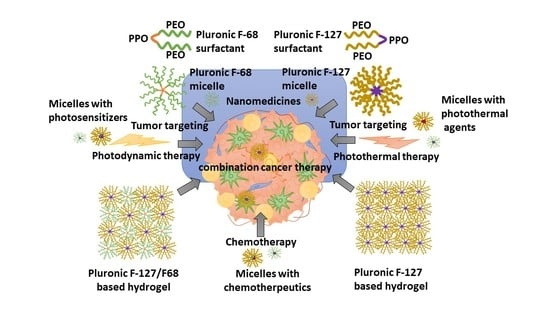
Pluronic F-68 and F-127 Based Nanomedicines for Advancing Combination Cancer Therapy
Abstract
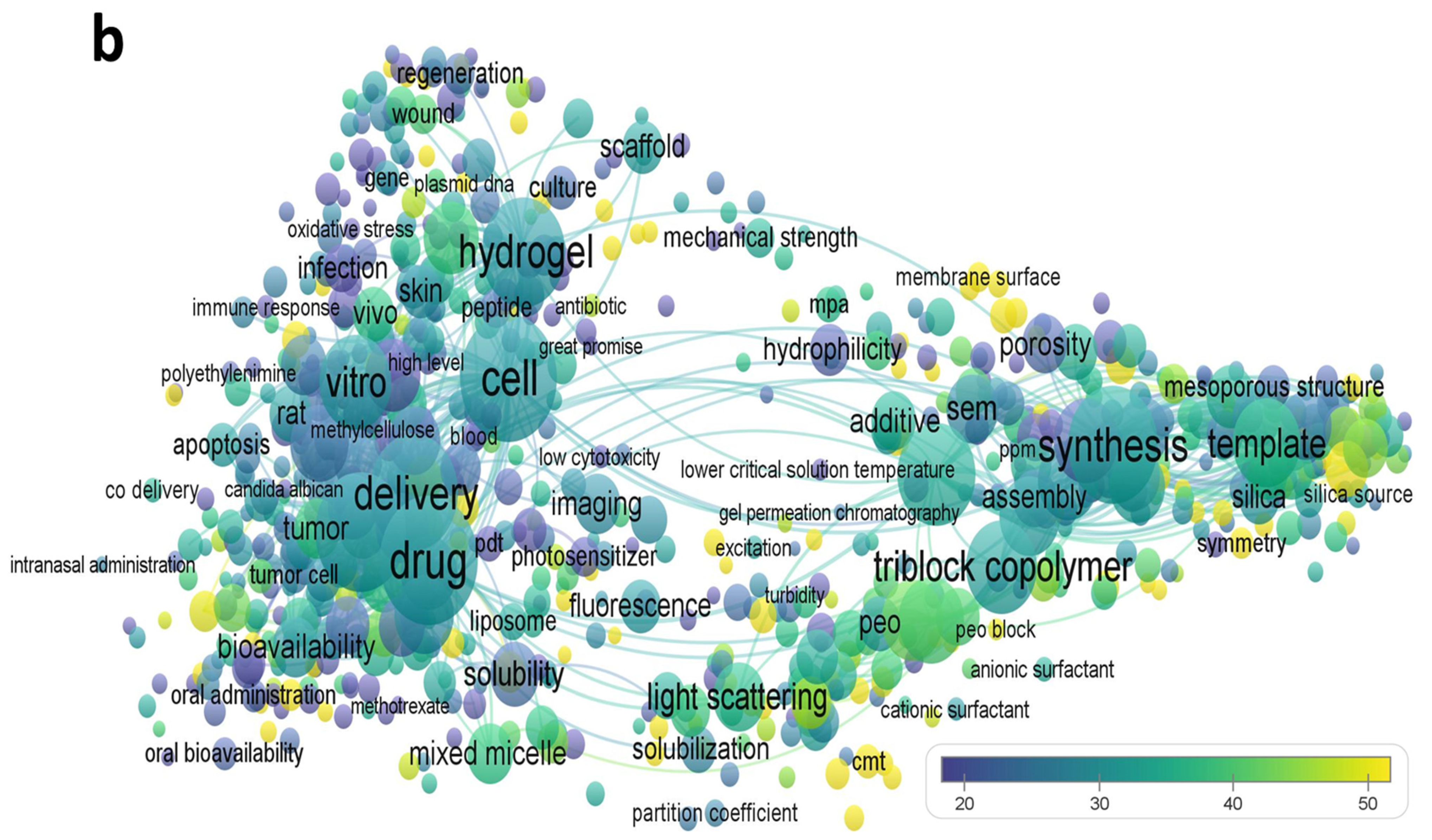
:1. Introduction
2. Properties of Pluronic F-68 and/or F-127
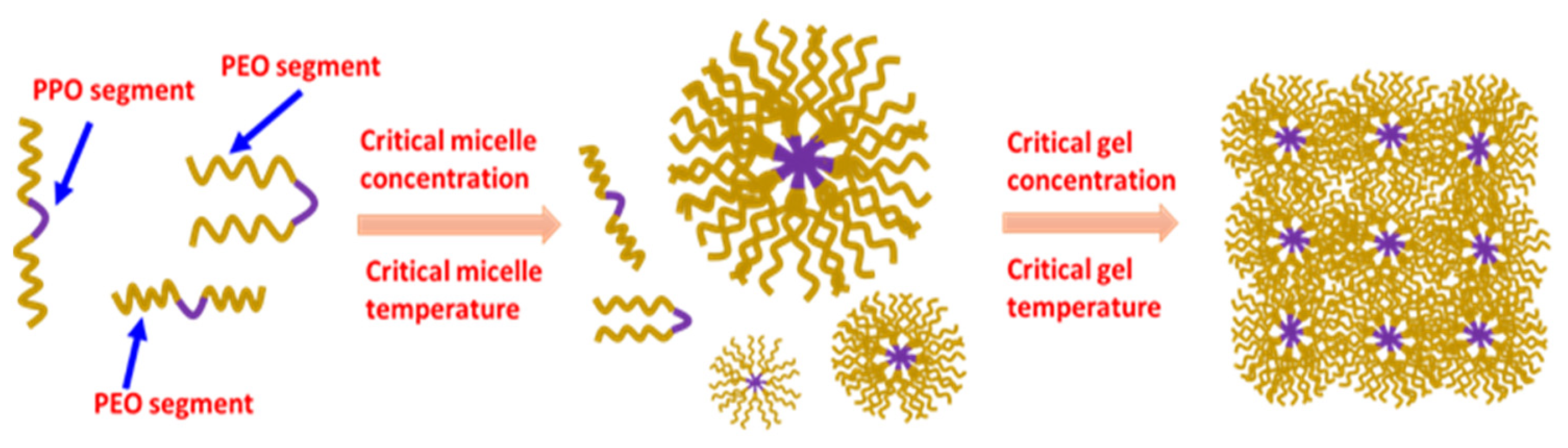
2.1. Preparation of Formulation
2.2. Solubility Enhancement of Poorly Water-Soluble Agents
2.3. Stabilization of Formulations
2.4. Strength of Gel
2.5. Adhesive Property
2.6. Solution to Gel Transformation Temperature
3. Pluronic F-68 and Pluronic F-127 for Targeted Delivery of a Single Chemotherapeutic
3.1. Pluronic-Based Micelles of Poorly Water-Soluble Anticancer Agents
3.2. Pluronic-Based Micelles of Water-Soluble Anticancer Agents
4. Pluronic F-68 and F-127 Nanomicelles as Anticancer Combination Therapy
5. Pluronic F-68 and Pluronic F-127 Nanomicelles for Chemo-Photodynamic Combination Therapy
6. Pluronic F-68- and Pluronic F-127-Based Hydrogels as Cancer Combination Therapy
7. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Varga, N.; Hornok, V.; Janovák, L.; Dékány, I.; Csapó, E. The effect of synthesis conditions and tunable hydrophilicity on the drug encapsulation capability of PLA and PLGA nanoparticles. Colloids Surf. B Biointerfaces 2019, 176, 212–218. [Google Scholar] [CrossRef] [Green Version]
- Varga, N.; Turcsányi, Á.; Hornok, V.; Csapó, E. Vitamin E-loaded PLA-and PLGA-based core-shell nanoparticles: Synthesis, structure optimization and controlled drug release. Pharmaceutics 2019, 11, 357. [Google Scholar] [CrossRef] [Green Version]
- Varga, N.; Bélteki, R.; Juhász, Á.; Csapó, E. Core-Shell Structured PLGA Particles Having Highly Controllable Ketoprofen Drug Release. Pharmaceutics 2023, 15, 1355. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, S.-H.; Huang, J.S. Small-Angle Neutron Scattering Analysis of the Structure and Interaction of Triblock Copolymer Micelles in Aqueous Solution. Macromolecules 1998, 31, 2236–2244. [Google Scholar] [CrossRef]
- Kabanov, A.V.; Nazarova, I.R.; Astafieva, I.V.; Batrakova, E.V.; Alakhov, V.Y.; Yaroslavov, A.A.; Kabanov, V.A. Micelle Formation and Solubilization of Fluorescent Probes in Poly(oxyethylene-b-oxypropylene-b-oxyethylene) Solutions. Macromolecules 1995, 28, 2303–2314. [Google Scholar] [CrossRef]
- Kabanov, A.V.; Batrakova, E.V.; Alakhov, V.Y. Pluronic® block copolymers for overcoming drug resistance in cancer. Adv. Drug Deliv. Rev. 2002, 54, 759–779. [Google Scholar] [CrossRef]
- Kabanov, A.V.; Batrakova, E.V.; Alakhov, V.Y. Pluronic® block copolymers as novel polymer therapeutics for drug and gene delivery. J. Control. Release 2002, 82, 189–212. [Google Scholar] [CrossRef]
- Lippens, E.; Swennen, I.; Gironès, J.; Declercq, H.; Vertenten, G.; Vlaminck, L.; Gasthuys, F.; Schacht, E.; Cornelissen, R. Cell survival and proliferation after encapsulation in a chemically modified Pluronic® F127 hydrogel. J. Biomater. Appl. 2013, 27, 828–839. [Google Scholar] [CrossRef]
- Alexandridis, P. Amphiphilic copolymers and their applications. Curr. Opin. Colloid Interface Sci. 1996, 1, 490–501. [Google Scholar] [CrossRef]
- Alexandridis, P. Poly(ethylene oxide)/poly(propylene oxide) block copolymer surfactants. Curr. Opin. Colloid Interface Sci. 1997, 2, 478–489. [Google Scholar] [CrossRef]
- Almgren, M.; Van Stam, J.; Lindblad, C.; Li, P.; Stilbs, P.; Bahadur, P. Aggregation of poly(ethylene oxide)-poly(propylene oxide)-poly(ethylene oxide) triblock copolymers in the presence of sodium dodecyl sulfate in aqueous solution. J. Phys. Chem. 1991, 95, 5677–5684. [Google Scholar] [CrossRef]
- Hurter, P.N.; Hatton, T.A. Solubilization of polycyclic aromatic hydrocarbons by poly(ethylene oxide-propylene oxide) block copolymer micelles: Effects of polymer structure. Langmuir 1992, 8, 1291–1299. [Google Scholar] [CrossRef]
- Su, Y.-L.; Wang, J.; Liu, H.-Z. Melt, Hydration, and Micellization of the PEO–PPO–PEO Block Copolymer Studied by FTIR Spectroscopy. J. Colloid Interface Sci. 2002, 251, 417–423. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, K.; Saito, Y.; Tokuoka, Y.; Abe, M.; Sato, T. Poly(ethylene oxide)/poly(propylene oxide)/poly(ethylene oxide) triblock copolymer as a sustained-release carrier for perfume compounds. J. Am. Oil Chem. Soc. 1997, 74, 55–59. [Google Scholar] [CrossRef]
- Yang, L.; Alexandridis, P.; Steytler, D.C.; Kositza, M.J.; Holzwarth, J.F. Small-Angle Neutron Scattering Investigation of the Temperature-Dependent Aggregation Behavior of the Block Copolymer Pluronic L64 in Aqueous Solution. Langmuir 2000, 16, 8555–8561. [Google Scholar] [CrossRef]
- Kozlov, M.Y.; Melik-Nubarov, N.S.; Batrakova, E.V.; Kabanov, A.V. Relationship between Pluronic Block Copolymer Structure, Critical Micellization Concentration and Partitioning Coefficients of Low Molecular Mass Solutes. Macromolecules 2000, 33, 3305–3313. [Google Scholar] [CrossRef]
- Alvarez-Lorenzo, C.; Sosnik, A.; Concheiro, A. PEO-PPO Block Copolymers for Passive Micellar Targeting and Overcoming Multidrug Resistance in Cancer Therapy. Curr. Drug Targets 2011, 12, 1112–1130. [Google Scholar] [CrossRef]
