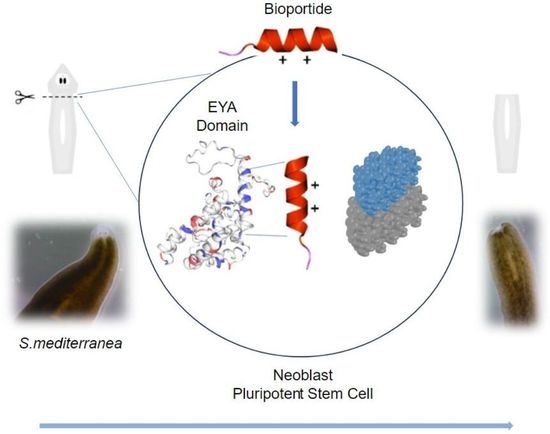
Stem Cell Bioengineering with Bioportides: Inhibition of Planarian Head Regeneration with Peptide Mimetics of Eyes Absent Proteins
Abstract
:1. Introduction
2. Materials and Methods
2.1. Peptide Synthesis and Secondary Structure Analysis
2.1.1. Microwave Enhanced Solid Phase Peptide Synthesis
2.1.2. Circular Dichroism
2.2. Schmidtea mediterranea Culture and Head Morphogenesis
2.2.1. Planarian Maintenance
2.2.2. Anterior Pole Regeneration and Eye Development Assay
2.3. Biological Characterization of Aib-Substituted Djeya1 Analogues
2.3.1. Cell Culture Maintenance
2.3.2. Qualitative Peptide Uptake Analyses
2.3.3. Quantitative Peptide Uptake Analyses
2.3.4. Cytotoxicity Assays
2.3.5. Cellular Proliferation
2.3.6. Cell Migration Assays
2.4. Graphical Representations and Statistical Analyses
3. Results
3.1. Site-Directed Bioengineering of the Djeya1 CPP
3.2. CD Spectral Analyses of Peptide α-Helicity

3.3. Exogenous Application of [Aib13]Djeya1 and [Aib16]Djeya1 Delays Anterior Pole Regeneration and Eye Development in S. mediterranea
3.4. Aib-Substituted Djeya1 Analogues Are Efficient CPPs
3.4.1. Qualitative Peptide Uptake Analyses
3.4.2. Quantitative Peptide Uptake Analyses
3.5. Cytotoxicity of Djeya1 Analogues
3.6. Impact of Djeya1 Analogues upon U251 Cellular Proliferation
3.7. Aib-Substituted Djeya1 Analogues Enhance Cell Migration
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kurrikoff, K.; Vunk, B.; Langel, Ü. Status update in the use of cell-penetrating peptides for the delivery of macromolecular therapeutics. Expert Opin. Biol. Ther. 2021, 21, 361–370. [Google Scholar] [CrossRef]
- Guidotti, G.; Brambilla, L.; Rossi, D. Cell-penetrating peptides: From basic research to clinics. Trends Pharmacol. Sci. 2017, 38, 406–424. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Zhang, H.; Dong, S.; Teng, L.; Lee, R.J.; Yang, Z. Cell-penetrating peptides in diagnosis and treatment of human diseases: From preclinical research to clinical application. Front. Pharmacol. 2020, 11, 697. [Google Scholar] [CrossRef] [PubMed]
- Howl, J.; Matou-Nasri, S.; West, D.S.; Farquhar, M.; Slaninová, J.; Östenson, C.-G.; Zorko, M.; Östlund, P.; Kumar, S.; Langel, Ü.; et al. Bioportide: An emergent concept of bioactive cell penetrating peptides. Cell. Mol. Life Sci. 2012, 69, 2951–2966. [Google Scholar] [CrossRef]
- Howl, J.; Jones, S. Insights into the molecular mechanisms of action of bioportides: A strategy to target protein-protein interactions. Expert Rev. Mol. Med. 2015, 17, e1. [Google Scholar] [CrossRef]
- Silva, J.V.; Freitas, M.J.; Santiago, J.; Jones, S.; Guimarães, S.; Vijayaraghavan, S.; Publicover, S.; Colombo, G.; Howl, J.; Fardilha, M. Disruption of protein phosphatase 1 complexes with the use of bioportides as a novel approach to target sperm motility. Fertil. Steril. 2021, 115, 348–362. [Google Scholar] [CrossRef]
- Aboobaker, A.A. Planarian stem cells: A simple paradigm for regeneration. Trends Cell. Biol. 2011, 21, 304–311. [Google Scholar] [CrossRef] [PubMed]
