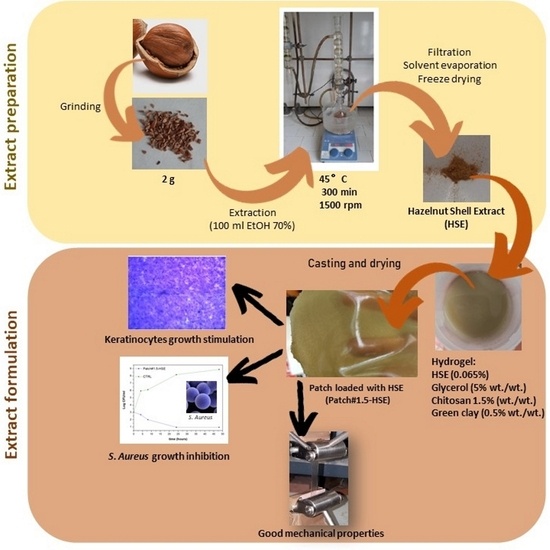
Polymeric Patches Based on Chitosan/Green Clay Composites and Hazelnut Shell Extract as Bio-Sustainable Medication for Wounds
Abstract
:1. Introduction
2. Materials and Methods
2.1. Extract Preparation
2.2. Composite Patch Preparation
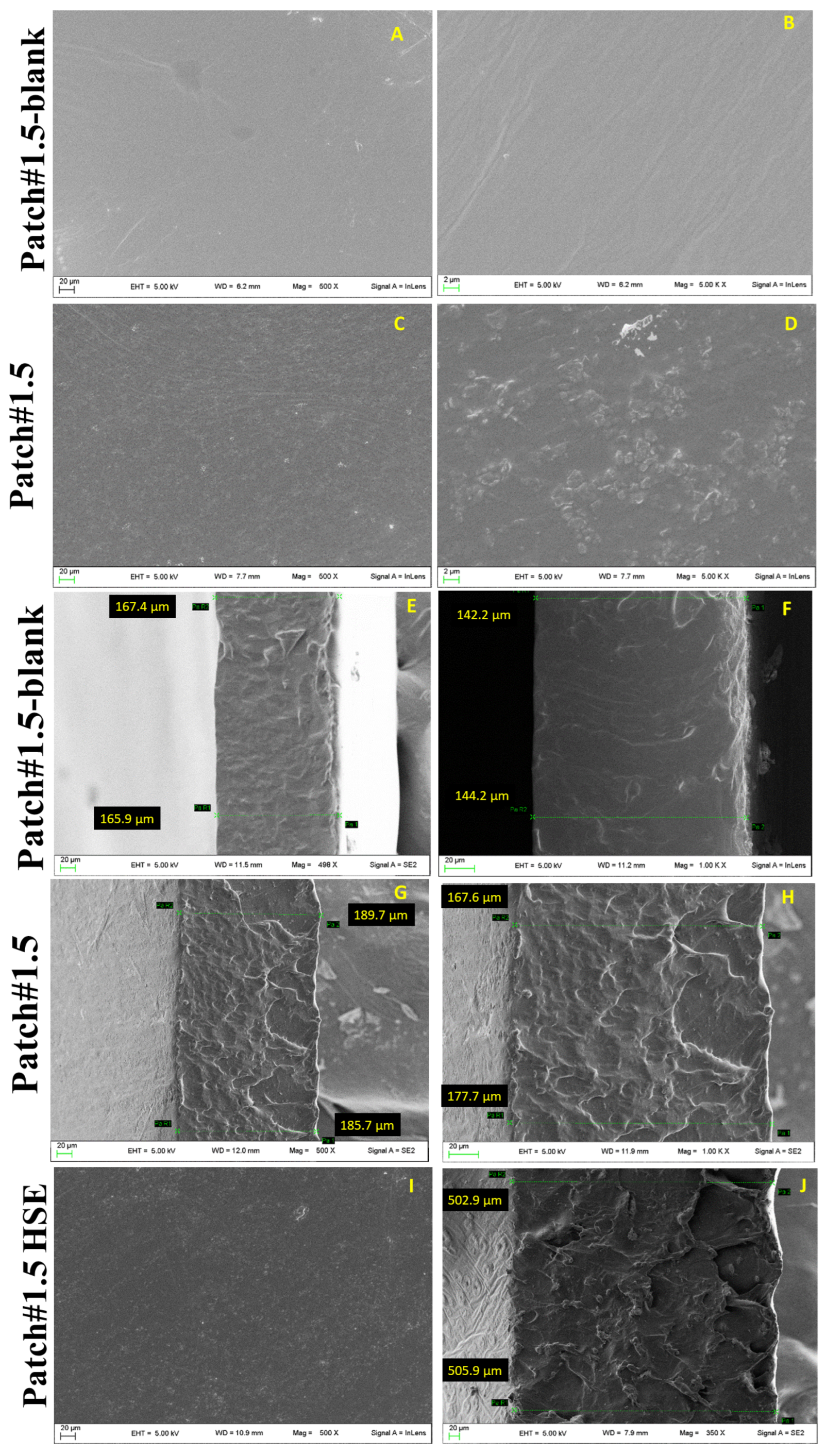
2.3. Morphology and Thickness
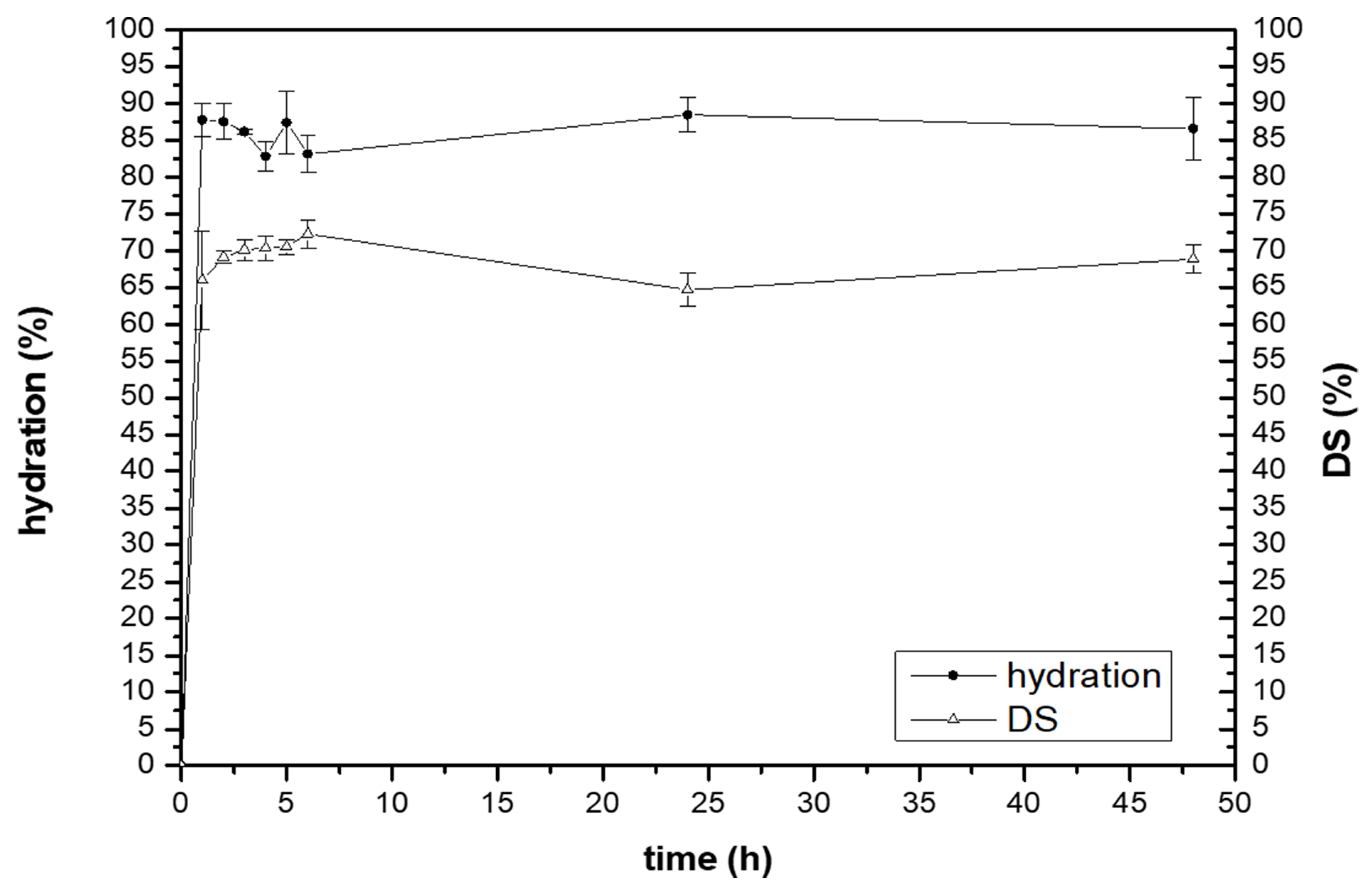
2.4. Water-Holding Studies
2.5. Ex Vivo Adhesion Studies
2.6. FT-IR Analysis
2.7. Mechanical Characterization
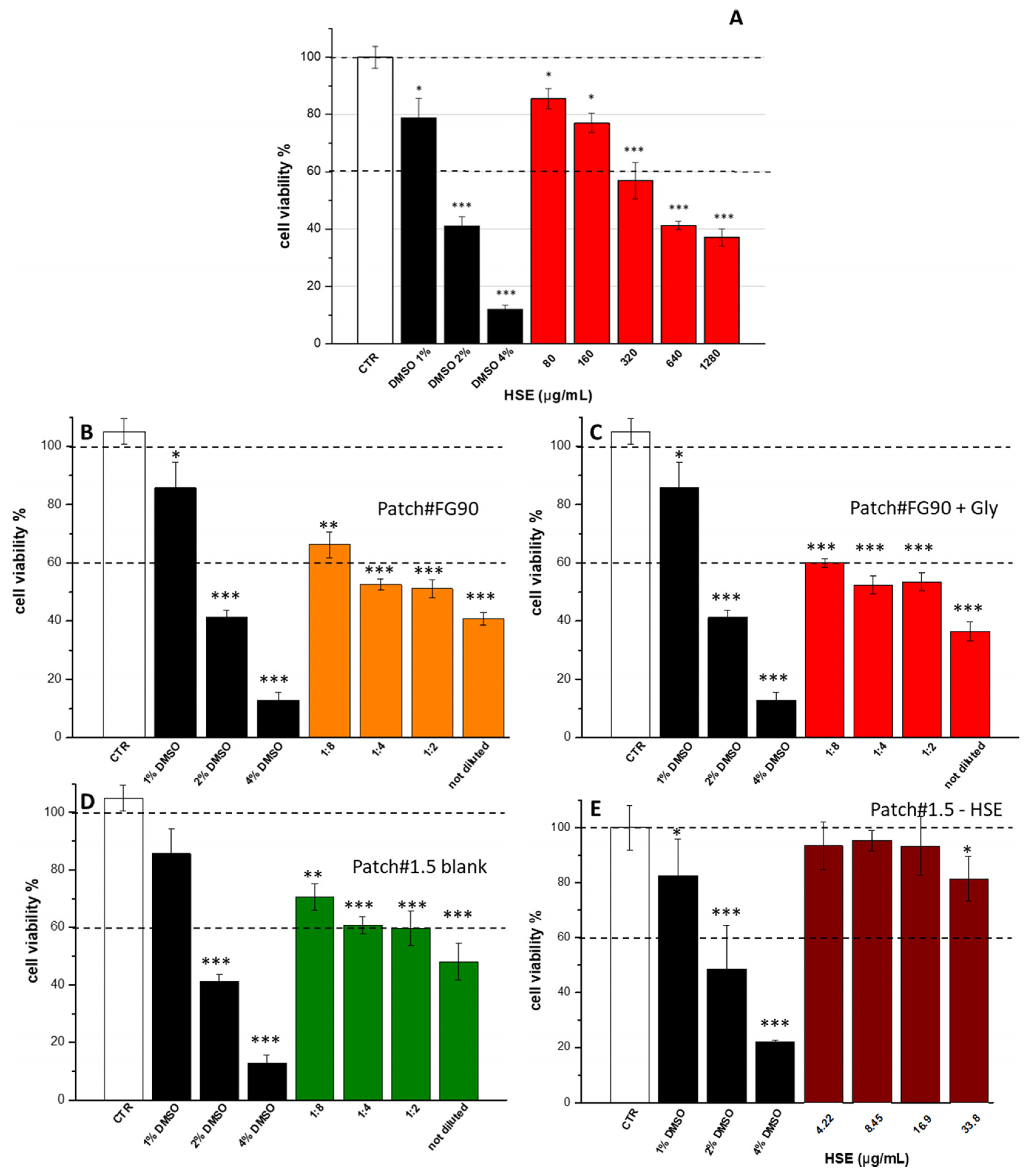
2.8. Cytotoxicity
2.9. Wound Healing Assay
2.10. Antimicrobial Activity Assay
- ∑C is the sum of the colonies counted on the two dishes from two successive dilutions;
- V is the volume of inoculum placed in each dish, in mL;
- d is the dilution corresponding to the first dilution retained.
2.11. Statistical Analysis
3. Results and Discussions
3.1. Patch Preparation and Characterization
3.2. Morphology and Thickness
3.3. Water-Holding Studies
3.4. Ex Vivo Adhesion Studies
3.5. Cytotoxic Activity
3.6. Loaded Patch Characterization
3.6.1. Morphology and Thickness
3.6.2. FT-IR Analysis
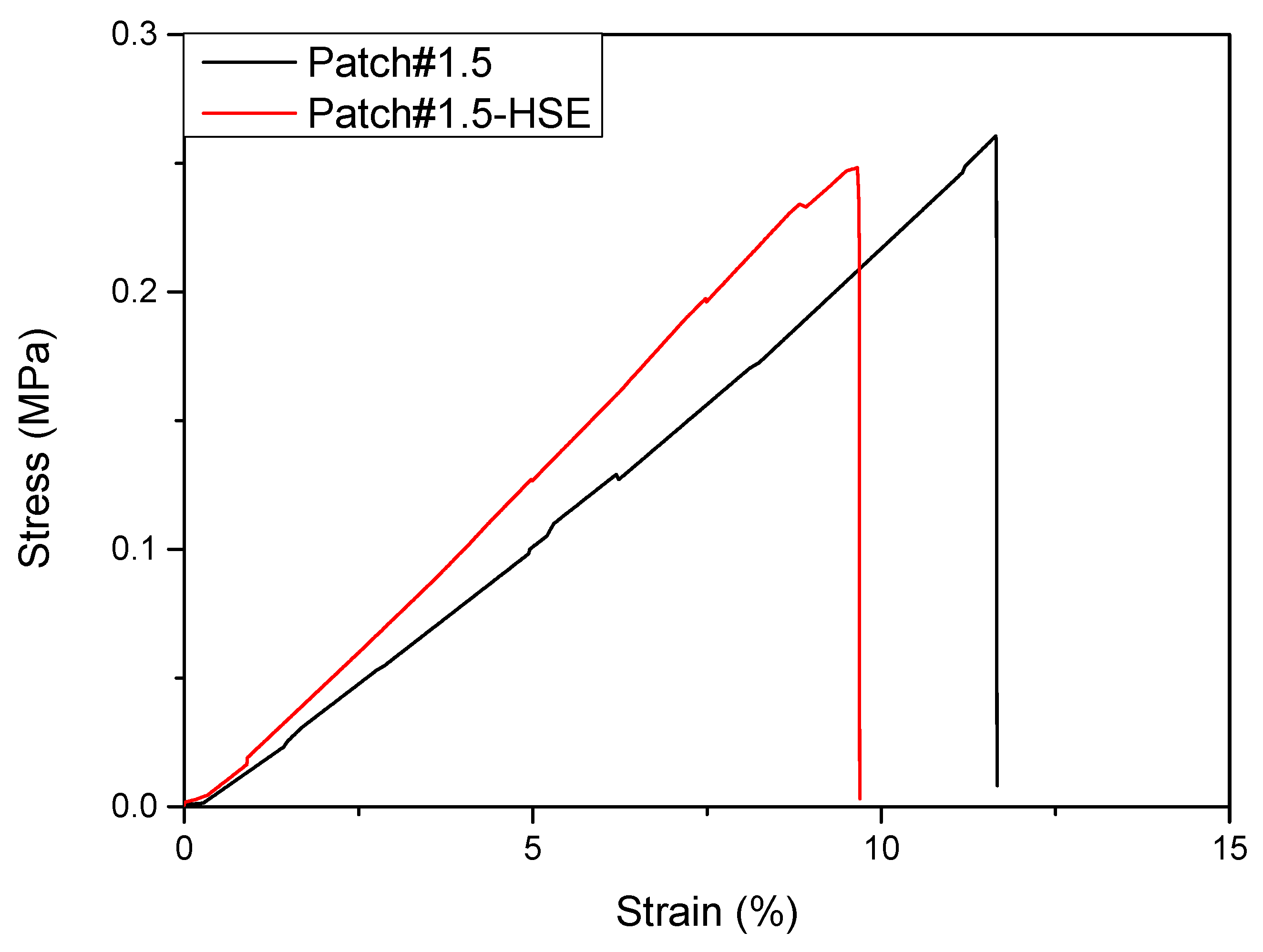
3.6.3. Mechanical Properties
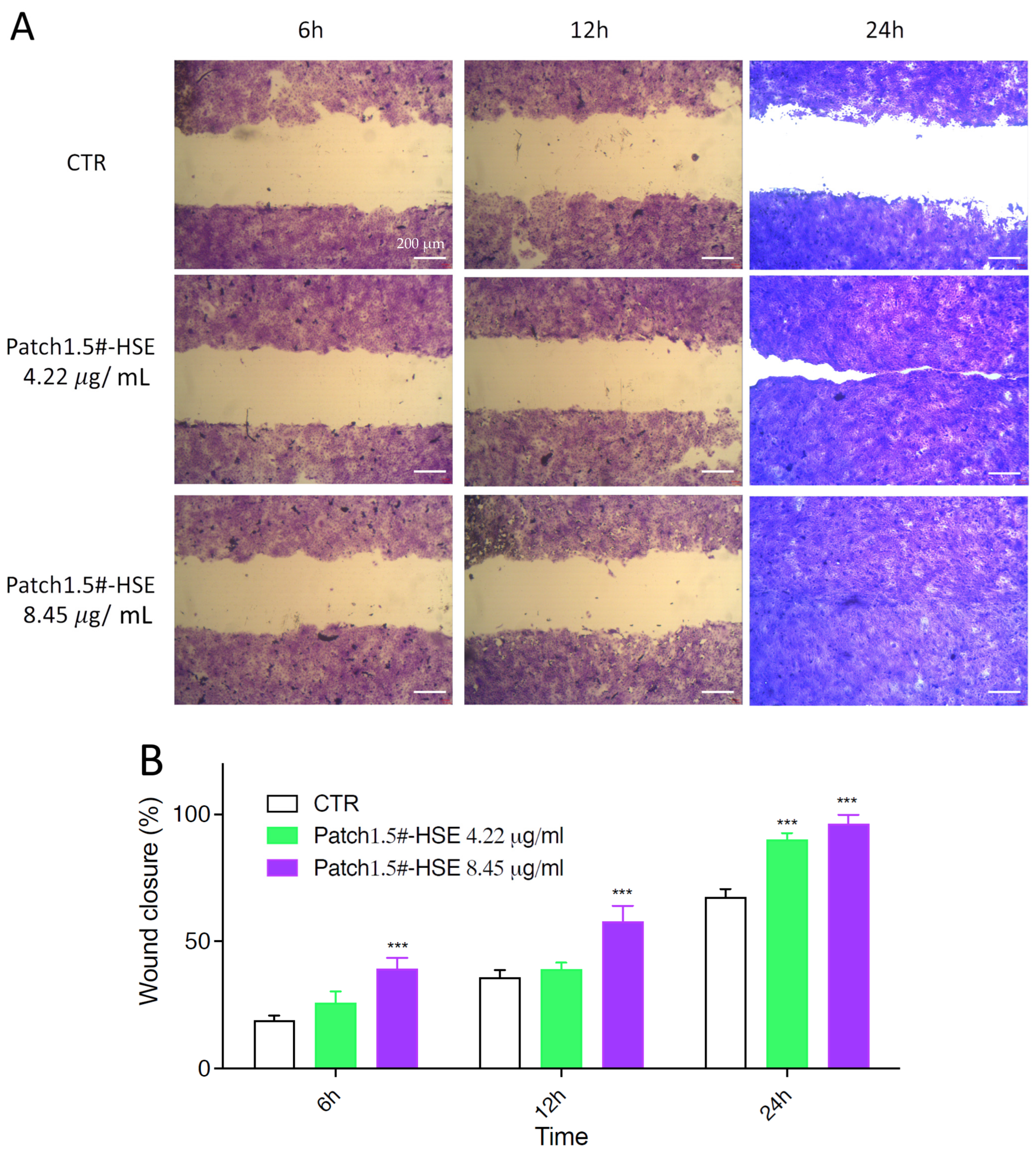
