- Gu, B.B.; Wu, W.B.; Xu, G.X.; Feng, G.X.; Yin, F.; Chong, P.H.J.; Qu, J.L.; Yong, K.T.; Liu, B. Precise Two-Photon Photodynamic Therapy using an Efficient Photosensitizer with Aggregation-Induced Emission Characteristics. Adv. Mater. 2017, 29, 1701076. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Lee, S.; Christensen, J.M.; Plotnikov, N.V.; Burgess, M.; Martínez, T.J.; Dlott, D.D.; Moore, J.S. Pressure-Induced Neutral-to-Ionic Transition in an Amorphous Organic Material. Chem. Mater. 2016, 28, 6446–6449. [Google Scholar] [CrossRef]
- Cao, S.P.; Abdelmoshen, L.K.E.A.; Shao, J.X.; van den Dikkenberg, J.; Mastrobattista, E.; Williams, D.S.; van Hest, J.C.M. pH-Induced Transformation of Biodegradable Multilamellar Nanovectors for Enhanced Tumor Penetration. ACS Macro Lett. 2018, 7, 1394–1399. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Luo, Y.; Wu, H.; Cao, S.; Abdelmohsen, L.K.E.A.; Shao, J.; van Hest, J.C.M. Inherently Fluorescent Peanut-Shaped Polymersomes for Active Cargo Transportation. Pharmaceutics 2023, 15, 1986. https://doi.org/10.3390/pharmaceutics15071986
Wang J, Luo Y, Wu H, Cao S, Abdelmohsen LKEA, Shao J, van Hest JCM. Inherently Fluorescent Peanut-Shaped Polymersomes for Active Cargo Transportation. Pharmaceutics. 2023; 15(7):1986. https://doi.org/10.3390/pharmaceutics15071986
Chicago/Turabian StyleWang, Jianhong, Yingtong Luo, Hanglong Wu, Shoupeng Cao, Loai K. E. A. Abdelmohsen, Jingxin Shao, and Jan C. M. van Hest. 2023. "Inherently Fluorescent Peanut-Shaped Polymersomes for Active Cargo Transportation" Pharmaceutics 15, no. 7: 1986. https://doi.org/10.3390/pharmaceutics15071986