- Owlarn, S.; Bartscherer, K. Go ahead, grow a head! A planarian’s guide to anterior regeneration. Regeneration 2016, 24, 139–155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robb, S.M.C.; Gotting, K.; Ross, E.; Sánchez Alvarado, A. SmedGD 2.0: The Schmidtea mediterranea genome database. Genesis 2015, 53, 535–546. [Google Scholar] [CrossRef] [Green Version]
- Sánchez Alvarado, A.; Newmark, P.A.; Robb, S.M.; Juste, R. The Schmidtea mediterranea database as a molecular resource for studying platyhelminthes, stem cells and regeneration. Development 2002, 129, 5659–5665. [Google Scholar] [CrossRef] [Green Version]
- Jones, S.; Osman, S.; Howl, J. The planarian Schmidtea mediterranea as a model system for the discovery and characterization of cell penetrating peptides and bioportides. Chem. Biol. Drug Des. 2019, 93, 1036–1049. [Google Scholar] [CrossRef]
- Futaki, S.; Suzuki, T.; Ohashi, W.; Yagami, T.; Tanaka, S.; Ueda, K.; Sugiura, Y. Arginine-rich peptides. An abundant source of membrane-permeable peptides having potential as carriers for intracellular protein delivery. J. Biol. Chem. 2001, 276, 5836–5840. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mannini, L.; Rossi, L.; Deri, P.; Gremigni, V.; Salvetti, A.; Saló, E.; Batistoni, R. Djeyes absent (Djeya) controls prototypic planarian eye regeneration by cooperating with the transcription factor Djsix-1. Dev. Biol. 2004, 269, 346–359. [Google Scholar] [CrossRef] [PubMed]
- Rayapureddi, J.P.; Kattamuri, C.; Steinmetz, B.D.; Frankfort, B.J.; Ostrin, E.J.; Mardon, G.; Hedge, R.S. Eyes absent represents a class of protein tyrosine phosphatases. Nature 2003, 426, 295–298. [Google Scholar] [CrossRef]
- Hedge, R.S.; Roychoudhury, K.; Pandey, R.M. The multi-functional eyes absent proteins. Crit. Rev. Biochem. Mol. Biol. 2020, 55, 372–385. [Google Scholar] [CrossRef]
- Eisenhoffer, G.T.; Kang, H.; Sánchez Alvarado, A. Molecular analysis of stem cells and their descendants during cell turnover and regeneration in the planarian Schmidtea mediterranea. Cell Stem Cell. 2008, 3, 327–339. [Google Scholar] [CrossRef] [Green Version]
- Baguñà, J. The planarian neoblast: The rambling history of its origin and some current black boxes. Int. J. Dev. Biol. 2012, 56, 19–37. [Google Scholar] [CrossRef] [Green Version]
- Sánchez Alvarado, A. Stem cells and the planarian Schmidtea mediterranea. Comptes Rendus Biol. 2007, 330, 498–503. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, P.X.; Zheng, W.; Luclef, C.; Maire, P.; Maas, R.L.; Peters, H.; Xu, X. Eya1 is required for the morphogenesis of mammalian thymus. Development 2002, 129, 3033–3034. [Google Scholar] [CrossRef]
- Jones, S.; Osman, S.; Howl, J. A high-throughput synthetic platform enables the discovery of proteomimetic cell penetrating peptides and bioportides. Int. J. Pept. Res. Ther. 2018, 25, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Subirs-Fumosas, R.; Prohens, R.; Barbas, R.; El-Faham, A.; Albericio, F. Oxyma: An efficient additive for peptide synthesis to replace the benzotrialzole-based HOBt and HOAt with a lower risk of explosion. Chemistry 2009, 15, 9394–9403. [Google Scholar] [CrossRef] [PubMed]