- Yamagata, T.; Kusuhara, H.; Morishita, M.; Takayama, K.; Benameur, H.; Sugiyama, Y. Effect of excipients on breast cancer resistance protein substrate uptake activity. J. Control. Release 2007, 124, 1–5. [Google Scholar] [CrossRef]
- Yamagata, T.; Kusuhara, H.; Morishita, M.; Takayama, K.; Benameur, H.; Sugiyama, Y. Improvement of the Oral Drug Absorption of Topotecan through the Inhibition of Intestinal Xenobiotic Efflux Transporter, Breast Cancer Resistance Protein, by Excipients. Drug Metab. Dispos. 2007, 35, 1142–1148. [Google Scholar] [CrossRef] [Green Version]
- Batrakova, E.V.; Li, S.; Alakhov, V.Y.; Elmquist, W.F.; Miller, N.W.; Kabanov, A.V. Sensitization of cells overexpressing multidrug-resistant proteins by pluronic P85. Pharm. Res. 2003, 20, 1581–1590. [Google Scholar] [CrossRef]
- Batrakova, E.V.; Li, S.; Vinogradov, S.V.; Alakhov, V.Y.; Miller, D.W.; Kabanov, A. Mechanism of pluronic effect on P-glycoprotein efflux system in blood-brain barrier: Contributions of energy depletion and membrane fluidization. J. Pharmacol. Exp. Ther. 2001, 299, 483–493. [Google Scholar]
- Batrakova, E.V.; Miller, D.W.; Li, S.; Alakhov, V.Y.; Kabanov, A.; Elmquist, W.F. Pluronic P85 enhances the delivery of digoxin to the brain: In vitro and in vivo studies. J. Pharmacol. Exp. Ther. 2001, 296, 551–557. [Google Scholar] [PubMed]
- Szakács, G.; Paterson, J.K.; Ludwig, J.A.; Booth-Genthe, C.; Gottesman, M.M. Targeting multidrug resistance in cancer. Nat. Rev. Drug Discov. 2006, 5, 219–234. [Google Scholar] [CrossRef] [PubMed]
- Regev, R.; Katzir, H.; Yeheskely-Hayon, D.; Eytan, G.D. Modulation of P-glycoprotein-mediated multidrug resistance by acceleration of passive drug permeation across the plasma membrane. FEBS J. 2007, 274, 6204–6214. [Google Scholar] [CrossRef]
- Sotoudegan, F.; Amini, M.; Faizi, M.; Aboofazeli, R. Nimodipine-loaded Pluronic® block copolymer micelles: Preparation, characterization, in-vitro and in-vivo studies. Iran. J. Pharm. Res. IJPR 2016, 15, 641. [Google Scholar]
- Naharros-Molinero, A.; Caballo-González, M.Á.; de la Mata, F.J.; García-Gallego, S. Direct and Reverse Pluronic Micelles: Design and Characterization of Promising Drug Delivery Nanosystems. Pharmaceutics 2022, 14, 2628. [Google Scholar] [CrossRef] [PubMed]
- Prasanthan, P.; Kishore, N. Self-assemblies of pluronic micelles in partitioning of anticancer drugs and effectiveness of this system towards target protein. RSC Adv. 2021, 11, 22057–22069. [Google Scholar] [CrossRef]
- Shaik, N.; Giri, N.; Elmquist, W.F. Investigation of the micellar effect of pluronic P85 on P-glycoprotein inhibition: Cell accumulation and equilibrium dialysis studies. J. Pharm. Sci. 2009, 98, 4170–4190. [Google Scholar] [CrossRef] [Green Version]
- Lee, C.-F.; Yang, C.-H.; Lin, T.-L.; Bahadur, P.; Chen, L.-J. Role of molecular weight and hydrophobicity of amphiphilic tri-block copolymers in temperature-dependent co-micellization process and drug solubility. Colloids Surf. B Biointerfaces 2019, 183, 110461. [Google Scholar] [CrossRef]
- Thanitwatthanasak, S.; Sagis, L.M.; Chitprasert, P. Pluronic F127/Pluronic P123/vitamin E TPGS mixed micelles for oral delivery of mangiferin and quercetin: Mixture-design optimization, micellization, and solubilization behavior. J. Mol. Liq. 2019, 274, 223–238. [Google Scholar] [CrossRef]
- Barba, A.A.; Lamberti, G.; Rabbia, L.; Grassi, M.; Larobina, D.; Grassi, G. Modeling of the reticulation kinetics of alginate/pluronic blends for biomedical applications. Mater. Sci. Eng. C 2014, 37, 327–331. [Google Scholar] [CrossRef] [PubMed]
- Dalmoro, A.; Barba, A.A.; Grassi, M.; Grassi, G.; Lamberti, G. In situcoronary stent paving by Pluronic F127-alginate gel blends: Formulation and erosion tests. J. Biomed. Mater. Res. Part B Appl. Biomater. 2016, 104, 1013–1022. [Google Scholar] [CrossRef] [PubMed]
- Al Khateb, K.; Ozhmukhametova, E.K.; Mussin, M.N.; Seilkhanov, S.K.; Rakhypbekov, T.K.; Lau, W.M.; Khutoryanskiy, V.V. In situ gelling systems based on Pluronic F127/Pluronic F68 formulations for ocular drug delivery. Int. J. Pharm. 2016, 502, 70–79. [Google Scholar] [CrossRef] [PubMed]
- Park, K.D.; Jung, H.H.; Son, J.S.; Rhie, J.W.; Park, K.D.; Ahn, K.D.; Han, D.K. Thermosensitive and cell-adhesive pluronic hydrogels for human adipose-derived stem cells. Key Eng. Mater. 2007, 342, 301–304. [Google Scholar] [CrossRef]
- Shaik, N.; Pan, G.; Elmquist, W.F. Interactions of pluronic block copolymers on P-gp efflux activity: Experience with HIV-1 protease inhibitors. J. Pharm. Sci. 2008, 97, 5421–5433. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bollenbach, L.; Buske, J.; Mäder, K.; Garidel, P. Poloxamer 188 as surfactant in biological formulations—An alternative for polysorbate 20/80? Int. J. Pharm. 2022, 620, 121706. [Google Scholar] [CrossRef]
- Akash, M.S.H.; Rehman, K. Recent progress in biomedical applications of Pluronic (PF127): Pharmaceutical perspectives. J. Control. Release 2015, 209, 120–138. [Google Scholar] [CrossRef]
- Shriky, B.; Kelly, A.; Isreb, M.; Babenko, M.; Mahmoudi, N.; Rogers, S.; Shebanova, O.; Snow, T.; Gough, T. Pluronic F127 thermosensitive injectable smart hydrogels for controlled drug delivery system development. J. Colloid Interface Sci. 2020, 565, 119–130. [Google Scholar] [CrossRef]