3.6.4. In Vitro Wound Healing Capacity
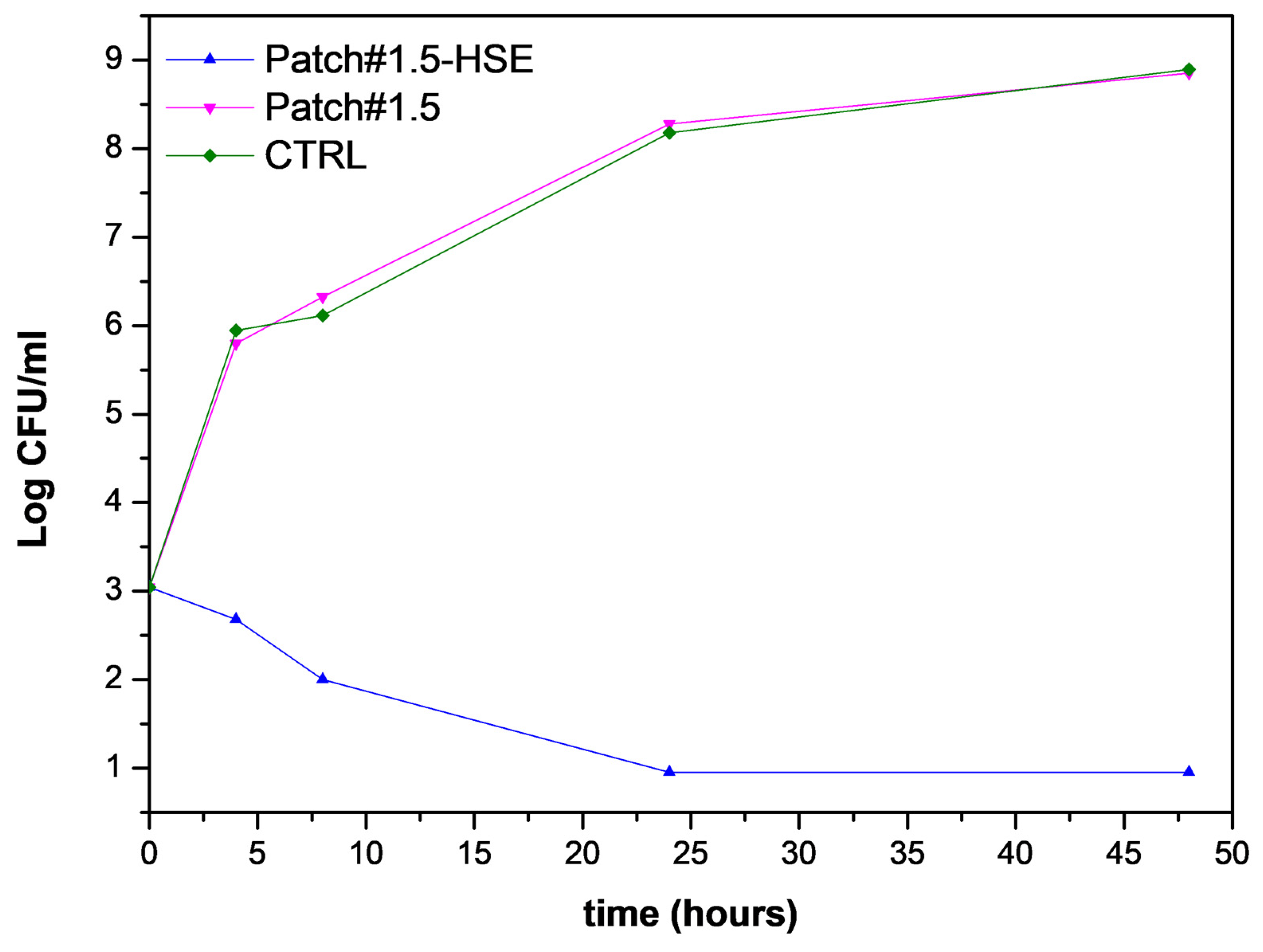
3.6.5. Antimicrobial Activity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, A.; Liu, X.; Zhang, H.; Wu, H.; Xu, D.; Li, B.; Zhao, C. Influence of pyrolysis temperature on bio-oil produced from hazelnut shells: Physico-chemical properties and antioxidant activity of wood vinegar and tar fraction. J. Renew. Sustain. Energy 2021, 13, 043102. [Google Scholar] [CrossRef]
- Shahidi, F.; Alasalvar, C.; Liyana-Pathirana, C.M. Antioxidant phytochemicals in hazelnut kernel (Corylus avellana L) and hazelnut byproducts. J. Agric. Food Chem. 2007, 55, 1212–1220. [Google Scholar] [CrossRef]
- Yuan, B.; Lu, M.; Eskridge, K.M.; Isom, L.D.; Hanna, M.A. Extraction, identification, and quantification of antioxidant phenolics from hazelnut (Corylus avellana L.) shells. Food Chem. 2018, 244, 7–15. [Google Scholar] [CrossRef]
- Di Michele, A.; Pagano, C.; Allegrini, A.; Blasi, F.; Cossignani, L.; Di Raimo, E.; Faieta, M.; Oliva, E.; Pittia, P.; Primavilla, S.; et al. Hazelnut shells as source of active ingredients: Extracts preparation and characterization. Molecules 2021, 26, 6607. [Google Scholar] [CrossRef] [PubMed]