- Jones, S.; Uusna, J.; Langel, Ü.; Howl, J. Intracellular target-specific accretion of cell penetrating peptides and bioportides: Ultrastructural and biological correlates. Bioconjugate Chem. 2016, 27, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Kelly, S.M.; Jess, T.J.; Price, N.C. How to study proteins by circular dichroism. Biochim. Biophys. Acta 2005, 1751, 119–139. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, F.; Forster, L.S.; Pal, P.K.; Rupley, J.A. The circular dichroism of lysozyme. J. Biol. Chem. 1975, 250, 6977–6982. [Google Scholar] [CrossRef]
- Johnson, M.P.; Ruban, A.V. Rethinking the existence of a steady-state Δψ component of the proton motive force across plant thylakoid membranes. Photosynth. Res. 2014, 119, 233–242. [Google Scholar] [CrossRef] [Green Version]
- Cebriá, F.; Newmark, P.A. Planarian homologs of netrin and netrin receptor are required for proper regeneration of the central nervous system and the maintenance of nervous system architecture. Development 2005, 132, 3691–3703. [Google Scholar] [CrossRef] [Green Version]
- Howl, J.; Howl, L.; Jones, S. The cationic tetradecapeptide mastoparan as a privileged structure for drug discovery: Enhanced antimicrobial properties of mitoparan analogues modified at position-14. Peptides 2018, 101, 95–105. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.; Zhao, L.; Song, Y. Eya2 is overexpressed in human prostate cancer and regulates docetaxel sensitivity and mitochondrial membrane potential through AKT/Bcl-2 signaling. BioMed. Res. Int. 2019, 2019, 3808432. [Google Scholar] [CrossRef] [Green Version]
- Wen, Z.; Liang, C.; Pan, Q.; Wang, Y. Eya2 overexpression promotes the invasion of human astrocytoma through the regulation of ERK/MMP9 signaling. Int. J. Mol. Med. 2017, 40, 1315–1322. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Qiu, R.; Qiu, X.; Tian, T. EYA4 promotes cell proliferation through downregulation of p27Kip1 in glioma. Cell. Physiol. Biochem. 2018, 49, 1856–1869. [Google Scholar] [CrossRef]
- Jones, S.; Martel, C.; Belzacq-Casagrande, A.S.; Brenner, C.; Howl, J. Mitoparan and target-selective chimeric analogues: Membrane translocation and intracellular redistribution induces mitochondrial apoptosis. Biochim. Biophys. Acta 2008, 1783, 849–863. [Google Scholar] [CrossRef]
- Carmichael, J.; DeGraff, W.G.; Gazdar, A.F.; Minna, J.D.; Mitchell, J.B. Evaluation of a tetrazolium-based semiautomated colorimetric assay: Assessment of chemosensitivity testing. Cancer Res. 1987, 47, 936–942. [Google Scholar]
- Tadjuidje, E.; Hedge, R.S. The eyes absent proteins in development and disease. Cell. Mol. Life Sci. 2013, 70, 1897–1913. [Google Scholar] [CrossRef] [Green Version]
- Jung, S.-K.; Jeong, D.G.; Chung, S.J.; Kim, J.H.; Park, B.C.; Tonks, N.K.; Ryu, S.E.; Kim, S.J. Crystal structure of ED-Eya2: Insight into dual roles as a protein tyrosine phosphatase and a transcription factor. FASEB J. 2010, 24, 560–569. [Google Scholar] [CrossRef] [Green Version]