- Leyva-Gómez, G.; Santillan-Reyes, E.; Lima, E.; Madrid-Martínez, A.; Krötzsch, E.; Quintanar-Guerrero, D.; Garciadiego-Cázares, D.; Martínez-Jiménez, A.; Morales, M.H.; Ortega-Peña, S.; et al. A novel hydrogel of poloxamer 407 and chitosan obtained by gamma irradiation exhibits physicochemical properties for wound management. Mater. Sci. Eng. C 2017, 74, 36–46. [Google Scholar] [CrossRef]
- Chou, H.-Y.; Weng, C.-C.; Lai, J.-Y.; Lin, S.-Y.; Tsai, H.-C. Design of an Interpenetrating Polymeric Network Hydrogel Made of Calcium-Alginate from a Thermos-Sensitive Pluronic Template as a Thermal-Ionic Reversible Wound Dressing. Polymers 2020, 12, 2138. [Google Scholar] [CrossRef]
- Mayol, L.; Quaglia, F.; Borzacchiello, A.; Ambrosio, L.; Rotonda, M. A novel poloxamers/hyaluronic acid in situ forming hydrogel for drug delivery: Rheological, mucoadhesive and in vitro release properties. Eur. J. Pharm. Biopharm. 2008, 70, 199–206. [Google Scholar] [CrossRef] [PubMed]
- Inal, O.; Yapar, E.A. Effect of Mechanical Properties on the Release of Meloxicam from Poloxamer Gel Bases. Indian, J. Pharm. Sci. 2013, 75, 700–706. [Google Scholar] [PubMed]
- Cho, K.Y.; Chung, T.W.; Kim, B.C.; Kim, M.K.; Lee, J.H.; Wee, W.R.; Cho, C.S. Release of ciprofloxacin from poloxamer-graft-hyaluronic acid hydrogels in vitro. Int. J. Pharm. 2003, 260, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Dumortier, G.; Grossiord, J.L.; Agnely, F.; Chaumeil, J.C. A Review of Poloxamer 407 Pharmaceutical and Pharmacological Characteristics. Pharm. Res. 2006, 23, 2709–2728. [Google Scholar] [CrossRef]
- Moore, T.; Croy, S.; Mallapragada, S.; Pandit, N. Experimental investigation and mathematical modeling of Pluronic® F127 gel dissolution: Drug release in stirred systems. J. Control. Release 2000, 67, 191–202. [Google Scholar] [CrossRef]
- Desai, S.D.; Blanchard, J. In Vitro Evaluation of Pluronic F127-Based Controlled-Release Ocular Delivery Systems for Pilocarpine. J. Pharm. Sci. 1998, 87, 226–230. [Google Scholar] [CrossRef]
- Dimitrova, E.; Bogdanova, S.; Mitcheva, M.; Tanev, I.; Minkov, E. Development of Model Aqueous Ophthalmic Solution of Indomethacin. Drug Dev. Ind. Pharm. 2000, 26, 1297–1301. [Google Scholar] [CrossRef]
- Veyries, M.L.; Couarraze, G.; Geiger, S.; Agnely, F.; Massias, L.; Kunzli, B.; Faurisson, F.; Rouveix, B. Controlled release of vancomycin from Poloxamer 407 gels. Int. J. Pharm. 1999, 192, 183–193. [Google Scholar] [CrossRef]
- Shaarani, S.; Hamid, S.S.; Kaus, N.H.M. The Influence of pluronic F68 and F127 nanocarrier on physicochemical properties, in vitro release, and antiproliferative activity of thymoquinone drug. Pharmacogn. Res. 2017, 9, 12–20. [Google Scholar] [CrossRef] [Green Version]
- Khaliq, N.U.; Park, D.Y.; Lee, J.Y.; Joo, Y.; Oh, K.S.; Kim, J.S.; Kim, J.-S.; Kim, I.-S.; Kwon, I.C.; Yuk, S.H. The multilayer nanoparticles for deep penetration of docetaxel into tumor parenchyma to overcome tumor microenvironment. Colloids Surf. B Biointerfaces 2016, 146, 833–840. [Google Scholar] [CrossRef]
- Barichello, J.M.; Morishita, M.; Takayama, K.; Chiba, Y.; Tokiwa, S.; Nagai, T. Enhanced rectal absorption of insulin-loaded Pluronic® F-127 gels containing unsaturated fatty acids. Int. J. Pharm. 1999, 183, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Barichello, J.M.; Morishita, M.; Takayama, K.; Nagai, T. Absorption of insulin from Pluronic F-127 gels following subcutaneous administration in rats. Int. J. Pharm. 1999, 184, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Strickley, R.G. Solubilizing excipients in oral and injectable formulations. Pharm. Res. 2004, 21, 201–230. [Google Scholar] [CrossRef] [PubMed]
- Chutimaworapan, S.; Ritthidej, G.C.; Yonemochi, E.; Oguchi, T.; Yamamoto, K. Effect of Water-Soluble Carriers on Dissolution Characteristics of Nifedipine Solid Dispersions. Drug Dev. Ind. Pharm. 2000, 26, 1141–1150. [Google Scholar] [CrossRef]
- Rogers, T.L.; Johnston, K.P.; Williams, R.O., III. Physical stability of micronized powders produced by spray-freezing into liquid (SFL) to enhance the dissolution of an insoluble drug. Pharm. Dev. Technol. 2003, 8, 187–197. [Google Scholar] [CrossRef]
- Rogers, T.L.; Nelsen, A.C.; Sarkari, M.; Young, T.J.; Johnston, K.P.; Williams, I.R.O. Enhanced Aqueous Dissolution of a Poorly Water Soluble Drug by Novel Particle Engineering Technology: Spray-Freezing into Liquid with Atmospheric Freeze-Drying. Pharm. Res. 2003, 20, 485–493. [Google Scholar] [CrossRef]
- Rogers, T.L.; Overhoff, K.A.; Shah, P.; Santiago, P.; Yacaman, M.J.; Johnston, K.P.; Williams III, R.O. Micronized powders of a poorly water soluble drug produced by a spray-freezing into liquid-emulsion process. Eur. J. Pharm. Biopharm. 2003, 55, 161–172. [Google Scholar] [CrossRef]
- Veyries, M.-L.; Faurisson, F.; Joly-Guillou, M.-L.; Rouveix, B.; Patel, R.; Piper, K.; Iii, F.R.C.; Steckelberg, J.M.; Yousten, A.A. Control of Staphylococcal Adhesion to Polymethylmethacrylate and Enhancement of Susceptibility to Antibiotics by Poloxamer 407. Antimicrob. Agents Chemother. 2000, 44, 705–709. [Google Scholar] [CrossRef] [Green Version]