- Ramalhosa, E.; Delgado, T.; Estevinho, L.; Pereira, J.A. Hazelnut (Corylus avellana L.) Cultivars and Antimicrobial Activity. In Nuts and Seeds in Health and Disease Prevention; Springer: Berlin/Heidelberg, Germany, 2011. [Google Scholar]
- Shataer, D.; Abdulla, R.; Ma, Q.L.; Liu, G.Y.; Aisa, H.A. Chemical Composition of Extract of Corylus avellana Shells. Chem. Nat. Compd. 2020, 56, 2455. [Google Scholar] [CrossRef]
- Salem, M.A.; Aborehab, N.M.; Al-Karmalawy, A.A.; Fernie, A.R.; Alseekh, S.; Ezzat, S.M. Potential Valorization of Edible Nuts By-Products: Exploring the Immune-Modulatory and Antioxidants Effects of Selected Nut Shells Extracts in Relation to Their Metabolic Profiles. Antioxidants 2022, 11, 462. [Google Scholar] [CrossRef]
- Esposito, T.; Sansone, F.; Franceschelli, S.; Del Gaudio, P.; Picerno, P.; Aquino, R.P.; Mencherini, T. Hazelnut (Corylus avellana L.) shells extract: Phenolic composition, antioxidant effect and cytotoxic activity on human cancer cell lines. Int. J. Mol. Sci. 2017, 18, 392. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Esposito, T.; Mencherini, T.; Del Gaudio, P.; Auriemma, G.; Franceschelli, S.; Picerno, P.; Aquino, R.P.; Sansone, F. Design and development of spray-dried microsystems to improve technological and functional properties of bioactive compounds from hazelnut shells. Molecules 2020, 25, 127. [Google Scholar] [CrossRef] [Green Version]