- Seelig, J. Thermodynamics of lipid–peptide interactions. Biochim. Biophys. Acta 2004, 1666, 40–50. [Google Scholar] [CrossRef] [Green Version]
- Luo, P.Z.; Baldwin, R.L. Mechanism of helix induction by trifluoroethanol: A framework for extrapolating the helix-forming properties of peptides from trifluoroethanol/water mixtures back to water. Biochemistry 1997, 36, 8413–8421. [Google Scholar] [CrossRef]
- Cammers-Goodwin, A.; Allen, T.J.; Oslick, S.L.; McClure, K.F.; Lee, J.H.; Kemp, D.S. Mechanism of stabilization of helical conformations of polypeptides by water containing trifluoroethanol. J. Am. Chem. Soc. 1996, 118, 3082–3090. [Google Scholar] [CrossRef]
- Greenfield, N.J. Using circular dichroism spectra to estimate protein secondary structure. Nat. Protoc. 2006, 1, 2876–2890. [Google Scholar] [CrossRef]
- Pelletieri, J.; Sánchez Alvarado, A. Cell turnover and adult tissue homeostasis: From humans to planarians. Ann. Rev. Genet. 2007, 41, 83–105. [Google Scholar] [CrossRef]
- Bohr, T.E.; Shiroor, D.A.; Adler, C.E. Planarian stem cells sense the identity of the missing pharynx to launch its targeted regeneration. eLife 2021, 10, e68830. [Google Scholar] [CrossRef]
- Abnave, P.; Aboukhatwa, E.; Kosaka, N.; Thompson, J.; Hill, M.A.; Aboobaker, A.A. Epithelial-mesenchymal transition transcription factors control pluripotent adult stem cell migration in vivo in planarians. Development 2017, 144, 3440–3453. [Google Scholar] [CrossRef] [Green Version]
- Lukanowska, M.; Howl, J.; Jones, S. Bioportides: Bioactive cell-penetrating peptides that modulate cellular dynamics. Biotechnol. J. 2013, 8, 918–930. [Google Scholar] [CrossRef]
- Zorko, M.; Langel, Ü. Studies of cell-penetrating peptides by biophysical methods. Q. Rev. Biophys. 2022, 55, e3. [Google Scholar] [CrossRef]
- Hadjicharalambous, A.; Bournakas, N.; Newman, H.; Skynner, M.J.; Beswick, P. Antimicrobial and Cell-Penetrating Peptides: Understanding Penetration for the Design of Novel Conjugate Antibiotics. Antibiotics 2022, 11, 1636. [Google Scholar] [CrossRef]
- Infield, D.T.; Rasouli, A.; Galles, G.D.; Chipot, C.; Tajkhorshid, E.; Ahern, C.A. Cation-π interactions and their functional roles in membrane proteins. J. Mol. Biol. 2021, 433, 167035. [Google Scholar] [CrossRef]
- Buller, C.; Xu, X.; Marquis, V.; Schwanke, R.; Xu, P.-X. Molecular effects of Eya1 domain mutations causing organ defects in BOR syndrome. Hum. Mol. Genet. 2001, 10, 2775–2781. [Google Scholar] [CrossRef] [Green Version]
- Lapan, S.W.; Reddien, P.W. Transcriptome analysis of the planarian eye identifies ovo as a specific regulator of eye regeneration. Cell Rep. 2012, 2, 294–307. [Google Scholar] [CrossRef] [Green Version]
- Zhou, H.; Zhang, L.; Vartuli, R.L.; Ford, H.L.; Zhao, R. The Eya phosphatase: Its unique role in cancer. Int. J. Biochem. Cell Biol. 2018, 96, 165–170. [Google Scholar] [CrossRef]







| Species | Sequence |
|---|---|