- Gilbert, P.; Jones, M.; Allison, D.; Heys, S.; Maira, T.; Wood, P. The use of poloxamer hydrogels for the assessment of biofilm susceptibility towards biocide treatments. J. Appl. Microbiol. 1998, 85, 985–990. [Google Scholar] [CrossRef]
- Ban, E.; Park, M.; Jeong, S.; Kwon, T.; Kim, E.-H.; Jung, K.; Kim, A. Poloxamer-Based Thermoreversible Gel for Topical Delivery of Emodin: Influence of P407 and P188 on Solubility of Emodin and Its Application in Cellular Activity Screening. Molecules 2017, 22, 246. [Google Scholar] [CrossRef] [Green Version]
- Ban, E.; Jang, D.-J.; Kim, S.-J.; Park, M.; Kim, A. Optimization of thermoreversible poloxamer gel system using QbD principle. Pharm. Dev. Technol. 2017, 22, 939–945. [Google Scholar] [CrossRef] [PubMed]
- Pec, E.; Wout, Z.; Johnston, T. Biological Activity of Urease Formulated in Poloxamer 407 after Intraperitoneal Injection in the Rat. J. Pharm. Sci. 1992, 81, 626–630. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.L.; Johnston, T.P. Enhanced stability of two model proteins in an agitated solution environment using poloxamer 407. J. Parenter. Sci. Technol. A Publ. Parenter. Drug Assoc. 1993, 47, 183–189. [Google Scholar]
- Bromberg, L.E. Interactions among proteins and hydrophobically modified polyelectrolytes. J. Pharm. Pharmacol. 2001, 53, 541–547. [Google Scholar] [CrossRef]
- Lin, W.J.; Huang, L.I. Influence of pluronics on protein-loaded poly (epsilon-caprolactone) microparticles. J. Microencapsul. 2001, 18, 191–197. [Google Scholar] [CrossRef]
- Woodle, M.; Newman, M.; Martin, F. Liposome leakage and blood circulation: Comparison of adsorbed block copolymers with covalent attachment of PEG. Int. J. Pharm. 1992, 88, 327–334. [Google Scholar] [CrossRef]
- Castile, J.D.; Taylor, K.M.; Buckton, G. A high sensitivity differential scanning calorimetry study of the interaction between poloxamers and dimyristoylphosphatidylcholine and dipalmitoylphosphatidylcholine liposomes. Int. J. Pharm. 1999, 182, 101–110. [Google Scholar] [CrossRef]
- Castile, J.D.; Taylor, K.M.; Buckton, G. The influence of incubation temperature and surfactant concentration on the interaction between dimyristoylphosphatidylcholine liposomes and poloxamer surfactants. Int. J. Pharm. 2001, 221, 197–209. [Google Scholar] [CrossRef]
- Grapentin, C.; Müller, C.; Kishore, R.S.; Adler, M.; ElBialy, I.; Friess, W.; Huwyler, J.; Khan, T.A. Protein-Polydimethylsiloxane Particles in Liquid Vial Monoclonal Antibody Formulations Containing Poloxamer 188. J. Pharm. Sci. 2020, 109, 2393–2404. [Google Scholar] [CrossRef]
- Yong, C.S.; Choi, J.S.; Quan, Q.-Z.; Rhee, J.-D.; Kim, C.-K.; Lim, S.-J.; Kim, K.-M.; Oh, P.-S.; Choi, H.-G. Effect of sodium chloride on the gelation temperature, gel strength and bioadhesive force of poloxamer gels containing diclofenac sodium. Int. J. Pharm. 2001, 226, 195–205. [Google Scholar] [CrossRef]
- Ryu, J.-M.; Chung, S.-J.; Lee, M.-H.; Kim, C.-K.; Shim, C.-K. Increased bioavailability of propranolol in rats by retaining thermally gelling liquid suppositories in the rectum. J. Control. Release 1999, 59, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.-G.; Lee, M.-K.; Kim, M.-H.; Kim, C.-K. Effect of additives on the physicochemical properties of liquid suppository bases. Int. J. Pharm. 1999, 190, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Le Ray, A.; Iooss, P.; Gouyette, A.; Vonarx, V.; Patrice, T.; Merle, C. Development of a “continuous-flow adhesion cell” for the assessment of hydrogel adhesion. Drug Dev. Ind. Pharm. 1999, 25, 897–904. [Google Scholar] [CrossRef] [PubMed]
- Bourre, L.; Thibaut, S.; Briffaud, A.; Lajat, Y.; Patrice, T. Potential efficacy of a delta 5-aminolevulinic acid thermosetting gel formulation for use in photodynamic therapy of lesions of the gastrointestinal tract. Pharmacol. Res. 2002, 45, 159–165. [Google Scholar] [CrossRef]
- Pisal, S.S.; Paradkar, A.R.; Mahadik, K.R.; Kadam, S.S. Pluronic gels for nasal delivery of Vitamin B12. Part I: Preformulation study. Int. J. Pharm. 2004, 270, 37–45. [Google Scholar] [CrossRef]
- Kim, E.-Y.; Gao, Z.-G.; Park, J.-S.; Li, H.; Han, K. rhEGF/HP-β-CD complex in poloxamer gel for ophthalmic delivery. Int. J. Pharm. 2002, 233, 159–167. [Google Scholar] [CrossRef]
- Chang, J.Y.; Oh, Y.-K.; Choi, H.-G.; Kim, Y.B.; Kim, C.-K. Rheological evaluation of thermosensitive and mucoadhesive vaginal gels in physiological conditions. Int. J. Pharm. 2002, 241, 155–163. [Google Scholar] [CrossRef]
- Shawesh, A.; Kallioinen, S.; Antikainen, O.; Yliruusi, J. Influence of storage time and temperature on the stability of indomethacin Pluronic F-127 gels. Die Pharm. 2002, 57, 690–694. [Google Scholar]
- Oh, S.H.; Kim, J.K.; Song, K.S.; Noh, S.M.; Ghil, S.H.; Yuk, S.H.; Lee, J.H. Prevention of postsurgical tissue adhesion by anti-inflammatory drug-loaded pluronic mixtures with sol-gel transition behavior. J. Biomed. Mater. Res. Part A 2005, 72, 306–316. [Google Scholar] [CrossRef]
- Lee, M.H.; Shin, G.H.; Park, H.J. Solid lipid nanoparticles loaded thermoresponsive pluronic-xanthan gum hydrogel as a transdermal delivery system. J. Appl. Polym. Sci. 2018, 135, 46004. [Google Scholar] [CrossRef]