- Gao, J.; Hu, J.; Hu, D.; Yang, X. A Role of Gallic Acid in Oxidative Damage Diseases: A Comprehensive Review. Nat. Prod. Commun. 2019, 14, 1934578X1987417. [Google Scholar] [CrossRef] [Green Version]
- Comino-Sanz, I.M.; López-Franco, M.D.; Castro, B.; Pancorbo-Hidalgo, P.L. The role of antioxidants on wound healing: A review of the current evidence. J. Clin. Med. 2021, 10, 3558. [Google Scholar] [CrossRef]
- Yang, D.J.; Moh, S.H.; Son, D.H.; You, S.; Kinyua, A.W.; Ko, C.M.; Song, M.; Yeo, J.; Choi, Y.H.; Kim, K.W. Gallic Acid Promotes Wound Healing in Normal and Hyperglucidic Conditions. Molecules 2016, 21, 899. [Google Scholar] [CrossRef] [Green Version]
- Singh, M.P.; Gupta, A.; Sisodia, S.S. Wound healing activity of Terminalia bellerica Roxb. And gallic acid in experimentally induced diabetic animals. J. Complement. Integr. Med. 2020, 17. [Google Scholar] [CrossRef]
- Karatas, O.; Gevrek, F. Gallic acid liposome and powder gels improved wound healing in wistar rats. Ann. Med. Res. 2019, 26, 2720–2727. [Google Scholar] [CrossRef]