| Dugesia japonica (Djeya 1) Lingula unguis Euprymna scolopes Euperipatoides kanangrensis Dryobates pubescens Helobdella robusta Branchiostoma floridae Branchiostoma lanceolatum Capitella teleta Melanaphis sacchari Schizaphis graminum Felis catus Canus familiarus Bos Taurus Homo sapiens Ornithodoros moubata | RKLAFRYRRIKELYNSYR RKLAFRYRRIKEIYNSYR RKLAFRYRRIKEIYNSYR RKLAFRYRRIKEIYNSYR RKLAFRYRRVKELYNTYR RKLAFRYRRIKELYSAYR RKLAFRYRRIKEIYNSYK RKLAFRYRRIKEIYNSYK RKLAFRYRRIKEIYSSYR RKLAFRYRRVKELYNQYR RKLAFRYRRVKELYNQYR RKLAFRYRRVKELYNTYK RKLAFRYRRVKELYNTYK RKLAFRYRRVKELYNTYK RKLAFRYRRVKELYNTYK RKLAFRYRRIKEIYNQYR |
| Peptide | Sequence |
|---|---|
| Djeya [Aib10]Djeya1 [Aib13]Djeya1 [Aib16]Djeya1 Tat C105Y Mitoparan | H-RKLAFRYRRIKELYNSYR-NH2 H-RKLAFRYRR(Aib)KELYNSYR-NH2 H-RKLAFRYRRIKE(Aib)YNSYR-NH2 H-RKLAFRYRRIKELYN(Aib)YR-NH2 H-GRKKRRQRRRPPQ-NH2 H-CSIPPEVKFNKPFVYLI-NH2 H-INLKKLAKL(Aib)KKIL-NH2 |
| (a) | ||||
| Peptide | α-helix | ß-Strand | ß-Turns | Unordered |
| Djeya1 | 43.00 ± 1.73 | 30.33 ± 0.58 | 6.67 ± 0.58 | 20.00 ± 1.73 |
| [Aib10]Djeya1 | 41.33 ± 1.15 | 31.33 ± 0.58 | 7.00 ± 0.00 | 20.67 ± 0.58 |
| [Aib13]Djeya1 | 40.67 ± 1.52 | 30.00 ± 1.00 | 7.00 ± 0.00 | 22.33 ± 1.52 |
| [Aib16]Djeya1 | 39.67 ± 4.04 | 31.67 ± 2.87 | 7.33 ± 0.58 | 21.00 ± 1.00 |
| (b) | ||||
| Peptide | α-helix | ß-Strand | ß-Turns | Unordered |
| Djeya1 | 45.50 ± 4.94 | 27.55 ± 3.53 | 7.00 ± 0.00 | 20.00 ± 1.41 |
| [Aib10]Djeya1 | 51.50 ± 0.07 | 21.00 ± 0.02 | 7.50 ± 0.71 | 20.00 ± 0.00 |
| [Aib13]Djeya1 | 40.00 ± 2.64 | 31.00 ± 2.08 | 7.33 ± 0.05 | 22.33 ± 1.50 |
| [Aib16]Djeya1 | 46.00 ± 1.41 | 27.50 ± 0.71 | 7.00 ± 0.00 | 19.50 ± 0.71 |
| (c) | ||||
| Peptide | α-helix | ß-Strand | ß-Turns | Unordered |
| Djeya1 | 44.00 ± 1.53 | 34.00 ± 2.12 | 8.00 ± 0.0 | 22.00 ± 0.00 |
| [Aib10]Djeya1 | 45.00 ± 1.58 | 35.00 ± 0.70 | 7.00 ± 1.41 | 22.00 ± 1.41 |
| [Aib13]Djeya1 | 42.00 ± 0.71 | 30.00 ± 0.71 | 8.00 ± 0.71 | 22.00 ± 0.71 |
| [Aib16]Djeya1 | 42.00 ± 0.71 | 30.00 ± 0.71 | 7.00 ± 0.71 | 22.00 ± 0.71 |
| (d) | ||||
| Peptide | α-helix | ß-Strand | ß-Turns | Unordered |
| Djeya1 | 47.00 ± 1.24 | 26.00 ± 1.28 | 8.00 ± 0.00 | 20.00 ± 0.00 |
| [Aib10]Djeya1 | 63.00 ± 2.82 | 15.00 ± 0.00 | 5.00 ± 0.00 | 16.00 ± 0.00 |
| [Aib13]Djeya1 | 42.00 ± 0.00 | 30.00 ± 0.71 | 7.00 ± 0.71 | 21.00 ± 0.71 |
| [Aib16]Djeya1 | 42.00 ± 0.00 | 30.00 ± 0.71 | 8.00 ± 0.71 | 22.00 ± 0.71 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jones, S.; Matos, B.; Dennison, S.; Fardilha, M.; Howl, J. Stem Cell Bioengineering with Bioportides: Inhibition of Planarian Head Regeneration with Peptide Mimetics of Eyes Absent Proteins. Pharmaceutics 2023, 15, 2018. https://doi.org/10.3390/pharmaceutics15082018
Jones S, Matos B, Dennison S, Fardilha M, Howl J. Stem Cell Bioengineering with Bioportides: Inhibition of Planarian Head Regeneration with Peptide Mimetics of Eyes Absent Proteins. Pharmaceutics. 2023; 15(8):2018. https://doi.org/10.3390/pharmaceutics15082018
Chicago/Turabian StyleJones, Sarah, Bárbara Matos, Sarah Dennison, Margarida Fardilha, and John Howl. 2023. "Stem Cell Bioengineering with Bioportides: Inhibition of Planarian Head Regeneration with Peptide Mimetics of Eyes Absent Proteins" Pharmaceutics 15, no. 8: 2018. https://doi.org/10.3390/pharmaceutics15082018