- Khaliq, N.U.; Oh, K.S.; Sandra, F.C.; Joo, Y.; Lee, J.; Byun, Y.; Kim, I.-S.; Kwon, I.C.; Seo, J.H.; Kim, S.Y.; et al. Assembly of polymer micelles through the sol-gel transition for effective cancer therapy. J. Control. Release 2017, 255, 258–269. [Google Scholar] [CrossRef] [PubMed]
- Khemtong, C.; Kessinger, C.W.; Gao, J. Polymeric nanomedicine for cancer MR imaging and drug delivery. Chem. Commun. 2009, 24, 3497–3510. [Google Scholar] [CrossRef]
- Kröger, N.; Achterrath, W.; Hegewisch-Becker, S.; Mross, K.; Zander, A.R. Current options in treatment of anthracycline-resistant breast cancer. Cancer Treat. Rev. 1999, 25, 279–291. [Google Scholar] [CrossRef]
- Naito, S.; Yokomizo, A.; Koga, H. Mechanisms of drug resistance in chemotherapy for urogenital carcinoma. Int. J. Urol. 1999, 6, 427–439. [Google Scholar] [CrossRef] [Green Version]
- Shapira, A.; Livney, Y.D.; Broxterman, H.J.; Assaraf, Y.G. Nanomedicine for targeted cancer therapy: Towards the overcoming of drug resistance. Drug Resist. Updat. 2011, 14, 150–163. [Google Scholar] [CrossRef]
- Gelperina, S.; Kisich, K.; Iseman, M.D.; Heifets, L. The Potential Advantages of Nanoparticle Drug Delivery Systems in Chemotherapy of Tuberculosis. Am. J. Respir. Crit. Care Med. 2005, 172, 1487–1490. [Google Scholar] [CrossRef] [Green Version]
- Haseeb, M.T.; Khaliq, N.U.; Yuk, S.H.; Hussain, M.A.; Bashir, S. Linseed polysaccharides based nanoparticles for controlled delivery of docetaxel: Design, in vitro drug release and cellular uptake. J. Drug Deliv. Sci. Technol. 2019, 49, 143–151. [Google Scholar] [CrossRef]
- Park, J.H.; Saravanakumar, G.; Kim, K.; Kwon, I.C. Targeted delivery of low molecular drugs using chitosan and its derivatives. Adv. Drug Deliv. Rev. 2010, 62, 28–41. [Google Scholar] [CrossRef]
- Oh, K.S.; Song, J.Y.; Cho, S.H.; Lee, B.S.; Kim, S.Y.; Kim, K.; Jeon, H.; Kwon, I.C.; Yuk, S.H. Paclitaxel-loaded Pluronic nanoparticles formed by a temperature-induced phase transition for cancer therapy. J. Control. Release 2010, 148, 344–350. [Google Scholar] [CrossRef]
- Chen, L.; Sha, X.; Jiang, X.; Chen, Y.; Ren, Q.; Fang, X. Pluronic P105/F127 mixed micelles for the delivery of docetaxel against Taxol-resistant non-small cell lung cancer: Optimization and in vitro, in vivo evaluation. Int. J. Nanomed. 2013, 8, 73–84. [Google Scholar] [CrossRef] [Green Version]
- Yuk, S.H.; Oh, K.S.; Cho, S.H.; Kim, S.Y.; Oh, S.; Lee, J.H.; Kim, K.; Kwon, I.C. Enhancement of the Targeting Capabilities of the Paclitaxel-Loaded Pluronic Nanoparticles with a Glycol Chitosan/Heparin Composite. Mol. Pharm. 2012, 9, 230–236. [Google Scholar] [CrossRef] [PubMed]
- Wen, P.; Ke, W.; Dirisala, A.; Toh, K.; Tanaka, M.; Li, J. Stealth and pseudo-stealth nanocarriers. Adv. Drug Deliv. Rev. 2023, 198, 114895. [Google Scholar] [CrossRef]
- Popovici, C.; Popa, M.; Sunel, V.; Atanase, L.I.; Ichim, D.L. Drug Delivery Systems Based on Pluronic Micelles with Antimicrobial Activity. Polymers 2022, 14, 3007. [Google Scholar] [CrossRef]
- Desai, V.G.; Herman, E.H.; Moland, C.L.; Branham, W.S.; Lewis, S.M.; Davis, K.J.; George, N.I.; Lee, T.; Kerr, S.; Fuscoe, J.C. Development of doxorubicin-induced chronic cardiotoxicity in the B6C3F1 mouse model. Toxicol. Appl. Pharmacol. 2013, 266, 109–121. [Google Scholar] [CrossRef]
- Kremer, L.C.; Caron, H.N. Anthracycline Cardiotoxicity in Children. N. Engl. J. Med. 2004, 351, 120–121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rebbaa, A.; Zheng, X.; Chou, P.M.; Mirkin, B.L. Caspase inhibition switches doxorubicin-induced apoptosis to senescence. Oncogene 2003, 22, 2805–2811. [Google Scholar] [CrossRef] [Green Version]
- Shim, M.K.; Yang, S.; Park, J.; Yoon, J.S.; Kim, J.; Moon, Y.; Shim, N.; Jo, M.; Choi, Y.; Kim, K. Preclinical development of carrier-free prodrug nanoparticles for enhanced antitumor therapeutic potential with less toxicity. J. Nanobiotechnol. 2022, 20, 436. [Google Scholar] [CrossRef]
- Jarak, I.; Varela, C.L.; da Silva, E.T.; Roleira, F.F.; Veiga, F.; Figueiras, A. Pluronic-based nanovehicles: Recent advances in anticancer therapeutic applications. Eur. J. Med. Chem. 2020, 206, 112526. [Google Scholar] [CrossRef]
- Valle, J.W.; Armstrong, A.; Newman, C.; Alakhov, V.; Pietrzynski, G.; Brewer, J.; Campbell, S.; Corrie, P.; Rowinsky, E.K.; Ranson, M. A phase 2 study of SP1049C, doxorubicin in P-glycoprotein-targeting pluronics, in patients with advanced adenocarcinoma of the esophagus and gastroesophageal junction. Investig. New Drugs 2011, 29, 1029–1037. [Google Scholar] [CrossRef]
- Rowe, R.C.; Sheskey, P.; Quinn, M. Handbook of Pharmaceutical Excipients; Libros Digitales-Pharmaceutical Press: London, UK, 2009. [Google Scholar]
- Lee, B.S.; Cho, Y.W.; Kim, G.C.; Lee, D.H.; Kim, C.J.; Kil, H.S.; Chi, D.Y.; Byun, Y.; Yuk, S.H.; Kim, K.; et al. Induced Phenotype Targeted Therapy: Radiation-Induced Apoptosis-Targeted Chemotherapy. J. Natl. Cancer Inst. 2015, 107, dju403. [Google Scholar] [CrossRef] [PubMed] [Green Version]
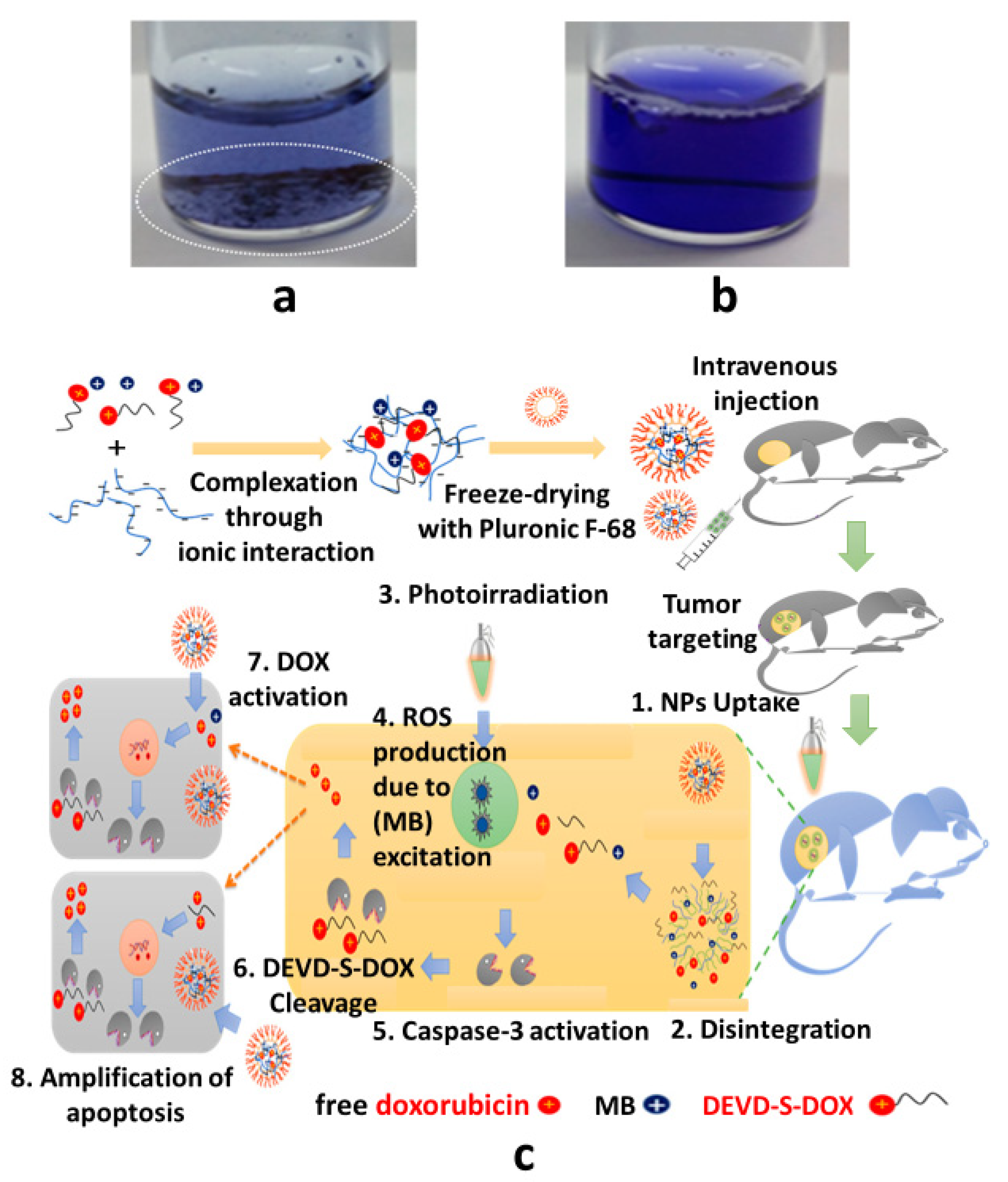
- Khaliq, N.U.; Sandra, F.C.; Park, D.Y.; Lee, J.Y.; Oh, K.S.; Kim, D.; Byun, Y.; Kim, I.-S.; Kwon, I.C.; Kim, S.Y.; et al. Doxorubicin/heparin composite nanoparticles for caspase-activated prodrug chemotherapy. Biomaterials 2016, 101, 131–142. [Google Scholar] [CrossRef] [PubMed]