- Patrulea, V.; Ostafe, V.; Borchard, G.; Jordan, O. Chitosan as a starting material for wound healing applications. Eur. J. Pharm. Biopharm. 2015, 97, 417–426. [Google Scholar] [CrossRef] [Green Version]
- Park, S.G.; Li, M.X.; Cho, W.K.; Joung, Y.K.; Huh, K.M. Thermosensitive gallic acid-conjugated hexanoyl glycol chitosan as a novel wound healing biomaterial. Carbohydr. Polym. 2021, 260, 117808. [Google Scholar] [CrossRef]
- Sun, X.; Dong, M.; Guo, Z.; Zhang, H.; Wang, J.; Jia, P.; Bu, T.; Liu, Y.; Li, L.; Wang, L. Multifunctional chitosan-copper-gallic acid based antibacterial nanocomposite wound dressing. Int. J. Biol. Macromol. 2021, 167, 10–12. [Google Scholar] [CrossRef]
- Kaparekar, P.S.; Pathmanapan, S.; Anandasadagopan, S.K. Polymeric scaffold of Gallic acid loaded chitosan nanoparticles infused with collagen-fibrin for wound dressing application. Int. J. Biol. Macromol. 2020, 165, 930–947. [Google Scholar] [CrossRef]
- Canada, T.E.; Cowan, M.E.; Wiencek, K.M. Wound Care Device Healing Fluid Transfer Proprieties. U.S. Patent No. 8,021,685, 20 September 2011. [Google Scholar]