- Butt, A.M.; Mohd Amin, M.C.I.; Katas, H. Synergistic effect of pH-responsive folate-functionalized poloxamer 407-TPGS-mixed micelles on targeted delivery of anticancer drugs. Int. J. Nanomed. 2015, 10, 1321–1334. [Google Scholar] [CrossRef] [Green Version]
- Soe, Z.C.; Kwon, J.B.; Thapa, R.K.; Ou, W.; Nguyen, H.T.; Gautam, M.; Oh, K.T.; Choi, H.-G.; Ku, S.K.; Yong, C.S.; et al. Transferrin-Conjugated Polymeric Nanoparticle for Receptor-Mediated Delivery of Doxorubicin in Doxorubicin-Resistant Breast Cancer Cells. Pharmaceutics 2019, 11, 63. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Yu, Y.; Ji, Q.; Qiu, L. Targeted delivery of anticancer drugs by aptamer AS1411 mediated Pluronic F127/cyclodextrin-linked polymer composite micelles. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 175–184. [Google Scholar] [CrossRef]
- Wang, H.; Williams, G.R.; Wu, J.; Wu, J.; Niu, S.; Xie, X.; Li, S.; Zhu, L.-M. Pluronic F127-based micelles for tumor-targeted bufalin delivery. Int. J. Pharm. 2019, 559, 289–298. [Google Scholar] [CrossRef]
- Russo, A.; Pellosi, D.S.; Pagliara, V.; Milone, M.R.; Pucci, B.; Caetano, W.; Hioka, N.; Budillon, A.; Ungaro, F.; Russo, G.; et al. Biotin-targeted Pluronic® P123/F127 mixed micelles delivering niclosamide: A repositioning strategy to treat drug-resistant lung cancer cells. Int. J. Pharm. 2016, 511, 127–139. [Google Scholar] [CrossRef] [Green Version]
- Tima, S.; Okonogi, S.; Ampasavate, C.; Pickens, C.; Berkland, C.; Anuchapreeda, S. Development and Characterization of FLT3-Specific Curcumin-Loaded Polymeric Micelles as a Drug Delivery System for Treating FLT3-Overexpressing Leukemic Cells. J. Pharm. Sci. 2016, 105, 3645–3657. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, X.; Lv, X.; Xu, J.; Zheng, Y.; Wang, X.; Tang, R. Pluronic micelles with suppressing doxorubicin efflux and detoxification for efficiently reversing breast cancer resistance. Eur. J. Pharm. Sci. 2020, 146, 105275. [Google Scholar] [CrossRef]
- Cheng, X.; Zeng, X.; Zheng, Y.; Fang, Q.; Wang, X.; Wang, J.; Tang, R. pH-sensitive pluronic micelles combined with oxidative stress amplification for enhancing multidrug resistance breast cancer therapy. J. Colloid Interface Sci. 2020, 565, 254–269. [Google Scholar] [CrossRef]
- Xu, C.; Xu, J.; Zheng, Y.; Fang, Q.; Lv, X.; Wang, X.; Tang, R. Active-targeting and acid-sensitive pluronic prodrug micelles for efficiently overcoming MDR in breast cancer. J. Mater. Chem. B 2020, 8, 2726–2737. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, W.; Bai, J.; Jin, L.; Kang, X.; Zhang, H.; Wang, Z. Pluronic P123 modified nano micelles loaded with doxorubicin enhanced tumor-suppressing effect on drug-resistant breast cancer cells. Aging 2020, 12, 8289–8300. [Google Scholar] [CrossRef] [PubMed]
- Gregoriou, Y.; Gregoriou, G.; Yilmaz, V.; Kapnisis, K.; Prokopi, M.; Anayiotos, A.; Strati, K.; Dietis, N.; Constantinou, A.I.; Andreou, C. Resveratrol loaded polymeric micelles for theranostic targeting of breast cancer cells. Nanotheranostics 2021, 5, 113–124. [Google Scholar] [CrossRef] [PubMed]
- Kamble, S.; Varamini, P.; Müllner, M.; Pelras, T.; Rohanizadeh, R. Bisphosphonate-functionalized micelles for targeted delivery of curcumin to metastatic bone cancer. Pharm. Dev. Technol. 2020, 25, 1118–1126. [Google Scholar] [CrossRef] [PubMed]
- Prasad Kushwaha, J.; Baidya, D.; Patil, S. Harmine-loaded galactosylated pluronic F68-gelucire 44/14 mixed micelles for liver targeting. Drug Dev. Ind. Pharm. 2019, 45, 1361–1368. [Google Scholar] [CrossRef] [PubMed]
- Turabee, H.; Jeong, T.H.; Ramalingam, P.; Kang, J.H.; Ko, Y.T. N,N,N-trimethyl chitosan embedded in situ Pluronic F127 hydrogel for the treatment of brain tumor. Carbohydr. Polym. 2019, 203, 302–309. [Google Scholar] [CrossRef]
- Gan, H.; Chen, L.; Sui, X.; Wu, B.; Zou, S.; Li, A.; Zhang, Y.; Liu, X.; Wang, D.; Cai, S.; et al. Enhanced delivery of sorafenib with anti-GPC3 antibody-conjugated TPGS-b-PCL/Pluronic P123 polymeric nanoparticles for targeted therapy of hepatocellular carcinoma. Mater. Sci. Eng. C 2018, 91, 395–403. [Google Scholar] [CrossRef]
- Dehghan Kelishady, P.; Saadat, E.; Ravar, F.; Akbari, H.; Dorkoosh, F. Pluronic F127 polymeric micelles for co-delivery of paclitaxel and lapatinib against metastatic breast cancer: Preparation, optimization and in vitro evaluation. Pharm. Dev. Technol. 2015, 20, 1009–1017. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, W.; Huang, Y.; Gao, F.; Sha, X.; Fang, X. Pluronic-based functional polymeric mixed micelles for co-delivery of doxorubicin and paclitaxel to multidrug resistant tumor. Int. J. Pharm. 2015, 488, 44–58. [Google Scholar] [CrossRef]
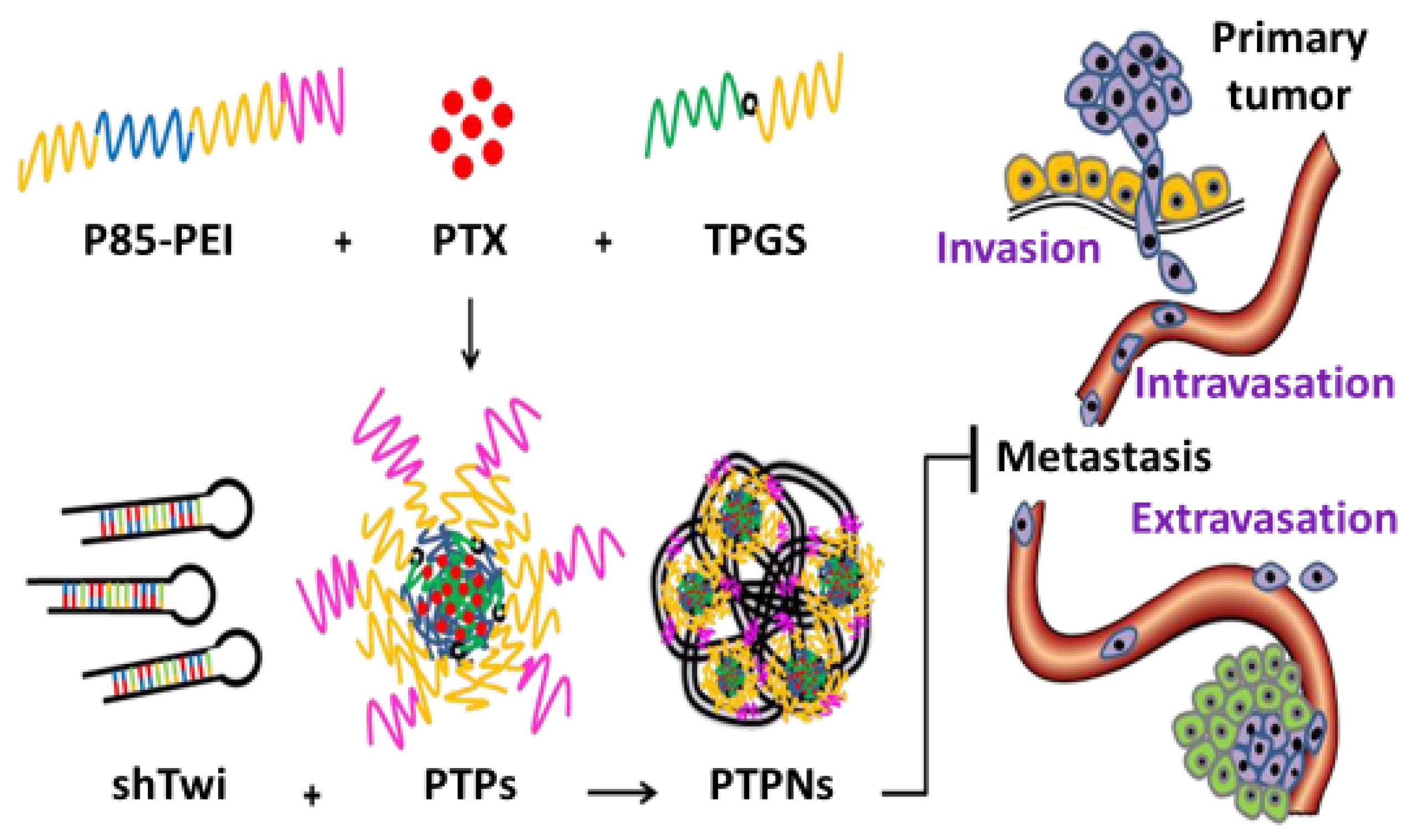
- Shen, J.; Sun, H.; Xu, P.; Yin, Q.; Zhang, Z.; Wang, S.; Yu, H.; Li, Y. Simultaneous inhibition of metastasis and growth of breast cancer by co-delivery of twist shRNA and paclitaxel using pluronic P85-PEI/TPGS complex nanoparticles. Biomaterials 2013, 34, 1581–1590. [Google Scholar] [CrossRef]