- Pagano, C.; Calarco, P.; Di Michele, A.; Ceccarini, M.R.; Beccari, T.; Primavilla, S.; Scuota, S.; Marmottini, F.; Ramella, D.; Ricci, M.; et al. Development of sodium carboxymethyl cellulose based polymeric microparticles for in situ hydrogel wound dressing formation. Int. J. Pharm. 2021, 602, 120606. [Google Scholar] [CrossRef]
- Pagano, C.; Luzi, F.; Ricci, M.; Di Michele, A.; Puglia, D.; Ceccarini, M.R.; Beccari, T.; Blasi, F.; Cossignani, L.; Schoubben, A.; et al. Wound Dressing: Combination of Acacia Gum/PVP/Cyclic Dextrin in Bioadhesive Patches Loaded with Grape Seed Extract. Pharmaceutics 2022, 14, 485. [Google Scholar] [CrossRef] [PubMed]
- Pagano, C.; Perioli, L.; Latterini, L.; Nocchetti, M.; Ceccarini, M.R.; Marani, M.; Ramella, D.; Ricci, M. Folic acid-layered double hydroxides hybrids in skin formulations: Technological, photochemical and in vitro cytotoxicity on human keratinocytes and fibroblasts. Appl. Clay Sci. 2019, 168, 382–395. [Google Scholar] [CrossRef]
- Ceccarini, M.R.; Vannini, S.; Cataldi, S.; Moretti, M.; Villarini, M.; Fioretti, B.; Albi, E.; Beccari, T.; Codini, M. In Vitro Protective Effects of Lycium barbarum Berries Cultivated in Umbria (Italy) on Human Hepatocellular Carcinoma Cells. Biomed. Res. Int. 2016, 2016, 7529521. [Google Scholar] [CrossRef] [Green Version]
- Hostanska, K.; Rostock, M.; Melzer, J.; Baumgartner, S.; Saller, R. A homeopathic remedy from arnica, marigold, St. John’s wort and comfrey accelerates in vitro wound scratch closure of NIH 3T3 fibroblasts. BMC Complement. Altern. Med. 2012, 12, 100. [Google Scholar] [CrossRef] [Green Version]
- Pagano, C.; Perioli, L.; Baiocchi, C.; Bartoccini, A.; Beccari, T.; Blasi, F.; Calarco, P.; Ceccarini, M.R.; Cossignani, L.; di Michele, A.; et al. Preparation and characterization of polymeric microparticles loaded with Moringa oleifera leaf extract for exuding wound treatment. Int. J. Pharm. 2020, 587, 119700. [Google Scholar] [CrossRef] [PubMed]
- Pagano, C.; Baiocchi, C.; Beccari, T.; Blasi, F.; Cossignani, L.; Ceccarini, M.R.; Orabona, C.; Orecchini, E.; Di Raimo, E.; Primavilla, S.; et al. Emulgel loaded with flaxseed extracts as new therapeutic approach in wound treatment. Pharmaceutics 2021, 13, 1107. [Google Scholar] [CrossRef] [PubMed]
- Perioli, L.; Ambrogi, V.; Pagano, C.; Scuota, S.; Rossi, C. FG90 chitosan as a new polymer for metronidazole mucoadhesive tablets for vaginal administration. Int. J. Pharm. 2009, 377, 120–127. [Google Scholar] [CrossRef] [PubMed]
- WHO lists bacteria that need to be targeted for antibiotic development. Pharm. J. 2017, 298, 7899.