- Yin, T.; Wang, P.; Li, J.; Wang, Y.; Zheng, B.; Zheng, R.; Cheng, D.; Shuai, X. Tumor-penetrating codelivery of siRNA and paclitaxel with ultrasound-responsive nanobubbles hetero-assembled from polymeric micelles and liposomes. Biomaterials 2014, 35, 5932–5943. [Google Scholar] [CrossRef] [PubMed]
- Rafael, D.; Martínez, F.; Andrade, F.; Seras-Franzoso, J.; Garcia-Aranda, N.; Gener, P.; Sayós, J.; Arango, D.; Abasolo, I.; Schwartz, S., Jr. Efficient EFGR mediated siRNA delivery to breast cancer cells by Cetuximab functionalized Pluronic® F127/Gelatin. Chem. Eng. J. 2018, 340, 81–93. [Google Scholar] [CrossRef]
- Khaliq, N.U.; Park, D.Y.; Lee, H.J.; Oh, K.S.; Seo, J.H.; Kim, S.Y.; Hwang, C.S.; Lim, T.-H.; Yuk, S.H. Pluronic/Heparin Nanoparticles for Chemo-Photodynamic Combination Cancer Therapy through Photoinduced Caspase-3 Activation. ACS Appl. Nano Mater. 2018, 1, 2943–2952. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, J.; Qin, Y.; Song, H.; Huang, P.; Wang, W.; Wang, C.; Li, C.; Wang, Y.; Kong, D. Co-delivery of doxorubicin and pheophorbide A by pluronic F127 micelles for chemo-photodynamic combination therapy of melanoma. J. Mater. Chem. B 2018, 6, 3305–3314. [Google Scholar] [CrossRef] [PubMed]
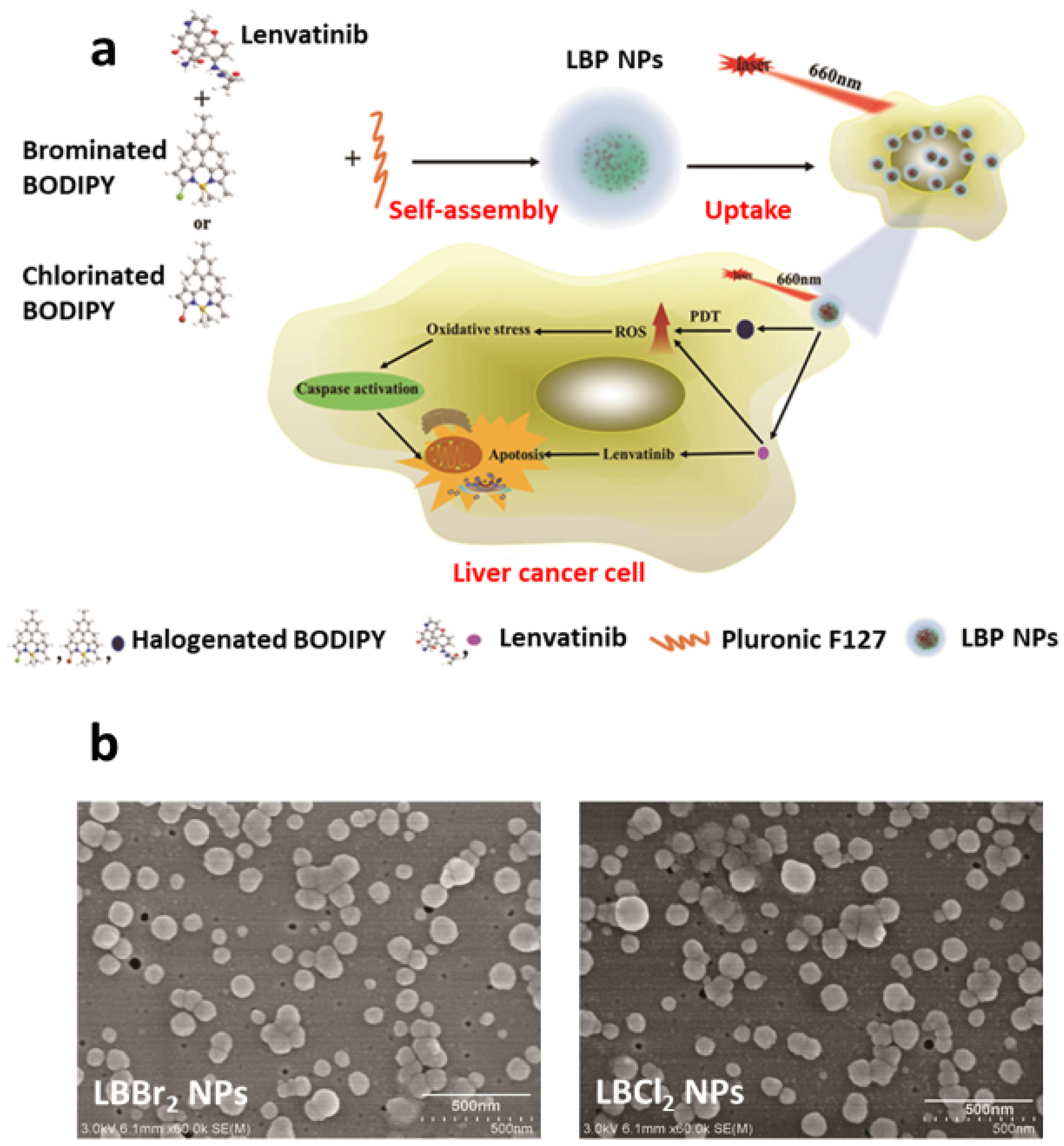
- Zong, J.; Peng, H.; Qing, X.; Fan, Z.; Xu, W.; Du, X.; Shi, R.; Zhang, Y. pH-Responsive Pluronic F127–Lenvatinib-Encapsulated Halogenated Boron-Dipyrromethene Nanoparticles for Combined Photodynamic Therapy and Chemotherapy of Liver Cancer. ACS Omega 2021, 6, 12331–12342. [Google Scholar] [CrossRef] [PubMed]
- Ma, M.; Cheng, L.; Zhao, A.; Zhang, H.; Zhang, A. Pluronic-based graphene oxide-methylene blue nanocomposite for photodynamic/photothermal combined therapy of cancer cells. Photodiagnosis Photodyn. Ther. 2020, 29, 101640. [Google Scholar] [CrossRef]
- Guan, J.; Lai, X.; Wang, X.; Leung, A.W.; Zhang, H.; Xu, C. Photodynamic action of methylene blue in osteosarcoma cells in vitro. Photodiagnosis Photodyn. Ther. 2014, 11, 13–19. [Google Scholar] [CrossRef]
- Gabrielli, D.; Belisle, E.; Severino, D.; Kowaltowski, A.J.; Baptista, M.S. Binding, Aggregation and Photochemical Properties of Methylene Blue in Mitochondrial Suspensions. Photochem. Photobiol. 2004, 79, 227–232. [Google Scholar] [CrossRef]
- Yang, K.; Feng, L.; Shi, X.; Liu, Z. Nano-graphene in biomedicine: Theranostic applications. Chem. Soc. Rev. 2013, 42, 530–547. [Google Scholar] [CrossRef]
- Chen, Y.; Tan, C.; Zhang, H.; Wang, L. Two-dimensional graphene analogues for biomedical applications. Chem. Soc. Rev. 2015, 44, 2681–2701. [Google Scholar] [CrossRef]
- de Melo-Diogo, D.; Lima-Sousa, R.; Alves, C.G.; Costa, E.C.; Louro, R.O.; Correia, I.J. Functionalization of graphene family nanomaterials for application in cancer therapy. Colloids Surf. B Biointerfaces 2018, 171, 260–275. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Dong, H.; Zhu, H.; Li, C.-W.; Li, Y.; Shi, D. Deposition of gadolinium onto the shell structure of micelles for integrated magnetic resonance imaging and robust drug delivery systems. J. Mater. Chem. B 2016, 4, 6094–6102. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Oh, K.S.; Park, D.Y.; Lee, J.Y.; Lee, B.S.; San Kim, I.; Kim, K.; Kwon, I.C.; Sang, Y.K.; Yuk, S.H. Doxorubicin/gold-loaded core/shell nanoparticles for combination therapy to treat cancer through the enhanced tumor targeting. J. Control. Release 2016, 228, 141–149. [Google Scholar] [CrossRef] [PubMed]
- De Castro, K.C.; Coco, J.C.; dos Santos, É.M.; Ataide, J.A.; Martinez, R.M.; do Nascimento, M.H.M.; Prata, J.; da Fonte, P.R.M.L.; Severino, P.; Mazzola, P.G.; et al. Pluronic® triblock copolymer-based nanoformulations for cancer therapy: A 10-year overview. J. Control. Release 2023, 353, 802–822. [Google Scholar] [CrossRef] [PubMed]
- Zielińska, A.; Eder, P.; Rannier, L.; Cardoso, J.C.; Severino, P.; Silva, A.M.; Souto, E.B. Hydrogels for modified-release drug delivery systems. Curr. Pharm. Des. 2022, 28, 609–618. [Google Scholar] [CrossRef]
- Kopeček, J. Hydrogel biomaterials: A smart future? Biomaterials 2007, 28, 5185–5192. [Google Scholar] [CrossRef] [Green Version]
- Peppas, N.A.; Hilt, J.Z.; Khademhosseini, A.; Langer, R. Hydrogels in Biology and Medicine: From Molecular Principles to Bionanotechnology. Adv. Mater. 2006, 18, 1345–1360. [Google Scholar] [CrossRef]
- Khaliq, N.U.; Park, D.Y.; Yun, B.M.; Yang, D.H.; Jung, Y.W.; Seo, J.H.; Hwang, C.S.; Yuk, S.H. Pluronics: Intelligent building units for targeted cancer therapy and molecular imaging. Int. J. Pharm. 2019, 556, 30–44. [Google Scholar] [CrossRef]
- Hu, H.; Lin, Z.; He, B.; Dai, W.; Wang, X.; Wang, J.; Zhang, X.; Zhang, H.; Zhang, Q. A novel localized co-delivery system with lapatinib microparticles and paclitaxel nanoparticles in a peritumorally injectable in situ hydrogel. J. Control. Release 2015, 220, 189–200. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Wen, P.; Li, J.; Kataoka, K. Targeted nanomedicine in cisplatin-based cancer therapeutics. J. Control. Release 2022, 345, 709–720. [Google Scholar] [CrossRef]
- Yuk, S.H.; Oh, K.S.; Koo, H.; Jeon, H.; Kim, K.; Kwon, I.C. Multi-core vesicle nanoparticles based on vesicle fusion for delivery of chemotherapic drugs. Biomaterials 2011, 32, 7924–7931. [Google Scholar] [CrossRef] [PubMed]
- Pachioni-Vasconcelos, J.d.A.; Apolinário, A.C.; Lopes, A.M.; Pessoa, A., Jr.; Barbosa, L.R.S.; Rangel-Yagui, C.d.O. Compartmentalization of therapeutic proteins into semi-crystalline PEG-PCL polymersomes. Soft Mater. 2021, 19, 222–230. [Google Scholar] [CrossRef]
- Tundisi, L.L.; Ataide, J.A.; Costa, J.S.R.; Coêlho, D.D.F.; Liszbinski, R.B.; Lopes, A.M.; Oliveira-Nascimento, L.; de Jesus, M.B.; Jozala, A.F.; Ehrhardt, C.; et al. Nanotechnology as a tool to overcome macromolecules delivery issues. Colloids Surf. B Biointerfaces 2022, 222, 113043. [Google Scholar] [CrossRef] [PubMed]
- Yau, A.; Lee, J.; Chen, Y. Nanomaterials for Protein Delivery in Anticancer Applications. Pharmaceutics 2021, 13, 155. [Google Scholar] [CrossRef]
- Pachioni-Vasconcelos, J.; Lopes, A.; Apolinario, A.; Valenzuela-Oses, J.; Costa, J.; Nascimento, L.; Pessoa, A.; Barbosa, L.; Rangel-Yagui, C. Nanostructures for protein drug delivery. Biomater. Sci. 2015, 4, 205–218. [Google Scholar] [CrossRef]
- Cui, R.; Wu, Q.; Wang, J.; Zheng, X.; Ou, R.; Xu, Y.; Qu, S.; Li, D. Hydrogel-By-Design: Smart Delivery System for Cancer Immunotherapy. Front. Bioeng. Biotechnol. 2021, 9, 723490. [Google Scholar] [CrossRef]
- Kimura, Y.; Aoyama, S.; Ueda, N.; Katayama, T.; Ono, K.; Nagahama, K. Covalent Cell-Loading Injectable Hydrogel Scaffold Significantly Promotes Tissue Regeneration In Vivo Compared with a Conventional Physical Cell-Loading Hydrogel Scaffold. Adv. Biol. 2021, 5, 2000106. [Google Scholar] [CrossRef]
- Fang, L.; Zhao, Z.; Wang, J.; Zhang, P.; Ding, Y.; Jiang, Y.; Wang, D.; Li, Y. Engineering autologous tumor cell vaccine to locally mobilize antitumor immunity in tumor surgical bed. Sci. Adv. 2020, 6, eaba4024. [Google Scholar] [CrossRef]
- Kim, J.; Francis, D.M.; Sestito, L.F.; Archer, P.A.; Manspeaker, M.P.; O’melia, M.J.; Thomas, S.N. Thermosensitive hydrogel releasing nitric oxide donor and anti-CTLA-4 micelles for anti-tumor immunotherapy. Nat. Commun. 2022, 13, 1479. [Google Scholar] [CrossRef]
- Jin, H.; Liao, S.; Yao, F.; Li, J.; Xu, Z.; Zhao, K.; Xu, X.; Sun, S. Insight into the Crosstalk between Photodynamic Therapy and Immunotherapy in Breast Cancer. Cancers 2023, 15, 1532. [Google Scholar] [CrossRef]
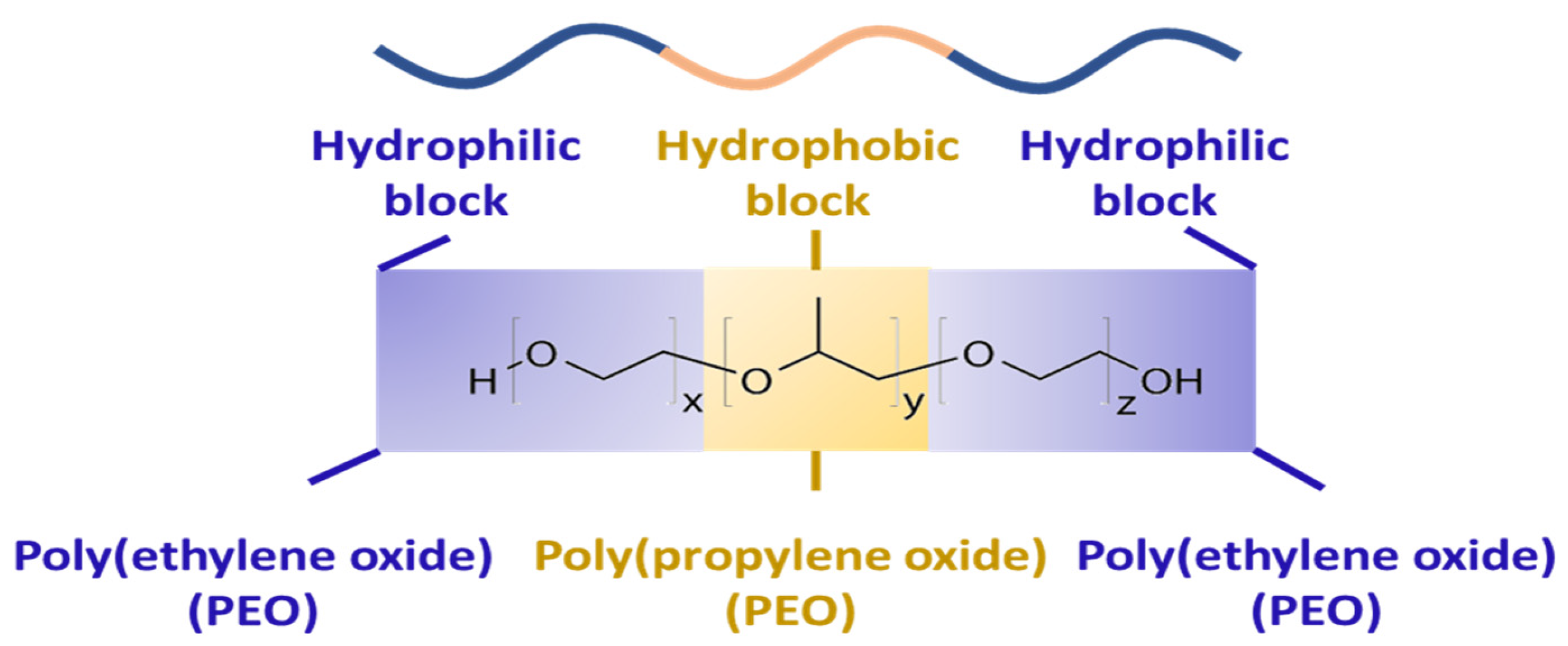
| Pluronic | Physical State | Mol. Weight (Average) | EO Segments (Each Segment; USP) | PO Segment (USP) | % Weight EO (USP) | Reference |
|---|---|---|---|---|---|---|
| F-68 | Solid | 7680–9510 | 12 | 27 | 81.8 ± 1.9 | [36] |
| F-127 | Solid | 9840–14,600 | 101 | 56 | 73.2 ± 1.7 | [36] |
| Pluronic-Based Nanomedicines | Drug Incorporated | Cancer Cell Treatment | Benefits | References |
|---|---|---|---|---|
| Pluronic F-127 and phenylboronic ester-grafted (PHE)-Pluronic P-123-based micelles | Doxorubicin | (MCF-7/ADR) Breast cancer cells | Doxorubicin efflux and detoxification for efficiently reversing breast cancer resistance | [109] |
| Pluronic P-123 modified with α-tocopheryl succinate-based micelles | Doxorubicin | (MCF-7/ADR) Breast cancer cells | Improved the delivery of fluorescent dyes and protein across the blood–brain barrier (BBB) | [110] |
| Pluronic F-127- and P-123-based micelles | Doxorubicin | (MCF-7/ADR) Breast cancer cells | Efficiently overcame MDR in breast cancer | [111] |
| Pluronic P-123-PEG2000-DSPE-based micelles | Doxorubicin | (MCF-7/ADR) Breast cancer cells | Enhanced tumor-suppressing effect on drug-resistant breast cancer cells | [112] |
| Pluronic P-127 and vitamin E-TPGS-based micelles | Resveratrol | (MCF-7 and MDA-MB-231) Breast cancer cells | Effective at selectively targeting aggressive forms of breast cancer | [113] |
| Pluronic F-127 conjugated to ALN-based micelles | Curcumin | Osteolytic tumors | Effective and targeted delivery of curcumin to osteolytic tumors in bone | [114] |
| Pluronic F-68-GL44 galactosylated-based micelles | Harmine | Liver cancer cells | Significant improvement in oral bioavailability of Harmine and drug targeting capability | [115] |
| Pluronic F-127/N,N,N trimethyl chitosan-based hydrogel | Docetaxel | (U87MG) Brain tumor cells | Enhancement in the sustained release behavior of DTX and inhibited the orthotropic glioblastoma tumor | [116] |
| Pluronic P-123-antiGPC3/TPGS-b-PCL Aptamer | Sorafenib | Hepatocellular carcinoma | Significantly improved the targeted therapy of liver cancer. | [117] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khaliq, N.U.; Lee, J.; Kim, S.; Sung, D.; Kim, H. Pluronic F-68 and F-127 Based Nanomedicines for Advancing Combination Cancer Therapy. Pharmaceutics 2023, 15, 2102. https://doi.org/10.3390/pharmaceutics15082102
Khaliq NU, Lee J, Kim S, Sung D, Kim H. Pluronic F-68 and F-127 Based Nanomedicines for Advancing Combination Cancer Therapy. Pharmaceutics. 2023; 15(8):2102. https://doi.org/10.3390/pharmaceutics15082102
Chicago/Turabian StyleKhaliq, Nisar Ul, Juyeon Lee, Sangwoo Kim, Daekyung Sung, and Hyungjun Kim. 2023. "Pluronic F-68 and F-127 Based Nanomedicines for Advancing Combination Cancer Therapy" Pharmaceutics 15, no. 8: 2102. https://doi.org/10.3390/pharmaceutics15082102