- Williams, L.B.; Haydel, S.E.; Giese, R.F.; Eberl, D.D. Chemical and mineralogical characteristics of French green clays used for healing. Clays Clay Miner. 2008, 56, 437–452. [Google Scholar] [CrossRef]
- Pagano, C.; Marinozzi, M.; Baiocchi, C.; Beccari, T.; Calarco, P.; Ceccarini, M.R.; Chielli, M.; Orabona, C.; Orecchini, E.; Ortenzi, R.; et al. Bioadhesive polymeric films based on red onion skins extract for wound treatment: An innovative and eco-friendly formulation. Molecules 2020, 25, 318. [Google Scholar] [CrossRef] [Green Version]
- Lih, E.; Lee, J.S.; Park, K.M.; Park, K.D. Rapidly curable chitosan-PEG hydrogels as tissue adhesives for hemostasis and wound healing. Acta Biomater. 2012, 8, 3261–3269. [Google Scholar] [CrossRef]
- Wongsa, P.; Phatikulrungsun, P.; Prathumthong, S. FT-IR characteristics, phenolic profiles and inhibitory potential against digestive enzymes of 25 herbal infusions. Sci. Rep. 2022, 12, 6631. [Google Scholar] [CrossRef]
- Madejová, J. FTIR techniques in clay mineral studies. Vib. Spectrosc. 2003, 31, 1–10. [Google Scholar] [CrossRef]
- Olivas, G.I.; Barbosa-Cánovas, G.V. Alginate-calcium films: Water vapor permeability and mechanical properties as affected by plasticizer and relative humidity. LWT 2008, 41, 359–366. [Google Scholar] [CrossRef]
- Zhang, W.; Li, X.; Jiang, W. Development of antioxidant chitosan film with banana peels extract and its application as coating in maintaining the storage quality of apple. Int. J. Biol. Macromol. 2020, 154, 1205–1214. [Google Scholar] [CrossRef]
- Balasubramaniam, M.P.; Murugan, P.; Chenthamara, D.; Ramakrishnan, S.G.; Salim, A.; Lin, F.-H.; Robert, B.; Subramaniam, S. Synthesis of chitosan-ferulic acid conjugated poly(vinyl alcohol) polymer film for an improved wound healing. Mater Today Commun. 2020, 25, 101510. [Google Scholar] [CrossRef]
- Rafieian, S.; Mahdavi, H.; Masoumi, M.E. Improved mechanical, physical and biological properties of chitosan films using Aloe vera and electrospun PVA nanofibers for wound dressing applications. J. Ind. Text. 2021, 50, 1456–1474. [Google Scholar] [CrossRef]
- Souza, V.G.L.; Fernando, A.L.; Pires, J.R.A.; Rodrigues, P.F.; Lopes, A.A.S.; Fernandes, F.M.B. Physical properties of chitosan films incorporated with natural antioxidants. Ind. Crops Prod. 2017, 107, 565–572. [Google Scholar] [CrossRef]


| Gels | CS FG90 % (w/w) | Green Clay % (w/w) | Glycerol % (w/w) | Aqueous Solution of Lactic Acid % (w/w) | Final Patch Name | Aspect of the Obtained Patch |
|---|---|---|---|---|---|---|
| H1 | 1.0 | - | - | 99.0 | F1 | rigid |
| H2 | 1.0 | - | 10.0 | 89.0 | F2 | sticky |
| H3 | 1.0 | - | 5.0 | 94.0 | F3 | fragile |
| H4 | 1.0 | - | 2.0 | 97.0 | F4 | rigid |
| H5 | 1.0 | 0.5 | 5.0 | 98.5 | F5 | flexible but fragile |
| H6 | 1.5 | 0.5 | 5.0 | 93.0 | F6 | flexible and manageable |
| Formulation | σB (MPa) | εB (%) | E (MPa) |
|---|---|---|---|
| Patch#1.5 | 0.29 ± 0.04 a | 11.63 ± 0.05 a | 1.95 ± 0.07 a |
| Patch#1.5-HSE | 0.32 ± 0.08 a | 11.54 ± 1.71 a | 2.03 ± 0.21 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pérez Gutíerrez, C.L.; Di Michele, A.; Pagano, C.; Puglia, D.; Luzi, F.; Beccari, T.; Ceccarini, M.R.; Primavilla, S.; Valiani, A.; Vicino, C.; et al. Polymeric Patches Based on Chitosan/Green Clay Composites and Hazelnut Shell Extract as Bio-Sustainable Medication for Wounds. Pharmaceutics 2023, 15, 2057. https://doi.org/10.3390/pharmaceutics15082057
Pérez Gutíerrez CL, Di Michele A, Pagano C, Puglia D, Luzi F, Beccari T, Ceccarini MR, Primavilla S, Valiani A, Vicino C, et al. Polymeric Patches Based on Chitosan/Green Clay Composites and Hazelnut Shell Extract as Bio-Sustainable Medication for Wounds. Pharmaceutics. 2023; 15(8):2057. https://doi.org/10.3390/pharmaceutics15082057
Chicago/Turabian StylePérez Gutíerrez, Carmen Laura, Alessandro Di Michele, Cinzia Pagano, Debora Puglia, Francesca Luzi, Tommaso Beccari, Maria Rachele Ceccarini, Sara Primavilla, Andrea Valiani, Camilla Vicino, and et al. 2023. "Polymeric Patches Based on Chitosan/Green Clay Composites and Hazelnut Shell Extract as Bio-Sustainable Medication for Wounds" Pharmaceutics 15, no. 8: 2057. https://doi.org/10.3390/pharmaceutics15082057